Abstract
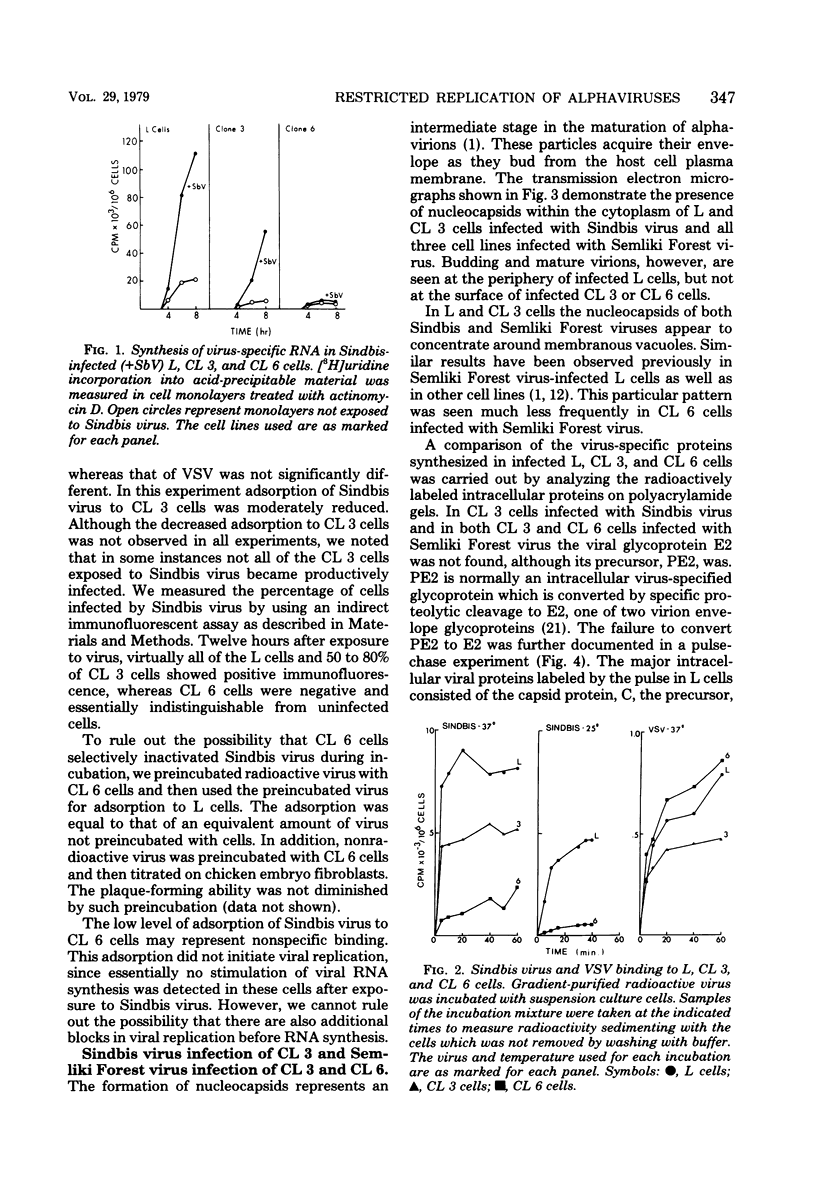
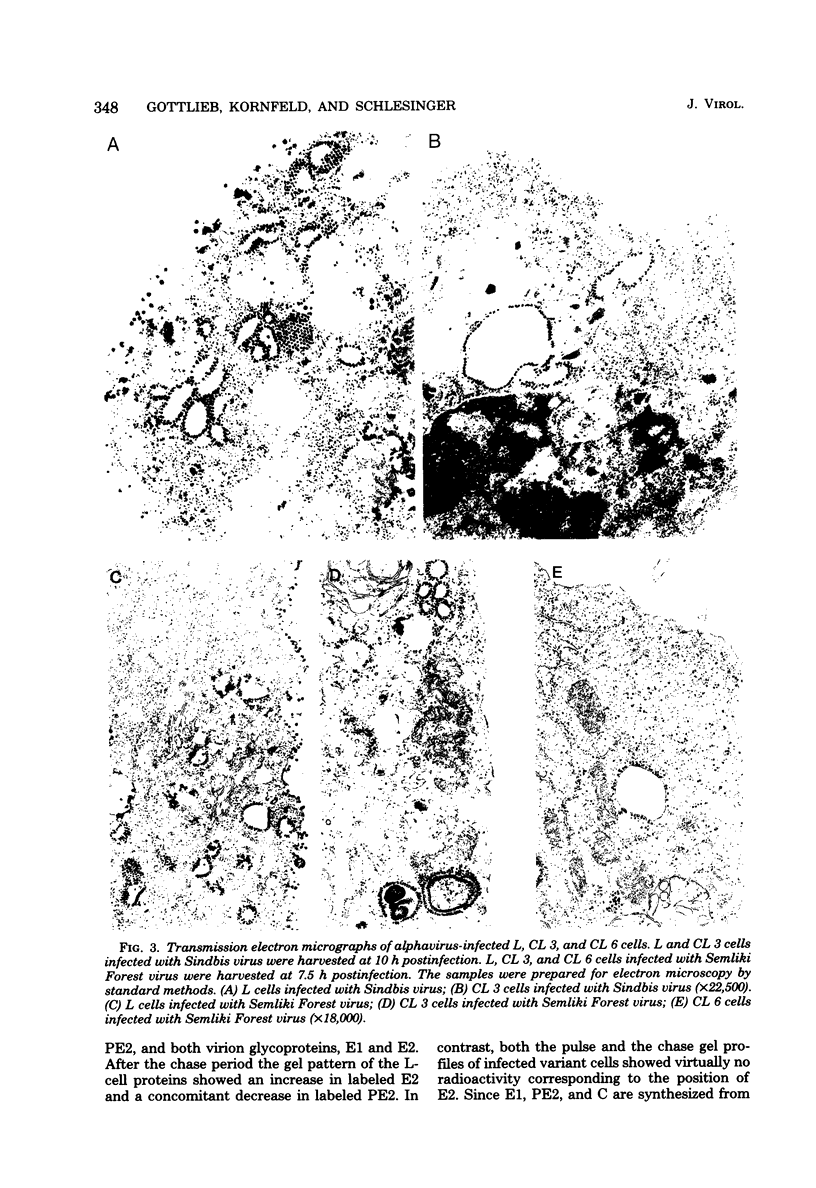
Two mouse L cell variant lines (CL 3 and CL 6) selected for resistance to the toxic plant lectin ricin were restricted in their ability to replicate the two alphaviruses Sindbis virus and Semliki Forest virus. CL 3 cells have been shown to exhibit increased CMP-sialic acid:glycoprotein sialyltransferase and GM3 synthetase activities, whereas CL 6 cells have been shown to contain decreased UDPgalactose:glycoprotein galactosyltransferase and UDP-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activities. The adsorption of Sindbis virus to CL 6 cells was considerably reduced, suggesting that the loss or inaccessibility of the receptors for Sindbis virus accounted for a major defect in virus production in these cells. In contrast, CL 3 synthesized Sindbis viral RNA and proteins but were unable to convert the precursor glycoprotein PE2 to the structural protein E2. The cleavage of PE2 to E2 was also blocked in both CL 3 and CL 6 cells infected with Semliki Forest virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Defects in RNA+ temperature-sensitive mutants of Sindbis virus and evidence for a complex of PE2-E1 viral glycoproteins. Virology. 1976 Oct 15;74(2):441–449. doi: 10.1016/0042-6822(76)90350-0. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Inhibition of Sindbis virus replication by zinc ions. Virology. 1976 Jul 1;72(1):272–277. doi: 10.1016/0042-6822(76)90330-5. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Smith J. F. Morphology of BHK-21 Cells Infected with Sindbis Virus Temperature-Sensitive Mutants in Complementation Groups D and E. J Virol. 1975 May;15(5):1262–1266. doi: 10.1128/jvi.15.5.1262-1266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Friedman R. M. A large glycoprotein of Moloney leukemia virus derived from interferon-treated cells. Biochem Biophys Res Commun. 1977 Jul 11;77(1):392–398. doi: 10.1016/s0006-291x(77)80210-6. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Jay F. T., Friedman R. M. Physical, morphological, and biochemical alterations in the membrane of AKR mouse cells after interferon treatment. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1859–1863. doi: 10.1073/pnas.75.4.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda E., Schlesinger M. J. Alterations in Sindbis viral enbelope proteins by treating BHK cells with glucosamine. J Virol. 1975 Feb;15(2):416–419. doi: 10.1128/jvi.15.2.416-419.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus grown in four mammalian cell lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4011–4014. doi: 10.1073/pnas.71.10.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Simons K., Renkonen O. Isolation and characterization of the membrane proteins of Semliki Forest virus. Virology. 1974 Oct;61(2):493–504. doi: 10.1016/0042-6822(74)90285-2. [DOI] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Kornfeld S. Isolation and characterization of two mouse L cell lines resistant to the toxic lectin ricin. J Biol Chem. 1976 Dec 25;251(24):7761–7768. [PubMed] [Google Scholar]
- Grimley P. M., Levin J. G., Berezesky I. K., Friedman R. M. Specific membranous structures associated with the replication of group A arboviruses. J Virol. 1972 Sep;10(3):492–503. doi: 10.1128/jvi.10.3.492-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Waite M. R., Bose H. R. Cleavage of a viral envelope precursor during the morphogenesis of Sindbis virus. J Virol. 1974 Apr;13(4):809–817. doi: 10.1128/jvi.13.4.809-817.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza G. Effect of impaired glycosylation on the biosynthesis of Semliki forest virus glycoproteins. J Virol. 1975 Sep;16(3):602–612. doi: 10.1128/jvi.16.3.602-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K., Sefton B., Burke D. Sindbis virus glycoproteins: effect of the host cell on the oligosaccharides. J Virol. 1975 Sep;16(3):613–620. doi: 10.1128/jvi.16.3.613-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J. Virus envelopes and plasma membranes. Annu Rev Biophys Bioeng. 1978;7:139–165. doi: 10.1146/annurev.bb.07.060178.001035. [DOI] [PubMed] [Google Scholar]
- Muller L. L., Jacks T. J. Rapid chemical dehydration of samples for electron microscopic examinations. J Histochem Cytochem. 1975 Feb;23(2):107–110. doi: 10.1177/23.2.1117127. [DOI] [PubMed] [Google Scholar]
- Robertson M. A., Etchison J. R., Robertson J. S., Summers D. F., Stanley P. Specific changes in the oligosaccharide moieties of VSV grown in different lectin-resistnat CHO cells. Cell. 1978 Mar;13(3):515–526. doi: 10.1016/0092-8674(78)90325-2. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Gottlieb C., Feil P., Gelb N., Kornfeld S. Growth of enveloped RNA viruses in a line of chinese hamster ovary cells with deficient N-acetylglucosaminyltransferase activity. J Virol. 1975 Jan;17(1):239–246. doi: 10.1128/jvi.17.1.239-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Keegstra K. Glycoproteins of Sindbis virus: priliminary characterization of the oligosaccharides. J Virol. 1974 Sep;14(3):522–530. doi: 10.1128/jvi.14.3.522-530.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Keränen S., Käriänen L. Identification of a precursor for one of the Semliki forest virus membrane proteins. FEBS Lett. 1973 Jan 15;29(2):87–91. doi: 10.1016/0014-5793(73)80532-0. [DOI] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Symington J., Schlesinger M. J. Isolation of a Sindbis virus variant by passage on mouse plasmacytoma cells. J Virol. 1975 Apr;15(4):1037–1041. doi: 10.1128/jvi.15.4.1037-1041.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]