Abstract
Miltefosine (hexadecylphosphocholine [HePC]) has proved to be a potent oral treatment for human visceral leishmaniasis due to Leishmania donovani. The molecular mechanisms that contribute to the antileishmanial activity of HePC are still unknown. We report that in wild-type promastigotes of Leishmania donovani HePC is able to induce a cell death process with numerous cytoplasmic, nuclear, and membrane features of metazoan apoptosis, including cell shrinkage, DNA fragmentation into oligonucleosome-sized fragments, and phosphatidylserine exposure. None of these changes were detected in an HePC-resistant clone treated with the same drug concentration. Therefore, HePC does not appear to kill L. donovani promastigotes by a direct toxic mechanism but, rather, kills the promastigotes by an indirect one. Pretreatment of wild-type promastigotes with two broad caspase inhibitors, z-Val-Ala-dl-Asp(methoxy)-fluoromethylketone and Boc-Asp(methoxy)-fluoromethylketone, as well as a broad protease inhibitor, calpain inhibitor I, prior to drug exposure interfered with DNA fragmentation but did not prevent cell shrinkage or phosphatidylserine externalization. These data suggest that at least part of the apoptotic machinery operating in wild-type promastigotes involves proteases. Identification of the death-signaling pathways activated in HePC-sensitive parasites appears to be essential for a better understanding of the molecular mechanisms of action and resistance in these parasites.
The protozoan parasites of the genus Leishmania are the causative agents of leishmaniasis, a complex of parasitic diseases that affect 12 million people worldwide and that threaten 350 million people worldwide. The pathology is manifested in visceral, mucocutaneous, or cutaneous forms. Visceral leishmaniasis (VL), the most severe form (which is usually fatal if patients are untreated), which is due to Leishmania donovani, is common in less developed countries, with an estimated 500,000 new cases each year (11, 20). Moreover, cases of Leishmania and human immunodeficiency virus coinfection are expected to rise in many countries, especially in sub-Saharan Africa and Asia, where highly active antiretroviral therapy is not available (39, 27). In the Indian subcontinent, a major area of endemicity of L. donovani VL, there is a worrying increment of resistance to pentavalent antimonial drugs, with more than 50% of infected people being unresponsive (52). In addition, the drugs recommended for the treatment of VL (pentavalent antimonials, amphotericin B, lipid formulations of amphotericin B) have limitations, including parenteral administration, long courses of treatment, toxic side effects, and high costs (20). Recently, miltefosine (hexadecylphosphocholine [HePC]), an alkylphosphocholine originally developed as an anticancer drug and currently used for topical treatment (Miltex) of skin metastases of breast cancer (59), has been proved to be the first effective and safe oral treatment (Impavido) for Indian VL, with cure rates of about 98% (25, 54). Moreover, HePC has been successfully used to treat patients with antimony-resistant VL (53, 55) as well as cutaneous leishmaniasis (51).
Numerous in vitro and in vivo experimental studies have shown that HePC is cytotoxic for both the promastigote and the amastigote stages of various species of Leishmania (7, 8, 15, 16, 29, 31, 58) as well as other protozoan parasites, including Trypanosoma cruzi (8, 32, 46, 47), Trypanosoma brucei (8, 28), Entamoeba histolytica (48), and Acanthamoeba spp. (64). Although the mechanism of the antiprotozoal activity of HePC is poorly understood, several hypotheses have been formulated, such as damage to the flagellar membrane (46), perturbation of alkyl-lipid metabolism and glycosylphosphatidylinositol anchor biosynthesis (33), interference with ether-lipid remodeling through the inhibition of the alkyl-lyso-phosphatidylcholine specific acyl coenzyme A acyltransferase (34), and inhibition of the de novo synthesis of phosphatidylcholine (32).
The antineoplastic activity of HePC has been linked to its capacity to induce apoptosis in numerous tumor cell lines (14, 22, 43, 45). HePC accumulates at the plasma membrane, due to its amphiphilic properties, thus affecting membrane fluidity (18, 61); but the molecular mechanisms implicated in its initiation of apoptotic signaling remain undefined. Suggested pathways include inhibition of phosphatidylcholine biosynthesis by depression of the translocation of CTP:choline phosphate cytidylyltransferase to membranes (5, 19, 21), a sustained increase in Ca2+ levels via calcium channels (23, 65), inhibition of sphingomyelin biosynthesis leading to increased levels of proapoptotic ceramide (67), mitogenic MAPK/ERK signaling inhibition, and proapoptotic SAPK/JNK pathway activation (44).
Apoptosis, a form of programmed cell death (PCD), is a genetically regulated physiological process of cell suicide that is central to the development, homeostasis, and integrity of multicellular organisms (2). Initially, it was assumed that PCD emerged with multicellularity and would have been counterselected in unicellular organisms (41). Recently, several studies have demonstrated that a process of PCD also operates in 10 different single-celled eukaryotic organisms whose phylogenetic origins arose 1 billion to 2 billion years ago (1, 6, 10, 42, 62, 66), including various species of Leishmania (4, 24, 35, 50, 69), among which is L. donovani (9, 30, 37). These studies have also shown that this type of cell death in kinetoplastids, slime mold, ciliates, and dinoflagellates induces some of the structural alterations characteristic of apoptosis in multicellular organisms.
The aim of the present work was to assess more precisely the cell death process induced by HePC in L. donovani promastigotes. We report that dying parasites share numerous cytoplasmic, nuclear, and membrane features with apoptotic metazoan cells, including cell shrinkage, DNA fragmentation into oligonucleosome-sized fragments, and phosphatidylserine exposure. Our findings also suggest that proteases are involved, at least in part, in the death machinery operating in L. donovani promastigotes in response to HePC.
MATERIALS AND METHODS
Materials
HePC (miltefosine) was obtained from Zentaris (Frankfurt, Germany). Adenosine, chloroform-isoamyl alcohol (24:1), dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), ethanol, hemin, medium 199, phenol, propidium iodide (PI), proteinase K, and trans-epoxysuccinyl-l-leucylamido-(4-guanido)butane (E64) were purchased from Sigma (St. Quentin Fallavier, France). Fetal bovine serum and HEPES buffer were purchased from Invitrogen (Eragny, France). The Annexin-V-FLUOS staining kit was obtained from Roche (Meylan, France). RNase A was from Amersham Biosciences (Orsay, France). z-Val-Ala-dl-Asp(methoxy)-fluoromethylketone (z-VAD-fmk) was from Bachem (Voisins le Bretonneux, France). Boc-Asp(methoxy)-fluoromethylketone (Boc-D-fmk; caspase inhibitor III) was from VWR International (Fontenay sous Bois, France). Calpain inhibitor I and lactacystin were from SA Coger (Paris, France).
In vitro culture of parasites
Promastigote forms of wild-type (WT) L. donovani (strain MHOM/ET/67/HU3/L82) and a derivative line, which is resistant to 40 μM HePC (designated HePC-R40) (49), were kindly provided by Simon L. Croft (Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom) and were grown in medium 199 supplemented with 40 mM HEPES, 100 μM adenosine, 0.5 mg of hemin per liter, 10% heat-inactivated fetal bovine serum, and 50 μg of gentamicin per ml at 26°C in a dark environment; and HePC-R40 promastigotes were continuously maintained in the presence of 40 μM HePC. All experiments were performed with parasite cultures in the logarithmic phase of growth.
Analysis of drug sensitivity by colorimetric MTT assay
Promastigotes, harvested in the exponential growth phase, were resuspended in fresh medium to achieve 106 parasites/ml and were seeded in 96-well culture plates. HePC, which was freshly prepared as a 10 mM stock solution before each experiment, was added in triplicate at final dilutions ranging from 1 to 100 μM. The plates were incubated at 26°C for 72 h in a 5% CO2 atmosphere, and promastigote viability was evaluated by using the quantitative colorimetric MTT assay. The conversion of MTT to the formazan product by the mitochondrial electron transport chain is an indicator of cell viability, and a decrease in the amount of MTT converted indicates toxicity to the cell. Briefly, the MTT labeling reagent (final concentration, 0.25 mg/ml) was added to each well; and after a 4-h incubation at 26°C, DMSO was added to dissolve the formazan crystals and obtain a homogeneous blue solution suitable for measurement of the absorbance with an enzyme-linked immunosorbent assay plate reader (wavelength, 540 nm). The percentage of surviving promastigotes versus the number of surviving control promastigotes was assessed by the formula 100 × (absorbance of treated cells/absorbance of control cells). The 50% inhibitory concentration (IC50), i.e., the drug concentration that decreases the rate of cell growth by 50%, was calculated by regression analysis; and the results are expressed as the means and standard deviations of five independent experiments.
DNA fragmentation assay by agarose gel electrophoresis
Qualitative analysis of DNA fragmentation was performed by agarose gel electrophoresis of total genomic DNA extracted from untreated and HePC-treated promastigotes. Cell pellets consisting of 108 promastigotes were lysed in Sarkosyl buffer (50 mM Tris, 10 mM EDTA, 0.5% [wt/vol] sodium-N-lauryl sarcosine [pH 7.5]) with proteinase K (100 μg/ml), vortexed, and allowed to be digested overnight at 50°C. RNase A (248 U/ml) was then added (1 h, 37°C), and the lysates were extracted with phenol-chloroform-isoamyl alcohol (25:24:1) and centrifuged at 16,000 × g for 5 min. The supernatants were treated with 0.1 volume of 3 M sodium acetate and 2 volumes of ethanol overnight at −20°C, spun at 16,000 × g for 10 min, and washed in 95% ethanol. The DNA was air dried, dissolved in 10 mM Tris-1 mM EDTA buffer, and quantified spectrophotometrically at 260/280 nm. The genomic DNA (10 μg) was run on a 2% agarose gel containing ethidium bromide for 1 h at 100 V and visualized under UV light.
Detection of phosphatidylserine exposure
Double staining for annexin V-fluorescein isothiocyanate (FITC)-PI was performed with the Annexin-V-FLUOS staining kit. In brief, untreated or HePC-treated promastigotes were washed twice in cold phosphate-buffered saline (PBS; 1.5 mM KH2PO4, 8.1 mM Na2HPO4, 136.9 mM NaCl, 2.6 mM KCl [pH 7.2]) and centrifuged at 14,000 × g for 10 min. The pellets were resuspended in 100 μl of annexin V-FITC in the presence of PI according to the instructions of the manufacturer. After 15 min of incubation in the dark at 26°C, samples were observed under a fluorescence microscope (magnification, ×400; Ariston; Leitz, Wetzlar, Germany). Alternatively, the intensity of annexin V-FITC labeling was recorded on a FACSCalibur flow cytometer (Becton Dickinson) and analyzed with CellQuest software, and the percentage of positive cells was assessed for each histogram.
Flow cytometry analysis of cell cycle
Untreated or HePC-treated promastigotes were washed twice in PBS (pH 7.2). Pelleted cells were fixed in 70% cold ethanol and incubated overnight at −20°C. After two washes in PBS, the promastigotes were resuspended in 0.5 ml of PI (100 μg/ml in PBS) containing RNase A (248 U/ml), and the mixture was incubated for 20 min in the dark at room temperature. The fluorescence intensity of PI was analyzed with a FACSCalibur flow cytometer and CellQuest software.
Protease inhibitors
Stock solutions of Boc-D-fmk (20 mM), z-VAD-fmk (20 mM), and lactacystin (1 mM) were prepared in DMSO; a stock solution of calpain inhibitor I (20 mM) was prepared in 100% ethanol; and a stock solution of E64 (20 mM) was prepared in water. The promastigotes were incubated for 2 h in the presence of various final concentrations of inhibitors or solvent alone prior to the addition of HePC.
RESULTS
Cytotoxic effect of HePC on L. donovani WT and HePC-R40 promastigotes
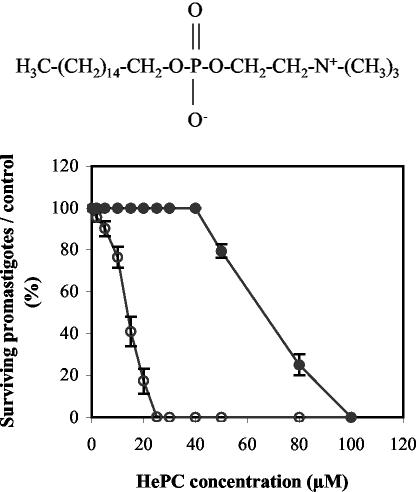
The effect of HePC (the chemical structure is shown in the top panel of Fig. 1) on L. donovani promastigote viability was assessed by the quantitative colorimetric MTT assay after 3 days of incubation. Treatment with HePC resulted in a concentration-dependent inhibition of WT promastigote viability (Fig. 1, bottom), with an IC50 of 13.6 ± 2.0 μM. In contrast, HePC at concentrations up to 40 μM had no cytotoxic effect on clone HePC-R40. This resistance, however, was not absolute, since doses of HePC higher than the 40 μM in which this clone is regularly maintained were found to be cytotoxic (Fig. 1, bottom), with an IC50 of 69.1 ± 1.1 μM.
FIG. 1.
(Top) Chemical structure of HePC; (bottom) cytotoxic activity of HePC on L. donovani promastigotes. The viability of WT (open circles) and HePC-R40 (closed circles) parasites after a 3-day incubation in the presence of graded concentrations of HePC was assessed by the colorimetric MTT assay and is expressed as the percentage of surviving promastigotes compared to the number of surviving untreated cells. Data are the means of five independent experiments performed in triplicate, and standard deviations are represented by error bars.
HePC induces cell shrinkage in L. donovani WT promastigotes
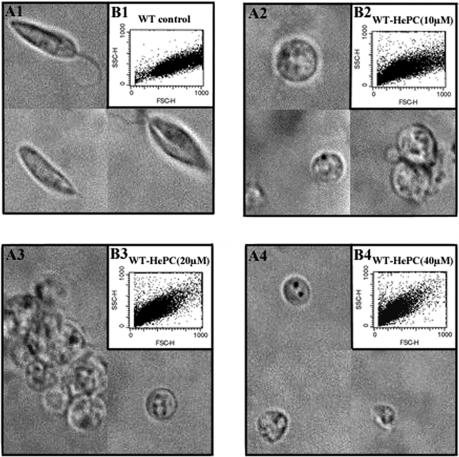
A visual inspection by light microscopy revealed that HePC induces morphological changes in WT promastigotes compared to the morphology of the untreated control group. WT promastigotes cultured for 24 h in fresh complete medium had elongated forms (Fig. 2A1). Cells exposed to 10 μM (Fig. 2A2), 20 μM (Fig. 2A3), or 40 μM (Fig. 2A4) HePC for 24 h showed rounded forms and cell shrinkage, particularly at the higher dose. Moreover, flow cytometry analysis of cell size confirmed that HePC causes cell shrinkage in WT promastigotes, measured by the decrease in forward scatter (compare Fig. 2B2, B3, and B4 to Fig. 2B1), which was more pronounced with higher doses of HePC. No rounding or shrinkage was observed in the resistant clone exposed to similar doses of HePC (data not shown).
FIG. 2.
Morphology and cell size of L. donovani WT promastigotes cultured for 24 h in fresh complete medium at 26°C in the absence or presence of different concentrations of HePC. The results of light microscopy (magnification, ×1,000) (A) and flow cytometry analysis (B) of the cell sizes of WT promastigotes either untreated (A1 and B1) or exposed to 10 μM (A2 and B2), 20 μM (A3 and B3), or 40 μM HePC (A4 and B4) are shown.
HePC induces DNA fragmentation in L. donovani WT promastigotes
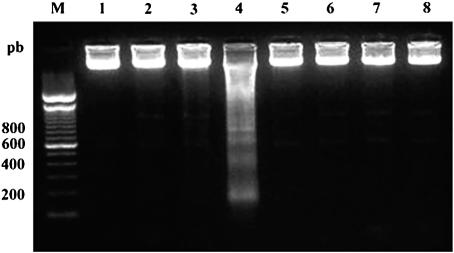
In mammalian cells, internucleosomal DNA fragmentation is one of the typical nuclear features of apoptosis (68). DNA analysis by agarose gel electrophoresis revealed DNA fragmentation into oligonucleosome-sized fragments (in multiples of 200 bp) in WT promastigotes treated with 40 μM HePC for 24 h (Fig. 3, lane 4), whereas untreated cells did not show any DNA fragmentation (Fig. 3, lane 1). No DNA fragmentation was visible in WT promastigotes treated with lower doses of HePC (10 or 20 μM) for 24 h (Fig. 3, lanes 2 and 3, respectively). Finally, no DNA fragmentation was detected in clone HePC-R40, either untreated or treated with 20, 40, or 80 μM HePC (Fig. 3, lanes 5, 6, 7, and 8, respectively).
FIG. 3.
DNA fragmentation analysis by agarose gel electrophoresis. The DNA profiles for untreated or HePC-treated L. donovani promastigotes after 24 h of incubation at 26°C are shown. Lane M, molecular size marker (pb, base pairs). WT promastigotes were untreated (lane 1) or were exposed to 10 μM (lane 2), 20 μM (lane 3), or 40 μM HePC (lane 4); HePC-R40 promastigotes were untreated (lane 5) or were exposed to 20 μM (lane 6), 40 μM (lane 7), or 80 μM HePC (lane 8). The results are representative of those from three independent experiments.
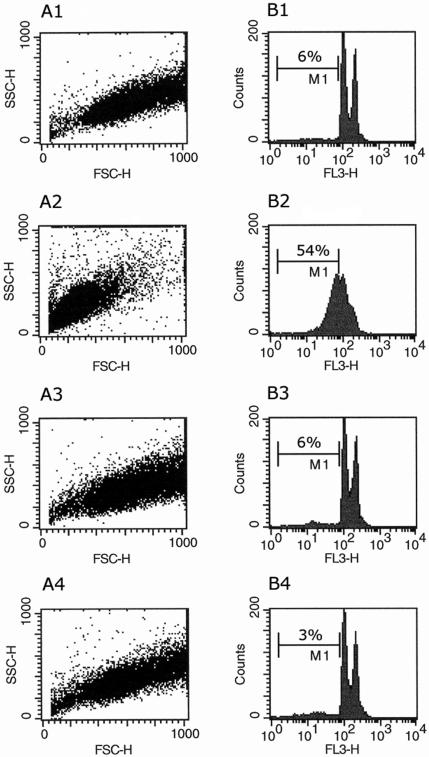
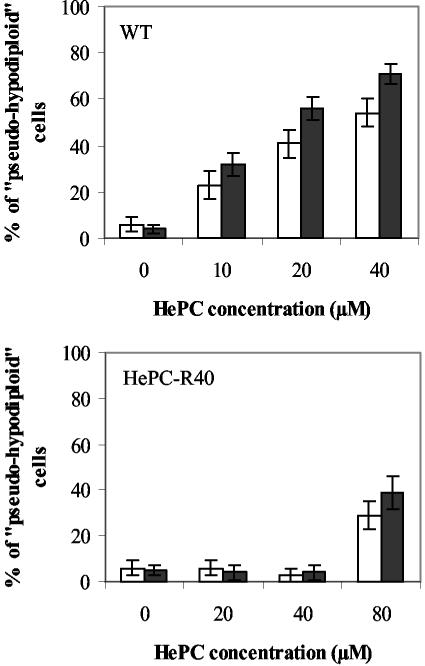
Flow cytometry analysis after cell permeabilization and labeling with PI was used to quantify the percentage of pseudohypodiploid cells. The amount of bound dye correlates with the DNA content in a given cell, and DNA fragmentation in apoptotic cells translates into a fluorescence intensity lower than that of G1 cells (sub-G1 peak) (38). After 24 h of incubation of WT promastigotes with 40 μM HePC, 54% of the cells were found in the sub-G1 peak region (Fig. 4B2) but only 6% of the control cells were found in the sub-G1 peak region (Fig. 4B1), thus confirming that HePC had induced DNA degradation in these cells. In contrast, no DNA degradation (Fig. 4B4) or cell shrinkage (Fig. 4A4) was observed in HePC-R40 promastigotes treated with the same dose of HePC for 24 h. HePC-induced DNA fragmentation, as quantified by flow cytometry in WT promastigotes, was concentration and time dependent (Fig. 5, top). Whereas HePC concentrations up to 40 μM did not induce alterations in the DNA of the resistant clone, a higher dose (80 μM) was able to cause DNA fragmentation in a moderate number of these cells (29% of pseudohypodiploid cells at 24 h and 39% of pseudohypodiploid cells at 48 h) (Fig. 5, bottom).
FIG. 4.
Cell size and analysis of DNA content by flow cytometry of untreated or HePC-treated L. donovani promastigotes after 24 h of incubation at 26°C. The light-scattering properties (A) and the DNA fragmentation (B) of WT promastigotes untreated (A1 and B1) or exposed to 40 μM HePC (A2 and B2) and of HePC-R40 promastigotes untreated (A3 and B3) or exposed to 40 μM HePC (A4 and B4) are shown. Cells containing amounts of DNA found in the sub-G1 peak region (pseudohypodiploid cells) are in region M1, and their percentages were calculated for each histogram. The data are representative of those from at least six independent experiments. SSC-H, side scatter; FSC-H, forward scatter; FL3-H, fluorescence intensity.
FIG. 5.
DNA fragmentation analysis by flow cytometry. The percentages of pseudohypodiploid cells of either WT promastigotes (top) or HePC-R40 promastigotes (bottom) were determined after 24 h (open bars) or 48 h (closed bars) of incubation at 26°C in the absence or presence of different concentrations of HePC. The data are the means of five independent experiments, and standard deviations are represented by errors bars.
HePC induces phosphatidylserine externalization in L. donovani WT promastigotes
During the early stages of mammalian cell apoptosis, an ubiquitous alteration is the translocation of phosphatidylserine from the inner side to the outer layer of the plasma membrane. Annexin V, a Ca2+-dependent phospholipid-binding protein with a high affinity for phosphatidylserine, is routinely used in a fluorescein-conjugated form to label externalized phosphatidylserine. Since annexin V-FITC can also label necrotic cells following the loss of membrane integrity, simultaneous addition of PI, which does not permeate cells with an intact plasma membrane, allows the discrimination between apoptotic cells (annexin V positive, PI negative), necrotic cells (annexin positive, PI positive), and surviving cells (annexin V negative, PI negative) (63). Observation by fluorescence microscopy of WT promastigotes treated with 40 μM HePC for 24 or 48 h indicated that rounded, condensed cells were labeled with annexin V-FITC, whereas untreated cells were negative (data not shown). Importantly, HePC exposure did not induce necrotic features, even after a prolonged incubation, as all the cells remained negative for PI. In contrast, no apoptotic or necrotic cells were detected in clone HePC-R40, either untreated or treated with the same dose of HePC for 24 or 48 h (data not shown).
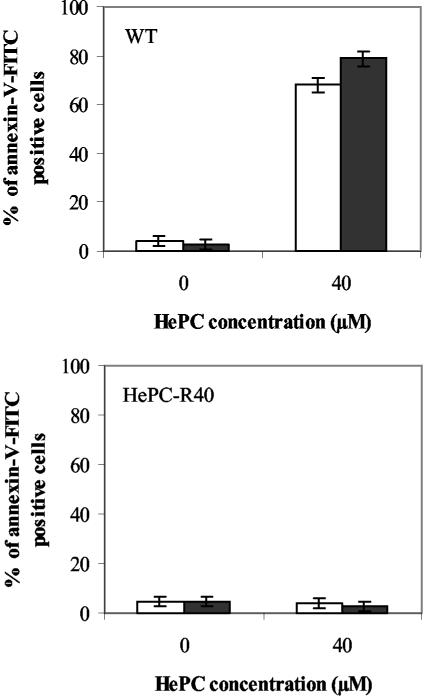
Flow cytometry analysis after labeling with annexin V-FITC was used to quantify the percentage of cells presenting this apoptotic feature (Fig. 6). Given the lack of PI labeling observed by fluorescence microscopy, PI was not used in these experiments. After 24 h of incubation of WT promastigotes with 40 μM HePC, 68% of the treated cells but only 4% of the control cells were annexin V-FITC positive (Fig. 6, top); and after 48 h the proportion of annexin V-FITC-positive cells increased to 79%, whereas no change was detected in the control group (3%). In contrast, no induction of phosphatidylserine exposure was observed in HePC-R40 promastigotes treated with the same dose of HePC for 24 or 48 h (Fig. 6, bottom).
FIG. 6.
Phosphatidylserine exposure analysis by flow cytometry. The percentages of annexin V-FITC-positive WT promastigotes (top) or HePC-R40 promastigotes (bottom) were determined after 24 h (open bars) or 48 h (closed bars) of incubation at 26°C in the absence or presence of 40 μM HePC. The data are the means of three independent experiments, and standard deviations are represented by errors bars.
Effect of protease inhibitors on HePC-induced DNA fragmentation in L. donovani WT promastigotes
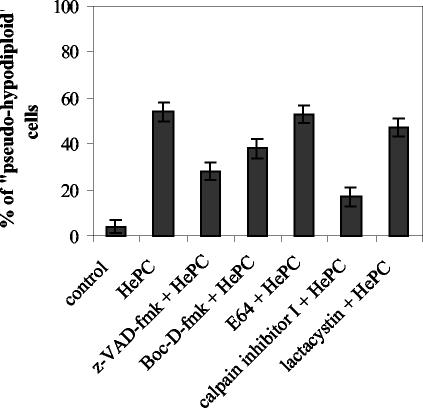
The family of cysteine proteases known as caspases, which specifically cleave their substrates after an aspartate residue, are the major effectors of apoptosis in metazoans (57), although other proteases have been also reported to be involved: cysteine proteases such as calpains and cathepsins, serine proteases, and the proteasome (26). As a first attempt to determine whether proteases could be involved in the induction of apoptosis-like death by HePC, two cell-permeant, irreversible, and specific caspase inhibitors (z-VAD-fmk [17] and Boc-D-fmk [12]), a specific calpain inhibitor (E64), a broad protease inhibitor (calpain inhibitor I), and an irreversible proteasome inhibitor (lactacystin) were used. Incubation with any of these inhibitors at the concentration used did not cause any cell alteration (data not shown). Microscopy and flow cytometry analysis showed that none of the inhibitors protected WT promastigotes against cell shrinkage or phosphatidylserine exposure induced by HePC (data not shown). However, quantitative analysis of pseudohypodiploid cells indicated that whereas E64 and lactacystin had a negligible effect, the presence of caspase inhibitors reduced the percentage of cells present in the sub-G1 peak region (Fig. 7). This effect was even more pronounced with calpain inhibitor I (Fig. 7).
FIG. 7.
Effects of protease inhibitors on HePC-induced DNA fragmentation in WT promastigotes. Parasites were incubated for 2 h in the presence or absence of the protease inhibitors z-VAD-fmk, Boc-D-fmk, or E64 (100 μM), calpain inhibitor I (20 μM), or lactacystin (50 μM) prior to the addition of HePC (40 μM). Promastigotes were incubated for an additional 24 h at 26°C. The percentage of pseudohypodiploid cells was determined by flow cytometry analysis. The data are the means of three independent experiments, and standard deviations are represented by errors bars.
DISCUSSION
In this work we show that addition of HePC to L. donovani WT promastigotes induces a type of death that shares most of the features associated with metazoan apoptosis, i.e., cell shrinkage, DNA oligonucleosomal digestion, and phosphatidylserine exposure with preservation of plasma membrane integrity. Interestingly, a clone derived from the same strain by selective pressure and resistant to HePC, clone HePC-R40, did not display any apoptosis-like changes when it was exposed to HePC concentrations sufficient to kill the WT strain (up to the 40 μM used for selection). From these data, we can conclude that HePC does not kill L. donovani by a direct mechanism, which would lead to necrotic cell death, but, rather, kills L. donovani by an indirect one, akin to apoptotic cell death. The existence of a form of PCD with apoptotic features has already been reported in various unicellular organisms, including Leishmania. Several agents or nutrient deficiencies known to induce apoptosis in higher eukaryotes were found to cause apoptosis-like changes in L. donovani promastigotes (hydrogen peroxide [9, 37], nutrient deprivation [30]) and Leishmania major promastigotes (staurosporine [4], serum removal [69]). Similar observations were made for another antileishmanial drug, amphotericin B, in L. donovani promastigotes (30). In most of these studies, as well as other published work on apoptosis in kinetoplastids, all the criteria defined in mammalian systems are not present. In this respect, HePC seems to induce a rather typical apoptotic phenotype in L. donovani.
L. donovani WT promastigotes exposed to HePC acquired rounded forms and displayed cell shrinkage, reminiscent of the cytoplasmic condensation detected in apoptotic cells of multicellular organisms. Digestion of DNA into oligonucleosomal fragments, a hallmark of classical apoptosis, was also found to occur in a concentration- and a time-dependent manner during drug exposure in WT promastigotes; and the percentage of cells undergoing this chromatin alteration, pseudohypodiploid cells, was assessed by flow cytometry. While staurosporine has been shown to cause some form of DNA degradation in L. major promastigotes, oligonucleosomal fragmentation was not detected (4). This typical nuclear feature, however, has been reported to occur in amphotericin B-treated L. donovani promastigotes and was associated with a decrease in the mitochondrial membrane potential (30). In the latter study, an early increase in plasma membrane permeability was observed, whereas membrane integrity is conserved during classical metazoan apoptosis. HePC-induced apoptotic cells do not undergo any major change in membrane permeability, even after prolonged incubation. In addition, during HePC treatment, translocation of phosphatidylserine residues to the outer layer of the plasma membrane, one of the most characteristic features of apoptotic cell death, was observed in dying promastigotes. Therefore, HePC appears to be remarkable in its ability to trigger in L. donovani promastigotes most of the changes associated with classical apoptosis. Taken together with the results of the studies mentioned above, our results imply that the components of the apoptotic machinery activated in Leishmania could vary according to the strain or the inducer used.
The biochemical pathways that mediate apoptosis-like death in kinetoplastids are still unknown. Caspases, a specific family of cysteine proteases, are the major components of the proteolytic machinery that mediate apoptosis in mammalian cells (57). The involvement of other cysteine proteases such as calpains and cathepsins, as well as serine proteases or the proteasome, has also been suggested (26). We used several inhibitors to investigate whether proteases could be involved in the apoptosis-like death observed in HePC-treated WT promastigotes. Treatment of L. donovani promastigotes with two cell-permeant and irreversible broad-spectrum caspase inhibitors, z-VAD-fmk and Boc-D-fmk, prevented DNA digestion but had no effect on the cytoplasmic (cell shrinkage) or membrane (phosphatidylserine exposure) features of apoptosis. Similar observations were made in L. major promastigotes exposed to staurosporine (4). These results suggest that nuclear events are under the control of proteases sensitive to caspase inhibitors. Indeed, caspases are directly implicated in the activation of the endonuclease that mediates oligonucleosomal DNA fragmentation in mammalian cells (13). However, to date no caspase homolog has been found in any unicellular eukaryote (3, 60). Recently, genes encoding for metacaspases, which belong to an ancestral metacaspase, paracaspase, and caspase superfamily, have been identified in numerous nonmetazoan eukaryotes (3, 60). Little is known about the function of these metacaspases, and their potential sensitivity to classical caspase inhibitors has not been tested. Five metacaspases have been cloned in T. brucei (56), and sequence alignment has revealed the existence of a comparable gene in L. major (36). Most interestingly, heterologous expression of a T. brucei metacaspase in yeast resulted in growth inhibition, mitochondrial dysfunction, and clonal cell death (56). The possibility that a metacaspase in L. donovani might be the target of caspase inhibitors deserves further investigation.
HePC-induced DNA digestion was not reduced in the presence of the broad-spectrum calpain and cysteine protease inhibitor E64. This result is at variance with the E64-mediated protection found in staurosporine-treated L. major promastigotes (4). Likewise, a proteasome inhibitor, lactacystin, was found to be ineffective in abrogating HePC-induced oligonucleosomal DNA fragmentation. However, lactacystin has been reported to be poorly permeant in Leishmania mexicana promastigotes compared to its ability to permeate amastigotes (40). If the same holds true for L. donovani, the use of an alternative proteasome inhibitor is clearly required before any final conclusion concerning a lack of involvement of this protease complex can be made. Calpain inhibitor I, which inhibits calpains, cathepsins, neutral cysteine proteases, as well as the proteasome, was the most efficient compound among all those tested and was capable of interfering with DNA fragmentation. These data further imply that proteases are most likely involved in the molecular signaling leading to nuclear changes, but the broad spectrum of activity of these inhibitors precludes any clear identification of their nature. Identification of the endonucleases(s) that mediates DNA oligonucleosomal fragmentation in L. donovani promastigotes could be helpful in defining the identities of the proteases responsible for their activation. A recent study (69) reported on the detection of 45- to 59-kDa nucleases with internucleosomal activities in L. major. Finally, as was the case for z-VAD-fmk, calpain inhibitor I had no preventive effect on cell shrinkage, indicating that inhibition of DNA fragmentation might not be sufficient for efficient cell protection. Thus, we are trying to determine whether a protease inhibitor capable of interfering with cell condensation and/or phosphatidylserine exposure can be found.
In conclusion, our findings indicate that HePC induces an apoptosis-like death in L. donovani WT promastigotes and that proteases are part of the cell death machinery. Better knowledge of the mechanism of action of HePC in L. donovani WT promastigotes, in addition to the identification of the major pathways involved in Leishmania apoptosis-like death, should be useful for elucidation of the molecular events underlying the resistance of the HePC-R40 clone. Moreover, given the impressive efficacy of HePC (Impavido) in clinical trials, HePC has become the major treatment option for VL. Promastigotes, which develop in the midgut of the insect vector, are not the clinical target of HePC, and extension of these studies to the amastigote form of L. donovani will allow us to better assess the relevance of our results.
Acknowledgments
This work was supported by EC grant QLRT-2000-01404.
We are grateful to Zentaris for providing the HePC used in this study and to Simon L. Croft for providing the promastigote forms of L. donovani WT (MHOM/ET/67/L82) and the derivative HePC-resistant line (HePC-R40).
REFERENCES
- 1.Ameisen, J. C. 1995. Apoptosis in a unicellular eukaryotes (Trypanosoma cruzi): implications for the evolutionary origin and role of programmed cell in the control of cell proliferation, differentiation and survival. Cell Death Differ. 2:285-300. [PubMed] [Google Scholar]
- 2.Ameisen, J. C. 2002. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 9:367-393. [DOI] [PubMed] [Google Scholar]
- 3.Aravind, L., V. M. Dixit, and E. V. Koonin. 2001. Apoptotic molecular machinery: vastly increased complexity in vertebrates revealed by genome comparisons. Science 291:1279-1284. [DOI] [PubMed] [Google Scholar]
- 4.Arnoult, D., K. Akarid, A. Grodet, P. X. Petit, J. Estaquier, and J. C. Ameisen. 2002. On the evolution of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinase activation and mitochondrion permeabilization. Cell Death Differ. 9:65-81. [DOI] [PubMed] [Google Scholar]
- 5.Boggs, K., C. O. Rock, and S. Jackowski. 1998. The antiproliferative effect of hexadecylphosphocholine toward HL60 cells is prevented by exogenous lysophosphatidylcholine. Biochim. Biophys. Acta 1389:1-12. [DOI] [PubMed] [Google Scholar]
- 6.Cornillon, S., C. Foa, J. Davoust, N. Buonavista, J. D. Gross, and P. Golstein. 1994. Programmed cell death in Dictyostelium. J. Cell Sci. 107:2691-2704. [DOI] [PubMed] [Google Scholar]
- 7.Croft, S. L., R. A. Neal, W. Pendergast, and J. H. Chan. 1987. The activity of alkylphosphocholines and related derivatives against Leishmania donovani. Biochem. Pharmacol. 36:2633-2636. [DOI] [PubMed] [Google Scholar]
- 8.Croft, S. L., D. Snowdon, and V. Yardley. 1996. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J. Antimicrob. Chemother. 38:1041-1047. [DOI] [PubMed] [Google Scholar]
- 9.Das, M., S. B. Mukherjee, and C. Shaha. 2001. Hydrogen peroxide induces apoptosis-like death in Leishmania donovani promastigotes. J. Cell Sci. 114:2461-2469. [DOI] [PubMed] [Google Scholar]
- 10.Davis, M. C., J. G. Ward, G. Herrick, and C. D. Allis. 1992. Programmed nuclear death: apoptotic-like degradation of specific nuclei in conjugating Tetrahymena. Dev. Biol. 154:419-432. [DOI] [PubMed] [Google Scholar]
- 11.Desjeux, P. 1996. Leishmaniasis. Public health aspects and control. Clin. Dermatol. 14:417-423. [DOI] [PubMed] [Google Scholar]
- 12.D'Mello, S. R., F. Aglieco, M. R. Roberts, K. Borodezt, and J. W. Haycock. 1988. A DEVD-inhibited caspase other than CPP32 is involved in the commitment of cerebellar granule neurons to apoptosis induced by K+ deprivation. J. Neurochem. 70:1809-1818. [DOI] [PubMed] [Google Scholar]
- 13.Enari, M., H. Sakahira, H. Yokoyama, K. Okawa, A. Iwamatsu, and S. Nagata. 1998. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43-50. [DOI] [PubMed] [Google Scholar]
- 14.Engelmann, J., J. Henke, W. Willker, B. Kutscher, G. Nossner, J. Engel, and D. Leibfritz. 1996. Early stage monitoring of miltefosine induced apoptosis in KB cells by multinuclear NMR spectroscopy. Anticancer Res. 16:1429-1439. [PubMed] [Google Scholar]
- 15.Escobar, P., S. Matu, C. Marques, and S. L. Croft. 2002. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH3 (edelfosine) and amphotericin B. Acta Trop. 81:151-157. [DOI] [PubMed] [Google Scholar]
- 16.Escobar, P., V. Yardley, and S. L. Croft. 2001. Activities of hexadecylphosphocholine (miltefosine), AmBisome, and sodium stibogluconate (Pentostam) against Leishmania donovani in immunodeficient scid mice. Antimicrob. Agents Chemother. 45:1872-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Calvo, M., E. P. Peterson, B. Leiting, R. Ruel, D. W. Nicholson, and N. A. Thornberry. 1998. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J. Biol. Chem. 273:32608-32613. [DOI] [PubMed] [Google Scholar]
- 18.Geilen, C. C., T. Wieder, A. Haase, W. Reutter, D. M. Morre, and D. J. Morre. 1994. Uptake, subcellular distribution and metabolism of the phospholipid analogue hexadecylphosphocholine in MDCK cells. Biochim. Biophys. Acta 1211:14-22. [DOI] [PubMed] [Google Scholar]
- 19.Geilen, C. C., T. Wieder, and W. Reutter. 1992. Hexadecylphosphocholine inhibits translocation of CTP:choline-phosphate cytidylyltransferase in Madin-Darby canine kidney cells. J. Biol. Chem. 267:6719-6724. [PubMed] [Google Scholar]
- 20.Guerin, P. J., P. Olliaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, P., M. K. Wasunna, and A. D. Bryceson. 2002. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 21.Haase, R., T. Wieder, C. C. Geilen, and W. Reutter. 1991. The phospholipid analogue hexadecylphosphocholine inhibits phosphatidylcholine biosynthesis in Madin-Darby canine kidney cells. FEBS Lett. 288:129-132. [DOI] [PubMed] [Google Scholar]
- 22.Henke, J., J. Engelmann, U. Flögel, J. Pfeuffer, B. Kutscher, G. Nobner, J. Engel, R. Voegeli, and D. Leibfritz. 1999. Apoptotic effects of hexadecylphosphocholine on resistant and non-resistant cells monitored by NMR spectroscopy. Drugs Today 34:37-50. [Google Scholar]
- 23.Henke, J., J. Engelmann, B. Kutscher, G. Nobner, J. Engel, R. Voegeli, and D. Leibfritz. 1999. Changes of intracellular calcium, fatty acids and phospholipids during miltefosine-induced apoptosis monitored by fluorescence- and 13C NMR-spectroscopy. Anticancer Res. 19:4027-4032. [PubMed] [Google Scholar]
- 24.Holzmuller, P., D. Sereno, M. Cavaleyrz, I. Mangot, S. Daulouede, P. Vincendeau, and J. L. Lemesre. 2002. Nitric oxide-mediated proteasome-dependent oligonucleosomal DNA fragmentation in Leishmania amazonensis amastigotes. Infect. Immun. 70:3727-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha, T. K., S. Sundar, C. P. Thakur, P. Bachmann, J. Karbwang, C. Fischer, A. Voss, and J. Berman. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. 341:1795-1800. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, D. E. 2000. Noncaspase proteases in apoptosis. Leukemia 14:1695-1703. [DOI] [PubMed] [Google Scholar]
- 27.The Joint United Nations Programme on HIV/AIDS. December 2002. AIDS epidemic update. [Online.] http://www.unaids.org.
- 28.Konstantinov, S. M., R. Kaminsky, R. Brun, M. R. Berger, and U. Zillmann. 1997. Efficacy of anticancer alkylphosphocholines in Trypanosoma brucei subspecies. Acta Trop. 64:145-154. [DOI] [PubMed] [Google Scholar]
- 29.Kuhlencord, A., T. Maniera, H. Eibl, and C. Unger. 1992. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob. Agents Chemother. 36:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, N., S. Bertholet, A. Debradant, J. Muller, R. Duncan, and H. L. Nakhasi. 2002. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 9:53-64. [DOI] [PubMed] [Google Scholar]
- 31.Le Fichoux, Y., D. Rousseau, B. Ferrua, S. Ruette, A. Lelievre, D. Grousson, and J. Kubar. 1998. Short- and long term-term efficacy of hexadecylphosphocholine against established Leishmania infantum infection in BALB/c mice. Antimicrob. Agents Chemother. 42:654-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lira, R., L. M. Contreras, R. M. Santa-Rita, and J. A. Urbina. 2001. Mechanism of action of anti-proliferative lysophospholipid analogues against the protozoan parasite Trypanosoma cruzi: potentiation of in vitro activity by the sterol biosynthesis inhibitor ketoconazole. J. Antimicrob. Chemother. 47:537-546. [DOI] [PubMed] [Google Scholar]
- 33.Lux, H., D. T. Hart, P. J. Parker, and T. Klenner. 1996. Ether lipid metabolism, GPI anchor biosynthesis, and signal transduction are putative targets for anti-leishmanial alkyl phospholipid analogues. Adv. Exp. Med. Biol. 416:201-211. [DOI] [PubMed] [Google Scholar]
- 34.Lux, H., N. Heise, T. Klenner, D. Hart, and F. R. Opperdoes. 2000. Ether-lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether-lipid analogues in Leishmania. Mol. Biochem. Parasitol. 111:1-14. [DOI] [PubMed] [Google Scholar]
- 35.Moreira, M. E., H. A. Del Portillo, R. V. Milder, J. M. Balanco, and M. A. Barcinski. 1996. Heat shock induction of apoptosis in promastigotes of the unicellular organism Leishmania (Leishmania) amazonensis. J. Cell. Physiol. 167:305-313. [DOI] [PubMed] [Google Scholar]
- 36.Mottram, J. C., M. J. Helms, G. H. Coombs, and M. Sajid. 2003. Clan CD cysteine peptidases of parasitic protozoa. Trends Parasitol. 19:182-187. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee, S. B., M. Das, G. Sudhandiran, and C. Shaha. 2002. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J. Biol. Chem. 277:24717-24727. [DOI] [PubMed] [Google Scholar]
- 38.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 39.Paredes, R., J. Munoz, I. Diaz, P. Domingo, M. Gurgui, and B. Clotet. 2003. Leismaniasis in HIV infection. J. Postgrad. Med. 49:39-49. [DOI] [PubMed] [Google Scholar]
- 40.Paugam, A., A. L. Bulteau, J. Dupouy-Camet, C. Creuzet, and B. Friguet. 2003. Characterization and role of protozoan parasite proteasomes. Trends Parasitol. 19:55-59. [DOI] [PubMed] [Google Scholar]
- 41.Raff, M. C. 1992. Social controls on cell survival and cell death. Nature 356:397-400. [DOI] [PubMed] [Google Scholar]
- 42.Ridgley, E. L., Z. H. Xiong, and L. Ruben. 1999. Reactive oxygen species activate a Ca2+-dependent cell death pathway in the unicellular organism Trypanosoma brucei brucei. Biochem. J. 340:33-40. [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiter, G. A., M. Verheij, S. F. Zerp, and W. J. Van Blitterswijk. 2001. Alkyl-lysophospholipids as anticancer agents and enhancers of radiation-induced apoptosis. Int. J. Radiat. Oncol. Biol. Phys. 49:415-419. [DOI] [PubMed] [Google Scholar]
- 44.Ruiter, G. A., S. F. Zerp, H. Bartelink, W. J. Van Blitterswijk, and M. Verheij. 1999. Alkyl-lysophospholipids activate the SAPK/ JNK pathway and enhance radiation-induced apoptosis. Cancer Res. 59:2457-2463. [PubMed] [Google Scholar]
- 45.Rybczynska, M., M. Spitaler, N. G. Knebel, G. Boeck, H. Grunicke, and J. Hofmann. 2001. Effects of miltefosine on various biochemical parameters in a panel of tumor cell lines with different sensitivities. Biochem. Pharmacol. 62:765-772. [DOI] [PubMed] [Google Scholar]
- 46.Santa-Rita, R. M., H. Santos Barbosa, M. N. Meirelles, and S. L. De Castro. 2000. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop. 75:219-228. [DOI] [PubMed] [Google Scholar]
- 47.Saraiva, V. B., D. Gibaldi, J. O. Previato, L. Mendonça-Previato, M. T. Bozza, C. G. Freire-De-Lima, and N. Heise. 2002. Proinflammatory and cytotoxic effects of hexadecylphosphocholine (miltefosine) against drug-resistant strains of Trypanosoma cruzi. Antimicrob. Agents Chemother. 46:3472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seifert, K., M. Duchene, W. H. Wernsdorfer, H. Kollaritsch, O. Scheiner, G. Wiedermann, T. Hottkowitz, and H. Eibl. 2001. Effects of miltefosine and other alkylphosphocholines on human intestinal parasite Entamoeba histolytica. Antimicrob. Agents Chemother. 45:1505-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seifert, K., S. Matu, F. J. Pérez-Victoria, S. Castanys, F. Gamarro, and S. L. Croft. 2003. Characterization of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int. J. Antimicrob. Agents 22:380-387. [DOI] [PubMed] [Google Scholar]
- 50.Sereno, D., P. Holzmuller, I. Mangot, G. Cuny, A. Ouaissi, and J. L. Lemesre. 2001. Antimonial-mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob. Agents Chemother. 45:2064-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soto, J., J. Toledo, P. Gutierrez, R. S. Nicholls, J. Padilla, J. Engel, C. Fischer, A. Voss, and J. Berman. 2001. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin. Infect. Dis. 33:E57-E61. [DOI] [PubMed] [Google Scholar]
- 52.Sundar, S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6:849-854. [DOI] [PubMed] [Google Scholar]
- 53.Sundar, S., L. B. Gupta, M. K. Makharia, M. K. Singh, A. Voss, F. Rosenkaimer, J. Engel, and H. W. Murray. 1999. Oral treatment of visceral leishmaniasis with miltefosine. Ann. Trop. Med. Parasitol. 93:589-597. [DOI] [PubMed] [Google Scholar]
- 54.Sundar, S., A. Makharia, D. K. More, G. Agrawal, A. Voss, C. Fischer, P. Bachmann, and H. W. Murray. 2000. Short-course of oral miltefosine for treatment of visceral leishmaniasis. Clin. Infect. Dis. 31:1110-1113. [DOI] [PubMed] [Google Scholar]
- 55.Sundar, S., F. Rosenkaimer, M. K. Makharia, A. K. Goyal, A. K. Mandal, A. Voss, P. Hilgard, and H. W. Murray. 1998. Trial of oral miltefosine for visceral leishmaniasis. Lancet 352:1821-1823. [DOI] [PubMed] [Google Scholar]
- 56.Szallies, A., B. K. Kubata, and M. Duszenko. 2002. A metacaspase of Trypanosoma brucei causes loss of respiration competence and clonal death in the yeast Saccharomyces cerevisiae. FEBS Lett. 517:144-150. [DOI] [PubMed] [Google Scholar]
- 57.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 58.Unger, C., T. Maniera, P. Kaufmann-Kolle, and H. Eibl. 1998. In vivo antileishmanial activity of hexadecylphosphocholine and other alkylphosphocholines. Drugs Today 34:133-140. [Google Scholar]
- 59.Unger, C., M. Peukert, H. Sindermann, P. Hilgard, G. Nagel, and H. Eibl. 1990. Hexadecylphosphocholine in the topical treatment of skin metastases in breast cancer patients. Cancer Treat. Rev. 17:243-246. [DOI] [PubMed] [Google Scholar]
- 60.Uren, A. G., K. O'Rourke, L. A. Aravind, M. T. Pisabarro, S. Seshagiri, E. V. Koonin, and V. M. Dixit. 2000. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell 6:961-967. [DOI] [PubMed] [Google Scholar]
- 61.Van Blitterswijk, W. J., H. Hilkmann, and G. A. Storme. 1987. Accumulation of an alkyl lysophospholipid in tumor cell membranes affects membrane fluidity and tumor cell invasion. Lipids 22:820-823. [DOI] [PubMed] [Google Scholar]
- 62.Vardi, A., I. Berman-Frank, T. Rozenberg, O. Hadas, A. Kaplan, and A. Levine. 1999. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress. Curr. Biol. 9:1061-1064. [DOI] [PubMed] [Google Scholar]
- 63.Vermes, I., C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis. Flow cytometry detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled annexin V. J. Immunol. Methods 184:39-51. [DOI] [PubMed] [Google Scholar]
- 64.Walochnik, J., M. Duchene, K. Seifert, A. Obwaller, T. Hottkowitz, G. Wiedermann, H. Eibl, and H. Aspock. 2002. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 46:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, Y. Z., Y. B. Chang, C. Xing, and D. Fu. 1998. The interference effects of hexadecylphosphocholine on proliferation and membrane phospholipid metabolism in human myeloid leukaemia cell lines. Int. J. Tissue React. 20:101-107. [PubMed] [Google Scholar]
- 66.Welburn, S. C., S. Lillico, and N. B. Murphy. 1999. Programmed cell death in procyclic form Trypanosoma brucei rhodesiense: identification of differentially expressed genes during Con A induced death. Mem. Inst. Oswaldo Cruz 94:229-234. [DOI] [PubMed] [Google Scholar]
- 67.Wieder, T., C. E. Orfanos, and C. C. Geilen. 1998. Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hexadecylphosphocholine. J. Biol. Chem. 273:11025-11031. [DOI] [PubMed] [Google Scholar]
- 68.Wyllie, A. H. 1980. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555-556. [DOI] [PubMed] [Google Scholar]
- 69.Zangger, H., J. C. Mottram, and N. Fasel. 2002. Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ. 9:1126-1139. [DOI] [PubMed] [Google Scholar]