Abstract
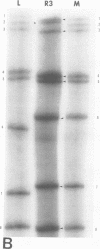
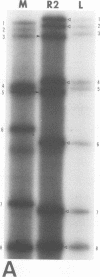
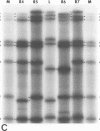
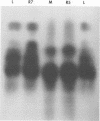
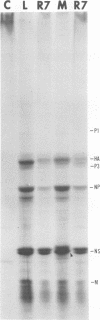
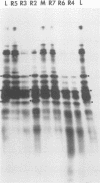
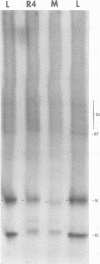
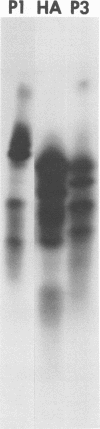
It was shown that all eight RNA segments of influenza B viruses are most likely monocistronic and code for eight virus-specific polypeptides. A genetic map of the influenza B virus genome was established, and six polypeptides (P1 protein, nucleoprotein, hemagglutinin, neuraminidase, M protein, and nonstructural protein) were unambiguously assigned to specific RNA segments. Molecular weight estimates of the eight individual genes are obtained by using the glyoxal method. These results suggest that each influenza B virus RNA segment has a greater molecular weight than the influenza A virus RNA segment which codes for the analogous gene product.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chakraverty P. Antigenic relationship between influenza B viruses. Bull World Health Organ. 1971;45(6):755–766. [PMC free article] [PubMed] [Google Scholar]
- Chakraverty P. Antigenic relationships between the neuraminidases of influenza B virus. Bull World Health Organ. 1972;46(4):473–476. [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Desselberger U., Palese P. Molecular weights of RNA segments of influenza A and B viruses. Virology. 1978 Jul 15;88(2):394–399. doi: 10.1016/0042-6822(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Francis T., Jr A NEW TYPE OF VIRUS FROM EPIDEMIC INFLUENZA. Science. 1940 Nov 1;92(2392):405–408. doi: 10.1126/science.92.2392.405. [DOI] [PubMed] [Google Scholar]
- Gentsch J., Wynne L. R., Clewley J. P., Shope R. E., Bishop D. H. Formation of recombinants between snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 Dec;24(3):893–902. doi: 10.1128/jvi.24.3.893-902.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNESSY A. V., MINUSE E., DAVENPORT F. M. A TWENTY-ONE-YEAR EXPERIENCE WITH ANTIGENIC VARIATION AMONG INFLUENZA B VIRUSES. J Immunol. 1965 Feb;94:301–306. [PubMed] [Google Scholar]
- Haslam E. A., Hampson A. W., Egan J. A., White D. O. The polypeptides of influenza virus. II. Interpretation of polyacrylamide gel electrophoresis patterns. Virology. 1970 Nov;42(3):555–565. doi: 10.1016/0042-6822(70)90302-8. [DOI] [PubMed] [Google Scholar]
- Haslam E. A., Hampson A. W., Radiskevics I., White D. O. The polypeptides of influenza virus. 3. Identification of the hemagglutinin, neuraminidase and nucleocapsid proteins. Virology. 1970 Nov;42(3):566–575. doi: 10.1016/0042-6822(70)90303-x. [DOI] [PubMed] [Google Scholar]
- Kendal A. P. A comparison of "influenza C" with prototype myxoviruses: receptor-destroycing activity (neuraminidase) and structural polypeptides. Virology. 1975 May;65(1):87–99. doi: 10.1016/0042-6822(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Cytoplasmic and nucleoplasmic viral RNPs in influenza virus-infected MDCK cells. Virology. 1972 Oct;50(1):103–113. doi: 10.1016/0042-6822(72)90350-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Lazdins I., Haslam E. A., White D. O. The polypeptides of influenza virus. VI. Composition of the neuraminidase. Virology. 1972 Sep;49(3):758–765. doi: 10.1016/0042-6822(72)90532-6. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford J. S. Polypeptide composition of Influenza B viruses and enzymes associated with the purified virus particles. J Virol. 1973 Oct;12(4):827–835. doi: 10.1128/jvi.12.4.827-835.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. P1 and P3 proteins of influenza virus are required for complementary RNA synthesis. J Virol. 1977 Mar;21(3):1187–1195. doi: 10.1128/jvi.21.3.1187-1195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Kilbourne E. D. RNAs of influenza A, B, and C viruses. J Virol. 1976 May;18(2):738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Difference in protein patterns of influenza A viruses. Virology. 1977 Jan;76(1):122–128. doi: 10.1016/0042-6822(77)90289-6. [DOI] [PubMed] [Google Scholar]
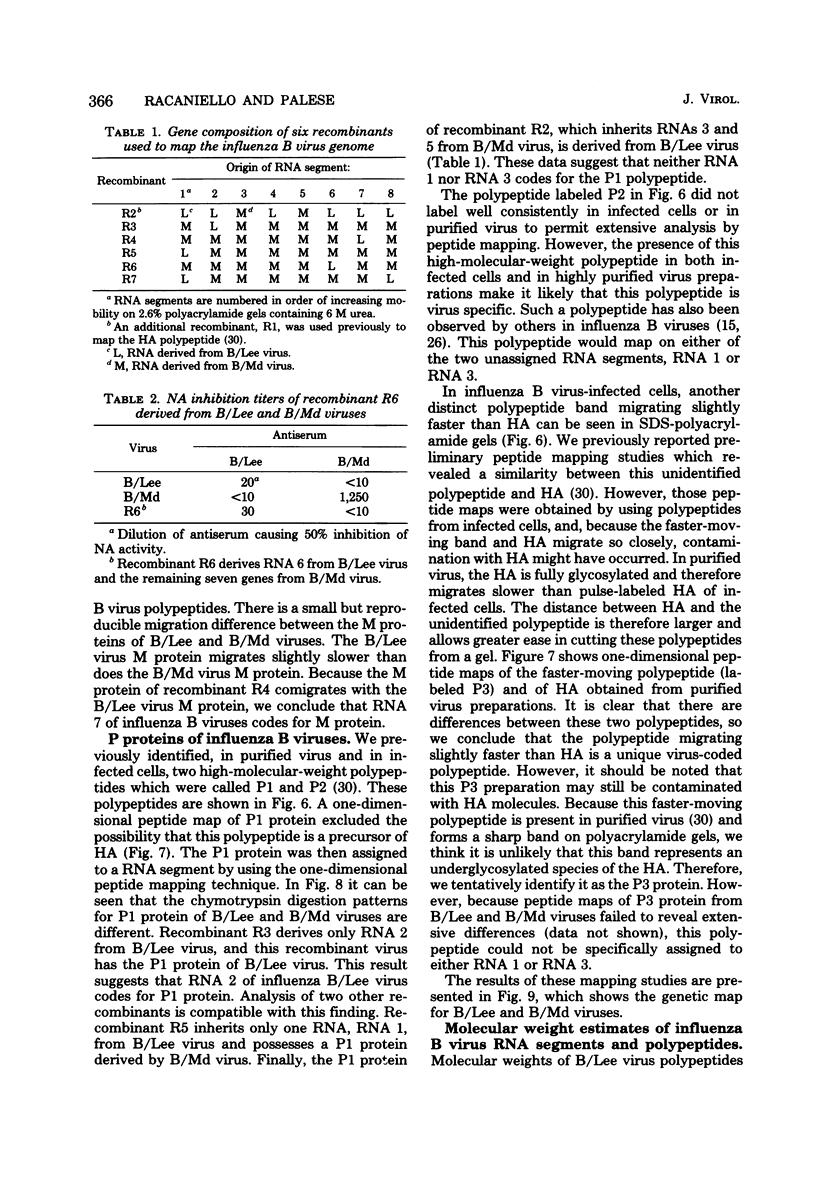
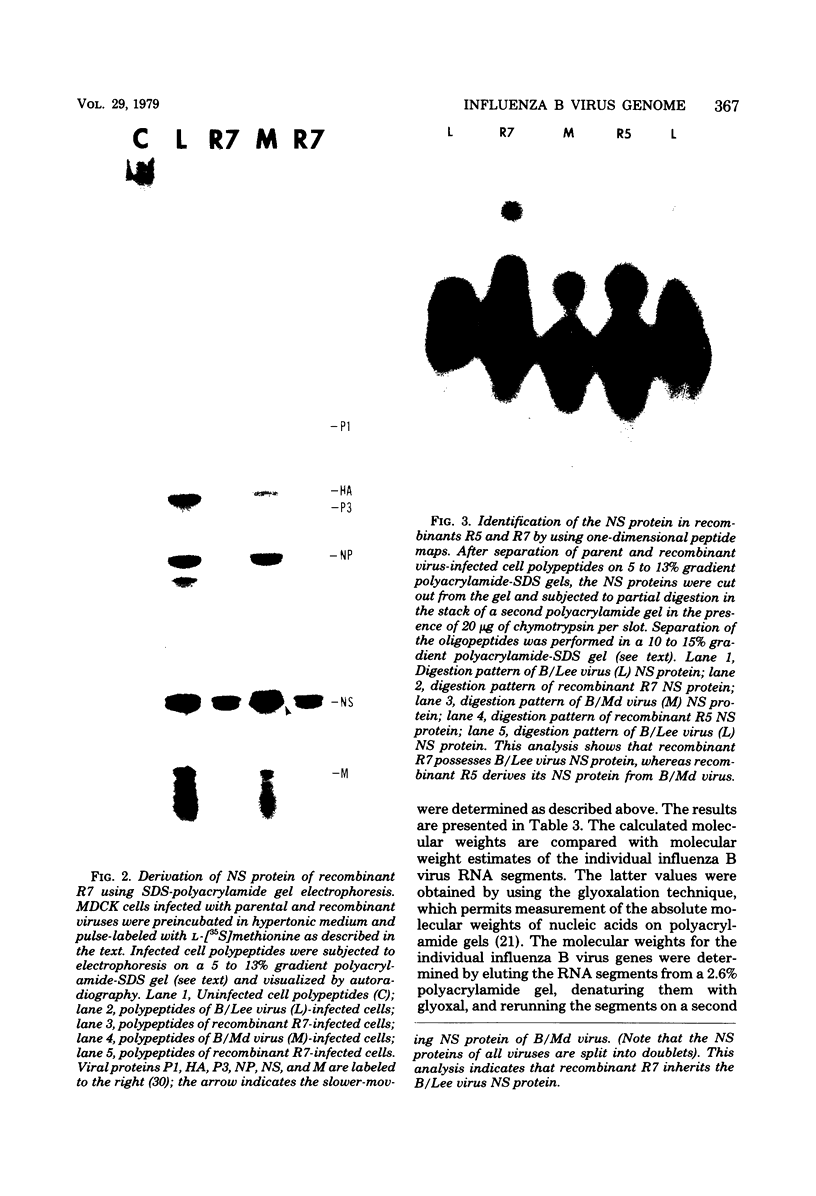
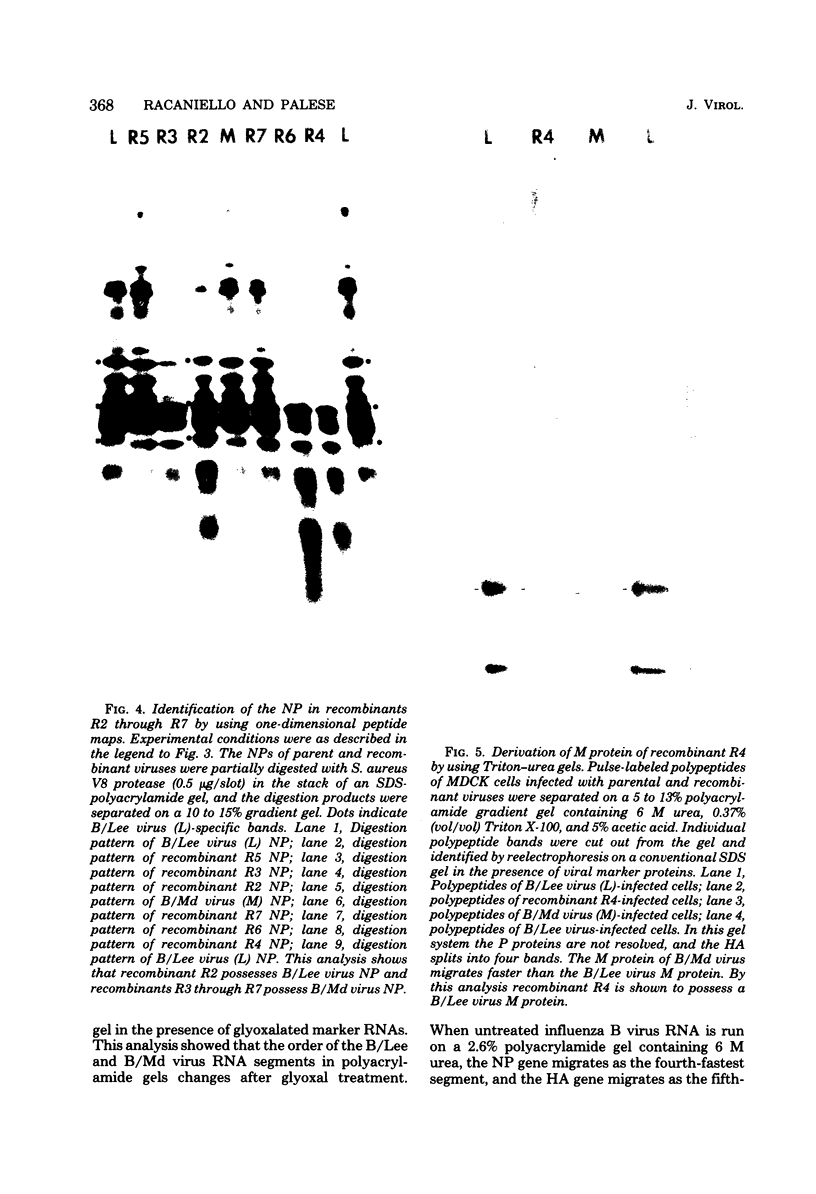
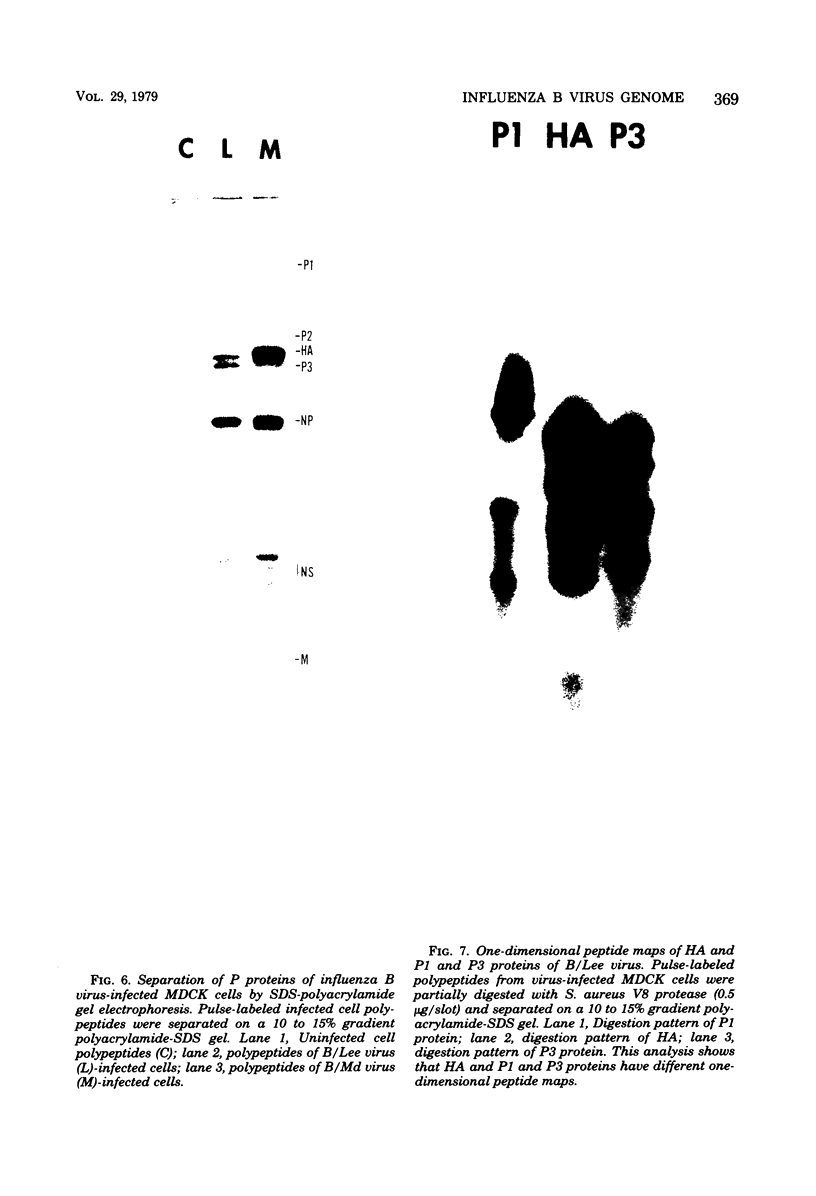
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild G. C., Pereira M. S., Chakraverty P., Coleman M. T., Dowdle W. R., Chang W. K. Antigenic variants of influenza B virus. Br Med J. 1973 Oct 20;4(5885):127–131. doi: 10.1136/bmj.4.5885.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J. L., Palese P. Selection and identification of influenza virus recombinants of defined genetic composition. J Virol. 1976 Oct;20(1):248–254. doi: 10.1128/jvi.20.1.248-254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Ramig R. F., Mustoe T. A., Fields B. N. A genetic map of reovirus. 1. Correlation of genome RNAs between serotypes 1, 2, and 3. Virology. 1978 Jan;84(1):63–74. doi: 10.1016/0042-6822(78)90218-0. [DOI] [PubMed] [Google Scholar]
- Tobita K., Kilbourne E. D. Structural polypeptides of antigenically distinct strains of influenza B virus. Arch Virol. 1975;47(4):367–374. doi: 10.1007/BF01347978. [DOI] [PubMed] [Google Scholar]
- Ueda M., Tobita K., Sugiura A., Enomoto C. Identification of hemagglutinin and neuraminidase genes of influenza B virus. J Virol. 1978 Feb;25(2):685–686. doi: 10.1128/jvi.25.2.685-686.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG S. C., KILBOURNE E. D. Changing viral susceptibility of a human cell line in continuous cultivation. I. Production of infective virus in a variant of the Chang conjunctival cell following infection with swine or N-WS influenza viruses. J Exp Med. 1961 Jan 1;113:95–110. doi: 10.1084/jem.113.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]