Abstract
Historic increase in urban population numbers in the face of shrinking urban economies and declining social services has meant that a large proportion of the urban population lives in precarious urban conditions, which provide the grounds for high urban health risks in low income countries. This study aims to identify, investigate, and contrast the spatial patterns of vulnerability and risk of two major causes of mortality, viz malaria and diarrhea mortalities, in order to optimize resource allocation for effective urban environmental management and improvement in urban health. A spatial cluster analysis of the observed urban malaria and diarrhea mortalities for the whole city of Accra was conducted. We obtained routinely reported mortality data for the period 1998–2002 from the Ghana Vital Registration System (VRS), computed the fraction of deaths due to malaria and diarrhea at the census cluster level, and analyzed and visualized the data with Geographic Information System (GIS, ArcMap 9.3.1). Regions of identified hotspots, cold spots, and excess mortalities were observed to be associated with some socioeconomic and neighborhood urban environmental conditions, suggesting uneven distribution of risk factors for both urban malaria and diarrhea in areas of rapid urban transformation. Case–control and/or longitudinal studies seeking to understand the individual level factors which mediate socioenvironmental conditions in explaining the observed excess urban mortalities and to establish the full range of risk factors might benefit from initial vulnerability mapping and excess risk analysis using geostatistical approaches. This is key to evidence-based urban health policy reforms in rapidly urbanizing areas in low income economies.
Keywords: Malaria, Diarrhea, Hotspots, Urban vulnerabilities, Health risk, Spatial autocorrelation, Cluster-level mortality
Introduction
Urban systems are not only characterized by a varied landscape, comprising a range of ecosystems and habitats, but may generally be viewed as a whole that comprises several mosaics of distinctive community areas or neighborhoods with differing levels of natural resource use and consumption and differing environmental quality conditions both in space and in time. A study of the urban systems to understand the interaction among these factors will not only help with their management, environmental risk reduction, disease control, and efficient allocation of limited resources within urban areas but may also help in understanding how urban systems function more broadly to influence human health.1
The dynamics of ecosystem change in and around urban centers are also influenced by a number of features characteristic of how urban landscapes change, examples of which include proliferation of alien species (e.g., insect vectors, rodents, etc.), high habitat diversity and fragmentation, and a high rate of (human-induced) habitat disturbance.2,3 Urban changes produce new challenges in terms of complex disease etiologies and risk factors, which make a careful study of the association between spatially varied urban structure and urban health outcomes (e.g., infectious disease transmission, mortality, etc.) scientifically relevant for health policy.
In Accra, where this study was conducted, imbalances in the provision of basic sanitation services have left the different residential spaces or neighborhoods at differing levels of environmental quality conditions.6 A combination of these urban environmental conditions and suitable temperatures provides good breeding opportunities for insect vector and microbial growth for both malaria and diarrhea transmission.5,5,7 While urbanization alters several component features of the urban environments, in this study, the focus was on the association between urban malaria and diarrhea, on the one hand, and urban environmental sanitation and socioeconomic conditions for which large data exist in the Ghana 2000 Census database, on the other hand. Moreover, despite clear dissimilarity of the etiologic agents, malaria and diarrhea both seem to share common risk factors, which are largely mediated by environmental sanitation infrastructure and socioeconomic conditions. Understanding the influence of urban ecological change processes on malaria and diarrhea mortalities in an urban area is not only crucial for devising effective disease control measures in rapidly urbanizing areas but will also provide insights for health-oriented urban environmental management.8
The main goal of this study was to map and describe the patterns of the observed urban malaria and diarrhea mortalities in an area with poor sanitation infrastructure and socioeconomic conditions and to identify the foci of excess malaria and diarrhea mortalities so that disease control efforts may appropriately be directed in a spatially explicit manner. The objectives of the study were: (i) to study the spatial patterns of the malaria and diarrhea mortalities in an urbanizing area with poor environmental quality and social services in a low income setting, (ii) to compare the spatial patterns of urban malaria and diarrhea mortalities, and (iii) to assess the distribution of risk for urban malaria and diarrhea mortalities.
Methodology
Study Area
This study was conducted in Accra, the capital city of Ghana. The city harbors over 30 % of the urban population and nearly 15 % of the country's total population.7 For the purpose of the 2000 census count, Accra was divided into 1,700 Enumeration Areas (EAs) which together make up the 70 census clusters used in this study, i.e., the basic units of the analysis (Figure 1).9,10 The boundaries of these clusters were digitized to produce a complete digital map of urban Accra.7
Figure 1.
Map of Ghana showing the ten administrative regions and Accra, the capital city and the study area. Accra is magnified to show the 70 census clusters employed in the study as the units of analysis. The map also shows lakes, rivers, and lagoons in blue, which drain the entire city.
The census clusters have been defined by the statistical system of Ghana as a group of geographically contiguous census EAs of fairly homogeneous populations based upon specified characteristics such as accessibility of population to enumerators, socioeconomic and cultural factors, etc..7,9,10
Data Collection
The census 2000 database held several cluster level measures of socioeconomic status including educational attainment, literacy rate, school enrolment, religion, ethnicity, marital status, employment status, type of employment, economic activity status (e.g., whether employable or not), and environmental variables such as, per capita waste generation, total waste generation, amount of solid wastes uncollected (waste deposition), volume of liquid wastes disposed through the sewer system, volume of liquid wastes by nonsewer disposal, number of households with pit latrines, number of households with toilet/bath facility in different house, number of households with pan latrines, etc., which were obtained by a written permission from the Government Statistician. We extracted and computed each of the relevant socioeconomic and environmental variables from the database as a proportion of the overall cluster level conditions in each of the 70 clusters in Accra. The health data (all-cause deaths) were obtained from the Ghana Births and Deaths Registry (i.e., the Ghana Vital Registration System, VRS) over a 5-year period spanning 1998 to 2002, inclusive. The total number of deaths recorded and coded according to ICD-9 in the registers over this period in urban Accra was 24,716 out of which 1,292 and 1,001 were deaths attributed to malaria and diarrhea, respectively. The death records were cleaned, validated, and allocated to the 70 clusters in Accra (Figure 1) according to the house address of normal place of residence prior to death. We linked the death records to the clusters via cluster codes in a Geographic Information System (GIS) (ArcMap 9.3.1) to provide information about spatial characteristics of the observed mortalities in Accra. The study analyzed existing data, and no ethical concerns were associated with the use of such data.
Analytical and Modeling Techniques
In all, we extracted a total of 118 socioeconomic and environmental variables from the 2000 national population database. For all the 70 clusters in Accra, we computed appropriate summary measures of the environmental and socioeconomic variables at an aggregate cluster level. The 118 variables were subgrouped into 11 subcategories, namely, waste generation, sanitation and water supply, housing type and construction materials, hygiene conditions, education level, occupation type, place of work, household energy use, economic activity, marital status, and ethnicity. In order to detect and deal with multicollinearity, we carried out a correlation analysis. We then examined the correlation matrix for variables, which showed strong collinearity by inspecting their variance inflation factor (VIF) (using Stata 9.0, College Station, TX). We, thus, modified or appropriately transformed those variables, which showed collinearity. In principal component analysis (PCA), we retained, for the final modeling, only variables with significant variability and explain over 30 % of the city socioeconomic and environmental conditions (i.e., variables with factor score greater than |0.3|).7 Furthermore, we excluded variables on household energy use, marital status, and ethnicity because they are unrelated to the outcome measure (i.e., malaria and diarrhea mortality). Given that the mortality data were beset with several data quality problems, including incomplete reporting, we computed a census cluster level metric—the fraction of deaths due to a specific mortality, in which we assumed the incompleteness factor in both the numerator and denominator cancelled out. In addition, we observed that misclassification and ascertainment biases would be nondifferential errors in nature and evenly distributed between the numerator and denominator and, therefore, cancelled out in the quotient, i.e., in the cause of death ascertainment, it is the fact of death rather the than cause of death that matters and no specific cause will be selectively over-misclassified compared to others except stigma-related HIV/AIDS. For this reason, we computed cluster level fraction of deaths due to a specific cause (PMRfd) as a derivative of cluster level Proportional Mortality Ratio (PMR) which robustly addressed the inherent quality issues. A PMR is defined as the observed proportion of deaths due to specific-cause mortality in an exposed population divided by the expected proportion of deaths due to that specific-cause mortality (i.e., the expected proportion being the number of deaths due to specific-cause over all-cause mortality in a standard or reference population). In the case of the current study, we used city-level all-cause death events as the reference population of death events, since we did not deal with actual population numbers (dead individuals other than those living). In addition, we linked the remaining aggregate level (cluster-level proportion of each variable) environmental and socioeconomic data (see full list in Box 1) to the health data at cluster level via cluster codes.
Box 1: List of variables extracted from the Accra census 2000 dataset
Using the cluster level fraction of deaths due to urban malaria and diarrhea (PMRfd) as the outcome measure and the environmental and socioeconomic variables as explanatory variables (covariates), we conducted a hierarchical spatial analysis using advanced frailty models (geostatistical approaches) in order to assess the patterns of spatial distribution of the observed and unobserved urban malaria and diarrhea mortalities as well as the pattern of excess mortalities due to the environmental and socioeconomic covariates as follows:
Mapped the spatial distribution of cluster level fraction of deaths due to malaria and diarrhea (PMRfd) to provide information about spatial characteristics of the observed malaria and diarrhea mortalities,
Checked for spatial autocorrelation at a global scale (Accrawide level) to detect general clusters or outliers using the Global Moran's I measure (ArcMap 9.3.1) based on inverse distance, inverse distance squared, queen and rook contiguity, and k nearest neighbors,11
Checked for spatial autocorrelation at a local level (cluster level) to detect small-scale clusters or outliers using univariate Local Indicators of Spatial Association (LISA) (GeoDa 0.9.5-i),12 and
Generated excess risk maps for malaria and diarrhea in relation to the urban environmental and socioeconomic variables (see Box 1) to provide a spatially explicit risk assessment for significantly influencing covariates on malaria and diarrhea mortality in an urban area in Africa (GeoDa 0.9.5-i).12
Excess risk maps show the relative risk to be affected by an event variable, in this case, the relationship between the outcome measure (PMRfd) and the base variables (i.e., the environmental or socioeconomic covariates). This risk is calculated as a ratio of the observed number of events over the expected number of events. The latter is computed by applying the average risk to the population at risk in each location. Excess risk values <1 show locations with fewer than expected events, and values >1 show locations where the number of events exceeds the expected number of events. The excess risk measure is a nonspatial measure and, therefore, ignores any effect of spatial autocorrelation.13,14
Results
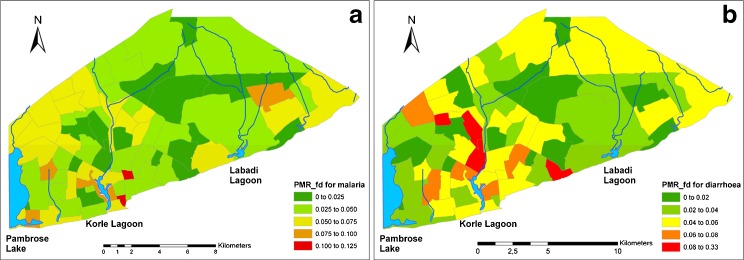
Using the PMRfd, we created mortality distribution maps separately for malaria and diarrheal deaths in Accra. The fraction of deaths due to each cause ranges from 0 to 0.12 for urban malaria and from 0 to 0.33 for urban diarrhea mortality (Figure 2). Whereas the distribution of diarrhea mortality was heterogeneous with high mortality ratios widely scattered around the Korle Lagoon (Figure 2a), malaria mortalities tended to be more homogenous with only a few hotspots in Accra West, distributed roughly radial around to the lagoon (Figure 2b). By visual inspection, the observed patterns of mortality distribution appeared to show the same degree of clustering for both urban malaria and diarrhea mortality.
Figure 2.
Spatial distribution of the a observed malaria deaths and b diarrheal deaths in Accra. The spatial patterns displayed in the figure represent cluster level fraction of deaths due to malaria and diarrhea and the different colors show different classification of the fraction of deaths due to malaria and diarrhea as defined by PMR_fd for malaria and PMR_fd for diarrhea, respectively. Green shows the lowest extreme, while red shows the highest extreme.
Spatial Autocorrelation: Global Moran's I
Having observed a similarity in degree clustering for malaria and diarrhea, subsequent autocorrelation analyses were carried out first on a global scale using global Moran's I which was followed by a local-scale LISA analysis. We conducted further spatial autocorrelation analysis, applying different types of neighborhood scenarios (inverse distance, inverse distance squared, queen and rook contiguity, and k nearest neighbors) to ensure a coverage of all possible and plausible neighborhood relationships. No evidence of significant (p ≥ 0.05) global clustering was detected for both malaria and diarrhea mortalities in any of the neighborhood scenarios considered at the global (city-wide) level.
Spatial Autocorrelation: Local Indicators of Spatial Association (LISA)
This section presents the results of further spatial analyses using univariate LISA which was conducted to check if a local scale of clustering for both malaria and diarrhea mortalities could be detected. In each case, a first-order queen contiguity was utilized as neighborhood condition. In this approach, the mean Local Moran value was 0.042 for malaria and −0.041 for diarrhea, respectively. The range of the Local Moran values was from −1.27 to 1.27 for malaria with a standard deviation of 0.39 and from −2.59 to 0.60 for diarrhea with a standard deviation of 0.35. In order to increase the power of cluster detection, we conducted spatial sampling in the order of 9,999 permutations.
The choropleths occupying the left half of both Figures 3 and 4 display those locations with a significant Local Moran statistic classified by type of spatial correlation. HH identifies those clusters with high malaria mortality lying near clusters with high malaria mortality, HL identifies those clusters with high malaria mortality lying near clusters with low malaria mortality, and LL identifies those clusters with low malaria mortality lying near clusters with low malaria mortality. Thus, HH and LL clusters indicate a clustering of similar values, whereas the HL and LH clusters give an indication of spatial outliers. Areas with plain white backgrounds are locations showing no significant Local Moran statistic (p ≥ 0.05). The choropleths occupying the right half of both figures show the level of statistical significance (i.e., p values or the probability of the distribution). The locations with a significant Local Moran statistic are shown in different coloration, depending on the significance level. Four significance levels were shown as p = 0.05, p = 0.01, p = 0.001, and p = 0.0001 with dark green shade being the strongest extreme of p = 0.0001 and red coloration being the lowest extreme of p = 0.05.
Figure 3.
Spatial clustering of malaria mortality and choropleth. a Different spatial clusters. The clusters shown in red are clusters for which high–high (HH) neighbors occur and represent hotspots. Clusters shown in blue are spatial clusters for which low–low (LL) neighbors occur and represent cold spots. The clusters shown in yellow are spatial clusters for which high–low (HL) or low–high (LH) neighbors occur and represent spatial outliers. The choropleth (b) shows the different levels of significance in different colors representing different ranges of p values. Green represents a p value less than 0.001, yellow green represents the p value range of 0.001–0.010, orange presents the p value range of 0.010–0.025, and red represents the p value range of 0.025–0.050.
Figure 4.
Spatial clustering of diarrhea mortality with choropleth a displaying locations of different spatial clusters. Blank or white clusters are clusters for which no clustering was observed. Blue clusters are spatial clusters for which low–low (LL) neighbors occur and represent cold spots. Yellowclusters are spatial clusters for which high–low (HL) or low–high (LH) neighbors occur and represent spatial outliers. Choropleth b shows the different levels of significance in different colors representing different ranges of p values. Green represents a p value less than 0.001, yellow green represents the p value range of 0.001–0.010, orange presents the p value range of 0.010–0.025, and red represents the p value range of 0.025–0.050.
Figure 3 shows a spatial choropleth for malaria mortality distribution displaying those locations with a significant Local Moran statistic classified by type of spatial correlation: bright red for the high–high (HH) association, bright blue for low–low (LL), white for no clustering and yellow for high–low (HL). One location with strong evidence (p < 0.0001) of clustering, i.e., a hotspot (high–high location) near the Korle Lagoon, three cold spots (low–low locations), two in south-central Accra, and one in the northeastern quadrant of Accra were observed (Figure 3a). In addition, there were two outliers in south-central Accra. A total of nine significant (p ≤ 0.05) clusters were observed in the study area for malaria mortality (Figure 3b). There were four HH clusters located adjacent to one another and bordering the eastern shoreline of the Korle Lagoon (p range from <0.001 to 0.05). Two of the three LL clusters were observed in southern central Accra (0.01 < p < 0.05), one adjacent to the coast, the other one slightly northwest of the Korle Lagoon. The third LL cluster (0.01 < p < 0.025) was located at the northeastern outskirt of the city. Two HL clusters (0.001 < p < 0.01) were observed in south central Accra and within close proximity to the coastline.
The choropleth for diarrhea mortality distribution displays four cold spots (low–low locations) showing slightly weaker evidence (p = 0.05) of clustering compared to malaria mortality and two outliers lying on opposite sides of one of the cold spots (Figure 4a). Although the choropleths for both malaria and diarrhea distributions showed clustering at the local level, that for malaria was slightly stronger compared to that for diarrhea mortality. Unlike the case for malaria mortality, LISA analysis of the fraction of deaths due to diarrhea showed evidence of six significant (p ≤ 0.05) but weakly clustered distribution within the study area (Figure 4b). Two of the four LL clusters which showed very strong evidence (0.01 < p < 0.05) of cold spots were detected in the east of the city while the other two, which were much smaller in size, were observed in the west. There were also two HL clusters (0.025 < p < 0.05) lying to the mideastern stretches of the city, sandwiching one of the large LL clusters.
Analysis of Excess Urban Mortalities
The results of the spatial frailty modeling showed no significant association between diarrhea mortality and the neighborhood urban environmental and socioeconomic covariates, and therefore, excess mortality maps were created for malaria mortality only. Values less than one (greenish colors) indicate locations with fewer than expected events and values larger than one (reddish colors) indicate locations with more than the expected events (Figure 5). The deep red and deep green colorations represent the upper and lower extreme values, respectively. Census clusters showing excess rates larger than one have a higher risk of mortality than the average risk in the whole study area for the particular explanatory variable or covariate used.
Figure 5.
Distribution of excess malaria mortality as influenced by the occupation of residents. Choropleth a shows the influence of the proportion of the administrative sector workforce on the distribution of excess malaria mortality, while choropleth b shows that of the proportion of the retail sector workforce on excess malaria mortality. Different coloration depicts the different levels of excess malaria mortality. Deep green shows regions with excess risk ratio of less than 0.25, whereas light green shows regions with excess risk ratio between 0.25 and 0.50. Pale green shows regions with excess risk ratio between 0.50 and 1.00, flesh-colored areas shows regions with excess risk ration between 1.00 and 2.00, orange shows regions with excess risk ratio between 2.00 and 4.00, and red shows regions with excess risk ration greater than 4.
The two choropleths in Figure 5 show excess malaria mortality with the occupation of residents as a covarying factor in Accra. The choropleth to the right shows retail sector workforce (Figure 5b) as the covariate for excess malaria mortality while that to the left, presents administrative sector workforce (Figure 5a) as the covariate for the same condition. As shown by the intensities of reddish coloration of the choropleth to the left, administrative sector workforce tended to contribute more to the observed excess malaria mortality than retail sector workforce which appeared to have neutral influence, i.e., locations with excess malaria mortality tended to occur in the western part of Accra with a relatively higher proportion of administrative sector workforce (Figure 5a). In contrast, the choropleth to the right showed that a lower than expected number of malaria deaths tended to occur in the western part of Accra, coinciding with locations which had a lower proportion of the retail sector workforce.
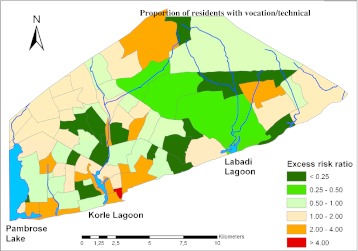
Figure 6 displays the excess malaria mortality with educational status as the covariate. As can be gleaned from the choropleth, only one small area close to the Korle Lagoon had excess mortality far above what was expected. The western part of Accra was observed to have elevated mortality although less pronounced compared to the case for which the administrative sector workforce was the base covariate and much the same pattern compared to the case for which the retail sector workforce was considered as base variable.
Figure 6.
Distribution of excess malaria mortality as influenced by the proportion of residents with vocational and technical education. Deep green shows regions with excess risk ratio of less than 0.25, whereas the yellow green shows regions with excess risk ratio between 0.25 and 0.50. Pale green color shows regions with excess risk ratio between 0.50 and 1.00, flesh-colored areas shows regions with excess risk ration between 1.00 and 2.00, orange shows regions with excess risk ratio between 2.00 and 4.00, and red color shows regions with excess risk ration greater than 4.
The choropleths in Figure 7 show excess malaria mortality considering the proportions of households with pipe-borne water supply source outside and those connected to water closet (WC) as the underlying covariates. Locations with excess malaria mortality were observed to be consigned to the western part of Accra when proportion of households connected to WC was imposed as the base variable (Figure 7a). On the other hand, when we imposed the proportion of households with pipe outside as the main covariate (Figure 7b), we observed fewer locations with excess malaria mortality than in the case for which the proportion of households with WC as the base variable. In both cases however, locations to the eastern parts of the city recorded fewer than expected malaria mortality, while several clusters in the western part of the city exhibited more than expected number of malaria deaths.
Figure 7.
Distribution of excess malaria mortality as influenced by hygiene, water, and sanitation conditions. Choropleth a shows the influence of the proportion of households with water pipe outside on the distribution of excess malaria mortality, while choropleth b shows that of the proportion of households with WC on excess malaria mortality. Different coloration depicts the different levels of excess malaria mortality. Deep green shows regions with excess risk ratio of less than 0.25, whereas yellow green shows regions with excess risk ratio between 0.25 and 0.50. Pale green shows regions with excess risk ratio between 0.50 and 1.00, flesh-colored areas shows regions with excess risk ration between 1.00 and 2.00, orange color shows regions with excess risk ratio between 2.00 and 4.00, and red shows regions with excess risk ration greater than 4.
Figure 8 shows an excess mortality map considering housing type as the underlying risk factor driving malaria mortality in Accra. Housing type is defined as the type of built units, i.e., compound units, single units, construction material types, etc. This represents the proportion of separate or stand-alone structure as the potential risk factor for malaria mortality. Areas with more than expected malaria mortality and those with less than expected mortality appeared to occur randomly, although clusters with more than expected malaria mortality tended to be more common in the western part of the city and those with lower than expected malaria mortality were more ubiquitous in the eastern half of the city.
Figure 8.
Distribution of excess malaria mortality as influenced by housing type. Deep green shows regions with excess risk ratio of less than 0.25, whereas yellow green shows regions with excess risk ratio between 0.25 and 0.50. Pale green shows regions with excess risk ratio between 0.50 and 1.00, flesh-colored areas color shows regions with excess risk ration between 1.00 and 2.00, orange shows regions with excess risk ratio between 2.00 and 4.00, and red shows regions with excess risk ration greater than 4.
Discussion
The main goal of this study was not only to map and describe the patterns of malaria and diarrhea mortalities in a rapidly urbanizing area in a low income country but also to identify the foci of excess malaria and diarrhea mortalities in an area with high heterogeneity of neighborhood environmental conditions in order to allow for comparison across space. A scrutiny of the excess malaria and diarrhea mortality maps showed that socioeconomic and environmental conditions in distinct urban areas very much explained the observed excess mortality patterns, thus suggesting that their deterioration could spell elevation in the risk of mortality in urban spaces. The association observed between excess mortality, and urban environmental and socioeconomic conditions are consistent with findings from several published studies15–19 and strengthens the case that disease control programs in urban centers in low income countries would benefit tremendously from environmental management.
Infectious diseases, especially malaria and diarrhea are widely reported to exhibit spatial clustering.8,15,16,18,20–22 In these high transmission areas, infectious diseases may tend to cluster with respect to socioeconomic factors or may cluster based on differing levels in environmental conditions.8,15,23–26 There are several published reports of studies which found excess urban diarrhea cases to occur in areas which lack access to potable water, poor hygiene conditions, and low income neighborhoods.15,16,18,22,23,27–29 Malaria is believed to have elevated incidences in areas with more breeding opportunities for the mosquito vector.17,18,29,30
In the current study, a “hotspot” of high malaria mortality was observed in the neighborhood of an open lagoon while two “cold spots” were far removed from the lagoonal influences, thus suggesting that large water bodies in urban spaces could exert some amount of influence on malaria mortality, an observation which was quite consistent with findings from other studies.15,19,29,30 This observation has an important implication for urban health, which means urban health policy could be strengthened if strategic objectives and program scope were broadened to encompass the larger urban structure and physical landforms, including surface water bodies and other social matrices and not just the conventional approach of focusing on only hygiene, water supply, and sanitation.
In order to lower the excess malaria mortality, it is reasonable to advocate for increased deployment of insecticide bednets in areas identified as hotspots. On the contrary, the observed pattern of diarrhea mortality was rather random and exhibited strong evidence of clustering of only low–low values (i.e., only cold spots) in the neighborhood of a closed freshwater lagoon in the eastern part of Accra. No evidence exists in the literature that demonstrates that closed freshwater bodies in urban environments are associated with lower malaria or diarrhea mortality, but the striking clustering of malaria and diarrhea mortality (i.e., malaria mortality hotspots near an open saline lagoon and diarrhea mortality cold spots near a closed freshwater lagoon) may provide leads for further investigation into influences of urban lagoons and wetlands on infectious disease mortality.27,31–35 Such further investigations would provide additional evidence for informed decision-making on health policy change in rapidly urbanizing areas in countries with limited health sector budgets.
Finally, the strong evidence of local level spatial autocorrelation and of the association between excess mortality maps, on the one hand, and socioeconomic and environmental quality conditions, on the other hand, suggest that both the socioeconomic and the environmental covariates may be important risk factors for urban malaria and diarrhea mortalities.
Bearing in mind that malaria and diarrhea both exhibit strong clustering, we deliberately chose geospatial statistical tools, which are better suited for such analyses. In hazard or risk analysis, random effects are assumed to represent different clusters which theoretically are assumed to be independent.36 However, random effects corresponding to clusters that are spatially arranged and in close proximity to each other might be similar in magnitude or might exhibit spatial interdependency.36,37 This means that the key assumption in ordinary frailty models may not be sufficient to deal with the complicated dependencies in spatial settings.38 In order to account for such spatial interdependence, the inclusion of a random effect (frailty) term in ordinary frailty models have become routinely necessary.36 For this analysis, we employed geostatistical approaches, which are an extension of ordinary frailty models to accommodate spatial correlations in modeling the observed and unobserved urban malaria and diarrhea mortalities in Accra.
The data used in the analysis were based on reported deaths, which were assigned to clusters on the basis of house address of the normal place of residence prior to death. It was understood that errors of house address would lead to cause-of-death misallocation which could then affect the study outcome and results. While it was not possible to verify house address by confirmation visits, having to deal with over 30,000 death records, we checked and corrected errors of house address by matching information on cause-of-death certificate with that recorded in registers at the Births and Deaths Registry. Generally speaking, although misdiagnosis was a problem for both malaria and diarrhea, it was more a problem in the diagnosis of malaria (febrile fevers) compared to diarrhea (fairly specific definition “passage of more than 3 loose watery stools per day”). Additionally, malaria could typically be overdiagnosed in the wet season compared to dry season in Ghana. However, we assumed that misdiagnosis was a nondifferential error/bias in terms of cluster allocation of death records, as it was deemed to affect all census clusters equally, i.e., there was no differential misdiagnosis by census cluster and by cause. All deaths except for still-births and some neonatal deaths occurring in urban areas were assigned cause-of-death and place address. In general, the validity of our analysis was dependent on the accuracy and reliability of the reported data, a function of the strength of the Ghanaian vital registration system. In order to address the numerous data problems fairly robustly, we computed the cluster level fraction of deaths due to a specific (PMRfds) as a derivative of PMR. However, an obvious limitation of our summary measure was that it was computed using all-cause mortality as the denominator, and so, if the risk of mortality for one of the components of all-cause mortality varied by cluster, then this could affect the summary/outcome measure PMRfd and, therefore, bias the final results of analysis. A way to deal with this problem will be to exclude such components, but unfortunately, we did not have the liberty of telling a priori if any of the components of all-cause mortality exhibited differential mortality risk by cluster, and therefore, this bias could not be controlled for. Another way to deal with this bias will be to use a specific cause of death known to have a uniform risk level across all clusters as the denominator for both the observed proportion and the expected proportion. Then again, this also requires knowledge of risk distribution by cause in the study area and which unfortunately was not available for the study area.
In conclusion, although socioeconomic and the environmental conditions may both be important risk factors for urban diarrhea mortalities, there are remarkable differences in how the different diseases are associated with urban conditions. Our opinion was that diarrheal control strategies may have worked effectively to bridge socioenvironmentally induced diarrhea inequalities than those of malaria. On the basis of the observed patterns of malaria hotspots and diarrhea cold spots around the biggest lagoons, we hypothesize that surface water bodies, which are media for mosquito larval development and the growth of pathogens, may be influencing infectious disease mortality in urban spaces. We, therefore, recommend further case–control and/or longitudinal studies to understand individual level factors, which mediate the socioenvironmental conditions in explaining the observed excess urban mortalities. This may help to channel resources appropriately at high-risk areas and the most vulnerable groups as well as to provide more convincing evidence for urban health policy reforms in rapidly urbanizing areas in low-income settings. Based on these findings, we support the use of small-scale mapping at all times in order to detect zones of vulnerability for an improved targeting of resources aimed at urban malaria and diarrhea control in rapidly urbanizing areas in low income settings.
Acknowledgments
Funding sources
Funding for this study was generously provided jointly by the Government of Ghana, through the GetFund Scholarship, and the German Government, through the DAAD Scholarship.
Authors' contribution
Conception and design by JNF, AK, and JM. Data collection and analysis were done by JNF, CL, TL, and WL. Drafting and revision of the manuscript were done all by authors.
References
- 1.Berkowitz AR, Nilon CH, Hollweg KS. Understanding Urban Ecosystems: a New Frontier for Science and Education. New York: Springer; 2003. p. 523. [Google Scholar]
- 2.Niemela J. Ecology and urban planning. Biodivers Conserv. 1999;8:119–131. doi: 10.1023/A:1008817325994. [DOI] [Google Scholar]
- 3.Rebelee F. Urban ecology and special features of urban ecosystems. Glob Ecol Biogeogr Lett. 1994;4:173–187. doi: 10.2307/2997649. [DOI] [Google Scholar]
- 4.Fobil JN, Armah NA, Hogarh JN, Carboo D. The influence of institutions and organizations on urban waste collection systems: an analysis of waste collection system 430 in Accra, Ghana (1985–2000). J Environ Manage. 2008; 86: 262–271. [DOI] [PubMed]
- 5.Fobil JN, Atuguba RA. Ghana: changing urban environmental ills in slum communities. Int J Environ Policy Law. 2004;34:206–215. [Google Scholar]
- 6.Fobil JN, Atuguba RA. Globalization and Urbanization in Africa. Trenton, NJ: Africa World; 2004. Ghana: migration and the African urban complex. [Google Scholar]
- 7.Fobil J, May J, Kraemer A. Assessing the relationship between socioeconomic conditions and urban environmental quality in Accra, Ghana. Int J Environ Res Public Health. 2010;7:125–145. doi: 10.3390/ijerph7010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bull World Health Organ. 2000;78:1401–1411. [PMC free article] [PubMed] [Google Scholar]
- 9.GSS Population and Housing Census 2000: summary of Final Results; Ghana Statistical Service: Accra; 2002.
- 10.GSS Preliminary Reports. Ghana Demographic and Health Survey 2003.; Ghana Statistical Service: Accra; 2004.
- 11.ESRI ArcGIS 9.3.1, 9.3.1; Redlands, 2009.
- 12.Anselin L. GeoDa 0.9.5-i Mesa, USA, 2004
- 13.Brunsdon C, Fotheringham AS, Charlton M. Geographically weighted regression—modelling spatial non-stationarity. Statistician. 1998;47:431–443. doi: 10.1111/1467-9884.00145. [DOI] [Google Scholar]
- 14.Nakaya T, Fotheringham AS, Brunsdon C, Charlton M. Geographically weighted Poisson regression for disease association mapping. Stat Med. 2005;24:2695–2717. doi: 10.1002/sim.2129. [DOI] [PubMed] [Google Scholar]
- 15.Baragatti M, Fournet F, Henry MC, Assi S, Ouedraogo H, Rogier C, Salem G. Social and environmental malaria risk factors in urban areas of Ouagadougou, Burkina Faso. Malar J. 2009;8:13. doi: 10.1186/1475-2875-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masendu HT, McClean D, Mushavave ST, Chinyowa D, Simbanegavi P, Chawarika C, Ndlovu F. Urban malaria transmission in Mutare City; an unlikely phenomenon. Cent Afr J Med. 2000;46:174–178. doi: 10.4314/cajm.v46i7.8552. [DOI] [PubMed] [Google Scholar]
- 17.Peterson I, Borrell LN, El-Sadr W, Teklehaimanot A. Individual and household level factors associated with malaria incidence in a highland region of Ethiopia: a multilevel analysis. AmJTrop Med Hyg. 2009;80:103–111. [PubMed] [Google Scholar]
- 18.Siri JG, Lindblade KA, Rosen DH, Onyango B, Vulule J, Slutsker L, Wilson ML. Quantitative urban classification for malaria epidemiology in sub-Saharan Africa. Malar J. 2008;7:34. doi: 10.1186/1475-2875-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai PJ, Lin ML, Chu CM, Perng CH. Spatial autocorrelation analysis of health care hotspots in Taiwan in 2006. BMC Public Health. 2009; 9. [DOI] [PMC free article] [PubMed]
- 20.Morrow RH, Jr. Epidemiological evidence for the role of falciparum malaria in the pathogenesis of Burkitt's lymphoma. IARC Sci Publ. 1985:177–186. [PubMed]
- 21.Biggar RJ, Nkrumah FK. Burkitt's lymphoma in Ghana: urban–rural distribution, time–space clustering and seasonality. Int J Cancer. 1979;23:330–336. doi: 10.1002/ijc.2910230310. [DOI] [PubMed] [Google Scholar]
- 22.Ajjampur SS, Gladstone BP, Selvapandian D, Muliyil JP, Ward H, Kang G. Molecular and spatial epidemiology of cryptosporidiosis in children in a semiurban community in South India. J Clin Microbiol. 2007;45:915–920. doi: 10.1128/JCM.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biritwum RB, Asante A, Amoo PK, Gyekye AA, Amissah CR, Osei KG, Appiah-Poku YA, Welbeck JE. Community-based cluster surveys on treatment preferences for diarrhoea, severe diarrhoea, and dysentery in children aged less than five years in two districts of Ghana. J Health Popul Nutr. 2004;22:182–190. [PubMed] [Google Scholar]
- 24.Henry MC, Alary M, Desmet P, Gerniers M, Muteteke D, Nku I, Mutombo L, Piot P. Community survey of diarrhoea in children under 5 years in Kinshasa, Zaire. Ann Soc Belg Med Trop. 1995;75:105–114. [PubMed] [Google Scholar]
- 25.Kandala NB, Magadi MA, Madise NJ. An investigation of district spatial variations of childhood diarrhoea and fever morbidity in Malawi. Soc Sci Med. 2006;62:1138–1152. doi: 10.1016/j.socscimed.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schirnding YE, Yach D, Blignault R, Mathews C. Environmental determinants of acute respiratory symptoms and diarrhoea in young coloured children living in urban and peri-urban areas of South Africa. S Afr Med J. 1991;79:457–461. [PubMed] [Google Scholar]
- 27.Lawoyin TO, Ogunbodede NA, Olumide EA, Onadeko MO. Outbreak of cholera in Ibadan, Nigeria. Eur J Epidemiol. 1999;15:367–370. doi: 10.1023/A:1007547117763. [DOI] [PubMed] [Google Scholar]
- 28.Samore MH, Venkataraman L, DeGirolami PC, Arbeit RD, Karchmer AW. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med. 1996;100:32–40. doi: 10.1016/S0002-9343(96)90008-X. [DOI] [PubMed] [Google Scholar]
- 29.Ye Y, Louis VR, Simboro S, Sauerborn R. Effect of meteorological factors on clinical malaria risk among children: an assessment using village-based meteorological stations and community-based parasitological survey. BMC Publ Health. 2007;7:101. doi: 10.1186/1471-2458-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson I, Borrell LN, El-Sadr W, Teklehaimanot A. A temporal–spatial analysis of malaria transmission in Adama, Ethiopia. AmJTrop Med Hyg. 2009;81:944–949. doi: 10.4269/ajtmh.2009.08-0662. [DOI] [PubMed] [Google Scholar]
- 31.Macintyre K, Keating J, Sosler S, Kibe L, Mbogo CM, Githeko AK, Beier JC. Examining the determinants of mosquito-avoidance practices in two Kenyan cities. Malar J. 2002;1:14. doi: 10.1186/1475-2875-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Liu M. [Current situation on the treatment modules of diarrhea cases in 12 counties/cities of Guangdong, Henan and Gansu provinces in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2008;29:989–993. [PubMed] [Google Scholar]
- 33.Bhandari N, Taneja S, Mazumder S, Bahl R, Fontaine O, Bhan MK. Adding zinc to supplemental iron and folic acid does not affect mortality and severe morbidity in young children. J Nutr. 2007;137:112–117. doi: 10.1093/jn/137.1.112. [DOI] [PubMed] [Google Scholar]
- 34.Nelson KC, Palmer MA, Pizzuto JE, Moglen GE, Angermeier PL, Hilderbrand RH, Dettinger M, Hayhoe K. Forecasting the combined effects of urbanization and climate change on stream ecosystems: from impacts to management options. J Appl Ecol. 2009;46:154–163. doi: 10.1111/j.1365-2664.2008.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graaf RE, Giesen NC, Ven FH. The closed city as a strategy to reduce vulnerability of urban areas for climate change. Water Sci Technol. 2007;56:165–173. doi: 10.2166/wst.2007.548. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee S, Wall MM, Carlin BP. Frailty modeling for spatially correlated survival data, with application to infant mortality in Minnesota. Biostatistics. 2003;4:123–142. doi: 10.1093/biostatistics/4.1.123. [DOI] [PubMed] [Google Scholar]
- 37.Zhao L. Mixtures of Polya trees for flexible spatial frailty survival modelling. Biometrika. 2009;96:263–276. doi: 10.1093/biomet/asp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Ryan L. Modeling spatial survival data using semiparametric frailty models. Biometrics. 2002;58:287–297. doi: 10.1111/j.0006-341X.2002.00287.x. [DOI] [PubMed] [Google Scholar]