Abstract
Whether automated estimated glomerular filtration rate (eGFR) reporting for patients is associated with improved provider recognition of chronic kidney disease (CKD), as measured by diagnostic coding of CKD in those with laboratory evidence of the disease, has not been explored in a poor, ethnically diverse, high-risk urban patient population. A retrospective cohort of 237 adult patients (≥20 years) with incident CKD (≥1 eGFR ≥60 ml/min/1.73 m2, followed by ≥2 eGFRs <60 ml/min/1.73 m2 ≥3 months apart)—pre- or postautomated eGFR reporting—was identified within the San Francisco Department of Public Health Community Health Network (January 2005–July 2009). Patients were considered coded if any ICD-9-CM diagnostic codes for CKD (585.x), other kidney disease (580.x–581.x, 586.x), or diabetes (250.4) or hypertension (403.x, 404.x) CKD were present in the medical record within 6 months of incident CKD. Multivariable logistic regression was used to obtain adjusted odds ratios (ORs) for CKD coding. We found that, pre-eGFR reporting, 42.5 % of incident CKD patients were coded for CKD. Female gender, increased age, and non-Black race were associated with lower serum creatinine and lower prevalence of coding but comparable eGFR. Prevalence of coding was not statistically significantly higher overall (49.6 %, P = 0.27) or in subgroups after the institution of automated eGFR reporting. However, gaps in coding by age and gender were narrowed post-eGFR, even after adjustment for sociodemographic and clinical characteristics: 47.9 % of those <65 and 30.3 % of those ≥65 were coded pre-eGFR, compared to 49.0 % and 52.0 % post-eGFR (OR = 0.43 and 1.16); similarly, 53.2 % of males and 25.4 % of females were coded pre-eGFR compared to 52.8 % and 44.0 % post-eGFR (OR 0.28 vs. 0.64). Blacks were more likely to be coded in the post-eGFR period: OR = 1.08 and 1.43 (Pinteraction > 0.05). Automated eGFR reporting may help improve CKD recognition, but it is not sufficient to resolve underidentification of CKD by safety net providers.
Keywords: Chronic kidney disease, Diagnostic coding, Estimated glomerular filtration rate, Female, African American
Introduction
Chronic kidney disease (CKD) is highly prevalent (up to 13 % of US adults),1 and the costs of care for related to prevalent CKD patients represented 14 % of the 2008 Medicare budget.2 While CKD is associated with excessive mortality and morbidity, provider awareness of CKD remains less than optimal.3–5 It is generally agreed that CKD recognition is necessary for optimization of CKD care, but identification of CKD may not be a priority in poor urban populations due to several factors, including the often silent nature of the disease in early stages; lack of resources to care for CKD, which often follows a protracted course; and the plethora of patient comorbid conditions that require immediate care by providers of these populations. However, it is well-established that financially disadvantaged populations are at higher risk of CKD development, progression, and mortality,6–10 independent of the risks conferred by demographic and clinical factors that are common in this population. Thus, patients who receive health care in an urban safety-net system may especially benefit from increased systemwide CKD interventions to increase identification.
Implementation of automated estimated glomerular filtration rate (eGFR) reporting side-by-side with serum creatinine values at clinical laboratories may improve CKD recognition in the urban poor overall and, particularly, among patients whose creatinine values are generally lower and less likely to be perceived as abnormal, i.e., older, female, or non-Black patients. Such standardization could help to decrease gaps in CKD identification and provide opportunities for earlier and better management in previously underrecognized populations with CKD.
Whether provider recognition of CKD improves after institution of automated laboratory reporting with serum creatinine measurement, and, particularly, whether changes over time differ by age, gender, and race/ethnicity in an underserved, high-risk urban population, remains unexplored. We sought to determine whether diagnostic coding for CKD differed overall and by sociodemographic characteristics, before and after the institution of automatic eGFR reporting, in incident CKD patients treated within the safety-net population of the San Francisco Department of Public Health Community Health Network.
Methods
Study Design and Population
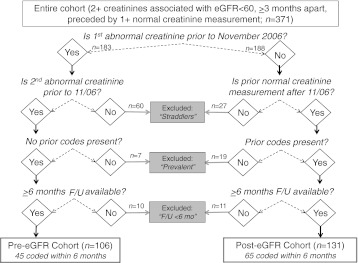
We assembled two cohorts, before (n = 183) and after (n = 188) the institution of automated eGFR reporting, among 371 adult (≥20 years) incident CKD patients within the San Francisco Department of Public Health Community Health Network, a safety-net health system of 12 full-service public health clinics with a special emphasis and commitment to serving the most poor, vulnerable, diverse population in the city. We examined provider recognition of CKD among these cohorts of patients, as defined by CKD coding within 6 months after CKD incidence as defined through laboratory testing. Patients were included in the cohorts if they had incident CKD after January 1, 2005, and before July 31, 2009, defined by at least two outpatient visits and two creatinine measurements associated with eGFR <60 ml/min/1.73 m2 over at least 3 months (CKD-defining measurements; median time between measurements, 152 days), preceded by at least one normal eGFR (≥60 ml/min/1.73 m2) prior to the first CKD-defining measurement. Mean eGFR <60 ml/min/1.73 m2 after the second CKD-defining measurement was also required to define incident CKD. Patients whose two CKD-defining creatinine measurements spanned the date of institution of automated eGFR reporting (November 1, 2006) were excluded (n = 60), as were patients whose prior normal creatinine and first CKD-defining measurement spanned this date (n = 27; Fig. 1a). The exclusion of these “straddlers” (Fig. 1b) allowed for similar cohort formation before and after institution of automated eGFR reporting. Also excluded were patients who had an existing code for CKD in the medical record prior to their first CKD-defining measurement (“prevalent,” presumably identified via eGFR prior to the start of the study or urine testing; n = 26) and patients who did not have at least 6 months of follow-up after their second defining creatinine during which the provider could code CKD (n = 21; Fig. 1a and b). The final cohort comprised 237 incident CKD patients.
Figure 1.
Assignment of incident CKD patients to pre- or post-eGFR cohort (before and after institution of automated eGFR reporting), coding status, and excluded status.
Measurements and Definitions
Predictor Variables
Patients were assigned to pre- and post-eGFR cohorts by whether both CKD-defining creatinine measurements fell before or after the laboratory instituted automated eGFR reporting with serum creatinine (Fig. 1a and b). Prior to this date, eGFR was only reported if specifically ordered by the provider. After this date, all serum creatinine assay results were accompanied by two eGFR values (for “African American” and “non-African American”). eGFR was calculated by the MDRD Study equation (four-variable),11 and both values were adjusted for patient gender. Age, gender, race/ethnicity, and poverty status were obtained from the medical record.
Outcome Variable
CKD coding was defined by the presence of an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnostic code in the medical record for CKD (585.x), other kidney disease (580.x–581.x, 586.x), or diabetes (250.4) or hypertension (403.x, 404.x) CKD, within 6 months of the second CKD-defining creatinine measurement. Specific CKD codes (585.x) were also examined separately.
Covariates
Serum creatinine was measured using an ADVIA chemistry analyzer (Siemens Healthcare Diagnostics, Deerfield, IL) by the Jaffe method (before June 2008) and by enzymatic assay after June 2008. Diagnosed diabetes and essential hypertension were defined by the presence of ICD-9-CM diagnostic codes (250.x and 401.x, respectively) in the medical record.
Statistical Analyses
Patient characteristics were compared across the pre- and post-eGFR cohorts. Additionally, crude means of serum creatinine and eGFR and crude percentages of patients who received a CKD code over follow-up were calculated by patient characteristic. Multivariable-adjusted logistic regression, with calculation of odds ratios (ORs), was used to assess the presence, direction, magnitude, and independence of an association between patient characteristics (age, gender, and race/ethnicity) and coding in the pre- and post-eGFR reporting enrollment periods. All analyses were performed with Stata v. 11.1 (StataCorp, College Station, TX). Statistical significance was set at P < 0.05.
Results
Patient Characteristics
Of the 237 patients included in the study, 106 (44.7 %) had their CKD-defining measurements documented prior to the institution of eGFR reporting (pre-eGFR reporting cohort). Those with CKD-defining measurements documented after eGFR reporting began (n = 131; post-eGFR cohort) were not statistically significantly different from those in the pre-eGFR reporting cohort in terms of sociodemographic or clinical variables (Table 1). The overall cohort was 37 % female, had a mean age of 56 years, and had a mean eGFR of 49 ml/min/1.73 m2 (Table 1). Most patients fell below the poverty line. Overall (pre- and post-eGFR reporting), 46 % had any code for kidney disease, whereas 43 % had a CKD-specific code of 585.x. Diabetes (17 %) and hypertension (30 %) were also commonly diagnosed.
Table 1.
Characteristics of incident CKD patients by study cohort
| Characteristic | Overall | Assigned cohort | ||
|---|---|---|---|---|
| Pre-eGFR | Post-eGFR | P | ||
| Number | 237 | 106 | 131 | – |
| Incidence per month | 4.4 | 4.6 | 4.2 | 0.25 |
| Sociodemographic | ||||
| Mean (SD) age (years) | 56.0 (13.3) | 57.1 (13.4) | 55.1 (13.2) | 0.24 |
| Gender | ||||
| % male | 63.3 | 61.3 | 64.9 | 0.57 |
| % female | 36.7 | 38.7 | 35.1 | |
| Race/ethnicity | ||||
| % Black | 27.9 | 29.3 | 26.7 | 0.23a |
| % not Black | 72.1 | 70.7 | 73.3 | |
| % White | 35.4 | 38.7 | 32.8 | 0.67b |
| % Hispanic | 12.7 | 13.2 | 12.2 | |
| % Asian | 21.5 | 15.1 | 26.7 | |
| % other | 2.5 | 3.8 | 1.5 | |
| Poverty status | ||||
| % PIR ≤ 100 | 67.6 | 63.4 | 70.2 | 0.30 |
| % PIR > 100 | 32.4 | 36.6 | 29.8 | |
| Clinical | ||||
| Mean (SD) serum creatinine (mg/dl) | 1.81 (1.18) | 1.86 (1.23) | 1.77 (1.14) | 0.54 |
| Mean (SD) eGFR (ml/min/1.73 m2) | 48.8 (15.5) | 46.9 (13.9) | 50.4 (16.5) | 0.09 |
| Mean (SD) no. outpatient visits | 26.9 (53.4) | 30.3 (55.8) | 24.3 (51.4) | 0.39 |
| Any CKD coding | ||||
| % with no code | 53.6 | 57.5 | 50.4 | 0.27 |
| % with code | 46.4 | 42.5 | 49.6 | |
| CKD (585.x) coding | ||||
| % with no code | 56.1 | 50.9 | 60.3 | 0.15 |
| % with code | 43.4 | 49.1 | 39.7 | |
| Diabetes | ||||
| % without diagnosis | 83.5 | 82.1 | 84.7 | 0.58 |
| % with diagnosis | 16.5 | 17.9 | 15.3 | |
| Hypertension | ||||
| % without diagnosis | 70.0 | 72.6 | 67.9 | 0.43 |
| % with diagnosis | 30.0 | 27.4 | 32.1 | |
Pre-eGFR prior to institution of automated eGFR reporting, Post-eGFR after the institution of eGFR reporting, PIR poverty index ratio, with federal poverty level = 100
aAcross all race/ethnicity categories
bFor Black vs. non-Black categories
The overall mean serum creatinine was 1.8 mg/dl, and the mean value and percentage with abnormal values varied substantially by age, gender, and race/ethnicity (Table 2). There were no differences in the pre- vs. post-eGFR cohorts in serum creatinine values, either overall or by subgroup (P > 0.3 for all; not shown in table). Notably, the percentage coded for CKD was lower among those with mean serum creatinine <1.3 vs. ≥1.3 mg/dl, both pre- (15.6 % vs. 54.1 %) and post-eGFR reporting (32.6 % vs. 58.8 %). Mean eGFR, on the other hand, was more consistent among subgroups, with values of 47–51 ml/min/1.73 m2 for all subgroups (Table 2).
Table 2.
Mean serum creatinine and eGFR and percentage coded for CKD over the entire study period among individuals with eGFR < 60 ml/min/1.73 m2 by patient characteristic
| Mean Scra (mg/dl) | % with abnormal Scrb | Mean eGFR, (ml/min/1.73 m2) | % coded in 6 months | P* | |
|---|---|---|---|---|---|
| Overall | 1.81 | 79.8 % | 48.8 | 46.4 % | – |
| Age | |||||
| 20–64 | 1.90 | 85.1 % | 48.2 | 48.4 % | 0.23 |
| ≥65 | 1.48 | 59.2 % | 51.3 | 38.8 % | |
| Gender | |||||
| Male | 2.01 | 87.3 % | 48.3 | 54.0 % | 0.002 |
| Female | 1.47 | 66.7 % | 49.7 | 33.3 % | |
| Race/ethnicity | |||||
| Black | 2.22 | 93.9 % | 47.3 | 53.0 % | 0.20 |
| Other | 1.65 | 74.3 % | 49.4 | 43.9 % |
Scr serum creatinine
aFrom first abnormal creatinine forward
bLaboratory abnormal thresholds: 1.3 mg/dl for males and 1.1 mg/dl for females
*P for % coded across subcategory by χ2 test
Association of Patient Characteristics with CKD Coding Pre- and Post-eGFR Reporting
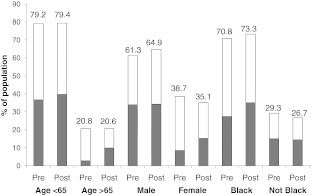
Those who were followed post-eGFR reporting were not statistically more likely to be coded, relative to those enrolled pre-eGFR reporting (OR = 1.34, 95 % CI, 0.80–2.24). In general, an increase in coding was seen in age and race subgroups immediately after the institution of automated eGFR reporting, with subsequent steady prevalence or slight declines in coding over time (data not shown). Despite the increases seen in all subgroups post-eGFR reporting, there appeared to be a persistent “coding gap,” with the proportion coded being less than the proportion of their counterparts who were coded, among older, female, and non-Black patients (Fig. 2). This gap was narrowed, but not eliminated, in the post-eGFR cohort, with the greatest narrowing in older and female patients (Fig. 2).
Figure 2.
Population distributions of patients by age, sex, and race/ethnicity, before (Pre) and after (Post) the institution of eGFR reporting. The shaded portions represent the proportion of the population that was coded for CKD; the white portions represent the noncoded proportion of the population.
The association of post- vs. pre-eGFR reporting with increased prevalence of coding was of greater magnitude among older (OR = 2.48, P = 0.14) and female (OR = 2.74, P = 0.04) patients, relative to their counterparts. Female gender was significantly associated with >70 % lower odds of being coded for CKD in the pre-eGFR period (Table 3); the association of gender with coding was no longer statistically significant in the post-eGFR period. Older age was also associated with >50 % lower odds of coding in the pre-eGFR period but not in the post-eGFR period, but the interaction between cohort and age were not statistically significant. Black race (Table 3) was not associated with significantly different odds of being coded in either time period. With adjustment for sociodemographic and clinical characteristics, those with diabetes were less likely to be coded than those without diabetes in the pre-eGFR period (39.6 % vs. 53.4 %) and more likely in the post-eGFR period (53.4 % vs. 49.0 %). For hypertension, the pattern was reversed: 55.3 % and 39.1 % of those with and without hypertension were coded pre-eGFR, compared to 43.0 % and 53.0 % post-eGFR. However, none of these differences by either condition or time period were statistically significant (not shown in table).
Table 3.
Association of patient characteristics with CKD coding by patient cohort
| Characteristic | Pre-eGFR | Post-eGFR | P*interaction | ||||
|---|---|---|---|---|---|---|---|
| % coded | OR (95 % CI) | % coded | OR (95 % CI) | ||||
| Age | <65 years | ≥65 years | ≥65 vs. <65 | <65 years | ≥65 years | ≥65 vs. <65 | |
| Unadjusted | 46.4 | 27.3 | 0.43 (0.15–1.21) | 50.0 | 48.1 | 0.93 (0.40–2.17) | 0.14 |
| Adjusted | |||||||
| +Sociodemographic | 47.8 | 30.9 | 0.46 (0.14–1.46) | 49.7 | 49.4 | 0.99 (0.42–2.34) | 0.31 |
| +Clinical | 47.9 | 30.3 | 0.43 (0.13–1.49) | 49.0 | 52.0 | 1.16 (0.43–3.13) | 0.58 |
| Gender | Male | Female | Female vs. male | Male | Female | Female vs. male | |
| Unadjusted | 55.3 | 22.0 | 0.23 (0.09–0.55) | 52.9 | 43.5 | 0.68 (0.33–1.41) | 0.06 |
| Adjusted | |||||||
| +Sociodemographic | 53.1 | 25.2 | 0.29 (0.10–0.81) | 53.0 | 43.3 | 0.67 (0.33–1.40) | 0.20 |
| +Clinical | 53.2 | 25.4 | 0.28 (0.10–0.80) | 52.8 | 44.0 | 0.64 (0.28–1.44) | 0.20 |
| Race/ethnicity | Other | Black | Black vs. other | Other | Black | Black vs. other | |
| Unadjusted | 38.7 | 51.6 | 1.69 (0.73–3.93) | 47.9 | 54.3 | 1.29 (0.59–2.81) | 0.64 |
| Adjusted | |||||||
| +Sociodemographic | 42.4 | 47.1 | 1.23 (0.45–3.35) | 47.8 | 54.6 | 1.32 (0.60–2.90) | 0.94 |
| +Clinical | 43.4 | 45.1 | 1.08 (0.38–3.10) | 47.8 | 55.0 | 1.43 (0.58–3.51) | 0.66 |
Pre-eGFR prior to institution of automated eGFR reporting, Post-eGFR after the institution of eGFR reporting, OR odds ratio, CI confidence interval, +Sociodemographic adjusted for all other sociodemographics listed in the table and poverty status, +Clinical additionally adjusted for mean eGFR, mean number of outpatient visits, diabetes, and hypertension
*P for interaction of characteristic with time (pre- vs. post-eGFR reporting)
Mean eGFR (adjusted OR = 1.57 per 10 ml/min/1.73 m2 decrease, P = 0.001) and number of visits (adjusted OR = 1.01 per visit, P = 0.06) were both associated with greater odds of CKD coding. However, these associations were similar pre- and post-eGFR reporting. Additionally, adjustment for mean eGFR and number of visits did not substantially change any other associations or effect modifications we explored. Restricting analyses to only those with mean eGFR <45 ml/min/1.73 m2, resulted in the following adjusted ORs in the pre- vs. post-eGFR reporting periods: age, 2.44 vs. 1.44 (Pinteraction = 0.65) and gender, 0.27 vs. 0.64 (Pinteraction = 0.58). Black race and eGFR < 45 perfectly predicted coding in the post-eGFR reporting period. Primary care provider specialty was associated with a difference in CKD coding prevalence (general internal medicine, 52.0 %; family practice, 29.6 %; and nurse practitioner/physician assistant, 40.7 %; P = 0.06). However, within specialty, no statistically significant differences for pre- vs. post-eGFR reporting were seen (data not shown).
Discussion
Prior to the institution of automated eGFR reporting, 42 % of this high-risk safety-net patient population with reduced kidney function had a documented diagnostic code for kidney disease within 6 months of detectable CKD. While less than optimal, this represents a far higher proportion of appropriate CKD coding than that seen in most other patient populations. Guessous et al.12 found that only 14 % of patients with eGFRs of 10–59 ml/min/1.73 m2 in a managed care population had codes for CKD. Our relatively high prevalence of detection relative to prior studies may reflect the academic setting and provider familiarity with the increased risk of CKD in a poor, ethnically diverse, urban patient population. Despite this relatively high prereporting prevalence, minimal (generally nonstatistically significant) improvements in the prevalence of CKD coding were seen overall and in patient subgroups after the implementation of automated eGFR reporting, with prevalence of coding for most groups remaining <50 % post-eGFR reporting,
We found that older and female patients had a decreased likelihood of having a documented CKD code, with females being half as likely as males with similar kidney function to have a code. In contrast, Black patients had higher prevalence of CKD coding than their non-Black counterparts. These findings may be partially due to lower abnormal thresholds for serum creatinine among older, female, and non-Black patients; in fact, we saw dramatically lower serum creatinine values among these patients. These differences in coding, while reduced in magnitude, persisted post-eGFR reporting, despite similar eGFRs in older and female patients, relative to their counterparts. Factors other than recognition of reduced kidney function may affect documentation of CKD including more attention to competing health issues, concern over unnecessary nephrology referrals, and provider's lack of knowledge about the risk of CKD. However, increases in coding, particularly of younger female and non-Black patients among the urban poor, may improve outcomes due to earlier detection and intervention.
While ICD-9-CM coding has been shown to detect relatively few cases of CKD,13 greater CKD documentation with ICD-9-CM codes has been associated with lower eGFR and a decrease in nonsteroidal antiinflammatory drug prescriptions, giving face validity to the use of ICD-9-CM codes as a measure of physician awareness of CKD.12 Indeed, we also found that coding was significantly associated with eGFR in our cohort. However, there are other markers of provider awareness that could not be examined here. For example, previous studies in the United Kingdom14 and Australia15 have shown that automated eGFR reporting with serum creatinine orders increased primary care provider recognition, as measured by nephrology referral rates. Similarly, a Canadian study16 demonstrated increased nephrology visits, with the greatest increases in female and older patients. Appropriate changes in drug dose and prescription, also not available here, could provide another marker of provider awareness of CKD. A recent literature review17 showed that nephrology referrals increased 13–270 % after institution of automated eGFR reporting, but change in the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (which can help prevent CKD progression as well as control blood pressure) only increased 0–6 %. Similarly, a retrospective cohort study of predialysis care showed that comanagement of CKD by primary care providers and nephrologists increased from 23 % to 49 % post-eGFR reporting, resulting in greater NSAID avoidance and phosphorus and parathyroid hormone testing, but angiotensin-converting enzyme inhibitors/angiotensin receptor blocker use or urinary albumin testing remained unchanged.18 Unfortunately, we did not have data on referrals or medications, so we could not compare the value of CKD coding relative to these other possible markers of provider awareness.
Many of these previous studies showing increased provider recognition of CKD (both overall and traditionally underrecognized populations) with automated eGFR reporting have been performed in far different healthcare systems and patient populations than the safety-net system serving the indigent patients we studied. Thus, our results showing an increase in coding postautomated eGFR reporting—without substantial narrowing of observed gaps in CKD coding—may reflect the inability of eGFR reporting to overcome challenges to CKD detection in a complex, resource-constrained healthcare system. Particularly, it may reflect the extent to which primary care providers do not feel empowered to actually slow CKD progression or it may reflect providers' unwillingness to overburden a safety-net system with more specialist referrals or treatments19 or label a patient with another diagnosis that may not change their management. Some prior studies coupled concomitant provider education and/or management programs with the institution of automated eGFR reporting, which was not performed at this site. Additionally, our results suggest that coding may vary substantially by primary care provider specialty. Thus, targeted provider education may be necessary to garner the attention of physicians who are busy managing a complex patient population and to alter possible preconceived notions about risk and prevalence of CKD in certain subpopulations. Such education efforts should include information about the high prevalence yet low awareness of CKD, particularly among populations that may require a higher index of suspicion, such as females; the major risk factors for CKD, including diabetes and hypertension, but also other factors that may not be currently be ascertained during routine clinic visits, such as family history; and clinical strategies for primary and secondary prevention of CKD, such as tight control of blood pressure, use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and closer oversight of the use of other potentially nephrotoxic prescription and over-the-counter medications used by these patients, with dosage adjustment as necessary.
This study has several other limitations, most notably the relatively small sample sizes in our incident CKD cohorts pre- and post-eGFR reporting, which limited our power to detect true differences in coding prevalence. The limitations of eGFR in estimating kidney function should also be considered. Compared to the MDRD equation, the CKD-EPI equation20 has been shown to be more accurate in the higher range of eGFR, resulting in fewer false-positive reports. However, this equation is not yet in routine use for automated laboratory reporting. Additionally, there is no race/ethnicity-based correction for eGFR, except the single indicator for Black race, for either the MDRD Study or the CKD-EPI equation. Thus, automated eGFR reporting is less likely to impact coding prevalence in non-Black ethnic populations, such as Hispanics and Asians, who represent nearly 50 % of our study population. Finally, there may have been secular trends resulting in increased uptake of CKD coding, e.g., the introduction and dissemination of stage-specific codes for CKD in October 2005, potentially masking the effect of eGFR reporting.
Despite these limitations, we have shown that appropriate CKD coding in this underserved urban population at high risk for CKD and its progression was relatively higher than the prevalence seen in other managed care populations, possibly due to provider knowledge of increased risk in indigent populations. However, even with available automated eGFR reporting, as many as half of the patients in this high-risk population with CKD remained possibly unrecognized and, thus, less likely to receive optimal CKD care to prevent progressive disease. Further, in this poor urban population, gaps existed in the prevalence of CKD coding, with older, female, and non-Black patients being less likely to be coded. Lower abnormal serum creatinine thresholds in these subpopulations likely partially explain this phenomenon. Additionally, the institution of automated eGFR reporting was associated with a higher prevalence of coding overall. Yet, eGFR reporting did not entirely eliminate these gaps in coding. Thus, automated eGFR reporting does not appear to be sufficient to increase provider detection and coding in similar patient populations. Targeted provider education regarding the importance of CKD recognition through the use of eGFR over serum creatinine may be necessary to achieve optimal management of CKD in a safety-net urban setting.
Acknowledgments
We thank the patients and providers of the San Francisco Department of Community Health Network. Ms. Plantinga and Dr. Powe were partially supported by K24DK02643, Dr. Hsu was partially supported by K24DK92291, and Drs. Hsu and Powe were partially supported by R34DK093992, all from the National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MD. Dr. Tuot was partially supported by UCSF KL2 RR024130 and an American Kidney Fund Clinical Scientist in Nephrology grant. Dr. Grubbs is supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Renal Diseases Diversity Supplement to R01 DK70939.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.USRDS 2010 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. [Google Scholar]
- 3.Boulware LE, Troll MU, Jaar BG, Myers DI, Powe NR. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis. 2006;48(2):192–204. doi: 10.1053/j.ajkd.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal V, Ghosh AK, Barnes MA, McCullough PA. Perception of indications for nephrology referral among internal medicine residents: a national online survey. Clin J Am Soc Nephrol. 2009;4(2):323–328. doi: 10.2215/CJN.03510708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lea JP, McClellan WM, Melcher C, Gladstone E, Hostetter T. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis. 2006;47(1):72–77. doi: 10.1053/j.ajkd.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Hall YN, Choi AI, Chertow GM, Bindman AB. Chronic kidney disease in the urban poor. Clin J Am Soc Nephro. 2010;5(5):828–835. doi: 10.2215/CJN.09011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis. 2010;55:992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClellan WM, Newsome BB, McClure LA, Howard G, Volkova N, Audhya P, Warnock DG. Poverty and racial disparities in kidney disease: the REGARDS study. Am J Nephrol. 2010;32:38–46. doi: 10.1159/000313883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce MA, Beech BM, Crook ED, Sims M, Wyatt SB, Flessner MF, Taylor HA, Williams DR, Akylbekova EL, Ikizler TA. Association of socioeconomic status and CKD among African Americans: the Jackson Heart study. Am J Kidney Dis. 2010;55:1001–1008. doi: 10.1053/j.ajkd.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehrotra R, Norris K. Hypovitaminosis D, neighborhood poverty, and progression of chronic kidney disease in disadvantaged populations. Clin Nephrol. 2010;74(Suppl 1):S95–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Guessous I, McClellan W, Vupputuri S, Wasse H. Low documentation of chronic kidney disease among high-risk patients in a managed care population: a retrospective cohort study. BMC Nephrol. 2009;10:25. doi: 10.1186/1471-2369-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grams ME, Plantinga LC, Hedgeman E, et al. Validation of CKD and related conditions in existing data sets: a systematic review. Am J Kidney Dis. 2011; 57: 44–54. [DOI] [PMC free article] [PubMed]
- 14.Richards N, Harris K, Whitfield M, et al. Primary care-based disease management of chronic kidney disease (CKD), based on estimated glomerular filtration rate (eGFR) reporting, improves patient outcomes. Nephrol Dial Transplant. 2008;23(2):549–555. doi: 10.1093/ndt/gfm857. [DOI] [PubMed] [Google Scholar]
- 15.Noble E, Johnson DW, Gray N, et al. The impact of automated eGFR reporting and education on nephrology service referrals. Nephrol Dial Transplant. 2008;23(12):3845–3850. doi: 10.1093/ndt/gfn385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmelgarn BR, Zhang J, Manns BJ, et al. Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. JAMA. 2010;303(12):1151–1158. doi: 10.1001/jama.2010.303. [DOI] [PubMed] [Google Scholar]
- 17.Kagoma YK, Weir MA, Iansavichus AV, et al. Impact of estimated GFR reporting on patients, clinicians, and health-care systems: a systematic review. Am J Kidney Dis. 2011; 57: 592–601. [DOI] [PubMed]
- 18.Abdel-Kader K, Fischer GS, Johnston JR, Gu C, Moore CG, Unruh ML. Characterizing pre-dialysis care in the era of eGFR reporting: a cohort study. BMC Nephrol. 2011;12(1):12. doi: 10.1186/1471-2369-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung CK, Bhandari S. Perspectives on eGFR reporting from the interface between primary and secondary care. Clin J Am Soc Nephrol. 2009;4(2):258–260. doi: 10.2215/CJN.05151008. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]