Abstract
AIDS Clinical Trials Group protocol 388 was designed to compare a three-drug regimen (indinavir with dual nucleosides) to a four-drug regimen (indinavir plus nelfinavir or indinavir plus efavirenz with dual nucleosides). Blood samples from patients taking indinavir and nelfinavir were collected over 8 to 12 h following a specified dose and were analyzed with high-performance liquid chromatography. Pharmacokinetic data were derived by using noncompartmental analysis. Following administration of indinavir every 8 h in the absence of nelfinavir (n = 8), the median predose indinavir concentration (C0) was 369 ng/ml (range, <10 to 949 ng/ml; one subject had a concentration of <10 ng/ml), and the concentration 8 h after administration of the study dose was 159 ng/ml (range, 85 to 506 ng/ml). In the group receiving 1,000 mg of indinavir every 12 h with nelfinavir (n = 10), the median indinavir C0 was <10 ng/ml (range, <10 to 3,740 ng/ml; six subjects had a value of <10 ng/ml), and the C12 h was 44 ng/ml (range, <10 to 4,236 ng/ml; five subjects had a value of <10 ng/ml), while the subjects who received 1,200 mg of indinavir every 12 h with nelfinavir (n = 7) had a C0 of 146 ng/ml (range, 58 to 5,215 ng/ml) and a C12 h of 95 ng/ml (range, 12 to 954 ng/ml). Indinavir clearance was significantly lower in the presence of nelfinavir (median [interquartile range], 34.1 liters/h [range, 22.6 to 45.8 liters/h] versus 47.9 liters/h [range, 42.7 to 70.3 liters/h]; P < 0.017). For subjects receiving 1,000 mg of indinavir every 12 h, the median C0 value for nelfinavir (n = 9) was 1,779 ng/ml (range, <187.5 to 4,579 ng/ml), and the C12 h was 1,554 ng/ml (range, <187.5 to 5,540 ng/ml). Due to the unacceptable number of undetectable indinavir trough concentrations, 1,200 mg of indinavir appears to be the preferred dose in a twice-daily regimen that includes nelfinavir.
Following the introduction of protease inhibitors and nonnucleoside reverse transcriptase inhibitors, various strategies were evaluated to determine optimal combinations to achieve viral suppression for human immunodeficiency virus (HIV)-infected patients. In addition, the strategy of combining protease inhibitors based on anticipated positive pharmacokinetic interactions was an area of active clinical investigation. To further study these clinical questions in antiretroviral-naive subjects, AIDS Clinical Trials Group protocol (ACTG) 388 was designed to compare a three-drug regimen (indinavir with dual nucleosides) to a four-drug regimen (either indinavir plus nelfinavir or indinavir plus efavirenz with dual nucleosides) (6). At the time that ACTG 388 was implemented, limited data were available describing the drug-drug interaction between indinavir and nelfinavir. For this protease inhibitor combination, early pharmacokinetic information suggested that the combination of the two protease inhibitors would lead to a favorable pharmacokinetic profile for each drug, allowing for every-twelfth-hour dosing, and one report has demonstrated antiviral activity of this protease inhibitor combination (11). The initial ACTG 388 indinavir-nelfinavir regimen included indinavir at a dosage of 1,000 mg every 12 h (q12h) but was subsequently changed to 1,200 mg q12h. These two pharmacokinetic studies form the basis of this report.
The rationale for combining indinavir and nelfinavir and employing prolonged administration intervals (e.g., q12h) was based on the premise that positive drug interactions would occur via competition for cytochrome P450 (CYP) enzymes, thereby resulting in greater drug exposure over a 12-h dosing interval (3; Viracept package insert; Agouron Pharmaceuticals). Therefore, the objective for these two substudies was to examine indinavir and nelfinavir disposition in antiretroviral-naive, HIV-infected subjects to determine protease inhibitor drug exposure during an extended dosing interval (q12h).
MATERIALS AND METHODS
Study design and subjects
Subjects were included according to the following criteria. (i) HIV-1 infection as documented by any licensed enzyme-linked immunosorbent assay test kit and subsequently confirmed by either Western blot (preferred), HIV culture, HIV antigen, plasma HIV RNA, or a second antibody test (other than enzyme-linked immunosorbent assay) at any time prior to study entry. (ii) A CD4 cell count of ≤200 cells/mm3 or a plasma HIV RNA level of ≥80,000 copies/ml (using the standard Roche Amplicor HIV-1 monitor assay) within 60 days prior to study entry. (iii) Zidovudine (ZDV), zalcitabine, didanosine, or stavudine therapy alone or in combination any time prior to study entry or no prior antiretroviral therapy (treatment naive). Subjects with intolerance to 600 mg of ZDV per day, defined as any grade of toxicity which resulted in a dose reduction or termination of ZDV, could substitute stavudine for ZDV at any time during the study. (iv) A Karnofsky performance status of ≥70 within 14 days prior to study entry. (v) The following laboratory parameters within 14 days prior to study entry: hemoglobin level of ≥9.1 g/dl for men and ≥8.9 g/dl for women, absolute neutrophil count of ≥850 cells/mm3, platelet count of ≥45,000 platelets/mm3, an aspartate aminotransferase (serum glutamic oxaloacetic transaminase)/alanine aminotransferase (serum glutamic pyruvic transaminase) ratio of ≤5.0 times the upper limit of normal (ULN), total serum amylase level of ≤1.5 times the ULN (if the serum amylase was more than 1.5 times the ULN, pancreatic amylase or lipase level must have been ≤1.5 times the ULN), serum creatinine level of ≤2.0 times the ULN. (vi) Age of at least 13 years.
All women of childbearing potential were required to use an acceptable form of birth control to prevent pregnancy while receiving study medications and for 3 months thereafter. Due to potential interactions between study medications and oral contraceptives or depo-progesterone, oral contraceptives or depo-progesterone was not used as the sole form of birth control. Women of childbearing potential had a negative serum or urine β-human chorionic gonadotropin within 14 days prior to study entry.
Subjects were excluded according to the following criteria. (i) Acute therapy for a serious infection or other serious medical illnesses that were potentially life threatening and that required systemic therapy and/or hospitalization within 14 days prior to study entry. (ii) Subjects with a serious infection or serious medical illness who had not completed acute therapy <14 days prior to study entry. (iii) Subjects with other infections or medical illnesses (e.g., vaginitis, folliculitis, bronchitis, pharyngitis, etc.) who completed acute therapy prior to study entry, with the exception of oral thrush, which had no restriction. (iv) Unexplained temperature of >38.5°C for any 7 days or chronic diarrhea (defined as >3 liquid stools per day persisting for 15 days and within 30 days prior to study entry). (iv) A malignancy which required systemic therapy. Subjects with minimal Kaposi's sarcoma, defined as no more than five cutaneous lesions and no visceral disease or tumor-associated edema, were allowed to enroll as long as they did not require systemic therapy for Kaposi's sarcoma.
Subjects were not receiving the following medications: erythropoietin, granulocyte colony-stimulating factor, or granulocyte-macrophage colony-stimulating factor within 30 days prior to study entry; interferons, interleukins, or HIV vaccines within 30 days prior to study entry; any experimental therapy within 30 days prior to study entry; or amiodarone, astemizole, cisapride, ergot alkaloids (or drugs containing derivatives of ergot alkaloids), itraconazole, midazolam, quinidine, rifampin, terfenadine, or triazolam within 14 days of study entry.
Women who were pregnant or breast-feeding were excluded. All subjects meeting eligibility criteria were randomly assigned to one of three treatment arms. Treatment was started within 72 h after randomization. Subjects who volunteered to participate in the substudies were registered for the main study at the same time that they were registered to a substudy. Subjects were assigned to treatment in the main study by using a permuted block randomization within each AIDS clinical trials unit with stratification by screening the CD4 cell count (≤50 cells/mm3 versus >50 cells/mm3), screening HIV-1 RNA (≤40,000 copies/ml versus >40,000 copies/ml), and prior antiretroviral treatment (no therapy versus any therapy). Prior to their participation in the substudy, all subjects were required to give informed consent in accordance with the consent process approved by each participating site's investigational review board.
Subjects enrolled in ACTG 733 and 5060 were instructed to take their study medications on a regular schedule and to then return to the study clinic on or after day 14 for an 8-h (indinavir, 800 mg, q8h) or 12-h (indinavir, 1,000 or 1200 mg, q12h [with nelfinavir, 1250 mg, q12h]) pharmacokinetic study. On the day of the pharmacokinetic study, the subjects arrived in a fasted state and a catheter was placed to allow venous access for serial blood sampling. A blood sample was collected from patients before they received their morning medications. The study medications were taken with water, and blood sampling continued at 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 10.0, and 12.0 h (for q12h regimens only). The subjects took no additional medications until the completion of the pharmacokinetic sampling period. Study medications were given at approximately 8:00 in the morning, with a light breakfast within 30 min of dosing. After a protocol-mandated increase in the indinavir dose from 1,000 mg every 12 h to 1,200 mg every 12 h, a second cohort of subjects were asked to repeat the pharmacokinetic study after at least 2 weeks on the increased indinavir dose. Blood samples were collected in a 5-ml heparinized tube and centrifuged for 10 min at 800 × g for use in the indinavir and nelfinavir assay. After plasma separation, the plasma was split into two equal aliquots. The samples were stored at −70°C until shipment by overnight delivery to an Adult AIDS Clinical Trials Group (AACTG) pharmacology support laboratory for high-performance liquid chromatography analysis.
Data analysis
Standard noncompartmental techniques using WinNonlin version 2.1 (Pharsight, Palo Alto, Calif.) were used to assess pharmacokinetic parameters. The area under the concentration-time curve (AUC) was determined by using the linear-trapezoidal method, and the maximum observed concentration (Cmax) and time to maximum observed concentration (Tmax) were determined by visual inspection. If the sample drawn at the end of the dosing interval was not available or increased in concentration compared to the previous time point, the concentration reported was determined by extrapolation using the estimated terminal elimination rate. Clearance (CL) was calculated as CL = dose/AUC. Pharmacokinetic parameters or their log transforms were compared among groups by using the two-sample t test in SAS software version 8 (SAS Institute, Cary, N.C.).
Drug assays
Indinavir and nelfinavir were measured in plasma with high-performance liquid chromatography in two AACTG pharmacology support laboratories (nelfinavir was measured at Stanford University [Stanford, Calif.]; indinavir was measured at the University of California, San Francisco) using methods validated within the AACTG quality assurance proficiency testing program. The lower limits of quantitation were <10 ng/ml and <187.5 ng/ml for indinavir and nelfinavir, respectively.
RESULTS
Of the subjects enrolling in ACTG 733, 8 were receiving dual nucleosides with 800 mg of indinavir q8h and 10 were receiving dual nucleosides with 1,000 mg of indinavir q12h and 1,250 mg of nelfinavir q12h. Seven subjects enrolled in AACTG 5060S after having their indinavir doses increased to 1,200 mg q12h with dual nucleosides and 1,250 mg of nelfinavir q12h. Two subjects had indinavir concentration-time profiles for both dosing regimens. Figure 1 displays the concentration-time profiles of each indinavir group, and pharmacokinetic parameters are listed in Table 1. In the group receiving 1,000 mg of indinavir q8h, the median predose indinavir concentration was 369 ng/ml (range, <10 to 949 ng/ml; one patient was <10 ng/ml) and the median concentration 8 h after the study dose (C8 h) was 159 ng/ml (range, 85 to 506 ng/ml). The median peak indinavir concentration (Cmax) was 5,508 ng/ml (range, 2,864 to 19,786 ng/ml), and the time to peak (Tmax) occurred at 0.5 to 2.0 h after the dose. The median AUC for an 8-h dosing interval and half-life values for subjects receiving 1,000 mg of indinavir every 8 h were 16,715 h · ng/ml (range, 5,705 to 28,084 h · ng/ml) and 1.3 h (range, 0.9 to 1.9 h), respectively.
FIG. 1.
Indinavir (IDV) plasma concentration profiles for 800 mg of indinavir q8h (top), 1,000 mg of indinavir q12h with 1,250 mg of nelfinavir q12h (middle), and 1,200 mg of indinavir q12h with 1,250 mg of nelfinavir q12h (bottom). Each symbol and associated line represent a subject. Concentrations less than 10 μg/liter were below the lower limit of detection and are therefore not included herein.
TABLE 1.
Pharmacokinetic parameters for indinavir and nelfinavira
| Regimenb | C0 (ng/ml) | Clast (ng/ml)c | Cmax (ng/ml) | AUC (h · ng/ml) | t1/2 (h)d |
|---|---|---|---|---|---|
| Indinavir 800 mg q8h | 369 (<10-949) | 159 (85-506) | 5,508 (2,864-19,786) | 16,715 (5,705-28,084) | 1.3 (0.9-1.9) |
| Indinavir dosage (with nelfinavir) | |||||
| 1,000 mg q12h | <10 (<10-3,740) | 44 (<10-4,236) | 10,740 (3,379-18,210) | 38,863 (10,623-135,665) | 1.8 (1.1-2.4) |
| 1,200 mg q12h | 146 (58-5,215) | 95 (12-954) | 9,675 (6,154-18,198) | 26,901 (19,139-63,595) | 1.3 (1.0-2.4) |
| Nelfinavir plus indinavir | |||||
| Nelfinavir 1,250 mg q12h and indinavir 1,000 mg q12h | 1,779 (<187.5-4,579) | 1,554 (<187.5-5,540) | 5,826 (2,437-9,337) | 33,106 (15,434-81,717) | 3.6 (1.8-31) |
| Nelfinavir 1,250 mg q12h and indinavir 1,200 mg q12h | 1,805 (611-8,307) | 534 (189-4,270) | 5,641 (1,869-9,974) | 33,269 (10,494-89,539) | 2.4 (1.9-10.9) |
Values are medians (ranges).
The numbers of subjects included in the indinavir 1,000 mg q8h, 1,000 mg q12h, and 1,200 mg q12h analyses were 8, 10, and 7, respectively, and the numbers of subjects included in the nelfinavir with indinavir 1,000 mg and 1,200 mg q12h analyses were 9 and 7, respectively.
Clast is the concentration 8 h after the dose (for the q8h regimen) and 12 h after the dose (for the q12h regimen).
t1/2, half-life.
In the group receiving 1,000 mg of indinavir every 12 h, the median predose indinavir concentration was <10 ng/ml (range, <10 to 3,740 ng/ml; six subjects had a value of <10 ng/ml) and the median concentration 12 h after the study dose (C12 h) was 44 ng/ml (range, <10 to 4,236 ng/ml; five subjects had a value of <10 ng/ml). The median Cmax was 10,740 ng/ml (range, 3,379 to 18,210 ng/ml), and Tmax occurred at 1.0 to 7.5 h after administration of the dose. The median dosing interval AUC (AUC12) and half-life values for subjects receiving 1,000 mg of indinavir every 12 h were 38,863 h · ng/ml (range, 10,623 to 135,665 h · ng/ml) and 1.8 h (range, 1.1 to 2.4 h), respectively. In the group receiving 1,200 mg of indinavir every 12 h, the median predose indinavir concentration was 146 ng/ml (range, 58 to 5,215 ng/ml) and the median C12 h was 95 ng/ml (range, 12 to 954 ng/ml). The median Cmax was 9,675 ng/ml (range, 6,154 to 18,198 ng/ml), and Tmax occurred at 0.5 to 2.0 h after the dose. The median AUC12 and half-life for subjects receiving 1,200 mg of indinavir every 12 h were 26,901 h · ng/ml (range, 19,139 to 63,595 h · ng/ml) and 1.3 h (range, 1.0 to 2.4 h), respectively. The higher incidence of predose and 12-h postdose concentrations of indinavir below the limit of detection in the group that received 1,000 mg every 12 h did not appear to be due to inappropriate sampling times. In the group receiving 1,000 mg every 12 h, the time since last dose for the predose and C12 h samples ranged from 8.8 to 14.2 h (one subject was 21.3 h) and 11.5 to 12.0 h, respectively.
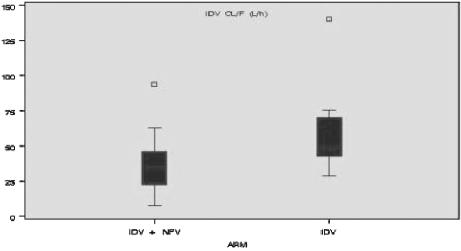
Nelfinavir appeared to decrease clearance of indinavir (Fig. 2). Median indinavir clearance was significantly lower (P < 0.017) for subjects who received nelfinavir (34.1 liters/h [interquartile range, 22.6 to 45.8 liters/h]) than for those who did not receive nelfinavir (47.9 liters/h [interquartile range, 42.7 to 70.3 liters/h]).
FIG. 2.
Indinavir (IDV) clearance with and without concomitant administration of nelfinavir (NFV). The median is represented by the horizontal line inside the box; the top and bottom of the box represent the third quartile (75th percentile) and the first quartile (25th percentile), respectively. Whiskers are drawn from the upper edge of the box to the maximum observation within the upper fence (1.5 times the interquartile range [IQR] above the third quartile) and from the lower edge of the box to the minimum observation within the lower fence (1.5 times the IQR below the first quartile). Observations that fall beyond the fences are identified individually with symbols.
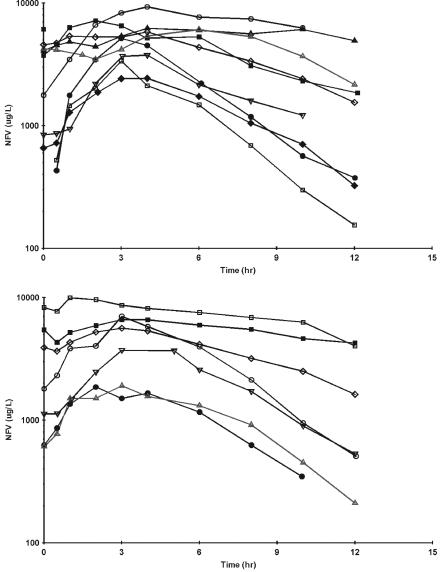
The concentration-time profiles for nelfinavir are shown in Fig. 2. Two subjects had nelfinavir concentration-time profiles for both indinavir dosing regimens. For the nine subjects receiving 1,250 mg of nelfinavir with 1,000 mg of indinavir every 12 h, the median predose nelfinavir concentration was 1,779 ng/ml (range, <187.5 to 4,579 ng/ml) and the median C12 h was 1,554 ng/ml (range, <187.5 to 5,540 ng/ml). The median (range) Cmax was 5,826 ng/ml (range, 2,437 to 9,337 ng/ml), and Tmax occurred at 2.0 to 6.0 h after administration of the dose. The median AUC for one 12-h dosing interval (AUC12) and half-life were 33,106 h · ng/ml (range, 15,434 to 81,717 h · ng/ml) and 3.6 h (range, 1.8 to 31 h), respectively. The following results are from seven subjects who received 1,250 mg of nelfinavir with 1,200 mg of indinavir every 12 h. The median predose nelfinavir concentration was 1,805 ng/ml (range, 611 to 8,307 ng/ml), and the median C12 h was 534 ng/ml (range, 189 to 4,270 ng/ml). The median Cmax was 5,641 ng/ml (range, 1,869 to 9,974 ng/ml), and Tmax occurred at 1.0 to 3.0 h after the dose. The median AUC12 and half-life values were 33,269 h · ng/ml (range, 10,494 to 89,539 h · ng/ml) and 2.4 h (range, 1.9 to 10.9 h), respectively. Four subjects, two from each substudy, had a markedly slow nelfinavir decline over the last 8 h of the interval (Fig. 3).
FIG. 3.
Nelfinavir (NFV) plasma concentration profiles in subjects receiving 1,000 mg of indinavir q12h with 1,250 mg of nelfinavir q12h (top) or 1,200 mg of indinavir q12h with 1,250 mg of nelfinavir q12h (bottom). Each symbol and associated line represent a subject. Two subjects in each substudy demonstrated a prolonged plasma concentration profile.
DISCUSSION
The combination of indinavir and nelfinavir used in ACTG 388 was developed based on the premise that these two protease inhibitors would compete for CYP 3A4, leading to increased exposure of both protease inhibitors. However, nelfinavir metabolism has been more clearly described since the design of this study. Nelfinavir is primarily metabolized by CYP 2C9 and 2C19, yielding the M8 metabolite, which has antiviral activity and is similar in potency to the parent compound. The M8 metabolite is, in turn, further oxidized via CYP 3A4, which is then eliminated (D. M. Burger, R. M. W. Hoetelmans, J. W. Mulder, P. L. Meenhorst, P. W. H. Hugen, K. Brinkman, and P. P. Koopmans, Abstr. 12th World AIDS Conf., abstr. 42275, 1998; 2, 9, 10, 11).
Our results indicate that the combination of indinavir and nelfinavir may not provide optimal exposure due to unpredictable interaction potential. If indinavir and nelfinavir are combined and administered every 12 h, 1,200 mg of indinavir is a more appropriate dose than 1,000 mg. The risks of low indinavir exposure when using a twice-daily indinavir regimen have been emphasized when comparing results of twice-daily to thrice-daily regimens (8). Although target indinavir trough concentrations remain under investigation, recent recommendations have been presented based on the growing amount of evidence supporting the relationship between protease inhibitor concentrations and clinical outcome (D. M. Burger, P. W. Hugen, J. Droste, A. D. Huitema, et al., Abstr. 2nd Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 6.2a and 6.2b, 2001; D. M. Burger et al., 12th World AIDS Conf.; C. V. Fletcher, T. Fenton, C. Powell, et al., Abstr. 8th Conf. Retrovir. Opportunistic Infect., abstr. 259, 2001; 1, 7). Since concentrations below the limit of detection for appreciable periods during a dosing interval are to be avoided, 1,200 mg of indinavir would be the preferred dose when relying on the pharmacokinetic boosting effects of nelfinavir in a twice-daily regimen.
The rationale for combining indinavir and nelfinavir with a goal of prolonging the dosing interval (e.g., to q12h) was based on limited clinical data. Subsequently, a small study demonstrated the antiviral effect of indinavir-nelfinavir combinations, with 45% of subjects achieving <400 viral copies/ml at week 72 (12). Interestingly, the pharmacokinetic profiles in the subjects we studied suggest that some type of interaction is at work when indinavir and nelfinavir are taken together. Nelfinavir coadministration resulted in significantly slower drug clearance (Fig. 2) and higher 24-h AUCs. This interaction was not evident in the only other report of this combination (12). For example, Fig. 1 illustrates subjects with a rapid decline in indinavir plasma concentrations over a 12-h dosing interval. In contrast, certain subjects had plasma indinavir concentrations that were sustained and exceed what are considered to be reasonable minimum plasma concentrations (e.g., 80 to 120 ng/ml) (1).
Nelfinavir is metabolized by 2C19; therefore, indinavir is not expected to act as a nelfinavir-boosting agent. Although we did not have a group that received nelfinavir alone for comparison, nelfinavir exposures were similar among substudy groups regardless of indinavir dose. Figure 3 illustrates concentration plots, including two types of subjects with markedly different nelfinavir plasma concentration profiles in each indinavir dosing group. One group had a rapid increase followed by a rapid decline in plasma concentrations, while the second group had an elevated trough at baseline, followed by a slow peak and slow decline. These different patterns suggest that an interaction may occur in certain individuals but not in others. Factors potentially contributing to this observation include those based on pharmacogenetics differences in drug disposition (4, 5). This observation needs to be further investigated in an attempt to identify patient factors that may be associated therewith.
In summary, the results of determining the pharmacokinetics of indinavir and nelfinavir when used in combination in this population of HIV-1-infected subjects indicate that indinavir at a dosage of 1,200 mg every 12 h is the preferred dose. More study is needed to determine whether the addition of a more potent boosting agent, such as ritonavir, will result in increased indinavir exposure. There also appears to be significant pharmacokinetic variability in exposure to nelfinavir when that drug is combined with indinavir.
Acknowledgments
The participation of the subjects in ACTG 388/733/5060 is appreciated. The conduct of the study by the clinical research staff at the AIDS clinical trial units and the Stanford and UCSF Pharmacology Support Laboratories is appreciated.
This work was supported by funding from the National Institute of Allergy and Infectious Diseases, University of Cincinnati AIDS Clinical Trials grant number AI-25897 (Judith Feinberg), University of Washington AIDS Clinical Trials grant number AI 27664 (Ann Collier), University of Miami AIDS Clinical Trials grant number AI27675 (M. Fischl), University at Buffalo Adult AIDS Pharmacology Support Laboratory grant number 200PC006 (G. Morse, R. Dicenzo, and A. Forrest), and by SDAC (grant number 5 U01 AI38855).
The Adult AIDS Clinical Trial Group 388/733/5060 Study Team (National Institute of Allergy and Infectious Diseases, Bethesda, Md.) comprises the following members: Robert DiCenzo (University at Buffalo, Buffalo, N.Y.), Alan Forrest (University at Buffalo), Margaret A. Fischl (University of Miami, Miami, Fla.), Ann Collier (University of Washington, Seattle), Judith Feinberg (University of Cincinnati, Cincinnati, Ohio), Heather Ribaudo (Harvard University, Boston, Mass.), Robin DiFrancecso (University at Buffalo), Gene D. Morse (University at Buffalo), Stefano Vella (Istituto Superiore di Sanita), Marjorie Dehlinger (National Institutes of Health), Kellye Maxwell (AACTG Operations Center), Sandra Dascomb (Frontier Science and Technology Research Foundation, Inc.), Ana Martinez (National Institutes of Health), Alejo Erice (University of Minnesota Medical School), Scott Hammer (Columbia University), Julie McElrath (Fred Hutchinson Cancer Research Center), Allan Rodriguez (University of Miami School of Medicine), Ernesto G. Scerpella (University of Miami), Alfred Saah (Merck & Co., Inc.), Rand Rhodes (Merck & Co., Inc.), Diane Goodwin (GlaxoWellcome, Inc.), Alex Rinehart (Virco, Inc.), Steven Schnittman (Bristol-Myers Squibb), Nancy Ruiz (DuPont Pharmaceuticals Co.), Laura J. Bessen (DuPont Pharmaceuticals Co.), Jeff Schouten, Pualani K. Kondo (University of Hawaii), Marlene Cooper (Frontier Science and Technology Research Foundation, Inc.), and Nick Hellmann (ViroLogic, Inc.).
REFERENCES
- 1.Acosta, E. P., J. G. Gerber, and the Adult Pharmacology Committee of the AIDS Clinical Trials Group. 2002. Position paper on therapeutic drug monitoring of antiviral agents. AIDS Res. Hum. Retrovir. 18:825-834. [DOI] [PubMed] [Google Scholar]
- 2.Baede-van Dijk, P. A., P. W. Hugen, C. P. Verweij-van Wissen, P. P. Koopmans, D. M. Burger, and Y. A. Hekster. 2001. Analysis of variation in plasma concentrations of nelfinavir and its active metabolite M8 in HIV-positive patients. AIDS 15:1057-1058. [DOI] [PubMed] [Google Scholar]
- 3.Capparelli, E. V., J. L. Sullivan, L. Mofenson, E. Smith, B. Graham, P. Britto, M. I. Becker, D. Holland, J. D. Connor, K. Luzuriaga, et al. 2001. Pharmacokinetics of nelfinavir in human immunodeficiency virus-infected infants. Pediatr. Infect. Dis. J. 20:746-751. [DOI] [PubMed] [Google Scholar]
- 4.Casado, J. L., S. Moreno, K. Hertogs, F. Dronda, A. Antela, P. Dehertogh, M. J. Perez-Elias, and A. Moreno. 2002. NELSANE study. Plasma drug levels, genotypic resistance, and virological response to a nelfinavir plus saquinavir-containing regimen. AIDS 16:47-52. [DOI] [PubMed] [Google Scholar]
- 5.Fellay J., C. Marzolini, E. R. Meaden, D. J. Back, T. Buclin, J. P. Chave, L. A. Decosterd, H. Furrer, M. Opravil, G. Pantaleo, D. Retelska, L. Ruiz, A. H. Schinkel, P. Vernazza, C. B. Eap, A. Telenti, et al. 2002. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet 359:30-36. [DOI] [PubMed] [Google Scholar]
- 6.Fischl, M. A., H. J. Ribaudo, A. C. Collier, A. Erice, M. Giuliano, M. Dehlinger, J. J. Eron, Jr., M. S. Saag, S. M. Hammer, S. Vella, G. D. Morse, J. E. Feinberg, L. M. Denter, S. H. Eshleman, and the Adult AIDS Clinical Trials Group 388 Study Team. A randomized trial of 2 different 4-drug antiretroviral regimens versus a 3-drug regimen, in advanced human immunodeficiency virus disease. J. Infect. Dis. 188:625-634. (Erratum, 188:1083.) [DOI] [PubMed]
- 7.Fletcher, C. V., P. L. Anderson, T. N. Kakuda, T. W. Schacker, K. Henry, C. R. Gross, and R. C. Brundage. 2002. Concentration-controlled compared with conventional antiretroviral therapy for HIV infection. AIDS 16:551-560. [DOI] [PubMed] [Google Scholar]
- 8.Haas, D. W., E. Arathoon, M. A. Thompson, R. D. Pedro, J. E. Gallant, D. E. Uip, J. Currier, L. M. Noriega, D. S. Lewi, P. Uribe, L. Benetucci, P. Cahn, D. Paar, A. C. White, Jr., A. C. Collier, C. H. Ramirez-Ronda, C. Harvey, M. O. Chung, D. Mehrotra, J. Chodakewitz, B. Y. Nguyen, et al. 2000. Comparative studies of two-times-daily versus three-times-daily indinavir in combination with zidovudine and lamivudine. AIDS 14:1973-1978. [DOI] [PubMed] [Google Scholar]
- 9.Hsyu, P. H., M. D. Schultz-Smith, J. H. Lillibridge, R. H. Lewis, and B. M. Kerr. 2001. Pharmacokinetic interactions between nelfinavir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and simvastatin. Antimicrob. Agents Chemother. 45:3445-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzolini, C., T. Buclin, L. A. Decosterd, J. Biollaz, and A. Telenti. 2001. Nelfinavir plasma levels under twice-daily and three-times-daily regimens: high interpatient and low intrapatient variability. Ther. Drug Monit. 23:394-398. [DOI] [PubMed] [Google Scholar]
- 11.Merry, C. 2001. Editorial comment on analysis of variation in plasma concentrations of nelfinavir and its active metabolite M8 in HIV-positive patients. AIDS 15:991-998. [DOI] [PubMed] [Google Scholar]
- 12.Riddler, S. A., D. Havlir, K. E. Squires, B. Kerr, R. H. Lewis, K. Yeh, L. H. Wynne, L. Zhong, Y. H. Peng, P. Deutsch, and A. Saah. 2002. Coadministration of indinavir and nelfinavir in human immunodeficiency virus type 1-infected adults: safety, pharmacokinetics, and antiretroviral activity. Antimicrob. Agents Chemother. 46:3877-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]