Abstract
A multiresistant Serratia marcescens strain, HD, isolated from a patient with a urinary tract infection, was resistant to amino-, carboxy-, and ureidopenicillins, ceftazidime, and cefepime and was susceptible to cefotaxime and ceftriaxone, according to the guidelines of the NCCLS. No synergy was found between expanded-spectrum cephalosporins and clavulanic acid, according to the double-disk synergy test. The blaAmpC gene of the strain was amplified by PCR and cloned into Escherichia coli DH10B, giving rise to high-level resistance to ceftazidime, cefepime, and cefpirome. Sequencing analysis revealed that the blaAmpC gene from S. marcescens HD had a 12-nucleotide deletion compared to the blaAmpC gene from reference strain S. marcescens S3, leading to a 4-amino-acid deletion located in the H-10 helix of the β-lactamase. Kinetic analysis showed that this enzyme significantly hydrolyzed ceftazidime, cefepime, and cefpirome. This work underlined that resistance to the latest expanded-spectrum cephalosporins may be mediated by structurally modified AmpC-type β-lactamases.
Serratia marcescens is a saprophytic, water-borne gram-negative rod (7) that possesses an inducible, chromosomally encoded AmpC-type β-lactamase (11) and is often involved in nosocomial infections (5, 8, 16, 19). Infections caused by cephalosporinase-producing Enterobacteriaceae may be difficult to treat due to their ability to confer resistance to a variety of β-lactams, including the expanded-spectrum cephalosporins cefotaxime and ceftazidime. This resistance may arise from at least three different mechanisms in S. marcescens: high-level production of the chromosomal AmpC-type cephalosporinase (9), acquisition of an Ambler class A extended-spectrum β-lactamase (21), and acquisition of metallo-β-lactamases (21). Cefepime and cefpirome, which contain quaternary nitrogen atoms at three different positions, remain active against overexpressed AmpC-producing enterobacteria. This activity results from both the stability of these molecules to attack by cephalosporinase (2, 6) and their rapid penetration through the outer membrane (15, 18).
We analyzed an S. marcescens clinical isolate harboring a chromosomally encoded β-lactamase with β-lactam hydrolysis extended to a cefepime that possessed a 4-amino-acid deletion compared to a wild-type cephalosporinase.
MATERIALS AND METHODS
Bacterial strains and plasmids
Clinical isolate S. marcescens HD was identified by using the API 20E system (bioMérieux, Marcy l'Etoile, France). Escherichia coli DH10B was used for the experiments (3). S. marcescens strain S3 was previously characterized as a wild-type AmpC producer (gift from H. Nikaido) (17).
Cloning of β-lactamase genes
Whole-cell DNAs of S. marcescens isolates HD and S3 were extracted as described previously (3). On the basis of the ampC gene sequence of S. marcescens S3 (17), primers PRESM1 (5′-ATACCCTGCAACCTAAGAGC-3′) and PRESM2 (5′-ATCGCTGGTAGGGGCGCCTC-3′) were designed to amplify a 1,195-bp fragment corresponding to ampC genes without their own promoter sequence. The amplification products were ligated into pBK-CMV phagemid (Stratagene, Amsterdam, The Netherlands) that had previously been digested with the restriction enzyme ScaI (Amersham Pharmacia Biotech, Orsay, France). Whole-cell DNA of S. marcescens HD was also digested by HindIII, and DNA fragments were ligated into HindIII-restricted phagemid vector pBK-CMV. Recombinant phagemids were transformed into E. coli strain DH10B by electroporation with a Gene Pulser II apparatus (Bio-Rad, Ivry-sur-Seine, France). Transformants were selected on Trypticase soy agar containing ampicillin (100 μg/ml) and kanamycin (30 μg/ml). Recombinant plasmids were purified with a plasmid Midi kit (QIAGEN, Courtaboeuf, France). The cloned β-lactamase genes were sequenced on both strands by using an Applied Biosystems sequencer (ABI 377). The nucleotide and deduced protein sequences were analyzed with software available over the Internet from the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov). The ClustalW program (www.infobiogen.fr) was used for the alignment of multiple protein sequences.
IEF analysis
The β-lactamase extracts from cultures of clinical isolates and purified enzymes were subjected to analytical isoelectric focusing (IEF) analysis as previously described (3) by using an ampholine polyacrylamide gel with a pH range of 3.5 to 9.5 (ampholine PAG plate; Amersham Pharmacia Biotech) for 90 min at 1,500 V, 50 mA, and 30 W. The focused β-lactamases were detected by overlaying the gel with a 1 mM nitrocefin solution (Calbiochem, Merck Eurolab SAS, Fontenay-sous-bois, France).
Antimicrobial agents and MIC determination
The antibiotic agents and their sources have been described elsewhere (3). MICs were determined by an agar dilution technique on Mueller-Hinton agar (Sanofi-Diagnostics Pasteur, Paris, France) with an inoculum of 104 CFU per spot and were interpreted according to the guidelines of the NCCLS (14).
β-Lactamase purification
Recombinant E. coli DH10B strains were grown overnight at 37°C in 4 liters of Trypticase soy broth containing amoxicillin (100 μg/ml) and kanamycin (30 μg/ml), resuspended in 40 ml of 100 mM phosphate buffer (pH 7), disrupted by sonication, and centrifuged at 20,000 × g for 1 h at 4°C as previously described (3). β-Lactamase extracts were filtered through a 0.45-μm-pore-size filter (Millipore, Saint-Quentin-en-Yvelines, France), dialyzed overnight at 4°C against 20 mM Tris (pH 7.5), and loaded onto a preequilibrated Q-Sepharose column (Amersham Pharmacia Biotech). The flowthrough fractions containing the β-lactamase were recovered and dialyzed against 50 mM phosphate buffer (pH 6) before being loaded onto a preequilibrated S-Sepharose column (Amersham Pharmacia Biotech). The enzymes were eluted by a linear NaCl gradient (0 to 1 M) in the same buffer. The eluted fractions with the highest β-lactamase activity (nitrocefin test) were pooled and dialyzed against 100 mM phosphate buffer (pH 7). To assess the purity of the extracts, purified enzymes were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (3).
Kinetic measurements
Purified β-lactamases AmpC S3 and AmpC HD were used for kinetic measurements (Km and kcat), which were made at 30°C in 100 mM sodium phosphate (pH 7.0). The rates of hydrolysis were determined with a Pharmacia ULTROSPEC 2000 spectrophotometer and were analyzed by using the SWIFT II software (Amersham Pharmacia Biotech). Km and kcat values were determined by analyzing β-lactam hydrolysis under initial rate conditions by using the Eadie-Hofstee linearization of the Michaelis-Menten equation as previously described (4). When the Km value was very low, the Ki value was determined from the initial rates at saturating substrate concentrations ([S] ≫ Km). Values were the means of three independent measures (the standard deviations of the values were within 15%).
Nucleotide sequence accession number
The nucleotide sequence of the blaAmpC HD gene from S. marcescens isolate HD has been submitted to the GenBank nucleotide database under the accession number AY336102.
RESULTS
Isolation of the clinical isolate
S. marcescens clinical strain HD was isolated from a urine specimen of a patient 7 days after admission into a surgical ward of the Laennec Hospital (Nantes, France) in September 2001. This isolate was selected for further study on the basis of its uncommon pattern of susceptibility to β-lactam antibiotics, including resistance to cefepime. S. marcescens HD was susceptible to aminoglycosides and cotrimoxazole but was resistant to fluoroquinolones. The urinary tract infection was treated successfully with cotrimoxazole for 7 days. No cefepime treatment was given prior to the isolation of this strain.
Cloning and sequence analysis of β-lactamase genes from S. marcescens isolates HD and S3
DNA sequence analysis revealed a lack of 12 nucleotides in the sequence of the blaAmpC HD gene compared to that of the blaAmpC S3 gene (wild-type blaAmpC gene of S. marcescens), leading to a 4-amino-acid deletion, MNGT, at positions 233 to 236 of the protein (Fig. 1). Cloning experiments using PCR products with each of the S. marcescens isolates as templates yielded recombinant strains E. coli DH10B(pBK-HD) and E. coli DH10B(pBK-S3). The alignment of the sequences of these AmpC-type β-lactamases with those of the Enterobacter cloacae P99 and E. cloacae CHE isolates revealed that the position of amino acids MNGT matched that of amino acids SKVALA, which were deleted in the sequence of the extended-spectrum AmpC-type β-lactamase CHE (2) compared to that of the cephalosporinase of E. cloacae P99 (Fig. 1). The cloning experiment using whole-cell DNA of S. marcescens HD digested with HindIII gave another recombinant clone, E. coli DH10B(pBK-TEM-1); the DNA sequence analysis of this insert showed its identity with the blaTEM-1 gene.
FIG. 1.
Alignment of the amino acid sequences of the AmpC β-lactamases of S. marcescens HD, S. marcescens clinical isolate S3, E. cloacae P99 (10), and E. cloacae CHE (2). Asterisks indicate amino acid residues identical to those of the cephalosporinase AmpC HD of S. marcescens HD. The N-terminal amino acid of the mature enzymes is designated position 1. The amino acid sequence from positions −22 to 1 is assumed to be the signal peptide. Dashes show a deletion or the absence of an amino acid residue when the sequences were aligned after optimal matching of the entire protein. The serine β-lactamase motif S-L-S-K, the conserved triad K-T-G, and the Ambler class C typical motif Y-X-N are shaded.
Susceptibility testing
The MICs of several β-lactams for S. marcescens isolates HD and S3 and their corresponding E. coli DH10B clones are reported in Table 1. Susceptibility data showed that S. marcescens HD was resistant to penicillins, narrow-spectrum cephalosporins, ceftazidime, and cefepime; had reduced susceptibility to cefoxitin; and was susceptible to cefotaxime, ceftriaxone, aztreonam, and imipenem. In comparison, S. marcescens S3 was resistant to amoxicillin and narrow-spectrum cephalosporins; had a decreased susceptibility to ticarcillin, piperacillin, and cefoxitin; and was susceptible to ceftazidime, cefotaxime, ceftriaxone, aztreonam, cefpirome, cefepime, and imipenem. E. coli DH10B(pBK-HD) remained susceptible to ticarcillin, aztreonam, and imipenem; had a reduced susceptibility to cefotaxime and ceftriaxone; and was resistant to the other β-lactams tested. MIC determinations for E. coli DH10B(pBK-S3) showed that the pattern of resistance of the recombinant strain mirrored that of the parental strain S3, with a better expression of resistance to ticarcillin, piperacillin, and cefoxitin. Recombinant E. coli DH10B that expressed the blaTEM-1 gene from S. marcescens HD had a typical clavulanate-inhibited phenotype (Table 1).
TABLE 1.
MICs of β-lactams for S. marcescens HD and S3 strains and for recombinant E. coli DH10B(pBK-HD) and E. coli DH10B(pBK-S3)
| β-Lactam(s)f | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| S. marcescens HDa | S. marcescens S3b | E. coli DH10B(pBK-HD)c | E. coli DH10B(pBK-S3)d | E. coli DH10B(pBK-TEM-1)e | E. coli DH10B | |
| Amoxicillin | >512 | >512 | >512 | >512 | >512 | 2 |
| Amoxicillin-CLA | 256 | >512 | 256 | 256 | 512 | 2 |
| Ticarcillin | >512 | 32 | 8 | 128 | >512 | 1 |
| Ticarcillin-CLA | 256 | 32 | 8 | 128 | >512 | 1 |
| Piperacillin | 512 | 16 | 256 | 256 | 64 | 1 |
| Piperacillin-TZB | 32 | 8 | 128 | 128 | 32 | 1 |
| Cephalothin | >512 | >512 | >512 | >512 | 8 | 4 |
| Cefoxitin | 8 | 16 | 128 | 128 | 2 | 2 |
| Cefuroxime | 128 | 512 | 512 | 128 | 0.5 | 0.5 |
| Ceftriaxone | 2 | 0.25 | 16 | 8 | <0.06 | <0.06 |
| Cefotaxime | 2 | 0.25 | 16 | 8 | <0.06 | <0.06 |
| Ceftazidime | 64 | 0.06 | 512 | 16 | <0.06 | <0.06 |
| Aztreonam | 0.5 | 0.06 | 0.25 | 0.06 | 0.06 | 0.06 |
| Cefepime | 32 | 0.06 | 512 | 2 | 0.06 | 0.06 |
| Cefpirome | 8 | 0.06 | 64 | 2 | 0.06 | 0.06 |
| Imipenem | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
S. marcescens isolate HD produced β-lactamases AmpC HD and TEM-1.
S. marcescens isolate S3 reference strain produced β-lactamase AmpC S3.
The recombinant E. coli DH10B(pBK-HD) strain produced β-lactamase AmpC HD.
The recombinant E. coli DH10B(pBK-S3) strain produced β-lactamase AmpC S3.
The recombinant E. coli DH10B(pBK-TEM-1) strain produced β-lactamase TEM-1.
CLA, clavulanic acid at 2 μg/ml; TZB, tazobactam at 4 μg/ml.
Biochemical analysis of β-lactamase AmpC HD from S. marcescens HD and β-lactamase AmpC S3 from S. marcescens S3
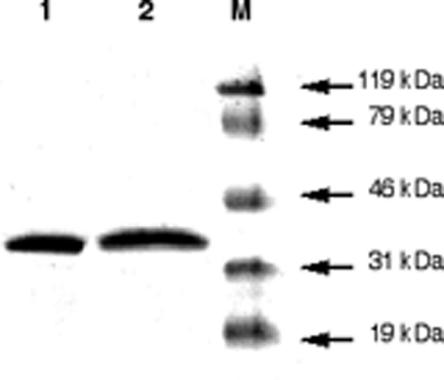
AmpC enzymes were purified to near homogeneity (>99%), as deduced from SDS-PAGE analysis (Fig. 2). The catalytic efficiency of the purified β-lactamase AmpC HD against the expanded-spectrum cephalosporins cefepime, cefpirome, and ceftazidime was at least 20-fold higher than that observed for the S. marcescens S3 enzyme (Table 2). This result was mostly due to a decrease in the Km values for those substrates. However, the affinity of AmpC HD toward benzylpenicillin, amoxicillin, ticarcillin, and piperacillin was decreased compared to that of AmpC S3 (higher Km values).
FIG. 2.
SDS-PAGE analysis of purified β-lactamases from cultures of E. coli DH10B(pBK-HD) and E. coli DH10B(pBK-S3) expressing AmpC β-lactamases HD and S3, respectively. Lane 1, purified AmpC HD; lane 2, purified AmpC S3; lane M, molecular mass markers (size indicated to the right of the figure).
TABLE 2.
Kinetic parameters of β-lactamases AmpC HD and AmpC S3
| β-Lactama | AmpC HD
|
AmpC S3
|
||||
|---|---|---|---|---|---|---|
| kcat (s−1) | Km (μM) | kcat/Km (mM−1 · s−1) | kcat (s−1) | Km (μM) | kcat/Km (mM−1 · s−1) | |
| Benzylpenicillin | 50 | 10 | 5,000 | 35 | 9 | 4,000 |
| Amoxicillin | 0.01 | 1 | 10 | 0.01 | 0.3 | 40 |
| Ticarcillin | <0.001 | 2 | 0.002 | 0.05 | 50 | |
| Piperacillin | 0.1 | 0.5 | 200 | 0.01 | 0.1 | 110 |
| Cephalothin | 95 | 2 | 48,000 | 1,200 | 125 | 9,600 |
| Cefoxitin | 0.01 | 3 | 2 | 0.002 | 1 | 2 |
| Cefuroxime | 4.5 | 5 | 1,000 | 2.5 | 7 | 350 |
| Ceftriaxone | 2 | 4 | 500 | 0.9 | 5 | 180 |
| Cefotaxime | 5 | 2 | 2,300 | 6 | 7 | 800 |
| Ceftazidime | 270 | 20 | 13,000 | 5 | >1,000 | <5 |
| Cefepime | 50 | 6 | 8,400 | 80 | >1,000 | <80 |
| Cefpirome | 270 | 100 | 2,800 | 120 | >1,000 | <120 |
| Aztreonam | <0.001 | <0.001 | ||||
| Imipenem | <0.001 | <0.001 | ||||
For those compounds with Km values less than 5 μM, Ki values were determined instead of Km, with cephalothin as the substrate. The values are the means of results from at least three independent experiments.
IEF analysis
IEF analysis of β-lactamase extracts of culture of S. marcescens HD gave two bands with pI values of 8.6 and 5.4 that comigrated with β-lactamases extracted from E. coli DH10B(pBK-HD), expressing the cephalosporinase variant, and E. coli DH10B(pBK-TEM-1), expressing TEM-1, respectively. Cultures of S. marcescens S3 and E. coli DH10B(pBK-S3) produced a single β-lactamase with a pI of 8.5 that corresponded to the wild-type cephalosporinase S3.
DISCUSSION
This report describes an AmpC-type β-lactamase from S. marcescens that conferred resistance to cefepime. Rare AmpC-type β-lactamases of S. marcescens with an expanded spectrum of hydrolysis have been reported. Raimondi et al. (17) characterized an enzyme with an isoleucine-for-threonine substitution at position 64, conferring resistance to ceftazidime and cefpirome, although its activity against cefepime was not studied. Matsumura et al. (12) have characterized AmpC SRT-1, which has a lysine-for-glutamate substitution at position 213 compared to wild-type AmpC SST-1, which confers resistance to oxyimino cephalosporins. We report here for the first time an S. marcescens strain that is highly resistant to cefepime and cefpirome because of its production of an AmpC-type β-lactamase and that it is also a clinical isolate.
Sequence analysis of AmpC HD indicated that a deletion of 4 amino acids was involved in resistance to ceftazidime, cefepime, and cefpirome. The catalytic efficiencies (kcat/Km) of AmpC HD for these β-lactams were increased compared to those of β-lactamase AmpC S3, taken as the wild-type enzyme, mostly resulting from an increase of the affinity for these substrates. Moreover, DNA sequence analysis revealed that blaAmpC HD was the only cephalosporinase-encoding gene harbored by the S. marcescens HD isolate, suggesting that it was likely the natural, chromosomally encoded blaAmpC gene of the S. marcescens strain that had undergone a 4-amino-acid deletion.
Four AmpC-type variants conferring resistance to cefepime have been described among AmpC β-lactamases of other enterobacterial species. The AmpC harbored by E. cloacae CHE had a 6-amino-acid deletion at positions 289 to 294 (2). A laboratory-obtained E. coli strain harboring AmpC-type β-lactamase of E. cloacae P99 had a proline-for-leucine substitution at position 293 (20). In vivo selection of an AmpC-type enzyme derived from the wild-type enzyme of E. cloacae strain MHN had a glutamic acid-for-valine substitution at position 298 (13). Finally, in vitro-obtained AmpC enzymes with several amino acid substitutions, including replacements at positions 292, 293, 294, 296, and 298 in the sequence of the plasmid-mediated CMY-2 β-lactamase (related to the chromosomally encoded AmpC of Citrobacter freundii), had resistance to cefepime (1). All these substitutions and deletions are located in the vicinity of the H-10 alpha helix, which is close to the C-terminal extremity of the protein and far from the active site located nearby the H-2 helix (10). These sequence changes may explain the extension of the substrate specificity, including that to cefepime, that was related to a decrease in the Km.
However, no crystallographic analysis of the AmpC β-lactamase of S. marcescens is available. Once the amino acid sequences of AmpC β-lactamases of S. marcescens HD and E. cloacae CHE were superimposed, we assumed that the deletion observed in AmpC HD was also located in the H-10 alpha helix (2). β-Lactamases AmpC HD of S. marcescens HD and AmpC CHE of E. cloacae CHE shared the same overall activity toward cephalosporins, providing an increased resistance to cefepime, cefpirome, and ceftazidime, whereas cefotaxime was hydrolyzed weakly. However, β-lactamase AmpC HD of S. marcescens conferred on an E. coli transformant a level of cefepime resistance (MIC > 512 μg/ml) that was higher than that of the AmpC CHE (MIC = 8 μg/ml). We assumed that this discrepancy was due to a 10-fold-higher catalytic efficiency of the AmpC HD β-lactamase.
This work provides further evidence that the substrate specificity of natural, chromosomally encoded AmpC may evolve to confer resistance to cefepime and cefpirome, which may no longer be considered optimal β-lactams for treating infections due to enterobacterial isolates with overexpressed cephalosporinases. However, the nature of the genetic rearrangement found in the β-lactamase gene (a 12-nucleotide deletion) makes the selection of such S. marcescens strains at high frequency unlikely. Finally, it remains to be determined why deletion as a source of extension of the hydrolysis spectrum has been reported for class C β-lactamases and not for β-lactamases belonging to the other Ambler classes.
Acknowledgments
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, Paris, France.
REFERENCES
- 1.Barlow, M., and B. G. Hall. 2003. Experimental prediction of the evolution of cefepime resistance from the CMY-2 AmpC β-lactamase. Genetics 164:23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnaud, G., R. Labia, L. Raskine, M. J. Sanson-Le Pors, A. Philippon, and G. Arlet. 2001. Extension of resistance to cefepime and cefpirome associated to a six amino acid deletion in the H-10 helix of the cephalosporinase of an Enterobacter cloacae clinical isolate. FEMS Microbiol. Lett. 195:185-190. [DOI] [PubMed] [Google Scholar]
- 3.Bellais, S., D. Aubert, T. Naas, and P. Nordmann. 2000. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing β-lactamases in Chryseobacterium meningosepticum. Antimicrob. Agents Chemother. 44:1878-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornish-Bowden, A. 1995. Fundamentals of enzyme kinetics, p. 30-37. Portland Press, Seattle, Wash.
- 5.Cox, C. E. 1985. Aztreonam therapy for complicated urinary tract infections caused by multidrug-resistant bacteria. Rev. Infect. Dis. 7:S767-S770. [DOI] [PubMed] [Google Scholar]
- 6.Hancock, R. E. W., and F. Bellido. 1992. Factors involved in the enhanced efficacy against Gram-negative bacteria of fourth-generation cephalosporins. J. Antimicrob. Chemother. 29:S1-S6. [DOI] [PubMed] [Google Scholar]
- 7.Hejazi, A., and F. R. Falkiner. 1997. Serratia marcescens. J. Med. Microbiol. 46:903-912. [DOI] [PubMed] [Google Scholar]
- 8.Körner, R. J., A. Nicol, D. S. Reeves, A. P. MacGowan, and J. Hows. 1994. Ciprofloxacin resistant Serratia marcescens endocarditis as a complication of non-Hodgkin's lymphoma. J. Infect. 29:73-76. [DOI] [PubMed] [Google Scholar]
- 9.Kuga, A., R. Okamoto, and M. Inoue. 2000. ampR gene mutations that greatly increase class C β-lactamase activity in Enterobacter cloacae. Antimicrob. Agents Chemother. 44:561-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lobkovsky, E., P. C. Moews, H. Liu, H. Zhao, J.-M. Frère, and J. R. Knox. 1993. Evolution of an enzyme activity: crystallographic structure at 2 Å resolution of cephalosporinase from the ampC gene of Enterobacter cloacae P99 and comparison with a class A penicillinase. Proc. Natl. Acad. Sci. USA 90:11257-11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahlen, S. D., S. S. Morrow, B. Abdalhamid, and N. D. Hanson. 2003. Analyses of ampC gene expression in Serratia marcescens reveal new regulatory properties. J. Antimicrob. Chemother. 51:791-802. [DOI] [PubMed] [Google Scholar]
- 12.Matsumura, N., S. Minami, and S. Mitsuhashi. 1998. Sequences of homologous β-lactamases from clinical isolates of Serratia marcescens with different substrate specificities. Antimicrob. Agents Chemother. 42:176-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morosini, M.-I., M.-C. Negri, B. Shoichet, M.-R. Baquero, F. Baquero, and J. Blazquez. 1998. An extended-spectrum AmpC-type β-lactamase obtained by in vitro antibiotic selection. FEMS Microbiol. Lett. 165:85-90. [DOI] [PubMed] [Google Scholar]
- 14.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. NCCLS, Wayne, Pa.
- 15.Nikaido, H., W. Liu, and E. Y. Rosenberg. 1990. Outer membrane permeability and β-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob. Agents Chemother. 34:337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowsky, B. E., C. Whitener, H. K. Bredenberg, L. A. Carson, S. Holt, L. Hutwagner, M. J. Arduino, and W. R. Jarvis. 2002. Serratia marcescens bacteremia traced to an infused narcotic. N. Engl. J. Med. 16:1529-1537. [DOI] [PubMed] [Google Scholar]
- 17.Raimondi, A., F. Sisto, and H. Nikaido. 2001. Mutation in Serratia marcescens AmpC β-lactamase producing high-level resistance to ceftazidime and cefpirome. Antimicrob. Agents Chemother. 45:2331-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders, C. C. 1993. Cefepime: the next generation? Clin. Infect. Dis. 17:369-379. [PubMed] [Google Scholar]
- 19.Sautter, R. L., H. L. Mattman, and R. C. Legaspi. 1984. Serratia marcescens meningitis associated with contaminated benzalkonium chloride solution. Infect. Control 5:223-225. [DOI] [PubMed] [Google Scholar]
- 20.Vakulenko, S. B., D. Golemi, B. Geryk, M. Suvorov, J. R. Knox, S. Mobashery, and S. A. Lerner. 2002. Mutational replacement of Leu-293 in the class C Enterobacter cloacae P99 β-lactamase confers increased MIC of cefepime. Antimicrob. Agents Chemother. 46:1966-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamasaki, K., M. Komatsu, T. Yamashita, K. Shimakawa, T. Ura, H. Nishio, K. Satoh, R. Washidu, S. Kinoshita, and M. Aihara. 2003. Production of CTX-M-3 extended-spectrum β-lactamase and IMP-1 metallo β-lactamase by five gram-negative bacilli: survey of clinical isolates from seven laboratories collected in 1998 and 2000, in the Kinki region of Japan. J. Antimicrob. Chemother. 51:631-638. [DOI] [PubMed] [Google Scholar]