Abstract
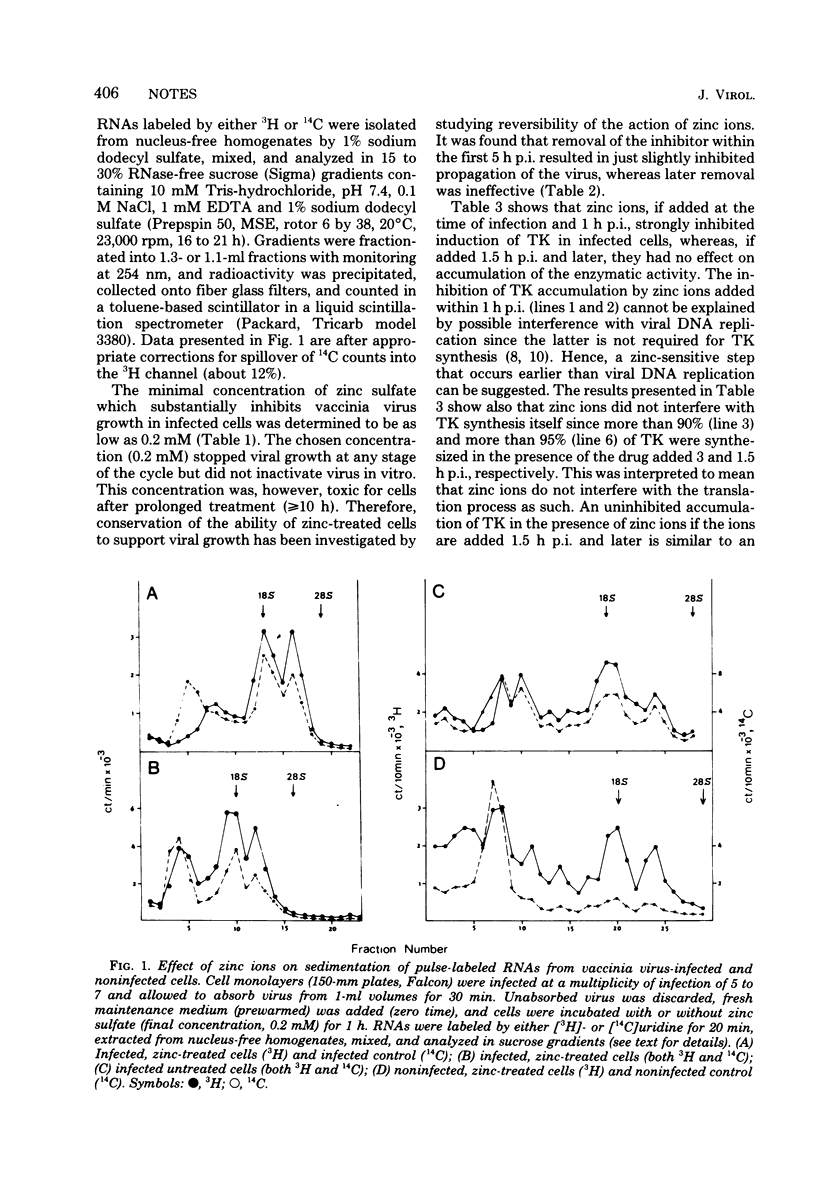
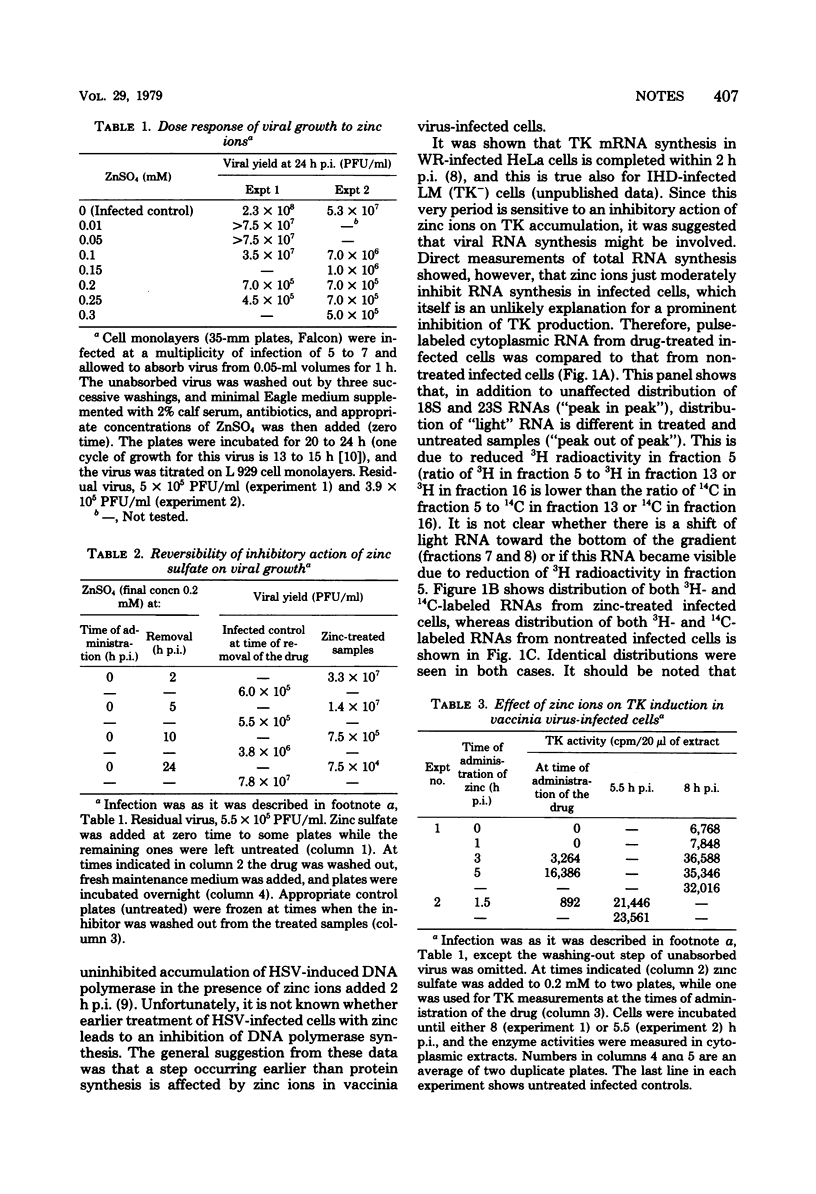
Accumulation of thymidine kinase activity in vaccinia virus-infected cells was severely inhibited by zinc ions if the drug was added within 1 h postinfection. If added later, zinc ions had no effect on the enzyme synthesis. A fraction of RNA which is normally synthesized in infected cells, was missing from a proper part of the gradient if the cells were treated with zinc ions within 1 h postinfection (as has been shown by cosedimentation of pulse-labeled RNAs in isokinetic gradients). It is suggested that a transcriptional (or posttranscriptional) step is involved in zinc-caused inhibition of vaccinia virus growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracha M., Schlesinger M. J. Inhibition of Sindbis virus replication by zinc ions. Virology. 1976 Jul 1;72(1):272–277. doi: 10.1016/0042-6822(76)90330-5. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Rapp F. Effect of zinc ions on synthesis of herpes simplex virus type 2-induced polypeptides. Proc Soc Exp Biol Med. 1976 Jul;152(3):455–458. doi: 10.3181/00379727-152-39417. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- KIT S., PIEKARSKI L. J., DUBBS D. R. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J Mol Biol. 1963 Jan;6:22–33. doi: 10.1016/s0022-2836(63)80078-9. [DOI] [PubMed] [Google Scholar]
- Kit S., Jorgensen G. N., Liav A., Zaslavsky V. Purification of vaccinia virus-induced thymidine kinase activity from [35S]methionine-labeled cells. Virology. 1977 Apr;77(2):661–676. doi: 10.1016/0042-6822(77)90490-1. [DOI] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- Shlomai J., Asher Y., Gordon Y. J., Olshevsky U., Becker Y. Effect of zinc ions on the synthesis of herpes simplex virus DNA in infected BSC-1 cells. Virology. 1975 Jul;66(1):330–335. doi: 10.1016/0042-6822(75)90204-4. [DOI] [PubMed] [Google Scholar]
- Zaslavsky V., Yakobson E. Control of thymidine kinase synthesis in IHD vaccinia virus-infected thymidine kinase-deficient LM cells. J Virol. 1975 Jul;16(1):210–213. doi: 10.1128/jvi.16.1.210-213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



