Abstract
Background
Microdialysis (MD) of the trapezius muscle has been an attractive technique to investigating small molecules and metabolites in chronic musculoskeletal pain in human. Large biomolecules such as proteins also cross the dialysis membrane of the catheters. In this study we have applied in vivo MD in combination with two dimensional gel electrophoresis (2-DE) and mass spectrometry to identify proteins in the extracellular fluid of the trapezius muscle.
Materials and Methods
Dialysate from women with chronic trapezius myalgia (TM; n = 37), women with chronic wide spread pain (CWP; n = 18) and healthy controls (CON; n = 22) was collected from the trapezius muscle using a catheter with a cut-off point of 100 kDa. Proteins were separated by two-dimensional gel electrophoresis and visualized by silver staining. Detected proteins were identified by nano liquid chromatography in combination with tandem mass spectrometry.
Results
Ninety-seven protein spots were identified from the interstitial fluid of the trapezius muscle; 48 proteins in TM and 30 proteins in CWP had concentrations at least two-fold higher or lower than in CON. The identified proteins pertain to several functional classes, e.g., proteins involved in inflammatory responses. Several of the identified proteins are known to be involved in processes of pain such as: creatine kinase, nerve growth factor, carbonic anhydrase, myoglobin, fatty acid binding protein and actin aortic smooth muscle.
Conclusions
In this study, by using in vivo microdialysis in combination with proteomics a large number of proteins in muscle interstitium have been identified. Several of the identified proteins were at least two-fold higher or lower in chronic pain patients. The applied techniques open up for the possibility of investigating protein changes associated with nociceptive processes of chronic myalgia.
Introduction
The risk for developing chronic trapezius myalgia (TM) is increased in several occupations with work tasks such as high exposure to awkward working positions, sustained static and repetitive movements of the arms and movements with high precision demands [1], [2]. Various but often ineffective treatment interventions are frequently implemented [3]. For some of the patients the pain will spread and a chronic widespread pain condition (CWP) can develop [4]. The pathophysiological mechanisms behind TM and CWP are poorly understood.
Microdialysis (MD) mimics the function of a capillary blood vessel by perfusing a thin dialysis tube implanted in the tissue with a physiological saline solution [5]. Substances can pass across the dialysis membrane along the concentration gradient. The dialysate is analyzed and reflects the composition of the extra cellular fluid where nociceptor free nerve endings terminate. Metabolic, algesic and potential nociception/pain inhibitory substances have been studied in chronic myalgia using MD [6]. Significant increases e.g., in serotonin, glutamate, lactate, pyruvate, N-stearoylethanolamine and palmitoylethanolamide have been reported; for a review see [6]. In the majority of hitherto performed studies one or a few small biochemical substances have been exclusively in focus. There are a few attempts to analyze larger molecules, e.g., proteins, with various results [7]–[10]. Proteins can act as signal substances, activate the formation of algesics and control nociceptive processes. Proteomics, defined as the science and the methodology of investigating the proteome [11] has been suggested as a useful technique in order to identify biomarkers of pain conditions [12]–[14]. Two-dimensional gel electrophoresis (2-DE) is a widely used technique to separate organic substances such as proteins in different tissues [15]. Proteomics have been applied to pain research to identify protein changes in different pain conditions. The proteome has been investigated in animals comparing inflammatory and neuropathic pain [16]. The proteome of the cerebrospinal fluid in healthy subjects, in patients with herniated disc and idiopathic back pain have been studied [17]–[19]. Also, nerve samples from patients with complex regional pain syndrome (CRPS) have been analyzed using proteomics in order to detect up/down regulated proteins [20]. Proteomics of serum have been used for the identification of possible biomarkers of chronic endometriosis [21], [22]. To our knowledge this technique has not been used for investigating potential alterations in muscle interstitium. In this study we have applied in vivo MD in combination with 2-DE and mass spectrometry to identify proteins in the interstitial fluid of the trapezius muscle in healthy subjects, in patients with chronic trapezius myalgia and in chronic widespread pain.
Materials and Methods
Subjects
37 women with chronic trapezius myalgia (TM), 18 women with chronic wide spread pain (CWP) and 22 healthy women (CON) were included in this study.Women were chosen as subjects in the present study due to the higher prevalence of chronic pain in women than in men according to community based epidemiological studies. Moreover the majority (approx. 65%) of patients referred to our clinical department (Pain- and rehabilitation Centre, University Hospital, Linköping, Sweden) are women. In future studies we will investigate men. The criteria for chronic trapezius myalgia have been described in our earlier studies [23]–[26]. The ACR criteria were used for the diagnosis of CWP [27]. No significant group differences in age (CON: 43.5±10.2 years, TM: 43.6±9.3 years and CWP: 47.9±9.6 years), height (CON: 168±7cm, TM: 166±6 cm and CWP: 167±5 cm) and weight (CON: 67.1±10.3 kg, TM: 67.0±12.2 kg and CWP: 75.8±17.3 kg) existed. The pain intensity was rated immediately before the experiment using a visual analogue scale (VAS) and as expected significant group differences existed (CON: 0±0 mm, TM: 15±16 mm and CWP: 43±22 mm; p<0.001).
Patients were instructed not to drink any beverages with caffeine on the day of the study, not to smoke and to avoid NSAID-medication the week before the study. The participants arrived at the clinic in the morning after having eaten breakfast. A brief interview was then made by one of the physicians checking that the instructions with respect to the different instructions had been followed. All subjects reported that they had followed the instructions. During the study, they were not allowed to eat, but they could drink water.
All participants gave their informed written consent before the start of the study. The study was approved by the Ethical Committee of Linköping University (Dnr: M10-08).
Microdialysis
The MD technique used has been described in detail in our previous studies [23]–[25], [28], [29]; here is given a summary. The skin and the subcutaneous tissues above, where the catheter entered were anaesthetized with a local injection (0.5 ml) of Xylocaine (20 mg/ml) without adrenaline, and care was taken not to anaesthetize the underlying muscle. As guidance the insertion of the microdialysis catheter, which was inserted in the middle third of the upper part of the trapezius muscle in the direction lateral to medial were preceded by ultrasound investigation of distance between the skin and the trapezius muscle and the width of the muscle. Using the SENIAM landmarks [30] the midpoint of the line between the spine of 7th cervical vertebra and the acromion was defined as the midpoint of the descending trapezius. The commercially available microdialysis catheter (CMA 71, cut-off points of 100 kDa, CMA Microdialysis AB, Solna, Sweden; membrane 30 mm length, 0.5 mm diameter) were inserted into the pars descendens of the trapezius muscle at half the distance between the processus spinosus of seventh cervical spine and the lateral end of the acromion. Typically, a brief involuntary contraction and change of resistance were perceived when the tip of the insertion needle of the catheter entered the fascia and the muscle. The catheters were placed in the trapezius muscle parallel to the muscle fibers and perfused with a high-precision syringe pump (CMA 107; CMA/Microdialysis AB, Stockholm, Sweden) at a rate of 5 µl/min with a Ringer acetate solution (Fresenius Kabi AB, Uppsala, Sweden) containing 3 mM glucose and 0.5 mM lactate in order to mimic the interstitial environment of the muscle [31].
Immediately after the insertion of catheters participants rested comfortably in an armchair for a 120 minutes trauma period to allow the tissue to recover from possible changes in the interstitial environment induced. Samples from trauma period were discarded and after the trauma period, sampling was performed at every 20 minutes in a period of 100 minutes. In the present study dialysate from the time period 140–200 min was used. The samples were stored on ice to prevent protease activation. The samples were then stored as aliquots in −70°C until analysis. All vials were weighted before the experiment started and after each 20 minutes interval in order to confirm that sampling and fluid recovery (FR) was working according to the perfusion rate set. Vials with visible sign of hemolysis were discarded.
Two dimensional gel electrophoresis (2-DE)
Dialysate samples from the time period of 140–200 minutes were used for 2-DE analysis. Between 10–20 µl of dialysate from each subjects in TM, CWP and CON group were pooled. Samples were desalted by gel filtration (PD-10 column, GE Healthcare) into 12 mM ammonium bicarbonate, pH 7.1. Proteins were lyophilized and dissolved in 0.20 ml urea sample solution according to Görg [15]. 2-DE was performed in a horizontal 2-DE setup (IPGphore and Multiphore from GE Healthcare), as described in detail previously [32] and essentially according to Görg [15]. The samples (containing 50 µg protein for analytical gels and 300 µg for preparative gel) were applied by in-gel rehydration (according to the manufacturer's instructions) for 12 h using low voltage (30 V) in pH 3–10 L IPGs. The proteins were then focused for up to 32 000 Vhs at a maximum voltage of 8000 V. IPGs were either used immediately for second dimensional analysis, or stored at −70°C until analyzed. The second dimension (SDS-PAGE) was carried out by transferring the proteins to gradient gels cast on GelBond PAG film (0.5/180/245 mm, 11–18%T, 1.5%C, 33–0% glycerol) running at 30 mA and up to 1000 V for about 5 h.
Staining and image analysis
In analytical gels separated proteins were detected by silver staining with a detection limit of about 5 ng/spot [33]. Proteins picked for MS analysis were mostly fluorescently stained with SYPRO Ruby. Fluorescent staining was performed according to the manufacturer's staining protocol (SYPRO Ruby protein gel stain web site: www.probes.com). After SDS-PAGE gels were fixed using 10% methanol/7% acetic acid solution for 30 min and then incubated in 400 ml SYPRO Ruby protein gel stain solution overnight. Gels were washed and placed in deionized water. All staining and washing steps were performed with continuous gentle agitation. The protein patterns of silver stained analytical gels were analyzed as digitized images, using a CCD camera in combination with a computerized imaging 12-bit system designed for evaluation of 2-DE patterns. The amount of protein in a spot was assessed as background corrected optical density, integrated over all pixels in the spot and expressed as integrated optical density (IOD). In order to correct for differences in total silver stain intensity between different 2-DE images, the amounts of the compared protein spots were quantified as optical density for individual spot per total protein intensity of all spots in the same gel. Thereby ppm-values (parts per million) for all proteins were generated that were evaluated for differences between the groups.
In-gel digestion by trypsin
In the gel used for protein identification 300 µg proteins were loaded and analyzed as above. Protein spots were excised using a homemade spot picker. The picked protein spots were digested with trypsin (Promega/SDS Biosciences, Falkenberg, Sweden). Briefly, the gel pieces were washed with a mixture of acetonitrile/ammonium bicarbonate, dehydrated with acetonitrile and incubated with 30 µl of 20 µg/ml trypsin overnight at 37°C. Silver stained protein spots were destained with 15 mM potassium ferricyanide/50 mM sodium thiosulfate as described previously [34] before trypsination. The supernatant was transferred to a new tube and the peptides further extracted from the gel by incubation in 50% acetonitrile/5% trifluoroacetic acid for about 3 hours at room temperature during constant mixing. The supernatant obtained by the two steps pooled, dried by SpeedVac.
Protein identification by LC-MS/MS
The dried tryptic samples from fluorescently stained proteins were dissolved in 6 µl of 0.1% formic acid. Peptides were analyzed using an on-line nano-flow HPLC system (EASY-nLC; Proxeon, Bruker Daltonics) in conjugation with the mass spectrometer HCTultra PTM Discovery System (Bruker Daltonics). A 100 mm×75 µm C18 column was used for separation at a flow rate 300 nL/min. The gradient buffers used were 0.1% formic acid in water (buffer A) and 0.1% formic acid in acetonitrile (buffer B) and a linear gradient from 0–100% buffer B in 40 min was used for separation. The automated online tandem MS analysis was performed using collision induced dissociation of peptide ions.
Protein identification by MALDI-TOF
The dried tryptic samples from silver stained proteins were dissolved in 4 µl of 0.1% trifluroacetic acid (TFA). The peptides were mixed 1∶1 with matrix solutions consisting of dihydroxybenzoic acid (DHB) (0.04 g/ml) in 70% ACN/0.3% TFA, and 1 µl was then spotted on the target plate (stainless-steel plate). Analysis of peptide masses was performed as described previously [25] using MALDI–TOF MS (Voyager-DE PRO, Applied Biosystems, Foster City, CA, USA).
Database searches
LC-MS/MS spectra were processed by Bruker Daltonics DataAnalysis 3.4 (Bruker Daltonics, Bremen, Germany) and resulting MS/MS data were searched in NCBInr and Swiss-Prot database on MASCOT server (www.matrixscience.com). Database search parameters were set as follows: the enzyme trypsin was used; up to one missed cleavage was allowed; fixed modification included were carbamidomethylation of cysteine and oxidation of methionine; mass tolerance for MS precursor ion was 0.8 Da and for MS/MS fragment ion was 0.6 Da; and charge states were varied. Criteria for identification of a protein were at least 3 peptides of the protein should be identified with a MASCOT score over 25 and an expectation value <1.
The mass list generated from the major peaks of the MALDI spectra was submitted to a database search (NCBI or SWISS-PROT) using MS-FIT search engines. Restrictions were placed on species (Human), mass tolerance (50 ppm), maximum missed cleavages by trypsin (up to 1) and cysteine modification by carbamidomethylation.
Statistics
For comparison of group differences concerning anthropometric data and pain intensity the Kruskal Wallis test was applied using IBM SPSS (version 20.0); p<0.05 was considered significant.
Results
Protein concentration
The total protein concentrations were measured before 2-DE analysis; 74 µg/ml in the TM pool, 55 µg/ml in the CWP pool and 59 µg/ml in the CON pool. The samples were desalted, concentrated and 50 µg protein from each group could be analyzed by 2-DE.
2-DE analysis
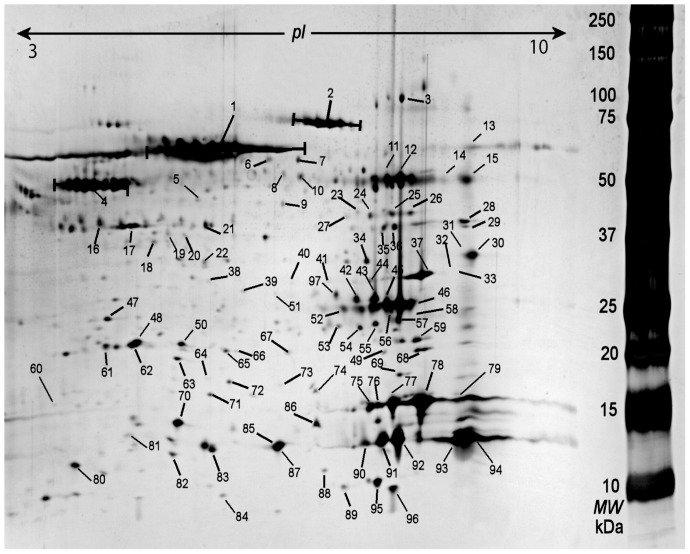
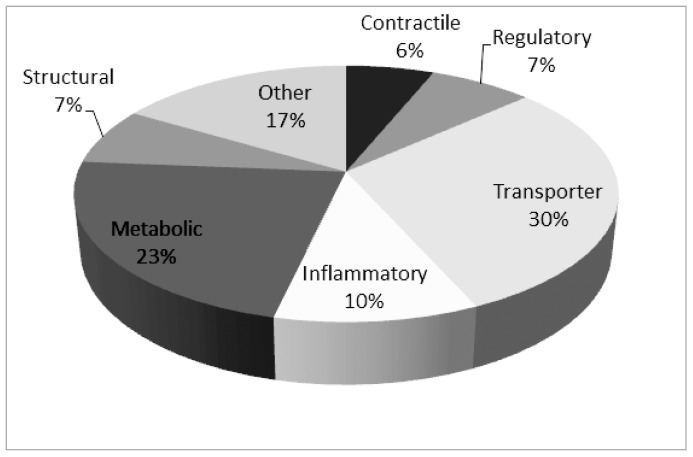
About 300 protein spots could be detected. 98 protein spots that were of good quality for identification were picked and in-gel digested for identification by nLC- MS/MS and MALDI-TOF mass spectrometer ( fig. 1 , Table 1 ). It was possible to identify 97 of the protein spots. The apparent molecular weight and isoelectric point (pI) determined from 2-DE pattern were generally in agreement with the theoretical values with the identified proteins. 50% of the proteins had different identity according to the accession numbers, i.e., many of the identified proteins were expressed as different isoforms. That could be explained by post translational modification and truncation of the proteins. The majority of identified proteins are known muscle proteins pertaining to several functional classes, i.e., metabolic, structural, regulatory, and contractile proteins and proteins that are involved in inflammatory responses ( fig. 2 ). Identified proteins known to be involved in nociceptive and pain processes were alpha-1 antitrypsin, creatine kinase, nerve grow factor, carbonic anhydrase, myoglobin, fatty acid binding protein and actin aortic smooth muscle.
Figure 1. Typical 2-DE pattern from interstitium of trapezius muscle.
The numbered protein spots are referred to the identified proteins in Table 1.
Table 1. Identified proteins in dialysate samples from microdialysis of the trapezius muscle using a catheter with 100 kDa cut-off.
| Spot no. | Protein | Molecular Function | Accession No. | Mw(kDa)/pI | Matched peptides | MS Score |
| Proteins that are involved in inflammatory processes | ||||||
| 4 | Alpha-1-antitrypsin | Serine protease inhibitor | P01009 | 46.9/5.4 | 17 | 600 |
| 5 | Protein S100-A14 | Calcium binding protein | Q9HCY8 | 11.7/5.2 | 2 | 21 |
| 49 | Phosphatidylethanolamine-binding protein 1 | Protease inhibitor/Serine protease inhibitor | P30086 | 21.1/7.0 | 2 | 89 |
| 64 | Haptoglobin | Serine protease homolog | P00738 | 45.9/6.1 | 2 | 98 |
| 66 | Protein DJ-1 | Protease | Q99497 | 20.0/6.3 | 1 | 53 |
| 68 | Phosphatidylethanolamine-binding protein 1 | Protease inhibitor | P30086 | 21.1/7.0 | 7 | 288 |
| 82 | Protein S100-A9 | Calcium binding protein | P06702 | 13.3/5.7 | 4 | 122 |
| 83 | Protein S100-A9 | Ca-binding protein | P06702 | 13.3/5.7 | 5 | 145 |
| 88 | Beta-2-microglobulin | Other | P61769 | 13.8/6.1 | 1 | 50 |
| 97 | Beta-nerve growth factor | Growth factor activity | P01138 | 27.0/9.8 | 5 | 218 |
| Transporter proteins | ||||||
| 1 | Serum albumin | Ca2+/Na+/K+/fatty acids/drug binding protein | P02768 | 71.3/5.9 | 25 | 761 |
| 2 | Serotransferrin | Iron binding protein | P02787 | 79.3/6.8 | 12 | 451 |
| 6 | Serum albumin | Ca2+/Na+/K+/fatty acids/drug binding protein | P02768 | 71.3/5.9 | 7 | 175 |
| 8 | Serum albumin | Ca2+/Na+/K+/fatty acids/drug binding protein | P02768 | 71.3/5.9 | 8 | 213 |
| 9 | Serum albumin | Ca2+/Na+/K+/fatty acids/drug binding protein | P02768 | 71.3/5.9 | 7 | 221 |
| 19 | Serum albumin | Ca2+/Na+/K+/fatty acids/drug binding protein | P02768 | 71.3/5.9 | 3 | 59 |
| 21 | Serum albumin | Ca2+/Na+/K+/fatty acids/drug binding protein | P02768 | 71.3/5.9 | 7 | 153 |
| 22 | Serum albumin | Ca2+/Na+/K+/fatty acids/drug binding protein | P02768 | 71.3/5.9 | 8 | 221 |
| 32 | Myoglobin | Muscle protein | P02144 | 17.2/7.1 | 5 | 193 |
| 34 | Vacuolar protein sorting-associated protein 28 homolog | Regulation of vesicular trafficking | Q9UK41 | 25.6/5.3 | 5 | 34 |
| 48 | Retinol-binding protein 4 | Other | P02753 | 23.3/5.8 | 2 | 47 |
| 56 | Serum albumin | Ca2+/Na+/K+/fatty acids/drug binding protein | P02768 | 71.3/5.9 | 6 | 161 |
| 61 | Apolipoprotein A-1 | Lipid metabolism | P02647 | 30.7/5.6 | 8 | 267 |
| 62 | Apolipoprotein A-1 | Lipid metabolism | P02647 | 30.7/5.6 | 8 | 331 |
| 70 | Transthyretin | Thyroid hormone-binding | P02766 | 16.0/5.5 | 4 | 186 |
| 75 | Myoglobin | Muscle protein | P02144 | 17.2/7.1 | 6 | 195 |
| 76 | Myoglobin | Muscle protein | P02144 | 17.2/7.1 | 4 | 135 |
| 77 | Myoglobin | Muscle protein | P02144 | 17.2/7.1 | 5 | 277 |
| 78 | Myoglobin | Muscle protein | P02144 | 17.2/7.1 | 7 | 432 |
| 79 | Myoglobin | Muscle protein | P02144 | 17.2/7.1 | 4 | 201 |
| 81 | Syntaxin-8 | Transport | Q9UNK0 | 27.0/4.9 | 1 | 31 |
| 85 | Fatty acid-binding protein | Lipid transport | P05413 | 14.9/6.3 | 7 | 227 |
| 87 | Fatty acid-binding protein | Lipid transport | P05413 | 14.9/6.3 | 10 | 312 |
| 89 | Hemoglobin subunit beta | Hypotensive agent | P68871 | 16.1/6.7 | 1 | 62 |
| 90 | Chain B, Deoxy hemoglobin | Hypotensive agnet | gi|27574248 | 15.9/6.7 | 4 | 193 |
| 91 | Hemoglobin subunit beta | Hypotensive agent | P68871 | 16.1/6.7 | 7 | 514 |
| 92 | Hemoglobin subunit beta | Hypotensive agent | P68871 | 16.1/6.7 | 12 | 724 |
| 93 | Hemoglobin subunit alpha | Other | P69905 | 15.3/8.7 | 5 | 803 |
| 94 | Hemoglobin subunit alpha | Other | P69905 | 15.3/8.7 | 6 | 229 |
| Contractile proteins | ||||||
| 10 | Actin, cytoplasmic 1 | Other | P60709 | 42.0/5.3 | 2 | 48 |
| 16 | Actin, aortic smooth muscle | Muscle protein | P62736 | 42.4/5.2 | 2 | 83 |
| 17 | Actin, aortic smooth muscle | Muscle protein | P62736 | 42.4/5.2 | 9 | 243 |
| 18 | Myosin light chain 5 | Muscle protein | Q02045 | 19.5/4.9 | 3 | 376 |
| 60 | Calponin-3 | Actin binding protein | Q15417 | 36.4/5.7 | 4 | 981 |
| 69 | Cofilin-1 | Other | P23528 | 18.7/8.2 | 1 | 27 |
| Regulatory proteins | ||||||
| 7 | Glomulin | Muscle cell differentiation | Q92990 | 68.9/5.2 | 1 | 47 |
| 41 | rRNA methyltransferase 1, mitochondrial | Methyltransferase | Q6IN84 | 38.6/8.2 | 11 | 1.06e+6 |
| 47 | Rho GDP-dissociation inhibitor2 | GTPase activation | P52566 | 23.0/5.1 | 5 | 166 |
| 50 | Glutathione S-transferase P | Transferase | P09211 | 23.6/5.4 | 5 | 275 |
| 73 | Transforming growth factor beta-1 | Growth factor/Mitogen | P01137 | 44.3/8.8 | 5 | 679 |
| 74 | Host cell factor C1 regulator 1 | Other | Q9NWW0 | 15.4/6.8 | 1 | 58 |
| 80 | 26S protease regulatory subunit 4 | ATP binding protein | P62191 | 49.2/5.9 | 6 | 104 |
| Structural proteins | ||||||
| 20 | Keratin, type I cytoskeletal 9 | Other | P35527 | 62.2/5.1 | 4 | 195 |
| 30 | Four and a half LIM domains protein 1 | Developmental protein | Q13642 | 38.0/9.2 | 2 | 86 |
| 31 | Four and a half LIM domains protein 1 | Developmental protein | Q13642 | 38.0/9.2 | 1 | 75 |
| 33 | Four and a half LIM domains protein 1 | Developmental protein | Q13642 | 38.0/9.2 | 1 | 68 |
| 37 | Four and a half LIM domains protein 1 | Developmental protein | Q13642 | 38.0/9.2 | 1 | 59 |
| 38 | Keratin, type I cytoskeletal 9 | Other | P35527 | 62.2/5.1 | 12 | 746 |
| 58 | Four and a half LIM domains protein 1 | Developmental protein | Q13642 | 38.0/9.2 | 1 | 48 |
| Metabolic proteins | ||||||
| 3 | Carbonic anhydrase 3 | Lyase | P07451 | 29.8/6.9 | 5 | 142 |
| 11 | Carbonic anhydrase 3 | Lyase | P07451 | 29.8/6.9 | 5 | 178 |
| 12 | Carbonic anhydrase 3 | Lyase | P07451 | 29.8/6.9 | 6 | 210 |
| 13 | Acyl-coenzyme A synthetase ACSM2A | Ligase | Q08AH3 | 64.8/8.3 | 1 | 37 |
| 23 | Beta-enolase | Lyase | P13929 | 47.2/7.6 | 4 | 223 |
| 25 | Beta-enolase | Lyase | P13929 | 47.2/7.6 | 4 | 211 |
| 26 | Beta-enolase | Lyase | P13929 | 47.2/7.6 | 5 | 211 |
| 28 | Phosphoglycerate kinase 1 | Transferase/Kinase | P00558 | 45.0/8.3 | 3 | 81 |
| 29 | Fructose-bisphosphate aldolase A | Lyase | P04075 | 39.8/8.3 | 7 | 257 |
| 35 | Creatine kinase M-type | Transferase/Kinase | P06732 | 43.3/6.8 | 9 | 386 |
| 36 | Creatine kinase M-type | Transferase/Kinase | P06732 | 43.3/6.8 | 10 | 476 |
| 42 | Carbonic anhydrase 1 | Lyase | P00915 | 28.9/6.6 | 6 | 174 |
| 43 | Carbonic anhydrase 1 | Lyase | P00915 | 28.9/6.6 | 8 | 276 |
| 44 | Glycosyltransferase 1 domain-containing protein 1 | Glycotransferase | Q96MS3 | 38.5/6.0 | 7 | 421 |
| 45 | Carbonic anhydrase 3 | Lyase | P07451 | 29.8/6.9 | 6 | 206 |
| 46 | Carbonic anhydrase 3 | Lyase | P07451 | 29.8/6.9 | 7 | 264 |
| 52 | Carbonic anhydrase 3 | Lyase | P07451 | 29.8/6.9 | 1 | 52 |
| 53 | Acetylcholine receptor subunit alpha | Ligand-gated ion channel, | P02708 | 54.5/5.8 | 1 | 39 |
| 54 | Triosephosphate isomerase | Isomerase | P60174 | 31.0/5.6 | 5 | 196 |
| 55 | Triosephosphate isomerase | Isomerase | P60174 | 31.0/5.6 | 5 | 302 |
| 57 | Carbonic anhydrase 3 | Lyase | P07451 | 29.8/6.9 | 1 | 42 |
| 84 | Acyl-protein thioesterase 2 | Hydrolase | O95372 | 25.1/6.7 | 1 | 37 |
| Others | ||||||
| 14 | Ig gamma-1 chain C region | Antigen binding | P01857 | 36.6/8.5 | 2 | 75 |
| 15 | Ig gamma-1 chain C region | Antigen binding | P01857 | 36.6/8.5 | 5 | 122 |
| 24 | Centromere protein M | Mitotic progression | Q9NSP4 | 19.7/6.7 | 2 | 12 |
| 27 | E3 ubiquitin-protein ligase RNF149 | Ligase | Q8NC42 | 43.1/6.1 | 1 | 40 |
| 39 | E3 ubiquitin-protein ligase RNF149 | Ligase | Q8NC42 | 43.1/6.1 | 1 | 36 |
| 40 | WD repeat domain phosphoinositide-interacting protein 3 | phosphatidylinositol-3,5-bisphosphate binding | Q5MNZ6 | 38.1/7.5 | 1 | 32 |
| 51 | Carboxypeptidase-like 3 | Metalloprotease | O75976 | 44.7/6.5 | 1 | 33 |
| 59 | Flavin reductase (NADPH) | Oxidoreductase | P30043 | 22.2/7.1 | 3 | 133 |
| 63 | Histone H2A.2 | DNA-binding | P04908 | 14.2/11 | 1 | 44 |
| 65 | DDB1- and CUL4-associated factor 8 | Other | Q5TAQ9 | 67.5/5.2 | 1 | 29 |
| 67 | Apoptosis-associated speck-like protein containing a CARD | Apoptosis | Q9ULZ3 | 21.7/5.9 | 1 | 87 |
| 71 | Superoxide dismutase [Cu-Zn] | Antioxidant | P00441 | 16.1/5.7 | 2 | 88 |
| 72 | Cysteine and glycine-rich protein 2 | Zinc ion binding proteins | Q16527 | 21.0/9.0 | 8 | 9981 |
| 86 | Unnamed protein product | Other | gi|29446 | 16.0/7.1 | 2 | 130 |
| 95 | ADP-ribosyl cyclase 1 | Hydrolase | P28907-2 | 13.8/9.0 | 1 | 35 |
| 96 | Ubiquitin-60S ribosomal protein L40 | Ribonucleoprotein | P62987 | 15.0/9.9 | 5 | 142 |
The proteins have been classified with respect to class and molecular function according to Swissprot. The accession number, molecular weight (Mw (kDa))/isoelectric point (pI),are according to Swissprot or NCBI database. The spot no are referred to numbered protein spots in figure 1.
Figure 2. Proportions (in per cent) of different classes for the identified 97 proteins.
The categorizations are based on protein function according to Swiss-Prot database.
2-DE protein patterns in TM, CWP and CON
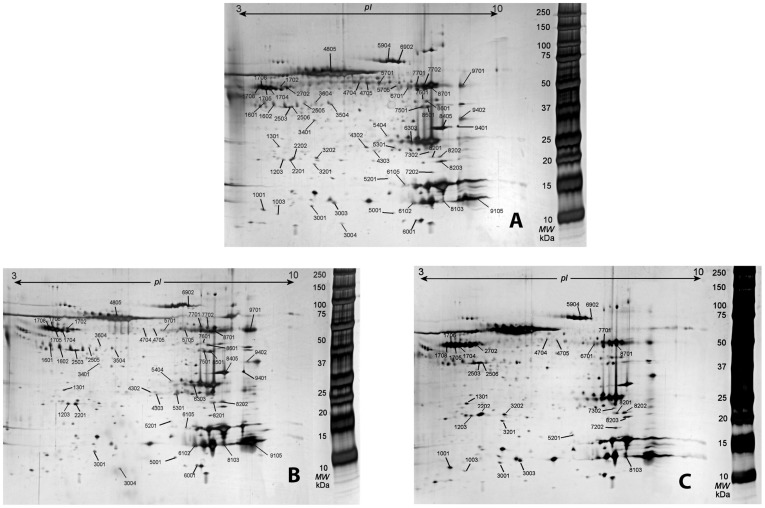
The protein pattern in the dialysate from CON was almost similar to the pattern in TM and in CWP ( fig. 3 ). Higher numbers of protein spots could be detected in TM (262) compared to CWP (196) gel and the healthy control gel (195).
Figure 3. Protein patterns of the muscle dialysate.
The 2-DE gels of controls (CON, panel A), chronic trapezius myalgia (TM; panel B), and chronic widespread pain (CWP, panel C). The marked spots are the differentially expressed proteins according to Table 2 and 3.
Forty-eight out of 262 proteins in TM ( Table 2 ) and 30 out of 196 proteins in CWP ( Table 3 ) had concentrations at least two-fold higher or lower than in CON. Seventeen of these proteins showed alterations in the concentrations both in TM and CWP when compared to CON. Twelve out of the 17 proteins were altered in a similar way (at least two-fold higher (↑) or lower (↓)) in both groups of patients compared to CON:
Table 2. The optical density for altered proteins (≥twofold up/down regulated) in dialysate from the interstitial fluid of the trapezius in chronic trapezius myalgia (TM) compared to healthy controls (CON).
| SSP | Protein | TM | CON |
| 1203 | Apolipoprotein AI | 1526.9 | 345.8 |
| 1301 | Rho GDP-dissociation inhibitor2 | 604.3 | 265.7 |
| 1601 | Actin, aortic smooth muscle | 2839.5 | 1411.2 |
| 1602 | Actin, aortic smooth muscle | 4033.9 | 1781.2 |
| 1702 | Alpha-1-antitrypsin | 2596.4 | 882.5 |
| 1704 | Alpha-1-antitrypsin | 10548.6 | 2477.5 |
| 1705 | Alpha-1-antitrypsin | 1835.1 | 4911.3 |
| 1706 | Alpha-1-antitrypsin | 8631.6 | 3950.6 |
| 1708 | Alpha-1-antitrypsin | 5425.6 | 2585.2 |
| 2201 | Apolipoprotein A-1 | 3042.8 | 891.0 |
| 2503 | Actin, aortic smooth muscle | 873.0 | 157.4 |
| 2505 | Serum albumin | 211.9 | 609.9 |
| 3001 | Protein S100-A9 | 940.2 | 463.9 |
| 3004 | Acyl-protein thioesterase 2 | 535.8 | 248.2 |
| 3401 | Serum albumin | 209.3 | 429.7 |
| 3504 | Serum albumin | 195.9 | 552.8 |
| 3604 | Keratin, type I cytoskeletal 9 | 211.6 | 807.1 |
| 4302 | Carbonic anhydrase 3 | 205.8 | 791.6 |
| 4303 | Acetylcholine receptor subunit alpha | 681.6 | 330.2 |
| 4704 | Serum albumin | 201.7 | 896.0 |
| 4705 | Serum albumin | 171.3 | 2356.3 |
| 4805 | Serum albumin | 4958.1 | 11312.5 |
| 5001 | Beta-2-microglobulin | 535.7 | 152.8 |
| 5201 | Host cell factor C1 regulator 1 | 273.0 | 79.1 |
| 5301 | Triosephosphate isomerase | 5092.9 | 624.9 |
| 5404 | Beta-nerve growth factor | 232.9 | 527.3 |
| 5701 | Actin, cytoplasmic 1 | 753.7 | 2737.6 |
| 5705 | Carbonic anhydrase 3 | 3655.2 | 1046.3 |
| 6001 | ADP-ribosyl cyclase 1 | 799.9 | 321.4 |
| 6102 | Chain B, Deoxy hemoglobin | 10021.0 | 2834.8 |
| 6105 | Myoglobin | 480.4 | 1075.2 |
| 6303 | Carbonic anhydrase 1 | 3526.5 | 8509.4 |
| 6902 | Serotransferrin | 3102.4 | 7660.9 |
| 7501 | Creatine kinase M-type | 2284.1 | 933.3 |
| 7601 | Beta-enolase | 303.4 | 1074.5 |
| 7701 | Carbonic anhydrase 3 | 4035.0 | 1253.1 |
| 7702 | Carbonic anhydrase 3 | 4615.4 | 10836.0 |
| 8103 | Hemoglobin subunit beta | 13345.5 | 1213.3 |
| 8201 | Flavin reductase (NADPH) | 1514.9 | 565.3 |
| 8202 | Flavin reductase (NADPH) | 2456.0 | 704.0 |
| 8405 | Four and a half LIM domains protein 1 | 1138.9 | 2494.5 |
| 8501 | Creatine kinase M-type | 4416.1 | 1596.1 |
| 8601 | Beta-enolase | 2580.2 | 1264.8 |
| 8701 | Carbonic anhydrase 3 | 15598.1 | 3562.7 |
| 9105 | Hemoglobin subunit alpha | 63347.9 | 29012.7 |
| 9401 | Four and a half LIM domains protein 1 | 1552.0 | 706.8 |
| 9402 | Four and a half LIM domains protein 1 | 1256.5 | 5112.5 |
| 9701 | Ig gamma-1 chain C region | 19206.2 | 6056.2 |
The SSP numbers are referred to the spot ide in figure 3A-C. The values are given in part per million (ppm).
Table 3. The optical density for altered proteins (≥twofold up/down regulated) in dialysate from the interstitial fluid of the trapezius in chronic widespread myalgia (CWP) compared to healthy controls (CON).
| SSP | Protein | CWP | CON |
| 1001 | Protein S100-A14 | 2347.4 | 849.4 |
| 1003 | Syntaxin-8 | 415.9 | 125.7 |
| 1203 | Apolipoprotein AI | 1200.8 | 345.8 |
| 1301 | Rho GDP-dissociation inhibitor2 | 1280.1 | 265.7 |
| 1704 | Alpha-1-antitrypsin | 98.5 | 2477.5 |
| 1705 | Alpha-1-antitrypsin | 17217.1 | 4911.3 |
| 1706 | Alpha-1-antitrypsin | 1672.9 | 3950.6 |
| 1708 | Alpha-1-antitrypsin | 7056.7 | 2585.2 |
| 2202 | Apolipoprotein A-1 | 4468.5 | 1366.7 |
| 2503 | Actin, aortic smooth muscle | 58.3 | 157.4 |
| 2506 | Serum albumin | 2552.5 | 1214.7 |
| 2702 | Alpha-1-antitrypsin | 7331.1 | 2935.8 |
| 3001 | Protein S100-A9 | 983.3 | 463.9 |
| 3003 | Protein S100-A9 | 2912.9 | 1308.2 |
| 3201 | Histone H2A.2 | 895.8 | 274.8 |
| 3202 | Glutathione S-transferase P | 1701.4 | 646.9 |
| 4704 | Serum albumin | 315.6 | 896.0 |
| 4705 | Serum albumin | 421.8 | 2356.3 |
| 5201 | Host cell factor C1 regulator 1 | 411.6 | 79.1 |
| 5904 | Serotransferrin | 19626.1 | 4499.9 |
| 6701 | Carbonic anhydrase 3 | 1182.2 | 2518.9 |
| 6902 | Serotransferrin | 1508.9 | 7660.9 |
| 7202 | Cofilin-1 | 818.8 | 380.7 |
| 7302 | Carbonic anhydrase 3 | 946.9 | 213.5 |
| 7701 | Carbonic anhydrase 3 | 3331.8 | 1253.1 |
| 8103 | Hemoglobin subunit beta | 4326.8 | 1213.3 |
| 8201 | Flavin reductase (NADPH) | 1133.7 | 565.3 |
| 8202 | Flavin reductase (NADPH) | 2208.9 | 704.0 |
| 8203 | Phosphatidylethanolamine-binding protein 1 | 1035.6 | 2450.5 |
| 8701 | Carbonic anhydrase 3 | 1485.2 | 3562.7 |
The SSP numbers are referred to the spot ide in figure 3A-C. The values are given in part per million (ppm).
Apolipoprotein AI (SSP 1203; ↑)
Rho GDP-dissociation inhibitor2 (SSP 1301; ↑)
Alpha-1-antitrypsin (SSP 1708; ↑)
Protein S100-A9 (SSP 3001; ↑)
Host cell factor C1 regulator 1(SSP 5201; ↑)
Carbonic anhydrase 3 (SSP 7701; ↑)
Hemoglobin subunit beta (SSP 8103; ↑)
Flavin reductase (NADPH) (SSP 8201; ↑)
Flavin reductase (NADPH) (SSP 8202; ↑)
Serum albumin (SSP 4704; ↓)
Serum albumin (SSP 4705; ↓)
Serotransferrin (SSP 6902; ↓)
The alterations of the remaining five proteins were different in the two groups (at least two-fold higher or lower) when compared to CON:
Alpha-1-antitrypsin (SSP 1704; CWP ↓, TM ↑)
Alpha-1-antitrypsin (SSP 1706; CWP ↓, TM ↑)
Actin, aortic smooth muscle (SSP 2503; CWP ↓, TM ↑) )
Carbonic anhydrase 3 (SSP 8701; CWP ↓, TM ↑))
Alpha-1-antitrypsin (SSP 1705;CWP ↑, TM ↓)
Discussion
We, for the first time to the best of our knowledge, have described the molecular pattern of protein expression in human muscle microdialysate by using two dimensional gel electrophoresis in combination with mass spectrometry. To investigate the possibility that specific proteins in muscle dialysate samples might be markers of the different pain conditions, we analyzed proteins that were changed two folds or more between the three groups. Pronounced alterations in the proteome in the myalgic muscle of the two common chronic pain conditions were found. Hence, major results were:
A large number of proteins (approx. 300) were found in the interstitium of human muscles using MD in combination with 2-DE analysis.
The identified proteins (n = 97) were proteins involved in inflammatory processes and metabolic, structural, regulatory, contractile and transporter proteins.
Considerable proportions of the identified proteins were at least two-fold higher or lower in TM (50%) and CWP (31%) than in CON.
The two groups of patients (TM and CWP) showed at least two-fold alterations in concentrations of the same proteins (18%) when compared to CON; approx 2/3 of these alterations were in the same direction.
There is a need to understand the activated nociceptive mechanisms at various levels of the pain systems in chronic myalgia. In the present study we have focused upon peripheral alterations and collected dialysate from the interstitium of the trapezius. Earlier MD studies have reported peripheral alterations in concentrations of algesic, metabolic and antinociceptive substances; see [6] for a review. However, these studies have, due to the small volumes of dialysate obtained from the muscle interstitium, only been able to analyze a few substances. The present technique, combining MD, 2-DE analysis and nLC-MS/MS, opens up for an explorative approach not focusing upon predetermined substances, in order to understand the involved mechanisms in the myalgic muscle. Proteins that are involved in pain are important to investigate due to the fact that they can act as signal substances, activate the formation of algesics and control nociceptive processes. The number of studies investigating the human proteome in body fluids and tissues in human chronic pain conditions are limited e.g., [17]–[22], [35]–[37]. There are also a few examples of proteomic studies in acute pain e.g., acute coronary syndrome [38], after eccentric exercises [39] and experimental cutaneous injury [40]. In the latter study details about the proteome of healthy human dermal dialysate in response to acute injury were reported. The authors suggested that the combination of microdialysis and proteomics has the potential to identify relevant, novel markers of injury and inflammation. The present results based upon two different groups of patients with chronic pain extend their suggestion to also include peripheral nociceptive processes and their consequences.
A number of previous proteomic studies of human skeletal muscle biopsies have reported identification of more proteins compared to this study [41], [42], but there are limitations to the analysis of the entire muscle proteome [39]. The high abundant proteins, i.e. contractile, structural and metabolic proteins, impair the detection of low abundant proteins such as inflammatory proteins. Using MD approximately 300 proteins were found in the dialysate of the muscle interstitium of the trapezius muscle. 10% of the identified proteins in this study are proteins that are involved in inflammatory processes ( fig. 2 and table 1 ). The total protein concentrations and the number of protein spots were higher in TM compared to CWP and CON. Several of the identified proteins presented in table 1 have previously been reported to be involved in nociceptive and pain processes (inflammatory and non-inflammatory pain) such as: creatine kinase [43], nerve growth factor [44], carbonic anhydrase [45], myoglobin [46], fatty acid binding protein [47] and actin aortic smooth muscle [48]. Hence, the presented methodology has a potential of identifying mechanisms involved in nociceptive processes in chronic muscle pain. Moreover, 30 proteins in CWP ( table 3 ) and 48 proteins in TM ( table 2 ) out of the 196 and 262 protein spots respectively, had concentrations at least twofold higher or lower than in CON. In other words a relatively prominent part of the identified proteins showed marked alterations in TM and CWP when compared to CON. This finding underscores that chronic myalgia, both as a part of a regional pain condition (TM) or a widespread pain condition (CWP), is associated with pronounced alterations in the proteome of the trapezius muscle. Some of these alterations are linked to chronic nociceptive processes while others reasonably are linked to the consequences of being in persistent pain e.g., deconditioning. Another alternative, very briefly reviewed by Gill et al, is that the insertion of microdialysis probes causes long-lasting tissue alterations [40]. In the present study the insertion procedure of the microdialysis probe was standardized and conducted in the same way in all three groups. Thus the differences reported in tables 2 and 3 are reasonably mainly due to the two former mechanisms. By combining tables 2 and 3 it was obvious that 17 of the identified proteins showed alterations in the concentrations both in TM and CWP when compared to CON; several of these were categorized as proteins involved in inflammatory processes ( table 1) In twelve out of the 17 proteins the direction of the alterations were similar in the two groups of patients with chronic myalgia and in the remaining five proteins the two groups had different signs of the alterations when compared to the gel of CON. These results indicate both common and different mechanisms in the two pain conditions.
Several of the altered spots were identified as the same protein, suggesting the presence of isoforms. This result is not surprising since many proteins are undergoing post translational modifications particularly involving glycosylation. Alpha-1 antitrypsin (A1AT) was one of the altered proteins that were detected as several different isoforms (SSP; 1704, 1705, 1706 and 1708) depending on the glycosylation [49]. The directions of alterations were different for the different isoforms. A1AT is an acute-phase serine proteinase inhibitor with a broad anti-inflammatory spectrum and it has been suggested that it controls the inflammatory component of musculoskeletal connective tissue associated with fibromyalgia [50].The results from two independent series of genotyping 3127 subjects for A1AT supported the hypothesis that the 13% of persons carrying A1AT polymorphisms may represent almost 40% of all cases of fibromyalgia [51].
Elevated levels of S100 protein family (S100-A8, S100-A9 and S100-A12) have been demonstrated in several inflammatory conditions, both locally at sites of inflammation and in the circulation [52]. These proteins are termed calgranulins, reflecting calcium-binding properties and high expression in granulocytes. A normalization of S100A8/9 levels in RA patients who achieved remission shortly after the initiation of conventional treatment have been demonstrated [53]; the decrease in S100A8/9 rather than CRP levels were associated with improvements in the total number of swollen joints over time.
N-acylethanolamines (NAEs) belong to a family of lipids that are able to activate different receptor systems, including nuclear PPAR-alpha receptors. Fatty acid binding proteins (FABPs) are essential for efficient intranuclear NAE trafficking and activation of PPAR-alpha receptors. FABPs also mediate endocannabinoid hydrolysis by FAAH [54]. FABP (spot no. 85 and 87) and phosphatidylethanolamine-binding protein (spot no 49 and 68) could be identified in the muscle dialysate expressed as two isoforms with different isoelectric points. We have previously reported elevated levels of NAEs in the trapezius dialysate in women with chronic trapezius myalgia [55]. In future studies it would be of high importance to investigate the expression levels of FABP and phosphatedylethanolamine-binding protein in dialysate from TM and CWP. Increased level of phosphatidylethanolamine-binding protein has been reported in the vastus lateralis muscle after 55 days of immobilization [56]. FABP has been suggested as a marker for the skeletal muscle injury induced by eccentric exercise [47].
We have also identified a number of proteins that are involved in the oxidative processes in muscle; carbonic anhydrase III, Superoxide dismutase [Cu-Zn], glutathione S-transferase P and flavin reductase (NADPH). Oxidative stress and contractile dysfunction of skeletal muscle induced by reactive oxygen species (ROS) result an imbalance of cellular redox potential and has profound effects on protein carbonylation [57] that leads to modifications in amino acid side chains that result in altered structure and/or functions of the proteins. Changes of the oxidation level of carbonic anhydrase III with muscle unloading have been reported [45]. NADPH is a main source of ROS generation inside skeletal muscle fibers and it has been suggested that the NADPH derived ROS play important physiological roles in regulating skeletal muscle signaling [58]. Superoxide dismutase [Cu-Zn] is an antioxidant protein present in the sarcoplasm of the skeletal muscle fibers and neutralizes ROS production to protect the skeletal muscle against oxidative stress. It has been reported that deficiency in superoxide dismutase [Cu-Zn] results in skeletal muscle weakness and functional innervation [59]. Glutathione S-transferase function also as an antioxidant agent in skeletal muscle, significantly higher level of the antioxidant has been observed in vastus lateralis biopsies from patient with chronic fatigue syndrome [60].
Further protein that was identified in muscle microdialysis and that was differentially expressed in TM and CWP compared to CON was Apolipoprotein AI (SSP no 1203, figure 3). Apolipoprotein AI is involved in lipid transport between cells during regeneration and degeneration of neurons [61] and has been suggested as a biomarker for neuropathic pain [11]. Proteomic study of skeletal muscle lipid droplets has shown that Apolipoprotein AI is expressed endogenously by skeletal muscle cells [62]. Apolipoprotein AI is also associated with acute-phase response and promotes anti-inflammatory and antioxidant effects [63], [64]. It has been identified as a biomarker of acute painful episodes [64].
Four and a half LIM domains protein 1 (FHL-1) is highly expressed in skeletal muscle and has been suggested to be a regulator of myogenesis and muscle growth [65], [66]. We identified this protein as intense spots (spot no. 30, 31, 33, 37) on the 2-DE proteome map of interstitium of trapezius muscle that can confirm that the identified proteins are typical muscle protein as FHL-1 is key skeletal muscle protein. FHL-1 has been identified as a regulator of skeletal muscle mass and suggested as a novel therapeutic target in muscle myopathy and atrophy [67] and it has also been identified in oxidative stress conditions in skeletal muscle [45].
There are limitations with the present technique of MD and 2-DE. The most important is the use of pooled samples from several participants to get the acquired amount of protein needed for 2-DE. Another limitation is that MD is an invasive technique although, according to our experience, most subjects perceive it as minimally invasive. In future studies it is important to optimize the relative recovery, the flow rate of the perfusate/dialysate and the composition of the perfusate in order to achieve a more complete picture of the proteome of the muscle interstitium. By extending the microdialysis time to collect more dialysate and proteins, this would be enough to make a separate analysis per participant making it possible to quantify individual differences. Another limitation is that food intake before the microdialysis not was controlled for. In future studies this can be achieved by a standardized overnight fasting and standardized breakfast before the experiment.
With respect to future perspectives proteins identified from this microdialysis study together with proteomic results from muscle biopsy could be a useful approach to find out the biological meaning of the identified proteins as valuable markers of different state of musculoskeletal pain. Further study on the protein alteration over time during the microdialysis experiment (before and after low force repetitive work) and of the recovery of the altered proteins would be the next intressting step.
In conclusion, 97 protein spots were identified from the interstitial fluid of the trapezius muscle and several of these are known to be involved in nociceptive processes. Forty-eight proteins in TM and 30 proteins in CWP had concentrations at least two-fold higher or lower than in CON. Investigating muscle dialysate using the applied techniques in combination (in vivo MD, 2-DE and mass spectrometry) open up for a) the possibility of investigating protein changes associated with different disease processes of musculoskeletal pain e.g., in chronic trapezius myalgia and in chronic widespread myalgia, b) a better understanding of the pathophysiological mechanisms of chronic muscle pain and c) to identify clinically applicably biomarkers.
Acknowledgments
We acknowledge research nurse Eva-Britt Lind for her valuable help during the microdialysis.
Funding Statement
This study was funded by the Swedish Research Council (K2011-69X-21874-01-6) and the Swedish Council for Working Life and Social Research 2010-0913). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bernard BP (1997) Musculosceletal disorders and workplace factors. A critical review of epidemiological evidence for work-related musculosceletal disorders of the neck, upper extremity, and low back. NIOSH: DHHS [Google Scholar]
- 2. Sluiter JK, Rest KM, Frings-Dresen MH (2001) Criteria document for evaluating the work-relatedness of upper-extremity musculoskeletal disorders. Scandinavian Journal of Work, Environment and Health 27 Suppl 1: 1–102. [PubMed] [Google Scholar]
- 3. Waling K, Jarvholm B, Sundelin G (2002) Effects of training on female trapezius Myalgia: An intervention study with a 3-year follow-up period. Spine (Phila Pa 1976) 27: 789–796. [DOI] [PubMed] [Google Scholar]
- 4. Larsson B, Balogh I (2005) Is there a relationship between fibromyalgia syndrome and work conditions? Journal of Musculoskeletal Pain 13: 5–14. [Google Scholar]
- 5. Gerdle B, Soderberg K, Salvador Puigvert L, Rosendal L, Larsson B (2010) Increased interstitial concentrations of pyruvate and lactate in the trapezius muscle of patients with fibromyalgia: a microdialysis study. J Rehabil Med 42: 679–687. [DOI] [PubMed] [Google Scholar]
- 6.Gerdle B, Larsson B (2012) Potential muscle biomarkers of chronic myalgia in humans - a systematic review of microdialysis studies. In: Khan T, editor. Biomarker INTECH open Access publisher.
- 7. Clough GF (2005) Microdialysis of large molecules. AAPS J 7: E686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Afinowi R, Tisdall M, Keir G, Smith M, Kitchen N, et al. (2009) Improving the recovery of S100B protein in cerebral microdialysis: implications for multimodal monitoring in neurocritical care. J Neurosci Methods 181: 95–99. [DOI] [PubMed] [Google Scholar]
- 9. Dahlin AP, Wetterhall M, Caldwell KD, Larsson A, Bergquist J, et al. (2010) Methodological aspects on microdialysis protein sampling and quantification in biological fluids: an in vitro study on human ventricular CSF. Anal Chem 82: 4376–4385. [DOI] [PubMed] [Google Scholar]
- 10. Takeda S, Sato N, Ikimura K, Nishino H, Rakugi H, et al. (2011) Novel microdialysis method to assess neuropeptides and large molecules in free-moving mouse. Neuroscience 186: 110–119. [DOI] [PubMed] [Google Scholar]
- 11. Niederberger E, Geisslinger G (2008) Proteomics in neuropathic pain research. Anesthesiology 108: 314–323. [DOI] [PubMed] [Google Scholar]
- 12. Marchi A, Vellucci R, Mameli S, Rita Piredda A, Finco G (2009) Pain biomarkers. Clin Drug Investig 29 Suppl 1: 41–46. [DOI] [PubMed] [Google Scholar]
- 13. Wilson R, Whitelock JM, Bateman JF (2009) Proteomics makes progress in cartilage and arthritis research. Matrix Biol 28: 121–128. [DOI] [PubMed] [Google Scholar]
- 14. Ruiz-Romero C, Blanco FJ (2010) Proteomics role in the search for improved diagnosis, prognosis and treatment of osteoarthritis. Osteoarthritis Cartilage 18: 500–509. [DOI] [PubMed] [Google Scholar]
- 15. Görg A, Drews O, Lück C, Weiland F, Weiss W (2009) 2-DE with IPGs. Electrophoresis 30: S122–S132. [DOI] [PubMed] [Google Scholar]
- 16. Kunz S, Tegeder I, Coste O, Marian C, Pfenninger A, et al. (2005) Comparative proteomic analysis of the rat spinal cord in inflammatory and neuropathic pain models. Neurosci Lett 381: 289–293. [DOI] [PubMed] [Google Scholar]
- 17. Finehout EJ, Franck Z, Lee KH (2004) Towards two-dimensional electrophoresis mapping of the cerebrospinal fluid proteome from a single individual. Electrophoresis 25: 2564–2575. [DOI] [PubMed] [Google Scholar]
- 18. Yuan X, Russell T, Wood G, Desiderio DM (2002) Analysis of the human lumbar cerebrospinal fluid proteome. Electrophoresis 23: 1185–1196. [DOI] [PubMed] [Google Scholar]
- 19. Liu XD, Zeng BF, Xu JG, Zhu HB, Xia QC (2006) Proteomic analysis of the cerebrospinal fluid of patients with lumbar disk herniation. Proteomics 6: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 20. Oki G, Wada T, Iba K, Aiki H, Sasaki K, et al. (2012) Metallothionein deficiency in the injured peripheral nerves of complex regional pain syndrome as revealed by proteomics. Pain 153: 532–539. [DOI] [PubMed] [Google Scholar]
- 21. Ferrero S, Gillott DJ, Remorgida V, Ragni N, Venturini PL, et al. (2008) Proteomics technologies in endometriosis. Expert Rev Proteomics 5: 705–714. [DOI] [PubMed] [Google Scholar]
- 22. Seeber B, Sammel MD, Fan X, Gerton GL, Shaunik A, et al. (2010) Proteomic analysis of serum yields six candidate proteins that are differentially regulated in a subset of women with endometriosis. Fertil Steril 93: 2137–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsson B, Rosendal L, Kristiansen J, Sjogaard G, Sogaard K, et al. (2008) Responses of algesic and metabolic substances to 8 h of repetitive manual work in myalgic human trapezius muscle. Pain 140: 479–490. [DOI] [PubMed] [Google Scholar]
- 24. Rosendal L, Kristiansen J, Gerdle B, Sogaard K, Peolsson M, et al. (2005) Increased levels of interstitial potassium but normal levels of muscle IL-6 and LDH in patients with trapezius myalgia. Pain 119: 201–209. [DOI] [PubMed] [Google Scholar]
- 25. Rosendal L, Larsson B, Kristiansen J, Peolsson M, Sogaard K, et al. (2004) Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain 112: 324–334. [DOI] [PubMed] [Google Scholar]
- 26. Ghafouri B, Larsson BK, Sjors A, Leandersson P, Gerdle BU (2010) Interstitial concentration of serotonin is increased in myalgic human trapezius muscle during rest, repetitive work and mental stress - an in vivo microdialysis study. Scand J Clin Lab Invest 70: 478–486. [DOI] [PubMed] [Google Scholar]
- 27. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160–172. [DOI] [PubMed] [Google Scholar]
- 28. Gerdle B, Hilgenfeldt U, Larsson B, Kristiansen J, Sogaard K, et al. (2008) Bradykinin and kallidin levels in the trapezius muscle in patients with work-related trapezius myalgia, in patients with whiplash associated pain, and in healthy controls - A microdialysis study of women. Pain 139: 578–587. [DOI] [PubMed] [Google Scholar]
- 29. Gerdle B, Lemming D, Kristiansen J, Larsson B, Peolsson M, et al. (2008) Biochemical alterations in the trapezius muscle of patients with chronic whiplash associated disorders (WAD) - A microdialysis study. European Journal of Pain 12: 82–93. [DOI] [PubMed] [Google Scholar]
- 30.Hermens H, Fredriks B (1997) SENIAM The state of the art on sensors and sensor placement procedures for surface elecromyography: A proposal for sensor placement procedures: Roessingh Research and Development bv.
- 31. Olausson P, Gerdle B, Ghafouri N, Karlsson L, Larsson B, et al. (2012) Relative recovery over time – an in vivo microdialysis study of human skeletal muscle. Scandinavian Journal of Clinical & Laboratory Investigation 0: 1–7. [DOI] [PubMed] [Google Scholar]
- 32. Ghafouri B, Stahlbom B, Tagesson C, Lindahl M (2002) Newly identified proteins in human nasal lavage fluid from non-smokers and smokers using two-dimensional gel electrophoresis and peptide mass fingerprinting. Proteomics 2: 112–120. [PubMed] [Google Scholar]
- 33. Swain M, Ross NW (1995) A silver stain protocol for proteins yielding high resolution and transparent background in sodium dodecyl sulfate-polyacrylamide gels. Electrophoresis 16: 948–951. [DOI] [PubMed] [Google Scholar]
- 34. Ghafouri B, Karlsson H, Mortstedt H, Lewander A, Tagesson C, et al. (2007) 2,5-Dihydroxybenzoic acid instead of alpha-cyano-4-hydroxycinnamic acid as matrix in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for analyses of in-gel digests of silver-stained proteins. Anal Biochem 371: 121–123. [DOI] [PubMed] [Google Scholar]
- 35. Hung PH, Chen YW, Cheng KC, Chou HC, Lyu PC, et al. (2011) Plasma proteomic analysis of the critical limb ischemia markers in diabetic patients with hemodialysis. Mol Biosyst 7: 1990–1998. [DOI] [PubMed] [Google Scholar]
- 36. Paulo JA, Lee LS, Banks PA, Steen H, Conwell DL (2011) Difference gel electrophoresis identifies differentially expressed proteins in endoscopically collected pancreatic fluid. Electrophoresis 32: 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu Y, Han S, Zhao H, Liang J, Zhai J, et al. (2011) Comparative analysis of serum proteomes of degenerative scoliosis. J Orthop Res 29: 1896–1903. [DOI] [PubMed] [Google Scholar]
- 38. Darde VM, de la Cuesta F, Dones FG, Alvarez-Llamas G, Barderas MG, et al. (2010) Analysis of the plasma proteome associated with acute coronary syndrome: does a permanent protein signature exist in the plasma of ACS patients? J Proteome Res 9: 4420–4432. [DOI] [PubMed] [Google Scholar]
- 39. Hody S, Leprince P, Sergeant K, Renaut J, Croisier JL, et al. (2011) Human muscle proteome modifications after acute or repeated eccentric exercises. Med Sci Sports Exerc 43: 2281–2296. [DOI] [PubMed] [Google Scholar]
- 40. Gill C, Parkinson E, Church MK, Skipp P, Scott D, et al. (2011) A qualitative and quantitative proteomic study of human microdialysate and the cutaneous response to injury. AAPS J 13: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hojlund K, Yi Z, Hwang H, Bowen B, Lefort N, et al. (2008) Characterization of the human skeletal muscle proteome by one-dimensional gel electrophoresis and HPLC-ESI-MS/MS. Mol Cell Proteomics 7: 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holloway KV, O'Gorman M, Woods P, Morton JP, Evans L, et al. (2009) Proteomic investigation of changes in human vastus lateralis muscle in response to interval-exercise training. Proteomics 9: 5155–5174. [DOI] [PubMed] [Google Scholar]
- 43. Hagberg M, Michaelson G, Ortelius A (1982) Serum creatine kinase as an indicator of local muscular strain in experimental and occupational work. Int Arch Occup Environ Health 50: 377–386. [DOI] [PubMed] [Google Scholar]
- 44. Mense S (2009) Algesic agents exciting muscle nociceptors. Exp Brain Res 196: 89–100. [DOI] [PubMed] [Google Scholar]
- 45. Chen CN, Ferrington DA, Thompson LV (2008) Carbonic anhydrase III and four-and-a-half LIM protein 1 are preferentially oxidized with muscle unloading. J Appl Physiol 105: 1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bailey DM, Davies B, Young IS, Hullin DA, Seddon PS (2001) A potential role for free radical-mediated skeletal muscle soreness in the pathophysiology of acute mountain sickness. Aviat Space Environ Med 72: 513–521. [PubMed] [Google Scholar]
- 47. Sorichter S, Mair J, Koller A, Pelsers MM, Puschendorf B, et al. (1998) Early assessment of exercise induced skeletal muscle injury using plasma fatty acid binding protein. Br J Sports Med 32: 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu JG, Thornell LE (2002) Desmin and actin alterations in human muscles affected by delayed onset muscle soreness: a high resolution immunocytochemical study. Histochem Cell Biol 118: 171–179. [DOI] [PubMed] [Google Scholar]
- 49. Packer NH, Lawson MA, Jardine DR, Sanchez JC, Gooley AA (1998) Analyzing glycoproteins separated by two-dimensional gel electrophoresis. Electrophoresis 19: 981–988. [DOI] [PubMed] [Google Scholar]
- 50. Blanco I, Canto H, de Serres FJ, Fernandez-Bustillo E, Rodriguez MC (2004) Alpha1-antitrypsin replacement therapy controls fibromyalgia symptoms in 2 patients with PI ZZ alpha1-antitrypsin deficiency. J Rheumatol 31: 2082–2085. [PubMed] [Google Scholar]
- 51. Schmechel DE, Edwards CL (2012) Fibromyalgia, mood disorders, and intense creative energy: A1AT polymorphisms are not always silent. Neurotoxicology [DOI] [PubMed] [Google Scholar]
- 52. Foell D, Frosch M, Sorg C, Roth J (2004) Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta 344: 37–51. [DOI] [PubMed] [Google Scholar]
- 53. Andres Cerezo L, Mann H, Pecha O, Plestilova L, Pavelka K, et al. (2011) Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent-onset rheumatoid arthritis. Arthritis Res Ther 13: R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG (2012) Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem 287: 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ghafouri N, Ghafouri B, Larsson B, Turkina MV, Karlsson L, et al. (2011) High levels of N-palmitoylethanolamide and N-stearoylethanolamide in microdialysate samples from myalgic trapezius muscle in women. PLoS One 6: e27257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moriggi M, Vasso M, Fania C, Capitanio D, Bonifacio G, et al. (2010) Long term bed rest with and without vibration exercise countermeasures: effects on human muscle protein dysregulation. Proteomics 10: 3756–3774. [DOI] [PubMed] [Google Scholar]
- 57. Barreiro E, Hussain SN (2010) Protein carbonylation in skeletal muscles: impact on function. Antioxid Redox Signal 12: 417–429. [DOI] [PubMed] [Google Scholar]
- 58. Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN (2002) Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med 165: 412–418. [DOI] [PubMed] [Google Scholar]
- 59. Larkin LM, Davis CS, Sims-Robinson C, Kostrominova TY, Remmen HV, et al. (2011) Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. Am J Physiol Regul Integr Comp Physiol 301: R1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fulle S, Mecocci P, Fano G, Vecchiet I, Vecchini A, et al. (2000) Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome. Free Radic Biol Med 29: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 61. Boyles JK, Notterpek LM, Anderson LJ (1990) Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I. J Biol Chem 265: 17805–17815. [PubMed] [Google Scholar]
- 62. Zhang H, Wang Y, Li J, Yu J, Pu J, et al. (2011) Proteome of skeletal muscle lipid droplet reveals association with mitochondria and apolipoprotein a-I. J Proteome Res 10: 4757–4768. [DOI] [PubMed] [Google Scholar]
- 63. Brouillette CG, Anantharamaiah GM, Engler JA, Borhani DW (2001) Structural models of human apolipoprotein A-I: a critical analysis and review. Biochim Biophys Acta 1531: 4–46. [DOI] [PubMed] [Google Scholar]
- 64. Tumblin A, Tailor A, Hoehn GT, Mack AK, Mendelsohn L, et al. (2010) Apolipoprotein A-I and serum amyloid A plasma levels are biomarkers of acute painful episodes in patients with sickle cell disease. Haematologica 95: 1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morgan MJ, Madgwick AJ (1999) The LIM proteins FHL1 and FHL3 are expressed differently in skeletal muscle. Biochem Biophys Res Commun 255: 245–250. [DOI] [PubMed] [Google Scholar]
- 66. Robinson PA, Brown S, McGrath MJ, Coghill ID, Gurung R, et al. (2003) Skeletal muscle LIM protein 1 regulates integrin-mediated myoblast adhesion, spreading, and migration. Am J Physiol Cell Physiol 284: C681–695. [DOI] [PubMed] [Google Scholar]
- 67. Cowling BS, McGrath MJ, Nguyen MA, Cottle DL, Kee AJ, et al. (2008) Identification of FHL1 as a regulator of skeletal muscle mass: implications for human myopathy. J Cell Biol 183: 1033–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]