Abstract
The Shigella resistance locus (SRL) pathogenicity island (PAI) in Shigella spp. mediates resistance to streptomycin, ampicillin, chloramphenicol, and tetracycline. It can be excised from the chromosome via site-specific recombination mediated by the P4-related int gene. Here, we show that SRL PAI attP is capable of RecA-independent, site-specific, int-mediated integration into two bacterial tRNA attB sites.
Shigella spp. are the causative agents of bacillary dysentery, a disease responsible for the deaths of over 1.1 million people annually (10). The infection, which is spread via the fecal-oral route, is commonly treated with a combination of rehydration and antimicrobial therapy. However, over the past few decades, treatment has become increasingly difficult as resistance to most of the widely used therapeutic antibiotics has emerged (9, 16).
Multiple-antibiotic resistance to Shigella spp. was first reported as early as the 1950s (21), and since that time, multiresistance gene clusters have been identified on R plasmids, transposons, and integrons. Recently, a 16-kb cluster of genes known as the Shigella resistance locus (SRL) was identified on the chromosome of Shigella flexneri 2a strain YSH6000. In this strain, the SRL, which confers resistance to the antibiotics streptomycin, ampicillin, chloramphenicol, and tetracycline, is present on a 66-kb pathogenicity island (PAI) designated the SRL PAI (11). Recent findings show that the SRL PAI is present in numerous Shigella strains, and it is hypothesized that the SRL PAI may be involved in the spread of multiple-antibiotic resistance in Shigella spp. (17).
PAIs, which are believed to be acquired by horizontal gene transfer, frequently carry phage-related integrase genes, are often integrated adjacent to tRNA genes, and are flanked by short direct repeats (DRs) which resemble phage attachment (att) sites (1). It is thought that the genetic similarities of PAIs and phages may extend to related mechanisms of integration and excision (7). However, genetic instability of PAIs has been observed in only a small number of cases, with only two PAIs having been shown to be mobilizable, and very little is known about mechanisms of PAI transfer in general (5). In contrast, the mechanism by which the SRL PAI is excised from the serX tRNA gene in S. flexneri 2a YSH6000 is known to be integrase mediated and site specific. These data, in conjunction with the presence of the SRL PAI in other Shigella strains (17), suggest that the SRL PAI, and therefore the antibiotic resistance genes it carries, may still be mobile. However, nothing is known of the excised form of the SRL PAI or its ability to integrate into a new host.
Circular form of the SRL PAI
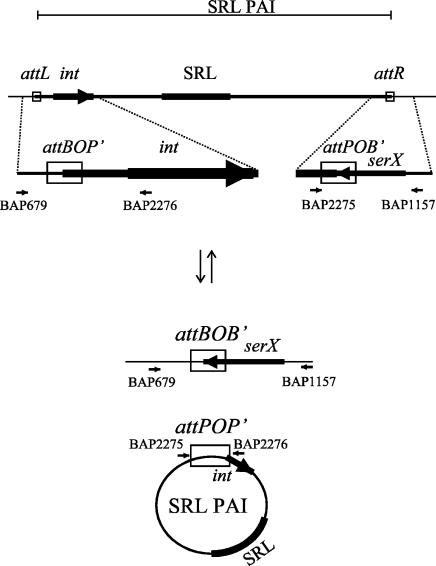
The SRL PAI of strain YSH6000 is flanked by 14-bp sequences that correspond to the terminal 3′ 14 bp of the serX tRNA gene at the right boundary and a directly repeated sequence at the left boundary. Integrase-mediated excision results in the loss of the PAI and one copy of the DR, while an intact serX gene and a single copy of the DR remain on the chromosome. Thus, the SRL PAI is excised via mechanisms that resemble the site-specific recombination exhibited by lambdoid phages (18). Lambdoid phages are also known to be integrated into and excised from their chromosomal attB sites via a circular extrachromosomal intermediate (4); thus, we tested whether the SRL PAI also formed a similar circular intermediate upon excision. The excision was predicted to take place via recombination of the attL (attBOP′) and attR (attPOB′) sites, which result in the reconstitution of the chromosomal attB (attBOB′) site (demonstrated previously with BAP679 and BAP1157 [18]) (Fig. 1) and also the formation of the attP (attPOP′) site carried on the circular form of the SRL PAI (Fig. 1). The attP junction on the SRL PAI circular intermediate was not detectable by PCR in YSH6000 (data not shown). Since the excised form of the SRL PAI does not replicate and the frequency of spontaneous PAI excision is low (10−5 to 10−6) (18), formation of the circle may be below the level of detection by PCR. For this reason, a YSH6000 derivative strain, AL325, which incorporates a suicide plasmid, pCACTUS (20), with a temperature-sensitive pSC101 ori as a single crossover in the fecI gene located on the SRL PAI, was constructed. To increase the sensitivity of the assay, hyperexcision of the SRL PAI was induced by the introduction of the rox gene, which increases the frequency of PAI excision, carried on pUC19Tp (S. N. Luck, unpublished data). Outward-facing primers BAP2275 (5′-CTTTAAGAATTCCTCCGCGCATATCACG-3′) and BAP2276 (5′-GAAAAATCTAGATCCCCCGCCCCTTTACTG-3′) were used in a PCR with an AL325 chromosomal template to successfully amplify the attP junction of an SRL PAI circular molecule formed upon recombination of the left (attL) and right (attR) PAI boundaries (Fig. 1). The resulting 553-bp fragment, designated attP due to the prophage-like organization of the SRL PAI, was cloned into the suicide vector pJP5603 (13) and sequenced. Sequence analysis of the resultant plasmid, pAL226, revealed that the SRL PAI attP (POP′) consisted of the left end of the SRL PAI (P′), an exact copy of the 14-bp DR (O), followed by the right end of the SRL PAI (P) (Fig. 1). These results are thus consistent with the presence of a circular form of the SRL PAI.
FIG. 1.
Model of the site-specific deletion of the SRL PAI YSH6000. The integrated SRL PAI is deleted via site-specific recombination to form a reconstituted attB site and an extrachromosomal circular form of the SRL PAI. Thin lines represent chromosomal DNA, and a thick line represents the SRL PAI. The SRL is represented by a filled box, and attL (attBOP′), attR (attPOB′), attP (attPOP′), and attB (attBOB′) sites are represented as open boxes. Integrase (int) and serX genes are represented as large arrows, and the position and orientation of primers used in amplifications are indicated with small arrows. The diagram is not drawn to scale.
To confirm that the SRL PAI was present as an extrachromosomal molecule, Eckhardt gel electrophoresis, an in-well lysis method which allows plasmid DNA to enter the gel matrix while retarding chromosomal DNA (14), was employed with strains AL325, YSH6000, and SBA573 (carrying the 95-kb plasmid R100-1) which served as a positive control. DNA was then analyzed by Southern hybridization with a probe corresponding to the cat gene which is harbored on R100-1 and the SRL PAI as described previously (17). Hybridization with the cat probe occurred in the lanes corresponding to AL325 and the positive-control SBA573, thus confirming an extrachromosomal location of the cat gene in both these strains. These results indicate that the SRL PAI, although present on the chromosome in YSH6000, is present extrachromosomally in the PAI-stabilized strain AL325, consistent with the presence of a stabilized, circular SRL PAI intermediate.
Site-specific recombination of the SRL PAI attP
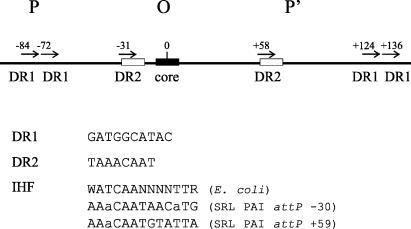
Sequence analysis of the SRL PAI attP site revealed the presence of several DRs (Fig. 2). Integration systems often require a combination of accessory elements, and the lambda attP site contains several DRs for the binding of integrase and the accessory protein integration host factor (IHF) (12). Sequences resembling an IHF binding site, WATCAANNNNTTR (where W is A or T, R is A or G, and N is A, G, C, or T) (6), are also present in the SRL PAI attP (Fig. 2). It is interesting that the positions of DR1 in the SRL PAI attP site (−84, −72, +124, and +136) (Fig. 2) show some similarity to the spacing of integrase binding sites in the lambda attP arm sites (+80, +70, +60, −110, and −140) (4).
FIG. 2.
Schematic representation of the SRL PAI attP (POP′) structure. The 14-bp O core is shown as a filled box. The numbers correspond to the positions of the DRs, with the center of the core region being considered position 0. Short horizontal arrows indicate DR1 and DR2, with sequences being given below the diagram. Sequences showing similarity to IHF binding sites are indicated with open boxes, alignments with the E. coli IHF binding site are shown below the diagram, and mismatched bases are shown in lowercase letters.
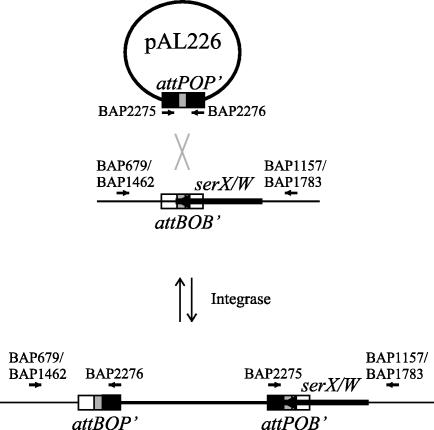
Previous analysis indicated that the SRL PAI is found adjacent to the identical paralogous tRNA genes serX and serW in a number of Shigella isolates (17). To determine if the integration mechanism of the SRL PAI resembled phage-like site-specific integration, the SRL PAI attP was tested for its ability to undergo recombination with serX and serW, which in this system represent the chromosomal attB sites (Fig. 3). pAL226, which carries the SRL attP site on the suicide plasmid pJP5603, was transferred to the mobilizing Escherichia coli strain S17-1 λpir to construct AL403 (Kanr). S17-1 λpir, which carries the RP4 conjugative-transfer genes, mobilized pAL226, which carries the mob site of RP4, to AL104 (Nalr Ampr), a RecA-deficient E. coli DH5α strain harboring the SRL PAI int gene on the plasmid pWSK29 (17). Transconjugants were selected by plating them on nalidixic acid plus ampicillin plus kanamycin and tested by PCR for the insertion of pAL226 into serX (using primer pairs BAP2276-BAP679 and BAP2275-BAP1157) or serW (using primer pairs BAP2276-BAP1462 and BAP2275-BAP1783) (Fig. 3). In all transconjugants tested, PCR product size and Southern hybridization results with serX or serW as a probe were consistent with the insertion of pAL226 into one or both of these sites. Sequencing of the BAP2276-BAP679 and BAP2276-BAP1462 products confirmed the precise insertion of the SRL PAI POP′, which results in an arrangement identical to that of wild-type YSH6000 attR (attPOB′) (Fig. 1 and 3). Thus, in the presence of int, the SRL PAI attP site is sufficient for site-specific recombination with the chromosomal attB sites partially contained within serX or serW.
FIG. 3.
Schematic representation of the integration of the SRL PAI attP (carried on plasmid pAL226) into the chromosomal attB sites. Chromosomal DNA is represented by thin lines, and the plasmid pAL226 is represented by thick lines. att sites are represented by black or white boxes, with the common O core site being represented by shaded boxes. Genes are represented as large arrows, and the positions and orientations of primers used in amplifications are indicated with small arrows. The diagram is not drawn to scale.
The SRL PAI attP × attB recombination requires int
To test the ability of the SRL PAI int to catalyze the attP × attB recombination, matings of the donor AL403 (harboring the SRL PAI attP site) were carried out with both an int-positive and an int-negative RecA-deficient DH5α recipient. Site-specific integration into the serX or serW tRNA gene was determined by PCR with the primer pairs BAP2275-BAP1157 and BAP2275-BAP1783 (Fig. 3). Integrants were obtained with a frequency of 2 × 10−4 per donor in the int-positive background. However, no integration was detected in the int-negative background (detection limit of 3.4 × 10−5). Thus, site-specific integration of the SRL PAI attP site requires the presence of the SRL PAI integrase.
Together with previous findings (18), these data indicate that the integration and excision mechanisms of the SRL PAI are closely related to those of lambdoid phages. First, the integrated SRL PAI forms a circular extrachromosomal intermediate through recombination of the left and right junctions. Second, integration via site-specific recombination occurs in a 14-bp sequence found both in the circular form of the SRL PAI and at the 3′ end of the tRNA genes serX and serW. Finally, both excision and integration of the SRL PAI require the P4 phage-related SRL PAI int gene.
Many PAIs and genomic islands are flanked by short DRs, are situated adjacent to tRNA genes, and carry integrase-related sequences. Thus, it has been suggested that, although in the majority of cases direct evidence of integration and excision is lacking, site-specific recombination is likely to play a role in the mobility of these elements (7). Certainly, an integrative module of the Yersinia HPI has been shown to undergo site-specific integration (15), and similarly, Staphylococcus SaPIbov2 undergoes site-specific, integrase-mediated integration and excision (19). Indeed, as the SRL PAI is able to undergo int-dependent, site-specific excision (18) and integration and is found in a number of Shigella isolates (17), it seems credible that site-specific recombination plays an essential role in the dissemination of the SRL PAI and therefore the dissemination of the SRL resistance determinants.
To date, site-specific recombination is known to be involved where mobility of genomic islands and PAIs has been demonstrated. The conjugative integrating Vibrio cholerae SXT element and several bacteriophages employ integrase-mediated, site-specific recombination to transfer as a circular intermediate (8). However, unlike these elements, sequence analysis indicates that the SRL PAI does not posses similarity to genes encoding conjugative-transfer or phage coat proteins (11). It therefore remains unclear how the transfer of the SRL PAI occurs. Interestingly, the Salmonella SaPI is encapsidated and mobilized by the staphylococcal phage 80α in a relationship that has been likened to that of coliphages P2 and P4 (18). It remains a possibility that the SRL PAI may be similarly mobilized, and future work will focus on the role of accessory elements in the mobilization of the PAI.
It is interesting that several genomic islands carrying multiresistance determinants are now thought to employ site-specific recombination as a means for lateral transfer. Such elements include the SXT element of V. cholerae (8), Salmonella genomic island 1 (2, 3), and the SRL PAI. Thus, the studies presented here not only are a useful tool in examining the mechanistic aspects of PAI dissemination but also provide an interesting insight into potential mechanisms for the spread of multiple-antibiotic resistance.
Acknowledgments
We acknowledge the expert technical assistance of Ian McPherson and Vicki Vallance.
This work was supported by the National Health and Medical Research Council, Canberra, Australia.
REFERENCES
- 1.Blum, G., M. Ott, A. Lischewski, A. Ritter, H. Imrich, H. Tschäpe, and J. Hacker. 1994. Excision of large DNA regions termed pathogenicity islands from tRNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infect. Immun. 62:606-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd, D., G. A. Peters, A. Cloeckaert, K. S. Boumedine, E. Chaslus-Dancla, H. Imberechts, and M. R. Mulvey. 2001. Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J. Bacteriol. 183:5725-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd, D. A., G. A. Peters, L.-K. Ng, and M. R. Mulvey. 2000. Partial characterization of a genomic island associated with the multidrug resistance region of Salmonella enterica Typhymurium DT104. FEMS Microbiol. Lett. 189:285-291. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, A. M. 1992. Chromosomal insertion sites for phages and plasmids. J. Bacteriol. 174:7495-7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, B. M., and M. K. Waldor. 2002. Mobile genetic elements and bacterial pathogenesis, p. 1040-1059. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 6.Friedman, D. I. 1988. Integration host factor: a protein for all reasons. Cell 55:545-554. [DOI] [PubMed] [Google Scholar]
- 7.Hacker, J., and J. B. Kaper. 1999. The concept of pathogenicity islands, p. 1-11. In J. B. Kaper and J. Hacker (ed.), Pathogenicity islands and other mobile virulence elements. ASM Press, Washington, D.C.
- 8.Hochhut, B., and M. K. Waldor. 1999. Site-specific integration of the conjugal Vibrio cholerae SXT element into prfC.Mol. Microbiol. 32:99-110. [DOI] [PubMed] [Google Scholar]
- 9.Khan, M. U. 1985. Fourteen years of shigellosis in Dhaka: an epidemiological analysis. Int. J. Epidemiol. 14:607-613. [DOI] [PubMed] [Google Scholar]
- 10.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. W. H. O. 77:651-665. [PMC free article] [PubMed] [Google Scholar]
- 11.Luck, S. N., S. A. Turner, K. Rajakumar, H. Sakellaris, and B. Adler. 2001. Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect. Immun. 69:6012-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash, H. A. 1996. Site-specific recombination: integration, excision, resolution, and inversion of defined DNA segments, p. 2363-2376. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 2. ASM Press, Washington D.C.
- 13.Penfold, R. J., and J. M. Pemberton. 1992. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene 118:145-146. [DOI] [PubMed] [Google Scholar]
- 14.Priefer, U. 1984. Characterization of plasmid DNA by agarose gel electrophoresis, p. 32-33. In A. Puhler and K. N. Timmis (ed.), Advanced molecular genetics. Springer-Verlag, New York, N.Y.
- 15.Rakin, A., C. Noelting, P. Schropp, and J. Heesemann. 2001. Integrative module of the high-pathogenicity island of Yersinia.Mol. Microbiol. 39:407-415. [DOI] [PubMed] [Google Scholar]
- 16.Salam, M. A., and M. L. Bennish. 1991. Antimicrobial therapy for shigellosis. Rev. Infect. Dis. 13:S332-S341. [DOI] [PubMed] [Google Scholar]
- 17.Turner, S. A., S. N. Luck, H. Sakellaris, K. Rajakumar, and B. Adler. 2003. Molecular epidemiology of the SRL pathogenicity island. Antimicrob. Agents Chemother. 47:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner, S. A., S. N. Luck, H. Sakellaris, K. Rajakumar, and B. Adler. 2001. Nested deletions of the SRL pathogenicity island of Shigella flexneri 2a. J. Bacteriol. 183:5535-5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ubeda, C., T. Angeles, C. Cucarella, P. Trotonda, T. J. Foster, I. Lasa, and J. R. Penades. 2003. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol. Microbiol. 49:193-210. [DOI] [PubMed] [Google Scholar]
- 20.Van den Bosch, L., P. Manning, and R. Morona. 1997. Regulation of O-antigen chain length is required for Shigella flexneri virulence. Mol. Microbiol. 23:765-775. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe, T. 1963. Infective heredity of multiple drug resistance in bacteria. Bacteriol. Rev. 27:87-115. [DOI] [PMC free article] [PubMed] [Google Scholar]