Abstract
Microdilution broth checkerboard techniques based on the National Committee for Clinical Laboratory Standards methodology were used to study double and triple antifungal combinations against clinical isolates of Aspergillus fumigatus and A. terreus. The influences of the end-point definition (partial or complete inhibition) and the mode of reading (visually or spectrophotometrically) were determined. Interactions between antifungal drugs were also evaluated by agar diffusion tests. Combinations of caspofungin with either amphotericin B or voriconazole were additive for all the isolates, and antagonism was not observed. The interaction between caspofungin and flucytosine was synergistic for 62% of the isolates. In contrast, the interaction between voriconazole and flucytosine was never synergistic and antagonism was noted for 93% of the isolates. The triple combination of caspofungin with flucytosine and amphotericin B was synergistic for all the isolates tested. The triple combination of caspofungin with flucytosine and voriconazole was also mostly synergistic; but complex interactions were obtained for some isolates, with synergy or antagonism depending on the concentrations of caspofungin and voriconazole. Analysis of the influence of the reading technique on the results showed that spectrophotometric reading was a good alternative to the recommended visual reading. The results of these in vitro tests suggest that the activity of flucytosine as part of a double combination with caspofungin and as part of a triple combination with caspofungin and amphotericin B against Aspergillus spp. warrants further investigations. Animal studies are needed to evaluate the in vivo efficacies of these combinations.
Invasive aspergillosis is an increasing problem that occurs primarily in immunocompromised patients (11). The mortality rate is particularly high in bone marrow transplant recipients and patients with disseminated infections (22). Until recently, amphotericin B and itraconazole were the drugs of choice for the treatment of these infections (44). Nevertheless, the overall success rate has been low, and recent reports of de novo and acquired resistance of Aspergillus fumigatus clinical isolates to itraconazole (7, 13) as well as in vivo resistance to amphotericin B (18) complicate the management of invasive aspergillosis. Moreover, some Aspergillus species such as Aspergillus terreus are less susceptible to amphotericin B (8). New drugs with activity against Aspergillus spp. include voriconazole and caspofungin. However, their therapeutic efficacies remain limited, with response rates of 45% for caspofungin in refractory cases (J. Maertens, I. Raad, G. Petrikkos, D. Selleslag, F. Petersen, C. Sable, N. Kartsonis, A. Ngai, A. Taylor, T. Patterson, D. Denning, and T. Walsh, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 868, 2002) and 48 to 53% for voriconazole as a first-line strategy (12, 17); the mortality rate among patients treated with voriconazole is about 30% at week 12. It is therefore mandatory to find new strategies for the treatment of invasive aspergillosis, among which combinations of drugs deserve evaluation. Combinations of more than two drugs are commonly used to treat various infections such as those caused by Mycobacterium tuberculosis and human immunodeficiency virus (HIV) (39, 47), but they have been poorly evaluated in medical mycology (35).
Azoles, echinocandins, flucytosine, and amphotericin B belong to different pharmacological classes and possess distinct mechanisms of action (16). As these drugs act on different targets, evaluation of their interactions is of potential interest for improving treatments for invasive aspergillosis. Azoles such as voriconazole act primarily by inhibition of 14α-demethylase (41), whereas echinocandins such as caspofungin inhibit the synthesis of beta-1,3-glucan (10). Flucytosine inhibits fungal RNA and DNA synthesis (46), and amphotericin B binds to ergosterol (16). All these drugs except flucytosine have activity against Aspergillus spp. Nevertheless, the use of flucytosine in combination with amphotericin B has been proposed for the treatment of cerebral aspergillosis (44). It must be noted that there is no standardized method for susceptibility testing of caspofungin, and various MICs of this drug for A. fumigatus have been obtained (2, 5, 15, 32).
The activities of combinations of echinocandins with amphotericin B against Aspergillus spp. have been evaluated in vitro (3) and in vivo in animals (23) and showed no antagonism. Moreover, it has been shown in a recent retrospective study (1) that the combination of caspofungin with liposomal amphotericin B is a safe and feasible option for the treatment of invasive fungal infections in patients with hematologic malignancies. Interactions between echinocandins and azole drugs have also been tested in vitro (24, 32, 40) and in animal models of aspergillosis (20, 23, 33), and it can be concluded that these combinations deserve consideration for the treatment of aspergillosis.
The activities of combinations of flucytosine with the new azoles or with echinocandins against Aspergillus spp. have not been tested so far. Although flucytosine is known to interact synergistically with azoles against some fungal pathogens such as Cryptococcus neoformans, antagonism against Candida glabrata (42, 43) and Candida lusitaniae (31) has also been reported.
The aim of this study was to investigate the in vitro interactions among caspofungin, amphotericin B, voriconazole, and flucytosine in double combinations as well as triple combinations against clinical isolates of A. fumigatus and A. terreus.
MATERIALS AND METHODS
Test isolates
A total of 35 clinical isolates of Aspergillus spp. (30 A. fumigatus isolates and 5 A. terreus isolates) were used in the study. Depending on the combination of drugs used, 12 to 35 of these isolates were tested in each experiment. For triple combinations, isolates were chosen to include those with which both synergistic and indifferent interactions were obtained in the studies with double combinations. All isolates were cultured from frozen stocks on Sabouraud dextrose agar (supplemented with 0.02% chloramphenicol) for 7 days at 30°C to ensure purity and viability. Two reference strains, Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019, were included to ensure quality control.
Medium
RPMI 1640 medium with l-glutamine but without sodium bicarbonate (Sigma-Aldrich, Saint Quentin Fallavier, France) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (Sigma-Aldrich) was used as the test medium.
Inoculum
The isolates were grown on Sabouraud dextrose agar for 7 days at 30°C, and stock spore suspensions were prepared by washing the surfaces of the slants with 2 ml of sterile saline containing 0.05% Tween 80. The spore suspensions were adjusted spectrophotometrically at 530 nm to optical densities ranging from 0.09 to 0.11 and were then diluted 1:50 (for double combinations) or 1:25 (for triple combinations) in RPMI 1640 medium to obtain two or four times the final concentration, respectively. Inoculum sizes were checked by quantitative determination of colony counts on Sabouraud dextrose agar.
Antifungal susceptibility testing
Drug combinations were tested by using the guidelines presented in document M38A of the National Committee for Clinical Laboratory Standards (NCCLS) (28), as modified for a broth microdilution checkerboard procedure.
The drugs tested included amphotericin B (Bristol-Myers Squibb, Paris, France), flucytosine (ICN Pharmaceuticals, Orsay, France), voriconazole (Pfizer Central Research, Sandwich, United Kingdom), and caspofungin (Merck & Co., Inc., Rahway, N.J.). For preparation of stock solutions, amphotericin B and voriconazole were dissolved in dimethyl sulfoxide and flucytosine and caspofungin were dissolved in water. The drug dilutions were prepared at four times the strength of the final concentration by following the additive drug dilution scheme of NCCLS (28). For the double combinations a two-dimensional checkerboard with twofold dilutions of each drug was used for the study of caspofungin with either voriconazole or amphotericin B. The final concentrations of the antifungal agents were 0.03 to 16 μg/ml for caspofungin (i.e., 10 twofold dilutions), 0.03 to 2 μg/ml for voriconazole (i.e., 7 twofold dilutions), and 0.06 to 4 μg/ml for amphotericin B (i.e., 7 twofold dilutions). For the double combinations of flucytosine with either caspofungin or voriconazole, the final concentrations of the antifungal agents were 2 to 128 μg/ml for flucytosine (i.e., 7 twofold dilutions), 0.03 to 16 μg/ml for caspofungin (i.e., 10 twofold dilutions), and 0.008 to 4 μg/ml for voriconazole (i.e., 10 twofold dilutions).
The triple combinations caspofungin-amphotericin B-flucytosine and caspofungin- voriconazole-flucytosine were tested by a three-dimensional checkerboard technique in the following manner. A checkerboard with twofold dilutions of caspofungin and either amphotericin B or voriconazole was set up as described above for the double combinations. Flucytosine was added at a single concentration per plate. Five plates were used for each isolate, including one plate without flucytosine. The final concentrations of the antifungal agents were 0.03 to 16 μg/ml for caspofungin, 0.03 to 2 μg/ml for amphotericin B, 0.015 to 1 μg/ml for voriconazole, and 2 to 128 μg/ml for flucytosine (i.e., five dilutions were tested: 0, 2, 8, 32, and 128 μg/ml).
Growth control wells containing medium plus 0.5% of the corresponding solvent were included. Microdilution trays were kept at −20°C for less than 1 month until the day of testing, and quality controls were included in each set of experiments to ensure maintenance of the potencies of the drugs during storage.
Incubation and MIC determination
On the day of the test, each well of the microtiter plates containing 100 μl of the diluted drug concentrations (150 μl for the triple combinations) was inoculated with 100 μl of the inoculum suspension (50 μl for the triple combinations). The microtiter plates were incubated at 35°C, and the MICs were determined after 48 h of incubation. The MICs were determined in two independent experiments, with similar results obtained in each experiment. The microtiter plates were read visually and spectrophotometrically.
(i) Visual MIC determination
The microtiter plates were read visually with the aid of a concave mirror, and the growth in each well was compared with that in the growth control well. Each well was then given a numerical score according to the NCCLS guidelines: 4, no reduction in growth; 3, growth reduction of 25%; 2, growth reduction of 50%; 1, growth reduction of 75% or more; and 0, absence of growth (optically clear). Visual readings were performed by one investigator and were always performed before the spectrophotometric reading.
(ii) Spectrophotometric MIC determination
Spectrophotometric readings were performed with an automated microtiter plate reader spectrophotometer (Multiscan RC-351; Labsystems Oy, Helsinki, Finland) at 450 nm. Blank optical density values for the uninoculated plates were subtracted from the optical density values, and the percentage of growth for each well was calculated by comparison with the growth for the drug-free control wells.
(iii) MEC determination
The minimal effective concentration (MEC) of caspofungin was determined by reading the microtiter plates with an inverted microscope and was defined as the minimal concentration that gave abnormal hyphal growth (2). Nevertheless, as the other antifungals were not responsible for the same pattern of microscopic modification of the hyphae, the MEC was not suitable for evaluation of the combined effects of the drugs. Therefore, we used the MIC instead of the MEC for evaluation of antifungal interactions, as reported previously (3, 32). From the 216 determinations performed in the present study, the median caspofungin MEC was 0.5 μg/ml (range, 0.25 to 2 μg/ml), which is in agreement with the results presented in previous reports (2, 3).
End-point determination
The MIC end points were defined as the lowest drug concentration (tested alone or in combination) which had a score of 0, as determined by visual reading (VisuMIC-0). Additionally, for all drugs the lowest drug concentration which had a score of 2 (VisuMIC-2) as well as the lowest concentrations that gave 50 and 90% inhibition by the spectrophotometric reading (SpecMIC50% and SpecMIC90%, respectively) was recorded.
Agar diffusion test
In order to visualize the interactions, the activities of the antifungal combinations against one of the isolates (A. fumigatus AF2) were also evaluated by agar diffusion tests. A disk diffusion assay was used in the first set of experiments. RPMI 1640 agar plates containing increasing concentrations of one of the antifungals were inoculated with a spore suspension by swabbing the agar surface. After the plates were allowed to dry, sterile paper disks impregnated with either 16 μg of caspofungin, 4 μg of voriconazole, or 128 μg of flucytosine were placed onto the agar surface. The inhibition zones were measured after incubation at 35°C for 48 h. Assays were run in duplicate. Etest (AB Biodisk, Solna, Sweden) was used to assess the interaction between voriconazole and flucytosine in the second set of experiments. Briefly, voriconazole Etest strips were placed on RPMI 1640 agar plates containing either 0, 4, or 32 μg of flucytosine per ml. Similarly, in other experiments, flucytosine Etest strips were placed on agar plates containing concentrations of voriconazole greater than the MIC for isolate AF2. The MICs were recorded after incubation at 35°C for 48 h.
Analysis
The VisuMIC-0s were used for the analysis. The MICs at which 50% (MIC50) and 90% (MIC90) of the isolates tested were inhibited as well as the geometric mean MICs were calculated. The fractional inhibitory concentrations (FICs) of each drug used in combination were calculated and added to obtain the FIC indices (6, 14), as follow: FIC index = (MICA in combination/MICA alone) + (MICB in combination/MICB alone) + (MICC in combination/MICC alone), where MICA, MICB, and MICC indicate the MICs of drugs A, B, and C, respectively. For double combinations, the third term of the equation was omitted. Drug interactions were defined as synergy if the lowest FIC index was ≤0.5, additivity (i.e., no interaction) if the lowest FIC index was >0.5 and ≤4, and antagonism if the highest FIC index was >4. For the calculations, the high off-scale MICs were converted to the next highest concentration and the low off-scale MICs were left unchanged.
To assess the influence of the end-point definition and the reading technique on the interaction modes between antifungals, the percent synergy, additivity, and antagonism obtained with VisuMIC-2, VisuMIC-0, SpecMIC50%, and SpecMIC90% were compared.
RESULTS
Combination of caspofungin with amphotericin B or voriconazole
Table 1 summarizes the MICs of caspofungin, amphotericin B, and voriconazole alone; the lowest FIC indices; and the corresponding MICs of the drugs in combination for the 35 isolates tested.
TABLE 1.
Interaction of caspofungin in combination with amphotericin B or voriconazole against 35 isolates of Aspergillus spp.a
| Isolate | MIC (μg/ml) of drug alone
|
MICs (μg/ml) of the drugs in combinationb
|
Lowest FIC index for the combination
|
||||
|---|---|---|---|---|---|---|---|
| CAS | AMB | VRZ | CAS-AMB | CAS-VRZ | CAS-AMB | CAS-VRZ | |
| A. fumigatus | |||||||
| AF1 | >16 | 0.5 | 0.5 | 0.03/0.5 | 0.03/0.5 | 1.00 | 1.00 |
| AF2 | >16 | 0.125 | 0.5 | 0.03/0.125 | 0.5/0.25 | 1.00 | 0.52 |
| AF3 | >16 | 0.25 | 0.25 | 0.03/0.25 | 0.03/0.25 | 1.00 | 1.00 |
| AF4 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF5 | >16 | 0.5 | 0.5 | 0.03/0.5 | 0.5/0.25 | 1.00 | 0.52 |
| AF6 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.5/0.125 | 1.00 | 0.52 |
| AF7 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.5/0.125 | 1.00 | 0.52 |
| AF8 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF9 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF10 | >16 | 0.25 | 0.5 | 0.03/0.25 | 0.5/0.25 | 1.00 | 0.52 |
| AF11 | >16 | 0.5 | 0.25 | 1/0.25 | 0.03/0.25 | 0.53 | 1.00 |
| AF12 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF13 | >16 | 0.5 | 0.5 | 0.5/0.25 | 0.03/0.5 | 0.52 | 1.00 |
| AF14 | >16 | 0.5 | 0.5 | 0.25/0.25 | 0.03/0.5 | 0.51 | 1.00 |
| AF15 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF16 | >16 | 0.5 | 0.5 | 0.03/0.5 | 0.5/0.25 | 1.00 | 0.52 |
| AF17 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF18 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF19 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF20 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF21 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF22 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF23 | >16 | 0.5 | 0.5 | 8/0.25 | 0.03/0.5 | 0.75 | 1.00 |
| AF24 | >16 | 0.25 | 0.5 | 0.03/0.25 | 0.03/0.5 | 1.00 | 1.00 |
| AF25 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF26 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF27 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF28 | >16 | 0.5 | 0.5 | 0.03/0.5 | 0.5/0.25 | 1.00 | 0.52 |
| AF29 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| AF30 | >16 | 0.5 | 0.25 | 0.03/0.5 | 0.03/0.25 | 1.00 | 1.00 |
| A. terreus | |||||||
| AT1 | >16 | 1 | 0.25 | 0.03/1 | 0.03/0.25 | 1.00 | 1.00 |
| AT2 | >16 | 1 | 1 | 0.03/1 | 1/0.5 | 1.00 | 0.53 |
| AT3 | >16 | 1 | 2 | 2/0.5 | 0.5/1 | 0.56 | 0.52 |
| AT4 | >16 | 2 | 0.5 | 0.03/2 | 0.03/0.5 | 1.00 | 1.00 |
| AT5 | >16 | 2 | 0.5 | 0.03/2 | 0.03/0.5 | 1.00 | 1.00 |
MICs were determined visually as the concentration that gave 100% inhibition (VisuMIC-0). CAS, caspofungin; AMB, amphotericin B; VRZ, voriconazole.
Corresponding to the lowest FIC index.
The geometric mean MIC and the MIC90 of amphotericin B alone for A. fumigatus were 0.45 and 0.5 μg/ml, respectively. Higher MICs of amphotericin B, ranging from 1 to 2 μg/ml, were found for the five A. terreus isolates. Caspofungin alone exhibited MICs of >16 μg/ml for all isolates. The geometric mean MIC and the MIC90 of voriconazole alone were 0.35 and 0.5 μg/ml, respectively.
The interaction between caspofungin and amphotericin B was additive for all isolates. Antagonism was not observed (the highest FIC indices ranged from 1.00 to 2.5 [data not shown]).
When caspofungin was combined with voriconazole, additive interactions were noted against all isolates. Antagonism was not observed (the highest FIC indices ranged from 1.00 to 1.5 [data not shown]).
Combination of caspofungin with flucytosine
Table 2 summarizes the MICs of caspofungin and flucytosine alone, the lowest FIC indices, and the corresponding MICs of the drugs in combination for the 14 isolates tested. The geometric mean MIC and the MIC90 of flucytosine alone were 210 and >128 μg/ml, respectively. The MICs of caspofungin for all isolates were >16 μg/ml.
TABLE 2.
Interaction of caspofungin in combination with flucytosine against 14 isolates of Aspergillus spp.a
| Isolate | MIC (μg/ml) of drug alone
|
MIC (μg/ml) of drugs in the combination CAS-5FCb | Lowest FIC index for the combination CAS-5FC | |
|---|---|---|---|---|
| CAS | 5FC | |||
| A. fumigatus | ||||
| AF2 | >16 | >128 | 2/64 | 0.31 |
| AF3 | >16 | >128 | 0.25/32 | 0.13 |
| AF4 | >16 | >128 | 4/128 | 0.63 |
| AF5 | >16 | >128 | 1/128 | 0.53 |
| AF6 | >16 | >128 | 4/128 | 0.63 |
| AF7 | >16 | >128 | 1/64 | 0.28 |
| AF8 | >16 | >128 | 0.5/64 | 0.27 |
| AF9 | >16 | >128 | 0.5/32 | 0.14 |
| AF11 | >16 | >128 | 0.5/32 | 0.14 |
| AF12 | >16 | >128 | 0.25/32 | 0.13 |
| AF17 | >16 | >128 | 0.5/128 | 0.52 |
| AF30 | >16 | >128 | 0.5/128 | 0.52 |
| A. terreus | ||||
| AT4 | >16 | 16 | 0.125/2 | 0.13 |
| AT5 | >16 | >128 | NDc/ND | ND |
MICs were determined visually as the concentration that gave 100% inhibition (VisuMIC-0). CAS, caspofungin; 5FC, flucytosine.
Corresponding to the lowest FIC index.
ND, not determined; the absence of inhibition at the highest concentrations used in combination precluded analysis of the results.
The interaction between caspofungin and flucytosine was synergistic for 7 of 12 A. fumigatus isolates (58%) and additive for 5 isolates (42%). Among the A. terreus isolates, the combination was synergistic for one isolate, but for the other isolate the absence of inhibition at the highest concentrations used in combination precluded analysis of the interaction. Antagonism was not observed (the highest FIC indices ranged from 0.62 to 1.0 [data not shown]). For the synergistic interactions the median concentration of caspofungin in the combination was 0.5 μg/ml (range, 0.125 to 2 μg/ml) and the median concentration of flucytosine in the combination was 32 μg/ml (range, 2 to 64 μg/ml).
Combination of voriconazole with flucytosine
Table 3 summarizes the mode of interaction between voriconazole and flucytosine. The geometric mean MIC and the MIC90 of voriconazole alone were 0.37 and 0.5 μg/ml, respectively. The interaction was antagonistic for all A. fumigatus isolates tested and for one A. terreus isolate. For the antagonistic interactions the concentrations of voriconazole and flucytosine in the combination were 1 to 4 and 2 to 128 μg/ml, respectively (data not shown).
TABLE 3.
Interaction of voriconazole in combination with flucytosine against 14 isolates of Aspergillus spp.a
| Isolate | MIC (μg/ml) of drug alone
|
MIC (μg/ml) of drugs in the combination VRZ-5FCb | Highest FIC index for the combination VRZ-5FC | |
|---|---|---|---|---|
| VRZ | 5FC | |||
| A. fumigatus | ||||
| AF2 | 0.5 | >128 | 2/128 | 4.5 |
| AF3 | 0.5 | >128 | 2/128 | 4.5 |
| AF4 | 0.5 | >128 | 4/128 | 8.5 |
| AF5 | 0.5 | >128 | 4/128 | 8.5 |
| AF6 | 0.25 | >128 | 2/128 | 8.5 |
| AF7 | 0.25 | >128 | 2/128 | 8.5 |
| AF8 | 0.25 | >128 | 2/128 | 8.5 |
| AF9 | 0.25 | >128 | 2/128 | 8.5 |
| AF11 | 0.25 | >128 | 2/128 | 8.5 |
| AF12 | 0.25 | >128 | 2/128 | 8.5 |
| AF17 | 0.5 | >128 | 2/128 | 4.5 |
| AF30 | 0.5 | >128 | 2/128 | 4.5 |
| A. terreus | ||||
| AT4 | 0.5 | >128 | 2/128 | 4.5 |
| AT5 | 0.5 | >128 | 1/128 | 2.5 |
MICs were determined visually as the concentration that gave 100% inhibition (VisuMIC-0). VRZ, voriconazole; 5FC, flucytosine.
Corresponding to the highest FIC index.
Triple combinations
Triple combinations that included caspofungin, flucytosine, and either amphotericin B or voriconazole were tested.
Table 4 presents the results of the three-dimensional checkerboard studies of the combination of caspofungin, flucytosine, and amphotericin B. Synergistic interactions were observed for all isolates tested, with FIC indices ranging from 0.04 to 0.41. Upon combination the median concentrations of caspofungin, flucytosine, and amphotericin B were 0.75, 128, and 0.03 μg/ml, respectively. Antagonism was not observed (the highest FIC indices ranged from 1.00 to 2.07 [data not shown]). The interaction between amphotericin B and flucytosine (the data were extracted from the results for the triple combination) was additive for all isolates tested.
TABLE 4.
Interactions of triple combination of caspofungin with flucytosine and amphotericin B against 12 isolates of Aspergillus spp.a
| Isolate | MIC (μg/ml) of drug alone
|
MICs (μg/ml) of the drugs in the combination CAS-AMB-5FCb | Lowest FIC index for the combination CAS-AMB-5FC | ||
|---|---|---|---|---|---|
| CAS | AMB | 5FC | |||
| A. fumigatus | |||||
| AF2 | >16 | 1 | >128 | 1/0.25/8 | 0.30 |
| AF4 | >16 | 1 | >128 | 4/0.03/128 | 0.41 |
| AF6 | >16 | 1 | >128 | 4/0.03/128 | 0.41 |
| AF7 | >16 | 1 | >128 | 4/0.03/128 | 0.41 |
| AF8 | >16 | 1 | >128 | 0.5/0.03/128 | 0.30 |
| AF9 | >16 | 1 | >128 | 2/0.03/32 | 0.16 |
| AF11 | >16 | 1 | >128 | 0.25/0.03/128 | 0.29 |
| AF12 | >16 | 1 | >128 | 0.25/0.03/128 | 0.29 |
| AF17 | >16 | 1 | >128 | 1/0.03/128 | 0.31 |
| AF30 | >16 | 1 | >128 | 0.5/0.03/128 | 0.30 |
| A. terreus | |||||
| AT4 | >16 | 2 | >128 | 0.25/0.03/8 | 0.04 |
| AT5 | >16 | 4 | >128 | 0.03/1/8 | 0.27 |
MICs were determined visually as the concentration that gave 100% inhibition (VisuMIC-0). AMB, amphotericin B; CAS, caspofungin; 5FC, flucytosine.
Corresponding to the lowest FIC index.
Table 5 summarizes the results for the triple combination of caspofungin, flucytosine, and voriconazole. Synergistic interactions were observed for 8 of 12 isolates (67%), and additivity was noted for 4 isolates (33%). The median concentrations of caspofungin, flucytosine, and voriconazole in combination required to achieve the lowest FIC index were 0.5, 128, and 0.016 μg/ml, respectively. Interestingly, antagonism was also observed for six isolates of A. fumigatus, indicating that two different modes of interaction can be observed with the same combination, depending on the concentrations used (data not shown). For these isolates, synergy was obtained with voriconazole at concentrations of 0.016 to 0.03 μg/ml, whereas antagonistic interactions were observed with higher concentrations of voriconazole (1 μg/ml).
TABLE 5.
Interactions of triple combination of caspofungin with flucytosine and voriconazole against 12 isolates of Aspergillus spp.a
| Isolate | MIC (μg/ml) of drug alone
|
MICs (μg/ml) of drugs in the combination CAS-VRZ-5FCb | Lowest FIC index for the combination CAS-VRZ-5FC | Highest FIC index for the combination CAS-VRZ-5FC | ||
|---|---|---|---|---|---|---|
| CAS | VRZ | 5FC | ||||
| A. fumigatus | ||||||
| AF2 | >16 | 0.5 | >128 | 8/0.016/128 | 0.53 | 2.56 |
| AF3 | >16 | 0.5 | >128 | 1/0.016/128 | 0.31 | 2.56 |
| AF5 | >16 | 0.5 | >128 | 0.5/1/2 | 2.02 | 2.52 |
| AF6 | >16 | 0.25 | >128 | 4/0.016/128 | 0.44 | 4.28 |
| AF7 | >16 | 0.25 | >128 | 0.5/0.25/2 | 1.02 | 4.75 |
| AF8 | >16 | 0.25 | >128 | 0.5/0.016/128 | 0.33 | 4.56 |
| AF9 | >16 | 0.25 | >128 | 0.25/0.016/128 | 0.32 | 4.56 |
| AF11 | >16 | 0.25 | >128 | 0.25/0.016/128 | 0.32 | 4.31 |
| AF12 | >16 | 0.25 | >128 | 0.5/0.016/128 | 0.33 | 4.56 |
| AF30 | >16 | 0.5 | >128 | 0.5/0.016/128 | 0.30 | 2.56 |
| A. terreus | ||||||
| AT4 | >16 | 0.5 | >128 | 0.25/0.016/32 | 0.10 | 2.56 |
| AT5 | >16 | 0.5 | >128 | 0.03/1/2 | 2.00 | 2.75 |
MICs were determined visually as the concentration that gave 100% inhibition (VisuMIC-0). AMB, amphotericin B; VRZ, voriconazole; 5FC, flucytosine.
Corresponding to the lowest FIC index.
Table 6 presents a summary of the results obtained with the different double and triple combinations.
TABLE 6.
Summary of drug interactions for the six combinations testeda
| Interaction mode | % of isolates for the following combination:
|
|||||
|---|---|---|---|---|---|---|
| CAS-5FC | VRZ-5FC | CAS-AMB | CAS-VRZ | CAS-AMB-5FC | CAS-VRZ-5FCb | |
| Synergy | 62 | 0 | 0 | 0 | 100 | 67 |
| Additivity (no interaction) | 38 | 7 | 100 | 100 | 0 | 33 |
| Antagonism | 0 | 93 | 0 | 0 | 0 | 50 |
MICs were determined visually as the concentration that gave 100% inhibition (VisuMIC-0). CAS, caspofungin; 5FC, flucytosine; VRZ, voriconazole; AMB, amphotericin B.
For this combination, different modes of interaction were detected for a given isolate, depending on the concentrations of the drugs.
Agar diffusion test
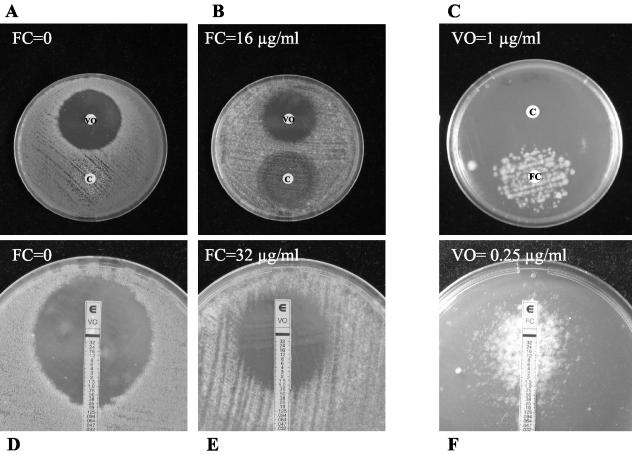
The results of the agar diffusion test for the combination of voriconazole or caspofungin and flucytosine for one isolate of A. fumigatus are shown in Fig. 1. Paper disks impregnated with antifungal drugs were used in the first set of experiments. A clear inhibition zone was observed with voriconazole on plain agar, whereas caspofungin gave partial inhibition. When flucytosine at 16 μg/ml was incorporated into the agar, the size of the inhibition zone around the voriconazole disk decreased from 35.0 to 29.9 mm and the size of the inhibition zone around the caspofungin disk increased from 27.3 to 33.2 mm, indicating antagonism and synergy, respectively. When voriconazole was added to the agar at 1 μg/ml (a concentration above the MIC), the growth of the strain was inhibited except around the flucytosine disk, indicating antagonism between the two drugs. Antagonism was not observed between caspofungin and voriconazole.
FIG. 1.
Agar diffusion test of the combination of flucytosine (FC) with voriconazole (VO) or caspofungin (C) against A. fumigatus AF2. (A and B) Paper disks impregnated with either 4 μg of voriconazole or 16 μg of caspofungin were placed on agar plates containing no antifungal (A) or flucytosine at 16 μg/ml (B). In the presence of flucytosine, the inhibition zone around the voriconazole disk decreased (indicating antagonism), whereas the inhibition zone around the caspofungin disk increased (indicating synergy). (C) Two paper disks impregnated with 16 μg of caspofungin (upper disk) or 128 μg of flucytosine (lower disk) were placed on an agar plate containing voriconazole at 1 μg/ml (concentration above the MIC). After incubation growth was apparent only around the flucytosine disk, indicating antagonism between voriconazole and flucytosine. Antagonism between caspofungin and voriconazole was not observed. (D and E) A voriconazole Etest strip was placed on agar plates containing either no antifungal (D) or flucytosine at 32 μg/ml (E). The voriconazole MIC increased from 0.19 μg/ml in the absence of flucytosine to 0.75 μg/ml in the presence of flucytosine, indicating antagonism between the two drugs. (F) A flucytosine Etest strip was placed on an agar plate containing voriconazole at 0.25 μg/ml (the MIC). After incubation an ellipse of growth was apparent around the strip, indicating antagonism between the two drugs.
The Etest strategy was also used. The voriconazole MIC increased from 0.19 μg/ml in the absence of flucytosine to 0.25 and 0.75 μg/ml in the presence of flucytosine at 4 and 32 μg/ml, respectively, indicating antagonism between the two drugs. Similarly, when voriconazole was added to the agar at 0.25 μg/ml (the MIC for the isolate), an ellipse of growth was apparent around the flucytosine strip, confirming the antagonism between voriconazole and flucytosine.
Influence of the MIC end point
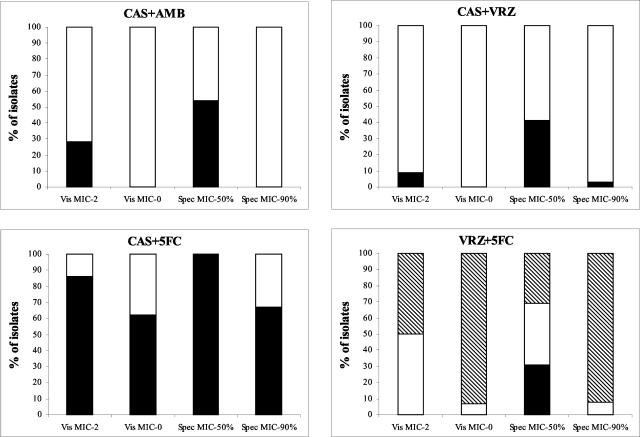
The influence of the MIC end point on the results was evaluated for all combinations. Four end points were used: VisuMIC-2, VisuMIC-0, SpecMIC-50%, and SpecMIC-90%. For the double combinations, a comparison of the modes of interaction between the antifungals obtained with the four end points is presented in Fig. 2. Similar results were obtained with triple combinations (data not shown). When 50% inhibition was used as the end point, the spectrophotometric reading led to higher percentages of synergy than visual reading for all four combinations. In contrast, when MIC end points were defined as complete growth inhibition (VisuMIC-0 and SpecMIC-90%), the percentages of synergy, additivity, and antagonism were almost identical by both reading techniques. Overall, whatever reading technique was used, visual or spectrophotometric, a lower percentage of synergy and a higher percentage of antagonism were obtained when a complete inhibition end point was used compared to the results obtained when a partial inhibition end point was used.
FIG. 2.
Influence of the MIC end point on the mode of interaction between antifungal drugs for double combinations of caspofungin (CAS) with either amphotericin B (AMB), voriconazole (VRZ), or flucytosine (5FC) and voriconazole with flucytosine. The MICs of the drugs alone and in combination were determined by visual reading by using 50% (VisuMIC-2) or complete (VisuMIC-0) inhibition and by spectrophotometric reading by using 50% (SpecMIC50%) or 90% (SpecMIC90%) inhibition. ▪, synergy; □, additivity; ▧, antagonism.
DISCUSSION
Despite recent advances in the treatment of invasive aspergillosis, particularly with the use of new antifungal drugs such as voriconazole (17) and caspofungin (19), the rate of mortality from these infections remains very high and new antifungal strategies are needed. Antifungal combination therapy is commonly used and is recommended for the treatment of some invasive yeast infections, such as cryptococcal meningitis (37). More recently it has been demonstrated that this approach could add some microbiological benefits to the treatment of candidemia in nonneutropenic patients (36). Nevertheless, the role of combination therapy for the management of filamentous fungal infections remains to be evaluated.
It must be noted that the relevance of data from in vitro studies of the activities of antifungal combinations against filamentous fungi has been poorly evaluated and is mainly unknown. This is related in part to the absence of a standardized method for studies of the activities of combinations of agents and to the influences of many parameters on the results. Nevertheless, it is possible to compare the activities of different combinations when the same methodology is used. Moreover, if the same mode of interaction between antifungals is obtained by different techniques (e.g., broth dilution and agar diffusion) for a given combination, one can suppose that the interaction is of clinical importance. However, animal studies are required to provide sufficient data before clinical trials with humans can be designed.
In the present study we evaluated the activities of four antifungal drugs in double combinations as well as triple combinations against A. fumigatus and A. terreus clinical isolates.
We found additive interactions between caspofungin and either amphotericin B or voriconazole against 35 isolates of Aspergillus spp., while antagonism was never observed. This is in accordance with the results of a previous in vitro study that reported additive to synergistic interactions between caspofungin and amphotericin B for more than half of 14 Aspergillus sp. isolates (3). A recent retrospective study with humans suggested favorable responses in patients with hematologic malignancies treated with caspofungin in combination with liposomal amphotericin B (1).
Caspofungin has also been shown to interact synergistically in combination with voriconazole in vitro against different Aspergillus species (32), and this combination was significantly more effective in terms of eliminating the fungal burden than either drug alone in an animal model of disseminated aspergillosis (20). It must be noted that the combination of caspofungin with other azoles seems to depend on the azole tested (24). Overall, these results show that the combination of an echinocandin with amphotericin B or an azole could be of interest for the treatment of aspergillosis.
Flucytosine, when used alone, has poor activity in vitro as well as in vivo against experimental aspergillosis (34). Nevertheless, the combination of flucytosine with amphotericin B or azoles has been shown to be additive to synergistic in vitro and in animal models of aspergillosis (4, 34, 35). Moreover, use of this combination has been advocated in some particularly difficult cases, such as central nervous system infections, due to the favorable pharmacokinetics properties of flucytosine. Because of this we tested flucytosine in combination with caspofungin and voriconazole. Synergy between flucytosine and caspofungin was demonstrated for more than 60% of the isolates and was confirmed with one A. fumigatus isolate by the agar diffusion test. As previously reported for the combination of flucytosine with caspofungin against C. neoformans (38), synergistic interactions were observed at concentrations of caspofungin and flucytosine that were in the range of the levels achievable in serum (45, 46). These data strongly suggest that this combination could be effective in vivo.
In contrast to the results obtained with caspofungin, when flucytosine was combined with voriconazole, the interactions were antagonistic for 93% of the isolates and synergy was never observed. This antagonism was also clearly demonstrated by agar diffusion tests either with disks or by Etest. The activities of the combination of flucytosine with azole drugs, particularly fluconazole, against C. neoformans have been extensively studied in vitro (29, 38), in animal models (21, 30), and in clinical trials (25). Overall, those studies demonstrated synergy in vitro and efficacy in vivo, and the combination of flucytosine with fluconazole can be recommended as an alternative therapy for the treatment of cryptococcosis in HIV-positive patients (37). Nevertheless, some studies have reported antagonistic interactions between flucytosine and azoles against specific fungal pathogens (31, 42, 43). It has been shown, for example, that the combination of flucytosine and miconazole was antagonistic against some strains of C. glabrata (43) and that the antagonism was possibly due to an increased quantity of the target enzyme of miconazole in the presence of flucytosine (42). In another study, in vitro antagonism between flucytosine and fluconazole was demonstrated against flucytosine-resistant strains of C. lusitaniae, and it was hypothesized that flucytosine is a competitive inhibitor of fluconazole uptake transport (31). It must be noted that the combination of terbinafine, which is another sterol biosynthesis inhibitor, with flucytosine was also antagonistic in vitro against Aspergillus spp. (27). The possible mechanism underlying the flucytosine-voriconazole antagonism against Aspergillus remains to be determined.
Combinations of more than two antimicrobial drugs are commonly used for the treatment of patients with tuberculosis (39) or HIV infection (47). In the field of medical mycology, triple antifungal therapy has seldom been used for humans and has been poorly evaluated experimentally either in vitro or in animal models. Combinations of amphotericin B with flucytosine and either fluconazole or itraconazole as a third partner drug have successfully been used to treat cryptococcal meningitis in AIDS patients in Europe and Asia (35). The activities of triple combinations that include the new drugs with activities against Aspergillus spp. have not yet been explored. In the present study we found synergistic interactions of the triple combination caspofungin-flucytosine-amphotericin B against 100% of the isolates. Each of the double combinations of these three drugs gave additive to synergistic interactions, and in no instance was antagonism observed. This could explain the synergy observed when the three drugs were combined together. Complex interactions were obtained with the triple combination caspofungin-flucytosine-voriconazole. Although synergistic or additive interactions were observed against all the isolates, antagonism was also present for 50% of the isolates. Antagonism was apparent at higher concentrations of voriconazole compared to the concentrations at which synergy was observed. Therefore, the antagonistic interactions in this triple combination are probably related to the antagonism between flucytosine and voriconazole. Animal studies should be performed to clarify the clinical relevance of the complex interactions observed in vitro.
The influence of the end-point definition on the modes of interaction between antifungal drugs was evaluated for the double and triple combinations. Overall, visual and spectrophotometric readings gave similar results for complete inhibition end points. MIC end-point determination by spectrophotometric reading is more objective and offers the potential for automation. This approach is particularly attractive for use when large sets of MICs must be determined. Spectrophotometric readings have previously been used for determination of the MICs of drugs alone for Aspergillus spp. (9) and also for determination of the MICs of combinations of antifungals for Scedosporium prolificans (26). The results of the present study show that this technique can be used to study the activities of combinations of antifungals against Aspergillus spp. The discrepancies between the visual and spectrophotometric methods obtained when the partial inhibition end point was used could reflect the difficulty of visual determination of 50% inhibition for the azoles and caspofungin.
In conclusion, our results indicate that the activities of the double combination of caspofungin with flucytosine and the triple combination of caspofungin and flucytosine with amphotericin B as the third partner drug are synergistic in vitro, as assessed by checkerboard studies and agar diffusion tests. In contrast, the combination of voriconazole and flucytosine can be antagonistic. Further investigation of these combinations in animal models of aspergillosis is warranted.
Acknowledgments
These studies were funded in part by a grant from ICN Pharmaceuticals.
REFERENCES
- 1.Aliff, T. B., P. G. Maslak, J. G. Jurcic, M. L. Heaney, K. N. Cathcart, K. A. Sepkowitz, and M. A. Weiss. 2003. Refractory Aspergillus pneumonia in patients with acute leukemia: successful therapy with combination caspofungin and liposomal amphotericin. Cancer 97:1025-1032. [DOI] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arroyo, J., G. Medoff, and G. S. Kobayashi. 1977. Therapy of murine aspergillosis with amphotericin B in combination with rifampin of 5-fluorocytosine. Antimicrob. Agents Chemother. 11:21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartizal, C., and F. C. Odds. 2003. Influences of methodological variables on susceptibility testing of caspofungin against Candida species and Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 7.Dannaoui, E., E. Borel, M. F. Monier, M. A. Piens, S. Picot, and F. Persat. 2001. Acquired itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 47:333-340. [DOI] [PubMed] [Google Scholar]
- 8.Dannaoui, E., E. Borel, F. Persat, M. A. Piens, and S. Picot. 2000. Amphotericin B resistance of Aspergillus terreus in a murine model of disseminated aspergillosis. J. Med. Microbiol. 49:601-606. [DOI] [PubMed] [Google Scholar]
- 9.Dannaoui, E., F. Persat, M. F. Monier, E. Borel, M. A. Piens, and S. Picot. 1999. Use of spectrophotometric reading for in vitro antifungal susceptibility testing of Aspergillus spp. Can. J. Microbiol. 45:871-874. [PubMed] [Google Scholar]
- 10.De Lucca, A. J., and T. J. Walsh. 1999. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob. Agents Chemother. 43:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 12.Denning, D. W., P. Ribaud, N. Milpied, D. Caillot, R. Herbrecht, E. Thiel, A. Haas, M. Ruhnke, and H. Lode. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. [DOI] [PubMed] [Google Scholar]
- 13.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliopoulos, G. M., and R. C. Moellering. 1991. Antimicrobial combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine. The Williams & Wilkins Co., Baltimore, Md.
- 15.Espinel-Ingroff, A. 2003. Evaluation of broth microdilution testing parameters and agar diffusion Etest procedure for testing susceptibilities of Aspergillus spp. to caspofungin acetate (MK-0991). J. Clin. Microbiol. 41:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgopapadakou, N. H., and T. J. Walsh. 1996. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 40:279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, E. M., K. L. Oakley, S. A. Radford, C. B. Moore, P. Warn, D. W. Warnock, and D. W. Denning. 2000. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J. Antimicrob. Chemother. 45:85-93. [DOI] [PubMed] [Google Scholar]
- 19.Keating, G. M., and B. Jarvis. 2001. Caspofungin. Drugs 61:1121-1129. [DOI] [PubMed] [Google Scholar]
- 20.Kirkpatrick, W. R., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen, R. A., M. Bauer, J. M. Weiner, D. M. Diamond, M. E. Leal, J. C. Ding, M. G. Rinaldi, and J. R. Graybill. 1996. Effect of fluconazole on fungicidal activity of flucytosine in murine cryptococcal meningitis. Antimicrob. Agents Chemother. 40:2178-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 23.Luque, J. C., K. V. Clemons, and D. A. Stevens. 2003. Efficacy of micafungin alone or in combination against systemic murine aspergillosis. Antimicrob. Agents Chemother. 47:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manavathu, E. K., G. J. Alangaden, and P. H. Chandrasekar. 2003. Differential activity of triazoles in two-drug combinations with the echinocandin caspofungin against Aspergillus fumigatus. J. Antimicrob. Chemother. 51:1423-1425. [DOI] [PubMed] [Google Scholar]
- 25.Mayanja-Kizza, H., K. Oishi, S. Mitarai, H. Yamashita, K. Nalongo, K. Watanabe, T. Izumi, J. Ococi, K. Augustine, R. Mugerwa, T. Nagatake, and K. Matsumoto. 1998. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin. Infect. Dis. 26:1362-1366. [DOI] [PubMed] [Google Scholar]
- 26.Meletiadis, J., J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosquera, J., A. Sharp, C. B. Moore, P. A. Warn, and D. W. Denning. 2002. In vitro interaction of terbinafine with itraconazole, fluconazole, amphotericin B and 5-flucytosine against Aspergillus spp. J. Antimicrob. Chemother. 50:189-194. [DOI] [PubMed] [Google Scholar]
- 28.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard. Document M38A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 29.Nguyen, M. H., F. Barchiesi, D. A. McGough, V. L. Yu, and M. G. Rinaldi. 1995. In vitro evaluation of combination of fluconazole and flucytosine against Cryptococcus neoformans var. neoformans. Antimicrob. Agents Chemother. 39:1691-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen, M. H., L. K. Najvar, C. Y. Yu, and J. R. Graybill. 1997. Combination therapy with fluconazole and flucytosine in the murine model of cryptococcal meningitis. Antimicrob. Agents Chemother. 41:1120-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noel, T., F. Francois, P. Paumard, C. Chastin, D. Brethes, and J. Villard. 2003. Flucytosine-fluconazole cross-resistance in purine-cytosine permease-deficient Candida lusitaniae clinical isolates: indirect evidence of a fluconazole uptake transporter. Antimicrob. Agents Chemother. 47:1275-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perea, S., G. Gonzalez, A. W. Fothergill, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson. 2002. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob. Agents Chemother. 46:3039-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petraitis, V., R. Petraitiene, A. A. Sarafandi, A. M. Kelaher, C. A. Lyman, H. E. Casler, T. Sein, A. H. Groll, J. Bacher, N. A. Avila, and T. J. Walsh. 2003. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J. Infect. Dis. 187:1834-1843. [DOI] [PubMed] [Google Scholar]
- 34.Polak, A. 1987. Combination therapy of experimental candidiasis, cryptococcosis, aspergillosis and wangiellosis in mice. Chemotherapy (Basel) 33:381-395. [DOI] [PubMed] [Google Scholar]
- 35.Polak, A. 1999. The past, present and future of antimycotic combination therapy. Mycoses 42:355-370. [DOI] [PubMed] [Google Scholar]
- 36.Rex, J. H., P. G. Pappas, A. W. Karchmer, J. Sobel, J. E. Edwards, S. Hadley, C. Brass, J. A. Vazquez, S. W. Chapman, H. W. Horowitz, M. Zervos, D. McKinsey, J. Lee, T. Babinchak, R. W. Bradsher, J. D. Cleary, D. M. Cohen, L. Danziger, M. Goldman, J. Goodman, E. Hilton, N. E. Hyslop, D. H. Kett, J. Lutz, R. H. Rubin, W. M. Scheld, M. Schuster, B. Simmons, D. K. Stein, R. G. Washburn, L. Mautner, T. C. Chu, H. Panzer, R. B. Rosenstein, and J. Booth. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221-1228. [DOI] [PubMed] [Google Scholar]
- 37.Saag, M. S., R. J. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, and W. E. Dismukes. 2000. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz, P., F. Dromer, O. Lortholary, and E. Dannaoui. 2003. In vitro interaction of flucytosine with conventional and new antifungals against Cryptococcus neoformans clinical isolates. Antimicrob. Agents Chemother. 47:3361-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sepkowitz, K. A., J. Raffalli, L. Riley, T. E. Kiehn, and D. Armstrong. 1995. Tuberculosis in the AIDS era. Clin. Microbiol. Rev. 8:180-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shalit, I., Y. Shadkchan, Z. Samra, and N. Osherov. 2003. In vitro synergy of caspofungin and itraconazole against Aspergillus spp.: MIC versus minimal effective concentration end points. Antimicrob. Agents Chemother. 47:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siau, H., and D. Kerridge. 1999. 5-Fluorocytosine antagonizes the action of sterol biosynthesis inhibitors in Candida glabrata. J. Antimicrob. Chemother. 43:767-775. [DOI] [PubMed] [Google Scholar]
- 43.Siau, H., and D. Kerridge. 1998. The effect of antifungal drugs in combination on the growth of Candida glabrata in solid and liquid media. J. Antimicrob. Chemother. 41:357-366. [DOI] [PubMed] [Google Scholar]
- 44.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey. 2000. Practice guidelines for diseases caused by Aspergillus. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 45.Stone, J. A., S. D. Holland, P. J. Wickersham, A. Sterrett, M. Schwartz, C. Bonfiglio, M. Hesney, G. A. Winchell, P. J. Deutsch, H. Greenberg, T. L. Hunt, and S. A. Waldman. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermes, A., H. J. Guchelaar, and J. Dankert. 2000. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 46:171-179. [DOI] [PubMed] [Google Scholar]
- 47.Yeni, P. G., S. M. Hammer, C. C. Carpenter, D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2002. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society—USA Panel. JAMA 288:222-235. [DOI] [PubMed] [Google Scholar]