Abstract
Purine nucleoside antimetabolites, such as clofarabine, are effective antileukemic agents. However, their effectiveness depends on an initial activation step in which they are monophosphorylated by deoxycytidine kinase (dCK). Some purine nucleoside antimetabolites and their monophosphate derivatives are exported by the ABC transporter ABCG2. Because clofarabine is a dCK substrate, and we show substantial variation in dCK and ABCG2 in myeloid leukemia, we hypothesized that the activity of dCK may modulate ABCG2-mediated resistance to clofarabine by regulating the formation of clofarabine monophosphate. We demonstrate that ABCG2 influence on clofarabine cytotoxicity was markedly influenced by dCK activity. When dCK expression was reduced by siRNA, clofarabine cytotoxicity was strongly reduced by enhanced ABCG2-mediated efflux. Conversely, dCK overexpression blunted ABCG2-mediated efflux of clofarabine by increasing the formation of clofarabine nucleotides. The use of an ABCG2 inhibitor confirmed that ABCG2 export of clofarabine is maximal when dCK levels are minimal. Analysis of intracellular clofarabine metabolites suggested that ABCG2 exported clofarabine more readily than clofarabine monophosphate. That ABCG2 primarily effluxes clofarabine, but not chlorfarabine-monophosphate was confirmed by HPLC analysis of drug exported from ABCG2 over expressing cells. Because the level and function of dCK and ABCG2 vary substantially among other types of cancer, these findings have important implications not only for clofarabine therapy but for purine nucleoside therapy in general. Therefore we propose that addition of ABCG2 inhibitors would effectively increase the anti-tumor efficacy of purine nucleosides by blocking drug efflux which may be a significant mode of resistance when dCK levels are low.
Keywords: ABC transporter, ABCG2, nucleoside, leukemia, transport, clofarabine
Introduction
Nucleoside antimetabolites are among the most widely used and effective classes of drugs for viral diseases and cancers (1, 2). Their cytotoxic effects and therapeutic efficacy require cellular uptake followed by phosphorylation to their nucleotide forms (3–5). Recent efforts have led to the development of several improved purine nucleoside analogs that are adenine derivatives; one such compound, clofarabine (2 – chloro-2 arabino – fluro-2 – deoxyadenosine, CAFdA) has broad cytotoxic activity showing therapeutic promise in xenograft models against human colon, renal, prostate, and leukemias (6–10). In humans it is currently approved for use in relapsed ALL (7). Clofarabine is readily phosphorylated by deoxycytidine kinase (dCK) and exerts cytotoxicity against both proliferating and nonproliferating cells via multiple mechanisms (4, 11, 12). Importantly, studies in leukemia cell lines show that clofarabine resistance is associated with reduced dCK activity (13), however other mechanisms, such as export of clofarabine and/or its mono-phosphate, may also impair response.
Conventional models of resistance to nucleoside derivatives have emphasized either impaired cellular uptake or reduced conversion to nucleoside monophosphates (2, 14–16). We and others (17–20) demonstrated a new mechanism of resistance to nucleoside analogues in which the ABC transporters, ABCC4 and ABCC5 (20, 21) export nucleoside monophosphates from cells. By reducing the concentration of nucleoside monophosphate, this mechanism leads to a reduction in higher phosphorylated nucleosides thereby reducing cytotoxicity. Notably recent studies have demonstrated that the ABC transporter ABCG2 (which is expressed in many tumor types (22)) exports both purine nucleoside monophosphates and nucleosides (18, 23). This suggests an ABC transporter could affect the intracellular nucleoside monophosphate concentration by reducing both the amount of nucleoside available for phosphorylation as well as the amount phosphorylated.
The finding that ABCG2 mediates the efflux of both purine nucleosides and nucleoside monophosphates (18, 23) and the fact that some nucleoside analogues are good dCK substrates (11) suggested that intracellular dCK activity might affect ABCG2-mediated drug export (Fig. 1A). Further, we found substantial variation in ABCG2 and dCK expression in myeloid leukemia cell lines (Fig 1B). Therefore we hypothesized that the activity of dCK may modulate ABCG2-mediated clofarabine resistance by increasing the formation of clofarabine monophosphate. Clofarabine was chosen as a representative nucleoside antimetabolite because it is a good dCK substrate (11, 24), is not an ABCC4 substrate (Supplementary Fig. 1), and is effective against multiple tumor types (6–10). We evaluated the relation between dCK expression and ABCG2 activity by engineering cell lines to overexpress ABCG2, wild-type dCK, or both; by “knocking down” endogenous dCK with siRNA; and by developing a new dCK antibody to confirm dCK protein levels. Taken together, our results indicate that clofarabine metabolism and cytotoxicity are strongly determined by the interplay between dCK and ABCG2; they also suggest that inhibition of ABCG2 can improve the tumor cytotoxicity of nucleosides especially when levels of dCK are low.
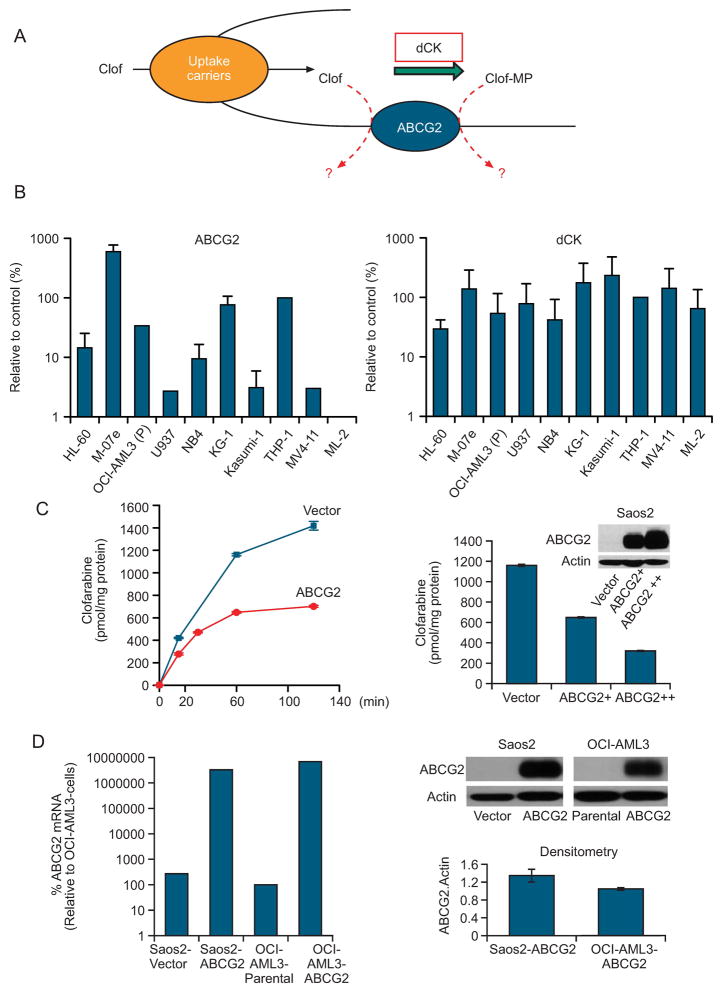
Figure 1. Expression and function of dCK and ABCG2.
A, Model illustrating dCK and ABCG2 interplay in the cellular metabolism of clofarabine. B, Expression of ABCG2 and dCK mRNA in prototypical myeloid leukemia cell lines as determined by real-time PCR (see Supplementary Information). C, Cells were incubated with 10 μM [3H] clofarabine for the indicated intervals (left); Cells were incubated for 60min with10 μM [3H] clofarabine. D, Immunoblot analysis of Saos2 and OCI-AML3 cells after transduction with an ABCG2 expression vector. The immunoblot was compiled by cropping a single immunoblot to remove unnecessary space.
Materials and Methods
Materials
The [3H]-clofarabine and clofarabine were obtained from Moravek Radiochemicals and Biochemicals (Brea, CA). The ABCG2 inhibitor fumitremorgin C (FTC) was kindly provided by Dr. Susan Bates (National Cancer Institute).
Cell culture
The Saos2 were obtained from ATTC and aliquoted and frozen at early passage number in all studies. No cell line authentication is carried out for these cells. The OCI-AML3-ABCG2 and OCI-AML3 cells were kindly provided by Dr. Brian Sorrentino (St. Jude Children’s Research Hospital, Memphis TN). The cells were tested and verified by comparative genomic hybridization (CGH). The human osteosarcoma cell line Saos2 was cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum and 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine. ABCG2-overexpressing Saos2 cells (Saos2-ABCG2) and the empty-vector control cell line Saos2-pcDNA3. 1 has previously been described (23). The HL60, M-07e, U937, NB4, KG-1, Kasumi-1, THP-1, MV4-11 and ML-2 cell lines were kindly provided by Dr. Sharyn Baker (St. Jude Children’s Research Hospital). No verification of these cell lines for authentication was carried out as they were used immediately.
Immunoblot analysis
As previously described, cells were analyzed by immunoblotting with antibodies to ABCG2 (BXP-53, Alexis Biochemicals, San Diego, CA) and β-actin. Protein concentration was quantified by using the Bio-Rad protein assay. Protein (50–100 μg) were separated on 12.5% or 10% polyacrylamide gel. The membrane was blocked with evaporated milk as described (23) and incubated first with the primary antibody at the appropriate dilution and then with an anti-rat, -mouse, or -rabbit IgG whole antibody conjugated to horseradish peroxidase (Amersham Bioscience, UK). Signal was detected by using the Amersham ECL detection system.
Intracellular accumulation of ABCG2 substrates
Saos2-ABCG2 cells and vector control cells were seeded at 5 × 105 cells/well in 6-well plates. OCI-AML3-ABCG2 cells and parental OCI-AML3 cells were grown in suspension at ~ 3.0 × 105 cells/ml. Cells were incubated with Hoechst 33342, mitoxantrone, or [3H] clofarabine as indicated. Radioactivity in samples was measured by liquid scintillation counting. Values were normalized to total cell protein content.
Efflux studies in intact cells
Cells containing empty vector, ABCG2 or both ABCG2 and-dCK we plated at a density of 5 × 105 cells/well on a 35 mm dish. Media was changed and cells incubated in warm ATP depleting media (DMEM w/o glucose supplemented with 10mM Sodium azide and 10mM 2-deoxy-glucose) for 30min. Afterward fresh ATP-depletion medium was added containing [3H]-clofarabine. Drug efflux was initiated after extensive washing with ice-cold PBS and the addition of warm DMEM containing glucose without FBS, but containing either no or 10 μM FTC. At the end of the efflux interval, the media was carefully aspirated and the cells lysed with 500μl 0.5M NaOH. The amount of radioactivity released into the media was determined after the one hour interval to obtain enough radioactive counts for HPLC analysis.
Cytotoxicity assay
Cells were seeded at 5000 (Saos2) or 10000 (OCI-AML3) cells per well in 96-well plates and cultured at 37 °C for 20 h, then were incubated with drugs for 72 h. Cell viability was determined by using the CellTiter 96 Non-Radioactive Cell Proliferation assay (Promega, WI). The concentration of drug that inhibited cell proliferation by 50% (IC50) was determined from cell viability data by using the online software Java Applets & Servlets for Biostatistics (programmed by H. Ono, Nagoya City University, Japan). Each assay included triplicate samples for each drug concentration, and all experiments were performed at least three times.
Cloning and mutagenesis of human deoxycytidine kinase
The open reading frame of human dCK was obtained by using a pair of primers targeted to the 5′- and 3′-ends (5-GAATTCCAGCAAGATCACAAAGTACTC-3 [EcoRI site is underlined)) and 5-GCTCGAGACTAAGGAATGGCCACCCCGCCCAAGAGAAGC-3 (XhoI) containing different restriction sites at the respective ends to permit directional cloning. The 792-bp dCK ORF was amplified by PCR using the human ovary cDNA library (Clontech) as template and after sequencing cloned into a pCR-TOPO vector and subsequently into the vector MSCV-IRES-GFP to create MSCV-IRES-GFP-hdCK. The dCK structure (25) was used to develop a mutant dCK with a disrupted P-loop. This site was mutated by changing the amino acid residues GK to GT, using Quikchange II XL site-directed mutagenesis kit (Agilent Technologies - Stratagene Products) according to the manufacturer’s instructions.
Anti-deoxycytidine kinase antibody
Human and murine dCK were compared to identify a conserved hydrophilic peptide (Hopp-Woods hydrophilicity) at the C-terminus of dCK. The human dCK sequence was: DUNEDFKDKYESLVEKVKEFL (the underlined base differed in mice). The peptide was KLH-conjugated and injected into rabbits. The resulting antisera were screened for dCK immunoreactivity against cells engineered to express dCK (see above).
Vesicle transport
Clofarabine HPLC
Real-time RT-PCR
Deoxycytidine kinase siRNA
Results
Possible influence of dCK expression level on ABCG2 modulation of cytotoxicity
Borst and coworkers recently showed that ABCG2 transports the purine nucleoside cladribine (structurally similar to clofarabine) and its nucleoside monophosphate (18). Because clofarabine is a good dCK substrate (11, 24), this finding suggested that intracellular dCK activity (i.e., the extent of clofarabine monophosphorylation) may modulate the effect of ABCG2 on clofarabine cytotoxicity (see model, Fig. 1A). Further, when we analyzed levels of ABCG2 and dCK mRNA in prototypical myeloid leukemia cell lines, ABCG2 mRNA levels varied more than 100-fold and dCK mRNA levels varied 10-fold (Fig. 1B). We tested several cell lines THP-1 (0.067μM) and KG-1 (0.037μM) clofaribine sensitivity and found that the IC50 values (in parentheses) were higher for THP-1 and KG-1 than the HL-60 (0.02 μM) consistent with elevated ABCG2. Therefore, we hypothesized that the activity of dCK may modulate ABCG2-mediated clofarabine resistance by increasing the formation of clofarabine monophosphate, a potentially poor substrate for ABCG2 based on the findings for cladribine-monophosphate (18).
ABCG2 reduces intracellular accumulation of clofarabine
We incubated cells with 10 μM clofarabine, a concentration that is very close to that found in patients undergoing chemotherapy (6, 7, 26). ABCG2 reduced the intracellular accumulation of [3H]-clofarabine in Saos2 cells (Fig. 1C, left panel). Moreover, the ABCG2-expressing cells reached a steady-state concentration of intracellular clofarabine after approximately 60 minutes, a finding suggesting further metabolism has been reduced, while the empty-vector cells continued accumulating clofarabine. We then incubated cells expressing ABCG2 at various levels (see insert) with [3H]-clofarabine for 60 minutes. The intracellular concentration of clofarabine was inversely proportional to the quantity of ABCG2 shown by immunoblotting (Fig. 1C, right panel).
ABCG2 expression and transport activity in Saos2 and OCI-AML3 cells
We compared ABCG2 expression and function in Saos2 cells and the human myeloid leukemia cell line OCI-AML3 to determine whether these parameters are affected by cell context. RT-PCR demonstrated slightly higher levels of endogenous ABCG2 mRNA in Saos2 cells than in OCI-AML3 cells. However, the total ABCG2 mRNA levels (endogenous and exogenous) were comparable in Saos2 and OCI-AML3 cells overexpressing ABCG2 (Fig. 1D, left panel). Analysis of ABCG2 protein expression, by immunoblot and densitometry, showed a slightly (30%) lower level of ABCG2 in OCI-AML3-ABCG2 cells than in Saos2-ABCG2 cells (Fig. 1D, right panel).
We next compared the functional activity of ABCG2 in Soas2 and OCI-AML3 cells by using two well known substrates (mitoxantrone and Hoechst 33342) (22) (Fig. 2A). Notably, regardless of the substrate, over 96 % of the ABCG2 cells have strongly reduced fluorescent intensity indicating strong ABCG2 dependent export activity. To assess transport of the fluorescent substrates, we compared the ratio of the mean fluorescence of vector-control and ABCG2-expressing Saos2 cells and of parental and ABCG2-expressing OCI-AML3 cells. With Hoechst 33342, the ratio was somewhat greater in Saos2 cells than in OCI-AML3 cells, indicating a tendency toward greater substrate efflux; with mitoxantrone, the ratio was greater in OCI-AML3 cells. Taken together, these results indicate that ABCG2 expression and transport activity are comparable in Saos2 and OCI-AML3 cells.
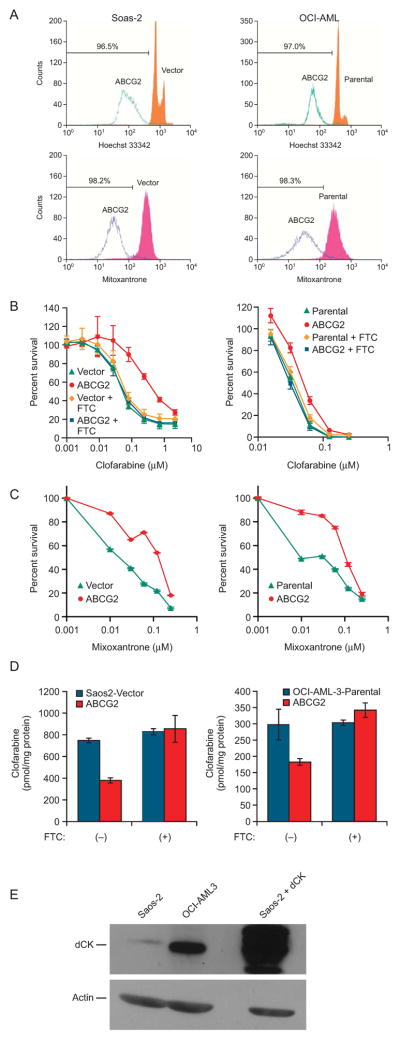
Figure 2. The cell context differentially affects ABCG2-mediated clofarabine resistance.
A, Efflux of the fluorescent ABCG2 substrates Hoechst 33342 and mitoxantrone is almost identical in Saos2 and OCI-AML3 cells. This is a representative experiment repeated several times with almost identical results. B, Clofarabine cytotoxicity was determined in Saos2 cells (left panel) and OCI-AML3 cells (right panel) in the absence and presence of fumitremorgin C (FTC) and is the average of 3 independent experiments conducted using triplicate determinations at each concentration. C, Saos2 and OCI-AML3 cells were exposed to mitoxatrone and cytotoxicity determined as described in the “Materials and Methods” the values are the average of a triplicate determination from a representative experiment repeated twice with similar results. The error bars are smaller than the symbol size. D, Intracellular accumulation of [3H]-clofarabine in Saos2 cells and OCI-AML3 cells. The values are the mean from four independent experiments with each point in duplicate. The bars represent one standard deviation. E, A representative dCK immunoblot of 100 μg of cytosol performed as described in the “Materials and Methods”. In panels A-D, the left hand column represents experiments conducted in Saos2 and the right hand column, OCI-AML3.
Impact of ABCG2 on clofarabine accumulation and cytotoxicity in Saos2 and OCI-AML3 cells
In Saos2 cells, overexpression of ABCG2 produced strong clofarabine resistance (>8-fold increase) that was readily overcome by the specific ABCG2 inhibitor, Fumitremorgin C (FTC) (22) treatment, demonstrating that the effect had been caused mainly by ABCG2 (Fig 2B, left panel). ABCG2 overexpression in OCI-AML3 cells induced only a modest increase in resistance (<2-fold) (Fig 2B, right panel), which was almost completely reversed by FTC. This differential effect was unexpected, because OCI-AML3 and Saos-2 cells expressing ABCG2 showed similar ABCG2 functional activity (comparable OCI-AML and Saos-2 cells have reduction of mitoxantrone and Hoechst 33342) (Fig. 2A). To determine if the differences in clofarabine cytotoxicity were attributed to intrinsic differences in the cells, we examined their sensitivity to mitoxantrone (Fig. 2C). The Saos2 cells require slightly higher concentrations of mitoxantrone to produce cytotoxicity, however the ratios of the IC50 for the ABCG2 expressing cells to their control counterparts are almost identical between Saos2 and OCI-AML3. The similar IC50 ratios and the nearly identical Hoechst and mitoxantrone efflux support the idea that ABCG2 is functionally equivalent in Saos2 and OCI-AML3 cells. However, the reduced impact of ABCG2 upon clofarabine cytotoxicity in OCI-AML3 cells suggests a factor independent of ABCG2 accounts for this difference.
Clofarabine accumulation was next compared in Saos2 and OCI-AML3 cells that did and did not carry an ABCG2 expression vector. The ratio of intracellular clofarabine content in the vector-control or parental cells to that in the ABCG2-overexpressing cells was greater at 2.0 for Saos2 cells and 1.6 for OCI-AML3 cells (Fig. 2D). Abrogation of ABCG2 actively with FTC eliminated this difference in both cell types. Therefore, ABCG2 reduces clofarabine accumulation in Saos2 and OCI-AML3 cells, but unexpectedly appears more functionally active in Saos2 cells.
The amount of dCK might impact the cytotoxicity of clofarabine by affecting clofarabine nucleotide formation. We developed an anti-body to dCK (see “Materials with Methods”) and evaluated the level of immunoactive dCK in cytosol from Saos2 and OCI-AML3 cells (Fig. 2E). The constituitive level of dCK in Saos2 is very low relative to OCI-AML3, and may account for the intrinsic sensitivity of the OCI-AML3 cells to clofarabine compared to the resistance of the Saos2 cells.
Effect of dCK on intracellular clofarabine metabolism
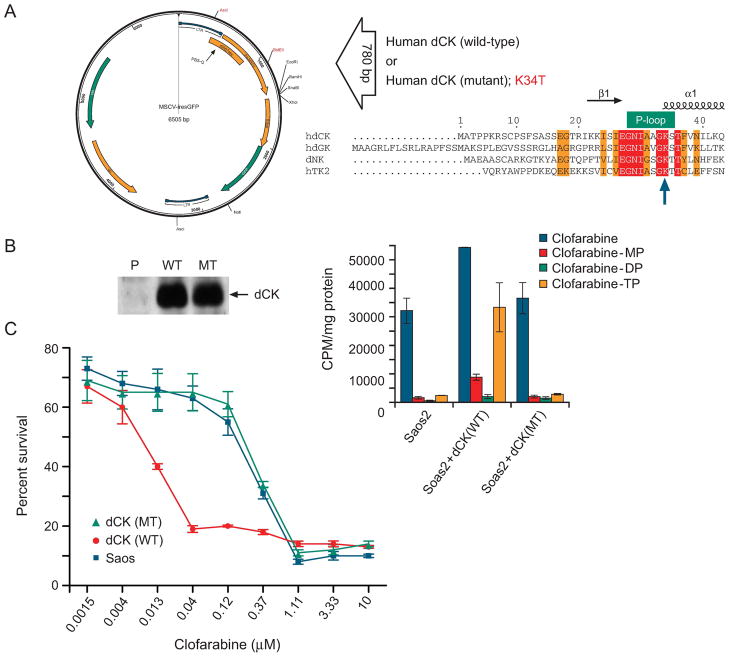
To test the role dCK on clofarabine cytotoxicity and intracellular metabolism, we developed expression vectors for wild-type dCK and mutant dCK containing an altered ATP-binding site which we predicted might represent a loss of function. The human dCK (25) cDNA was PCR amplified from a human ovary cDNA library. The dCK mutant was created on the basis of the dCK structure (25), with the P-loop lysine residue, typically required to coordinate ATP binding in the nucleotide binding fold (22), changed to a threonine. The wild-type and mutant dCKs were then subcloned into the retrovical expression plasmid, MSCV-IRES-GFP (Fig. 3A), which allowed us to transduce populations of cells, and then select pools of cells (expressing either dCK or mutant dCK) based upon equivalent levels of GFP fluorescence. Immunoblot analysis demonstrated nearly equivalent amounts of mutant or wild-type dCK (Fig. 3B, left panel). To determine dCK function, these cells were then incubated with radiolabeled clofarabine, and intracellular clofarabine metabolites were assayed by HPLC to assess dCK activity (Fig. 3B, right panel). The parental Saos2 cells convert a small proportion of clofarabine to phosphorylated metabolites. In contrast, the ratio of phosphorylated clofarabine metabolites to unchanged clofarabine was substantially greater in the Saos2 cells overexpressing wild-type dCK (0.8) than in cells containing the mutant dCK (0.15), a finding demonstrating high activity of dCK. The intracellular metabolism of chlofarabine in the mutant dCK cells and the parental cells was virtually indistinguishable (Fig 3B, right panel), suggesting the mutant dCK is non-functional. To further confirm this and rule out possible dominant negative effects of the mutant dCK upon endogenous dCK, we evaluated clofarabine sensitivity in the parental Saos2 and Saos2 cells expressing either mutant dCK, or wild-type dCK (Fig 3C). By using higher concentrations and a shorter duration exposure, we found that dCK overexpression strongly sensitized cells to clofarabine, and reduced the IC50 from ~0.2μM to 0.006, a thirty-fold reduction. Notably, the cells expressing mutant dCK did not have a significant change in the IC50 compared to parental Saos2. These studies indicate disruption of the P-loop in dCK produces a non-functional dCK that does not alter chlorfarabine metabolism to nucleotides or sensitivity to chlofarabine. Moreover, increasing the level of dCK markedly increases sensitivity of cells to clofarabine.
Figure 3. dCK function affects intracellular clofarabine metabolism.
A, Diagram of dCK expression vector and P-loop mutation. B, Immunoblot analysis of Saos2 cells expressing either no dCK, wild-type dCK, or mutant dCK (left panel), the ratio of phosphorylated clofarabine metabolites to unchanged clofarabine is substantially greater in parental Saos2 cells expressing wild-type dCK than in cells containing the mutant dCK (right panel). MP, monophosphate; DP, diphosphate; TP, triphosphate. C, Saos2 cells overexpressing wild-type dCK are more sensitive to clofarabine while cells harboring either mutant dCK or empty vector are of similar sensitivity. Cytotoxicity was assessed as described in the “Materials and Methods”. Each value is an average of three-independent determinations from a representative experiment repeated three times with similar results.
The interrelated affect of ABCG2 and dCK levels on clofarabine cytotoxicity and metabolism
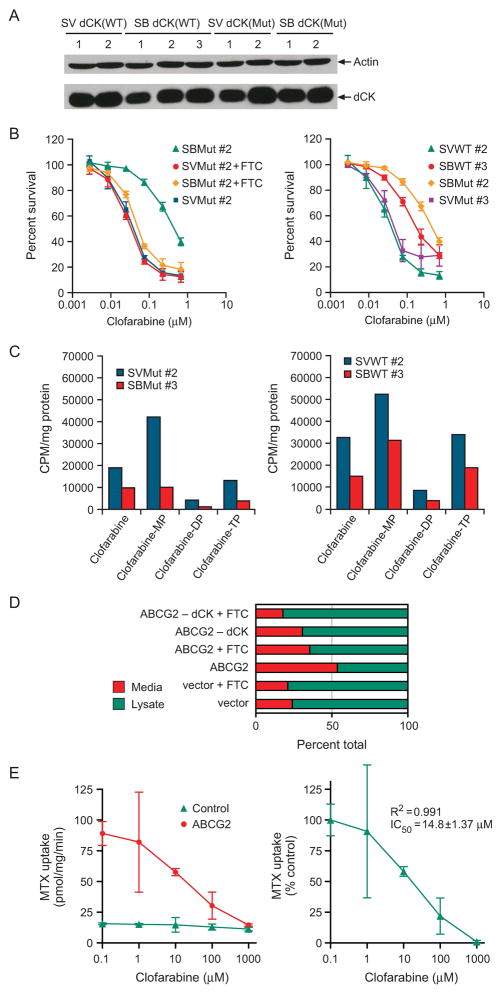
We retrovirally transduced vector-control and ABCG2-overexpressing Saos2 cells with viral supernatants containing retroviruses expressing either wild-type or mutant dCK, (cell populations with equal GFP fluorescence, were selected by fluorescence activated cell sorting) and dCK expression was determined by immunoblot analysis (Fig. 4A). We then analyzed the cytotoxicity of clofarabine to individually transduced cell pools with similar levels of dCK (Fig. 4B). The clofarabine IC50 was 12 times as high in ABCG2-overexpressing cells harboring a nonfunctional mutant dCK as in cells with mutant dCK but lacking ABCG2 (Fig. 4B, left panel). The specific ABCG2 inhibitor FTC almost completely reversed this clofarabine resistance, confirming that ABCG2 caused the clofarabine resistance in these cells. It is notable that at the low concentrations of clofarabine used to measure cytotoxicity, the difference in IC50 for clofarabine is < 2-fold for cells expressing mutant or wild-type dCK. However, when ABCG2 is present, the impact of dCK is clear: higher levels of dCK reduce ABCG2 ability to confer clofarabine resistance. Because ABCG2 exports the structurally related purine, cladribine and cladribine monophosphate, we assessed the intracellular metabolism of clofarabine, but used a higher concentration of clofarabine to determine if differences in metabolism occurred in the absence and presence of ABCG2 and fuctional dCK. Formation of clofarabine monophosphate, diphosphate, and triphosphate are often determinants of nucleoside cytotoxicity. ABCG2-overexpressing cells expressing mutant dCK showed the expected reduction in clofarabine level but also a substantial reduction (4-fold) in clofarabine monophosphate (Fig. 4C). This finding suggests ABCG2 either mediates the reduction in the pool of intracellular clofarabine available for monophosphorylation or that ABCG2 exports clofarabine-monophosphate.
Figure 4. ABCG2-mediated efflux is greater in cells expressing mutant dCK than in cells with enhanced dCK expression.
A, A representative immunoblot analysis of wild-type (WT) and mutant (MT) dCK in vector-control (SV) and ABCG2-expressing (SB) Saos2 cells. B, Survival of Saos2 vector- or ABCG2-transduced cells expressing mutant (MT) or wild-type (WT) dCK. The values are the average of three-independent experiments performed in triplicate. The bars represent one standard deviation. C, Clofarabine metabolites were determined by HPLC. Arabic numerals (#) indicate individual cell pools; D, Efflux of clofarabine was determined from the ratio of the proportion of drug in the media after a 60 min efflux period and the proportion of drug within the cell. This is a representative of an experiment repeated three times with similar results. E, Various concentrations of clofarabine were added to vesicles expressing either no ABCG2 or ABCG2 and 10μM [3H] methotrexate the values are means of triplicate determinations from a representative experiment.
ABCG2 exports clofarabine more readily than clofarabine monophosphate
To determine if clofarabine and/or clofarabine-monophosphate was effluxed from ABCG2 overexpressing cells in a transporter dependent fashion we evaluated clofarabine efflux after loading cells with 10μM (3H) - clofarabine under ATP-depleting conditions (see “Materials and Methods”). After a 1h incubation cells were extensively washed to remove extracellular drug and resuspended into warmed media containing either the ABCG2 inhibitor, FTC or its solvent. This analysis reveals that ABCG2 increases the efflux of clofarabine derived radioactivity by over 2.2-fold over Saos2 cells harboring the empty vector. Moreover, FTC strongly reduced efflux in the ABCG2 overexpressing cells (> 35%), while producing a minimal effect on the vector cells (<3%). Unlike our drug uptake assays, FTC appears less capable of inhibiting chlofarabine efflux. It is likely the inhibition by FTC under these conditions may be suboptimal, because the drug efflux is initiated concurrent with FTC addition, a circumstance that may allow drug efflux before FTC achieves full inhibition. It is notable that the proportion of clofarabine derived radioactivity is not enhanced in ABCG2 cells expressing dCK. We next performed HPLC analysis on the effluxed radioactivity to determine what forms of clofarabine were effluxed (Supplementary Fig 3.) (Fig 4D). We found unexpectedly for ABCG2 cells and ABCG2 cells overexpressing dCK that clofarabine was exclusively effluxed, however in two of our 12 HPLC runs we did detect some clofarabine-monophosphate, but because it was only a few percent of the total radioactivity we attribute this to some lysed cells producing clofarabine-monophosphate contamination. To confirm that there were not additional clofarabine metabolites we performed a “radioactive-balance” analysis of the total amount of radioactivity injected into the HPLC vs. the amount recovered in the eluate. This analysis confirmed that only chlorfarabine was effluxed. These studies indicate that in intact cells clofarabine is the primary substrate for ABCG2 in cells. To confirm that clofarabine directly interacts with ABCG2, we performed studies with sf9 membrane vesicles programmed to express either ABCG2 or the empty vector (Fig 4E). We evaluated the impact of chlorfarabine on the transport of methotrexate, a well known ABCG2 substrate. We performed these indirect studies because, like cladribine, clofarabine can either rapidly leak out of vesicles or be transported by equilabrative transporters. We estimated the IC50 for clofarabine at approximately 14 μM (Fig 4E, right panel). These findings together with our whole cell export studies indicate clofarabine is a good ABCG2 substrate.
Effect of dCK and ABCG2 function on intracellular clofarabine metabolism
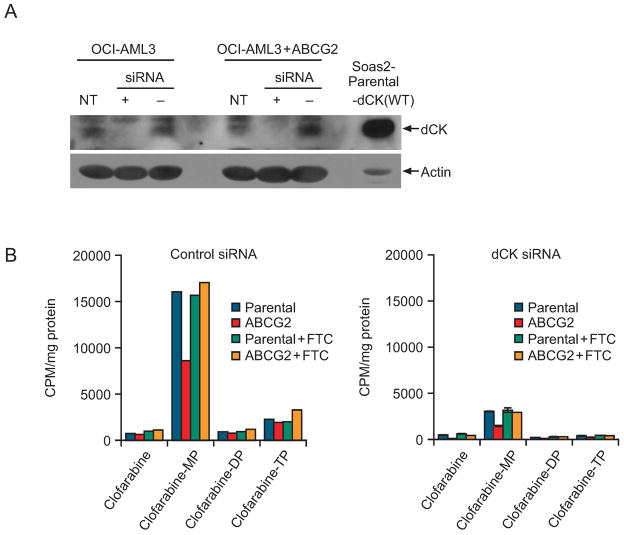
We developed siRNA conditions to knock down dCK mRNA, which was suppressed by more than 80% in parental OCI-AML3 and OCI-AML3-ABCG2 cells 48 hours after transient introduction of the siRNA (Supplementary Fig. 2). While dCK mRNA was not completely reduced by the siRNA, the dCK protein was undetectable by immunoblot analysis at 72 hours suggesting dCK mRNA and protein are not co-regulated (Fig. 5A).
Figure 5. Knockdown of dCK enhances ABCG2-mediated clofarabine resistance by reducing clofarabine phosphorylation in ABCG2-overexpressing cells.
A, Immunoblot analysis of dCK protein after transfection with dCK siRNA. This is a representative blot from an experiment repeated three separate times. B, Intracellular clofarabine metabolites after siRNA treatment. This is a representative HPLC analysis of clofarabine intracellular metabolites t from average values in this experiment repeated three times with similar results.
To understand how the intracellular metabolism of clofarabine is affected by ABCG2 after a reduction of endogenous dCK, we determined the intracellular clofarabine metabolites in the presence and absence of FTC in OCI-AML3 cells expressing dCK at normal levels or attenuated by a dCK siRNA. In cells with normal dCK expression, more than 80% of intracellular clofarabine was in the monophosphate form (Fig. 5B, left panel). Notably, ABCG2 overexpression appeared to substantially reduce the concentration of clofarabine monophosphate, but produced only a minimal affect on the concentration of clofarabine, suggesting that with these levels of dCK clofarabine is rapidly phosphorylated after uptake and unavailable for interaction with ABCG2. In contrast, ABCG2 reduced intracellular clofarabine levels by a factor of 3 in cells deficient in dCK by siRNA (Fig 5B, right panel); this reduction was not observed when the ABCG2 inhibitor FTC was added. These results demonstrate that when dCK is at low level, ABCG2 reduces the concentration of clofarabine leading to strongly reduced amounts of clofarabine available to form clofarabine metabolites.
Discussion
The formation of phosphorylated metabolites is an essential determinant of purine nucleoside cytotoxicity (4, 10, 12). We have demonstrated a new interactive mechanism in which ABCG2 efflux of clofarabine reduces the quantity of clofarabine available for phosphorylation by dCK, thereby reducing cytotoxicity. Because clofarabine is effective against multiple tumor types (colon, renal, prostate) in model systems and therapy for AML and ALL (6–10), we investigated the interplay between ABCG2 and the amount of dCK in both Saos2 osteosarcoma cells (because they have inherently low dCK (this paper and ABCG2) and the myeloid leukemia, OCI-AML3 cells containing high endogenous dCK levels (this paper). In Saos2 cells, ABCG2 restricted the formation of phosphorylated clofarabine metabolites because ABCG2 reduced the amount of intracellular clofarabine available for phosphorylation. The results of our dCK siRNA knockdown studies in OCI-AML3 (which have high endogenous dCK) were also consistent with this mechanism: in the absence of detectable dCK protein, ABCG2 strongly reduced intracellular clofarabine even at very low intracellular concentrations suggesting clofarabine is a very good substitute for ABCG2. In this dynamic system, the ABCG2-mediated reduction in clofarabine produced a proportionate reduction in clofarabine monophosphate. The role of ABCG2 was confirmed by using an ABCG2 inhibitor (FTC), which restored both clofarabine and clofarabine monophosphate concentrations. Moreover, our membrane vesicle transport findings demonstrate that ABCG2 strongly interacts with clofarabine, we speculate that this interaction has the potential to override nucleoside uptake processes when dCK levels are low leading to reduced nucleotide formation (Fig 1A). Thus, because reduced dCK is a well-known mechanism of resistance (14–16) it is conceivable that ABCG2 expression is an additional factor producing clofarabine resistance.
Although our studies depict a close relationship between dCK and ABCG2, the exact kinetic relationship between clofarabine efflux by ABCG2 and clofarabine phosphorylation by dCK remains to be elucidated. The clofarabine efflux step can be explored by in vitro transport studies. A recent study using membrane vesicles to study the kinetics of ABCG2-mediated transport of cladribine, a structurally similar purine anti-metabolite, was impeded by rapid leakage of cladribine from the vesicles (18). These authors used an indirect approach to show that cladribine inhibits the transport of a known ABCG2 substrate, suggesting that cladribine is an ABCG2 substrate (18). We also used this indirect approach to demonstrate potent inhibition of methotrexate transport by clofarabine (IC50 ~14μM). These findings in combination with our intracellular analysis provided a “snapshot” of how ABCG2 impacts intracellular clofarabine metabolism. Moreover, when coupled with the use of an ABCG2 inhibitor, our efflux data indicates that inhibition of ABCG2 primarily increases the intracellular concentration of clofarabine. Furthermore, our efflux studies show that clofarabine-monosphosphate is not exported in cells that have both high levels of dCK and ABCG2. In total, these findings suggest that ABCG2 has a much greater affinity for clofarabine than for clofarabine monophosphate. One implication is that tumors with high levels of ABCG2 and low dCK will be insensitive to clofarabine, while those that have high levels of dCK despite ABCG2 will be sensitive to clofarabine.
The extent to which ABCG2 confers resistance to clofarabine appears to be strongly influenced by dCK activity. Our findings indicate that, by enhancing clofarabine monophosphate formation, dCK minimizes the extent to which ABCG2 confers resistance. Such a mechanism would be consistent with our findings. Our studies show that even when clofarabine-monophosphate (typically the main intracellular metabolite of clofarabine), reaches high intracellular concentrations (e.g. cells with high dCK), it is refractory to ABCG2-mediated export. Early studies demonstrated that clofarabine monophosphate is the major intracellular clofarabine metabolite in an acute lymphoblastic leukemia cell line (10–12). This finding suggests that downstream phosphorylation steps are slow in tumor cells and/or that clofarabine monophosphate is a poor substrate for the next downstream step, i.e., nucleoside diphosphate kinase–mediated phosphorylation. Therefore, we propose that the addition of ABCG2 inhibitors (e.g., FTC, KO143, or tyrosine kinase inhibitors such as sorafenib (27)) might increase the efficacy of clofarabine in ABCG2-expressing tumor cells by reducing efflux of clofarabine to further enhance the formation clofarabine nucleotides.
Supplementary Material
Acknowledgments
We thank Dr. Susan Bates (National Cancer Institute) for kindly providing fumitremorgin C (FTC). We thank Sharon Naron for the excellent editorial assistance. We thank Alice Gibson for excellent technical assistance. We thank Betsy Williford and Julie Groff for preparation of the illustrations and figures. This work was supported by NIH Research Grants CA77545, GM60346, ES058571, and HL67366; by Cancer Center Support Grant P30 CA21745; and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Borst P, Balzarini J, Ono N, et al. The potential impact of drug transporters on nucleoside-analog-based antiviral chemotherapy. Antiviral Res. 2004;62:1–7. doi: 10.1016/j.antiviral.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Rose JB, Coe IR. Physiology of nucleoside transporters: back to the future. Physiology (Bethesda) 2008;23:41–8. doi: 10.1152/physiol.00036.2007. [DOI] [PubMed] [Google Scholar]
- 3.Carson DA, Wasson DB, Esparza LM, Carrera CJ, Kipps TJ, Cottam HB. Oral antilymphocyte activity and induction of apoptosis by 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine. Proc Natl Acad Sci U S A. 1992;89:2970–4. doi: 10.1073/pnas.89.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker WB, Shaddix SC, Chang CH, et al. Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5′-triphosphate. Cancer Res. 1991;51:2386–94. [PubMed] [Google Scholar]
- 5.Robertson LE, Chubb S, Meyn RE, et al. Induction of apoptotic cell death in chronic lymphocytic leukemia by 2-chloro-2′-deoxyadenosine and 9-beta-D-arabinosyl-2-fluoroadenine. Blood. 1993;81:143–50. [PubMed] [Google Scholar]
- 6.Gandhi V, Plunkett W, Bonate PL, et al. Clinical and pharmacokinetic study of clofarabine in chronic lymphocytic leukemia: strategy for treatment. Clin Cancer Res. 2006;12:4011–7. doi: 10.1158/1078-0432.CCR-05-2664. [DOI] [PubMed] [Google Scholar]
- 7.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–9. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–86. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 9.Lindemalm S, Liliemark J, Gruber A, et al. Comparison of cytotoxicity of 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine (clofarabine) with cladribine in mononuclear cells from patients with acute myeloid and chronic lymphocytic leukemia. Haematologica. 2003;88:324–32. [PubMed] [Google Scholar]
- 10.Parker WB, Secrist JA, 3rd, Waud WR. Purine nucleoside antimetabolites in development for the treatment of cancer. Curr Opin Investig Drugs. 2004;5:592–6. [PubMed] [Google Scholar]
- 11.Bonate PL, Arthaud L, Cantrell WR, Jr, Stephenson K, Secrist JA, 3rd, Weitman S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5:855–63. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi V, Plunkett W. Clofarabine and nelarabine: two new purine nucleoside analogs. Curr Opin Oncol. 2006;18:584–90. doi: 10.1097/01.cco.0000245326.65152.af. [DOI] [PubMed] [Google Scholar]
- 13.Lotfi K, Mansson E, Spasokoukotskaja T, et al. Biochemical pharmacology and resistance to 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine, a novel analogue of cladribine in human leukemic cells. Clin Cancer Res. 1999;5:2438–44. [PubMed] [Google Scholar]
- 14.Damaraju VL, Damaraju S, Young JD, et al. Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene. 2003;22:7524–36. doi: 10.1038/sj.onc.1206952. [DOI] [PubMed] [Google Scholar]
- 15.Mansson E, Spasokoukotskaja T, Sallstrom J, Eriksson S, Albertioni F. Molecular and biochemical mechanisms of fludarabine and cladribine resistance in a human promyelocytic cell line. Cancer Res. 1999;59:5956–63. [PubMed] [Google Scholar]
- 16.Lamba JK, Crews K, Pounds S, et al. Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther. 2007;323:935–45. doi: 10.1124/jpet.107.128595. [DOI] [PubMed] [Google Scholar]
- 17.Adachi M, Sampath J, Lan LB, et al. Expression of MRP4 confers resistance to ganciclovir and compromises bystander cell killing. J Biol Chem. 2002;277:38998–9004. doi: 10.1074/jbc.M203262200. [DOI] [PubMed] [Google Scholar]
- 18.de Wolf C, Jansen R, Yamaguchi H, et al. Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008;7:3092–102. doi: 10.1158/1535-7163.MCT-08-0427. [DOI] [PubMed] [Google Scholar]
- 19.Wielinga PR, Reid G, Challa EE, et al. Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol. 2002;62:1321–31. doi: 10.1124/mol.62.6.1321. [DOI] [PubMed] [Google Scholar]
- 20.Wijnholds J, Mol CA, van Deemter L, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97:7476–81. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuetz JD, Connelly MC, Sun D, et al. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048–51. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 22.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 23.Takenaka K, Morgan JA, Scheffer GL, et al. Substrate overlap between Mrp4 and Abcg2/Bcrp affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res. 2007;67:6965–72. doi: 10.1158/0008-5472.CAN-06-4720. [DOI] [PubMed] [Google Scholar]
- 24.King KM, Damaraju VL, Vickers MF, et al. A comparison of the transportability, and its role in cytotoxicity, of clofarabine, cladribine, and fludarabine by recombinant human nucleoside transporters produced in three model expression systems. Mol Pharmacol. 2006;69:346–53. doi: 10.1124/mol.105.015768. [DOI] [PubMed] [Google Scholar]
- 25.Sabini E, Ort S, Monnerjahn C, Konrad M, Lavie A. Structure of human dCK suggests strategies to improve anticancer and antiviral therapy. Nat Struct Biol. 2003;10:513–9. doi: 10.1038/nsb942. [DOI] [PubMed] [Google Scholar]
- 26.Kantarjian HM, Jeha S, Gandhi V, Wess M, Faderl S. Clofarabine: past, present, and future. Leuk Lymphoma. 2007;48:1922–30. doi: 10.1080/10428190701545644. [DOI] [PubMed] [Google Scholar]
- 27.Hu S, Chen Z, Franke R, et al. Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin Cancer Res. 2009;15:6062–9. doi: 10.1158/1078-0432.CCR-09-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.