Abstract
Glutaminase-deficient mice (GLS1 hets), with reduced glutamate recycling, have a focal reduction in hippocampal activity, mainly in CA1, and manifest behavioral and neurochemical phenotypes suggestive of schizophrenia resilience. To address the basis for the hippocampal hypoactivity, we examined synaptic plastic mechanisms and glutamate receptor expression. While baseline synaptic strength was unaffected in Schaffer collateral inputs to CA1, we found that long-term potentiation was attenuated. In wild-type mice, GLS1 gene expression was highest in the hippocampus and cortex, where it was reduced by about 50% in GLS1 hets. In other brain regions with lower wild-type GLS1 gene expression there were no genotypic reductions. In adult GLS1 hets, NMDA receptor NR1 subunit gene expression was reduced, but not AMPA receptor GluR1 subunit gene expression. In contrast, juvenile GLS1 hets showed no reductions in NR1 gene expression. In concert with this, adult GLS1 hets showed a deficit in hippocampal-dependent contextual fear conditioning while juvenile GLS1 hets did not. These alterations in glutamatergic synaptic function may partly explain the hippocampal hypoactivity seen in the GLS1 hets. The maturity-onset reduction in NR1 gene expression and in contextual learning supports the premise that glutaminase inhibition in adulthood should prove therapeutic in schizophrenia.
Keywords: hippocampus, glutamate, GLS1, NR1, schizophrenia
Introduction
Altered glutamate signaling has been strongly implicated in schizophrenia (Gaspar et al., 2009; Harrison and Weinberger, 2005; Javitt and Zukin, 1991; Lang et al., 2007). Blockade of NMDA glutamate receptors by drugs such as phencyclidine (PCP) and ketamine induces a schizophrenia-like state and exacerbates the symptoms of patients with schizophrenia (Javitt and Zukin, 1991). Mice with a knockdown of the NMDA receptor subunit 1 (NR1 hypomorphs) phenotypically mimic many aspects of schizophrenia (Duncan et al., 2009; Halene et al., 2009; Mohn et al., 1999), in contrast to NR2A deficient mice or rats treated with selective NR2B antagonists, that phenotypically mimic aspects of affective disorders (Higgins et al., 2003; Inta et al., 2010). Mounting evidence suggests that it is blockade of NMDA receptors on interneurons (Grunze et al., 1996; Homayoun and Moghaddam, 2007), leading to decreased inhibition, excitation of glutamatergic pyramidal neurons, and broader activation of AMPA receptors, that engenders schizophreniform symptoms (Anand et al., 2000; Deakin et al., 2008; Homayoun et al., 2005; Lewis and Moghaddam, 2006; Maeshima et al., 2007; Moghaddam and Adams, 1998). Indeed, selective deletion of NR1 in cortico-hippocampal interneurons is sufficient to engender a schizophrenia-like phenotype in mice (Belforte et al., 2010); notably, the deletion must be made in early life, arguing that circuit abnormalities associated with NMDA receptor hypofunction engender a hyperglutamatergic vulnerability, which may underlie the onset of schizophrenia in early adulthood (Lisman et al., 2010). This suggests that, rather than restoring NMDA receptor function, therapeutic intervention in schizophrenia should focus on tempering excessive glutamate release. Results from clinical studies with mGluR2/3 agonists support the therapeutic potential of this strategy (Patil et al., 2007).
Inhibiting phosphate-activated glutaminase (gene GLS1) offers a novel way to inhibit excessive glutamate release. Moreover, there is postmortem evidence that glutaminase expression is increased in schizophrenia (Bruneau et al., 2005; Gluck et al., 2002). In the brain, glutaminase is a neuron-specific enzyme that converts glutamine to glutamate, and is the rate-limiting step in the neuronal-glial glutamate-glutamine recycling pathway, the main source of neurotransmitter glutamate (Curthoys and Watford, 1995; Hertz and Zielke, 2004; Kvamme et al., 2001; Sibson et al., 2001). Consistent with its essential recycling role, GLS1 knockout neurons grown in culture show an activity-dependent reduction in glutamatergic synaptic transmission, and GLS1 knockout mice show abnormal patterned activity in brainstem respiratory centers, hypoventilate and die shortly after birth (Masson et al., 2006).
We have found that GLS1 hets, heterozygous mice with only one GLS1 allele, show about a 50% reduction in GLS1 gene expression, and similar reductions in glutaminase protein and enzymatic activity (Gaisler-Salomon et al., 2009a; Masson et al., 2006); overall glutamate content is modestly but significantly reduced in the hippocampus and frontal cortex. Despite their overt normalcy, GLS1 hets respond less to ketamine and amphetamine (dopamine-releasing psychotomimetic drugs), and display an antipsychotic drug-like profile in the latent inhibition test (Gaisler-Salomon et al., 2009a), commonly used as a screening assay for antipsychotic drugs (Barak and Weiner, 2011; Shadach et al., 2000). Moreover, in vivo imaging revealed that relative cerebral blood volume (rCBV) is decreased in the hippocampus of GLS1 hets —a pattern inverse of that seen in patients with schizophrenia (Schobel et al., 2009). Taken together, these findings suggested that GLS1 hets model schizophrenia resilience, with the implication that reducing GLS1 activity might be a new therapeutic strategy for the treatment of schizophrenia (Gaisler-Salomon et al., 2009b).
Our previous findings showed that a constitutive, global GLS1 deficiency mainly impacts the hippocampus; in hippocampal CA1, we found a selective reduction in spontaneous excitatory input and reduced strength of Schaffer collateral input in the GLS1 hets (Gaisler-Salomon et al., 2009a), consistent with diminished hippocampal glutamatergic synaptic function. We also found a marked reduction in contextual – but not cued — fear conditioning in the GLS1 hets, which may be an effect of altered hippocampal function. We have now asked whether hippocampal glutamatergic synaptic plasticity is affected in GLS1 hets, whether there are alterations in NMDA and AMPA receptor subunit gene expression, and whether alterations in glutamate receptor gene expression and hippocampal dependent fear conditioning show a maturational onset.
Materials and Methods
Animals
We used GLS1 het mice (Masson et al., 2006) in which transcription and expression of GLS1 were blocked by the insertion of a floxed PGKneo-Stop cassette inserted ahead of the transcription initiation site in exon 1 of the GLS1 gene (Entrez Gene 14660). The GLS1 mutant colony was kept on a mixed 129SvEv/J-C57BL6/J background. Mice were bred from crosses of GLS1 hets with wild type (WT) mice. WT littermates were used as controls. Adult mice were 3 to 5 months of age; juvenile mice were 30 to 45 days old.
Previous electrophysiological and behavioral studies of GLS1 hets showed no significant sex differences (Gaisler-Salomon et al., 2009b), so the sex of the mice used in the present studies was determined by animal availability. We used males exclusively for baseline synaptic strength recordings and females exclusively for synaptic plasticity studies. We used males exclusively for gene expression studies, in both adult and juvenile studies. We used male mice for fear conditioning studies in adults. Only fear conditioning in juveniles was conducted in both male and female mice; we ruled out sex differences by statistical analysis using sex as a between-subject factor.
Procedures involving mice and their care were conducted in conformitywith the institutional guidelines of the National Institutesof Health Guide for the Care and Use of Laboratory Animals and performed under protocols approved by the InstitutionalAnimal Care and Use Committees of Columbia University and New York State Psychiatric Institute. Animals were group housed and maintained on a 12-h light/dark cycle; all experiments were performed during the light phase. Food and water were provided ad libitum.
Electrophysiology
Patch clamp recordings in brain slice were done at ~22 °C (room temperature). Hippocampal slices, 400 μm transverse, were prepared from adult GLS1 het mice and WT littermates, and whole cell recordings were performed from CA1 pyramidal neurons as described previously (Gaisler-Salomon et al., 2009a). Briefly, slices were mounted in a recording chamber perfused with ACSF (concentration in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2 and 25 glucose, pH 7.4, saturated with 95% O2-5% CO2. The GABAA selective antagonist SR95531 (10 μM) was continuously applied via perfusion. Synaptic responses were recorded under voltage clamp at holding potentials of −75, −45, −15, +15 and +40 mV using an Axopatch 200B (Molecular Devices, Sunnyvale, CA). Recording patch pipettes were fabricated from standard wall borosilicate capillaries and had resistances of 3–5 MΩ. Composition of the pipette solution was (in mM): 140 Cs+-gluconate, 10 HEPES, 0.1 CaCl2, 2 MgCl2, 1 EGTA, 2 ATP-Na2 and 0.1 GTP-Na2, pH 7.3. To block unclamped and antidromic Na+ currents, 5 mM QX-314 was added to the pipette solution. Drugs were obtained from Sigma-Aldrich (St. Louis, MO).
Evoked EPSCs were induced by focal stimulation of Schaffer collaterals with a fine bipolar electrode (World Precision Instruments, Sarasota, FL) with 200 μsec duration, 200–400 μA pulses at 0.1 Hz. D-(−)-2-amino-5-phosphonopentanoic acid (D-AP5, 100 μM) was used to isolate AMPA receptor responses. EPSC strength was measured as charge transfer; for this, 10 consecutive traces were averaged and the EPSC integrated over a time window of 5 to 105 ms post-stimulus. The AMPA:NMDA ratio was measured in two ways: ratio of charge transfer and ratio of peak amplitude at −75 mV and +40 mV. For charge transfer, the AMPA response was measured from traces with D-APV application, and the NMDA response was determined by subtraction of the AMPA response from the control response. For peak amplitude, the peak amplitude of the AMPA response was measured at a holding potential of −75 mV; the NMDA response was measured as the amplitude at 80 ms post-stimulus at a holding potential of +40 mV. For current-voltage relationships, the measured amplitudes of AMPA and NMDA currents were plotted versus holding potential.
Field EPSP (fEPSP) recordings were done at ~28 °C. The Schaeffer collateral-CA1 fEPSP was recorded as described previously (Gaisler-Salomon et al., 2009a; Yano et al., 2006). Briefly, CA1 fEPSPs were recorded by placing both stimulating and recording electrodes in CA1 over the stratum radiatum. LTP was induced by a train of 10 short-burst stimulations (4 pulses at 100 Hz) delivered at 5 Hz. LTP was expressed as per cent of baseline, averaged over the preceding 15 min. Experiments were done genotype blind. Data were digitized and analyzed using Axograph X (Axograph Scientific, Sydney, Australia) and statistics were performed using SPSS (Version 17 or 18; IBM, Armonk, NY). Significance of effects was analyzed using a two-way repeated measures ANOVA and unpaired t-tests. Data are presented as mean ± s.e.m.
Gene Expression by RT-PCR
Mice were sacrificed by cerebral dislocation. Brains were removed, and dissected in cold PBS to obtain tissue from the hippocampus, frontal cortex, striatum, thalamus and cerebellum. All samples were taken from the left hemisphere. Samples were immediately placed in 300 μL of Qiazol (Qiagen, Valencia, CA), then homogenized and suspended in a total of 1 mL Qiazol. RNA was extracted using a Lipid Tissue Mini Extraction kit (Qiagen), following the manufacturer’s protocol. Quantities were standardized to 2 μg in 19 μL using a NanoDrop 1000 Spectrophometer (Thermo Scientific, Waltham, MA). The 260:280 nm absorbance ratio was measured to assess RNA quality; samples were excluded if the ratio was outside the range of 2.0 ± 0.2, or if the RNA concentration was too low. DNA digestion was carried out with 1 μL DNase (Promega, Madison, WI), incubated at 37 °C for 30 minutes, and then at 65 °C for 10 minutes. Reverse transcription was carried out using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) following the manufacturer’s protocol. Reverse transcription product (cDNA) was diluted to 1 mL in water.
Quantitative PCR (qPCR) was performed as previously described (Sibille et al., 2007). Briefly, 80–120 bp sequences were amplified in triplicate using a Opticon 2 DNA Engine (Bio-Rad, Hercules, CA), using universal PCR conditions (65–59 °C touch down, followed by 40 cycles (95°C for 15 sec, 59° C for 10 sec, 72° C for 10 sec). cDNA was amplified in a 25 μL reaction (10 μL primers, 12.5 μL 2x SYBR Green qPCR Master Mix (SABiosciences, Frederick, MD), 1 μL cDNA and 1.5 μL ddH2O). Primers (see Table 1) were designed using Primer3 (Rozen and Skaletsky, 2000) and Integrated DNA Technologies OligoAnalyzer software, and synthesized by Integrated DNA Technologies (Coralville, IA). Primer suitability was determined using standard curve analysis, melting curve analysis, and linearity at threshold (Bookout and Mangelsdorf, 2003; Pfaffl et al., 2004). Primer dimers were assessed in amplifications without cDNA. Genomic DNA carryover was ruled out in control amplifications without reverse transcriptase.
Table 1.
Primer sequences for qPCR
| Gene | forward primer | reverse primer |
|---|---|---|
| GAPDH | aactcccactcttccacct | caccaccctgttgctgta |
| GluR1 | ccacagccaaaccctatt | agccaactgccatgctat |
| NR1 | agactccaagagggctga | gtacaaggttgggtgagtga |
| GLS1 | gtacagtctctgtggcttgg | cagttagcggctcattcac |
The ΔΔCt method was used; intensity values for each gene were normalized to GAPDH (ΔCt). Fold change was calculated as 2−ΔΔCt relative to adult hippocampus. Samples were excluded if Ct values within triplicates were not within 1 cycle. Mice with fewer than 3 brain regions were excluded from the analysis. Regional differences in WT mice and genotypic differences were analyzed using a linear mixed-model analysis of variance (ANOVA) with an unstructured covariance matrix. A mixed-model ANOVA has been shown to be preferable over repeated-measures ANOVA, and can best accommodate an unbalanced design with missing values (Howell, 2010; Little and Rubin, 2002). Mouse number was set as the subject variable and brain region was set as the repeated variable. For analysis of brain region differences in WT mice, brain, age and brain x age interaction were fixed effects; for analysis of genotypic differences, brain, age, genotype and brain x age x genotype interaction were fixed effects. Individual mice were random effects in both analyses. Post-hoc pairwise comparisons with Bonferroni adjustments were used to detect specific differences between regions within each age group, or between genotypes within each brain region and age group. Statistical analyses were performed in SPSS, with significance set at p < 0.05.
Preparation of sub-cellular fractions
Brains were removed from WT and GLS1 het mice (age range P80 – P100). The hippocampus was immediately dissected and quick-frozen on dry ice. Protein was extracted according to the sub-cellular fraction method described by Schilström et al. (2006). Samples were homogenized in ice-cold homogenization buffer (320 mM sucrose, 10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, protease inhibitor mixture, and phosphatase inhibitor mixture). Homogenates (H) were centrifuged at 1000 x g to remove nuclei and large debris. The supernatant was then centrifuged at 10,000 x g to obtain a crude synaptosomal fraction, lysed hypoosmotically and centrifuged at 25,000 x g to pellet a synaptosomal membrane fraction (LP1). The supernatant above the crude synaptosomal fraction was centrifuged at 165,000 x g to obtain a cytosolic fraction (S3). Pellets were suspended in solubilization buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, protease inhibitor mixture, and phosphatase inhibitor mixture, 10% SDS). The H fraction corresponds most closely to the whole tissue preparation for RT-PCR. The LP1 membrane fraction includes the synaptic postsynaptic density and so should be enriched in NR1, while the S3 fraction includes cytosol, and so should not contain membrane proteins.
Western blotting
Changes in NR1 protein in homogenates and fractions were measured by Western blotting. Protein content was measured in triplicate using a BCA protein assay kit (Thermo Scientific). Samples were stored at −20°C prior to analysis. For H, LP1 and S3 fractions, 10, 6 or 8 μg of protein were mixed, respectively, with sample buffer x 5 (300 mM Tris-HCl pH 6.8, 50% glycerol, 25% βME, 10% SDS), incubated at 95 °C for 5 minutes, loaded in a 10% SDS-PAGE gel and separated at 190 V for 1 h. Protein samples were electrophoretically transferred onto Whatman nitrocellulose paper (GE Healthcare, Piscataway, NJ) at 350 mA for 1h. Since the target proteins are found at different sizes (NR1 at ~120 kDa and GAPDH at ~38 kDa), the nitrocellulose membrane was cut at approximately 70 kDa so that NR1 and GAPDH could be stained simultaneously without stripping and re-probing. The membranes were blocked with 3% milk in TBS-T (1x TBS buffer from Bio-Rad, with 0.01% Tween-20) for 20 minutes, and then washed in TBS-T for 5 minutes three times. Membranes were incubated at 4 °C overnight with gentle shaking in the appropriate primary antibody diluted in 3% milk in TBS-T: rabbit-anti-NMDAR1 at 1:1000 (Millipore, Billerica, MA) and mouse-anti-GAPDH at 1:10,000 (Millipore). Membranes were washed three times in TBS-T, then incubated for 60 minutes at room temperature with the appropriate secondary antibody diluted in TBS-T: HRP-conjugated goat anti-rabbit IgG at 1:1000 (Cell Signaling, Danvers, MA) or HRP-conjugated horse anti-mouse IgG at 1:10,000 (Cell Signaling). Membranes were washed 3 times for 10 min in TBS-T and soaked in Super Signal West Pico ECL reagent (Thermo Scientific) for 1.5 min. Immunoreactivity was detected using the Bio-Rad ChemiDoc XRS System with Quantity One software. Images were processed using ImageJ (Abramoff et al., 2004) quantifying peak areas to determine band intensity. NR1 intensity was normalized to GAPDH for each sample. GLS1 het values are expressed as a percentage of WT. Genotypic differences were determined using a two-tailed t-test, with significance set at p < 0.05.
Fear conditioning to tone and context
Fear conditioning was performed following standard procedures, as described previously (Gaisler-Salomon et al., 2009b; Saxe et al., 2007) in 20×6×9 cm sound- and light-attenuated chambers (Med Associates, St. Albans, VT). Behavior was recorded under ambient illumination (house light) with a digital video camera mounted above the conditioning chamber, and analyzed with FreezeFrame software (Actimetrics, Evanston, IL), which assesses freezing by measuring changes in pixel intensity between successive video frames. The fear conditioning procedure was conducted over 3 days. On day 1, mice received 3 pairings between a tone conditioned stimulus (CS; 20 sec, 80 dB, 2 KHz) and a co-terminating shock (unconditioned stimulus, US; 1 sec, 0.4–0.7 mA). Tones started at 120, 290, 400 s. Chambers were cleaned with 70% isopropanol before each set of mice and scented with a paper towel dabbed in limonene solution placed beneath the chamber floor. Freezing was scored during the 20 s of each tone presentation. On day 2, the procedure and context were changed in several ways to test conditioned fear to the tone CS in the absence of contextual cues associated with shock; the walls of the chamber were covered with dark green plastic inserts; the chamber was scented with rosemary (adult experiment) or anise (juvenile experiment); between runs, chambers were cleaned with a non-alcohol disinfectant. Each mouse was placed in the chamber for 240 s. The tone was presented twice for 20 s at 120 s and 200 s after the start of the session. No shocks were administered. Percent freezing during the 120 s period before of the first tone (pre-tone freezing) was compared to freezing during the average of the tone periods (tone-period freezing). On day 3, mice were tested for conditioned fear to the training context. The testing procedure and context were identical to those used on day 1, but without the tone CS. Mice were placed in the chamber for 240 s, and the entire session was scored for freezing behavior. A few sessions were lost due to apparatus failure. For Days 1 and 2, a repeated-measures ANOVA was conducted with genotype as the between-subject factor, and tone presentation as the within-subject factor. On Day 3, differences in freezing between genotypes were compared using an independent two-sample t-test. Statistical analyses were performed in SPSS, with significance set at p < 0.05.
Results
Schaeffer collateral-CA1 synaptic response in GLS1 het mice
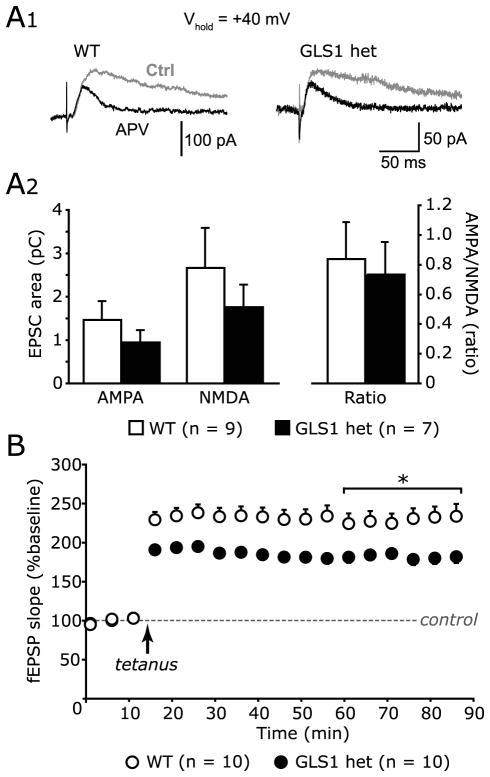
In hippocampal CA1, GLS1 hets show reduced spontaneous excitatory activity and an overall reduction in the input-output curve at higher stimulus intensities (Gaisler-Salomon et al., 2009b), suggesting that the impact of the genetic reduction in GLS1 expression is activity dependent. To explore this further, we recorded evoked synaptic responses of Schaeffer collateral input to CA1 to determine the effect of GLS1 knockdown on baseline synaptic transmission in the HIPP. We confirmed that integrated EPSCs (charge transfer through glutamate receptors) measured during a 100 ms window at −75 mV in WT and GLS1 hets (2.86 ± 0.47 pC (n = 16 cells) and 2.61 ± 0.61 pC (n = 12 cells), respectively) were unaffected (p = 0.74, un-paired t-test).
To examine baseline synaptic strength further, we measured AMPA:NMDA ratios (Rao and Finkbeiner, 2007). We separated AMPA and NMDA responses by application of the NMDA antagonist D-AP5 (100 μM). EPSCs were integrated at +40 mV to determine the maximum NMDA response (Fig 1A1). The integrated AMPA EPSC in WT was 1.47 ± 0.42 pC (n = 9 cells), and in GLS1 hets was 0.94 ± 0.27 pC (n = 7 cells; Fig 1A2 left). The integrated NMDA EPSC (control minus D-AP5) in WT was 2.66 ± 0.91 pC (n = 9 cells) and in GLS1 het was 1.76 ± 0.50 pC (n = 7 cells; Fig 1A2 middle). The AMPA:NMDA ratio (Fig 1A2 right) was 0.84 ± 0.25 in WT (n = 9 cells) and 0.73 ± 0.22 in GLS1 hets (n = 7 cells). There were no significant genotypic differences (integrated AMPA, p = 0.19; NMDA, p = 0.21; AMPA:NMDA ratio, p = 0.74; un-paired t-test). We also measured the AMPA:NMDA ratio using the peak amplitude of EPSCs at −75 mV (mostly AMPA) and at 70 ms post-stimulus at +40 mV (mostly NMDA). The ratios were not significantly different between WT (2.69 ± 0.41, n = 16 cells) and GLS1 hets (2.20 ± 0.30, n = 12 cells; p = 0.59, un-paired t-test).
Fig 1. Evoked synaptic responses in hippocampus slices.
(A) Schaeffer collateral input to CA1 pyramidal neurons recorded with whole cell voltage clamp. AMPA and NMDA responses were measured at +40 mV from 5–105 ms post-stimulus as charge transfer (integrated EPSC). (A1) Sample control traces (gray) and after D-APV 100 μM application (black) are shown from WT (left) and GLS1 het (right) cells. Traces shown are the averages of 10 responses. (A2) The AMPA receptor response (left) was measured from traces after D-APV application. The NMDA receptor response (middle) was calculated by subtracting the response after D-APV from control. The ratio of AMPA to NMDA charge transfer is shown on the right. There were no significant genotypic differences. (B) LTP of Schaeffer collateral-CA1 fEPSP. fEPSP slope data are shown as per cent of baseline (determined as the average of the preceding 15 min). Each point indicates a 5 min average. The dashed line indicates the control baseline (100%). The tetanus was delivered at the arrow. * p < 0.05 between genotypes. There was a significant genotypic reduction in LTP in GLS1 hets.
These observations suggest that the baseline synaptic strength of the Schaeffer collateral inputs to CA1 was not affected by the GLS1 knockdown. To look for more subtle differences in AMPA or NMDA receptor function in GLS1 hets we looked for genotypic differences in the current-voltage relationships for glutamatergic responses by measuring the amplitude of the peak response (AMPA) and at 70 ms from artifact (NMDA) over a range of holding potentials. There was no difference in the current-voltage relationships for AMPA (p = 0.46, F = 0.57, two-way repeated measures ANOVA) or NMDA (p = 0.25, F = 1.41, two-way repeated measures ANOVA; data not shown). Thus, the GLS1 knockdown did not affect the Schaeffer collateral input to CA1 at baseline.
To examine the effect of GLS1 knockdown on activity-dependent synaptic responses, we measured long-term potentiation (LTP) of Schaeffer collateral input to CA1. LTP (1 hour post-tetanus) was significantly reduced in GLS1 hets (178.8 ± 1.3 % of baseline, n = 10 slices; Fig 1B) compared to WT (231.6 ± 1.2 % of baseline, n = 10 slices, p < 0.01; un-paired t-test). Taken together with our previous observation that genotypic differences in synaptic strength of Schaeffer collateral input to CA1 were only evident with more intense stimulation (Gaisler-Salomon et al., 2009a), the present findings indicate further that GLS1 knockdown produces an activity-dependent reduction in synaptic transmission.
Gene Expression Analysis
GLS1 gene expression in WT mice
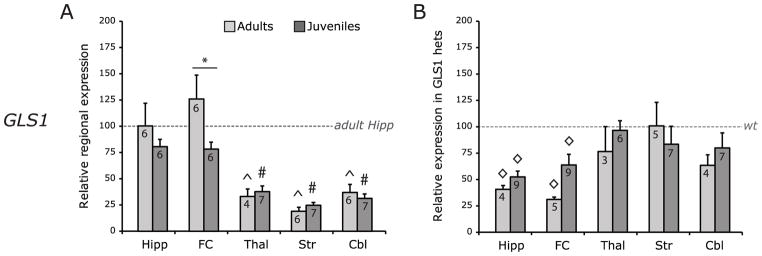
To determine brain regions most likely to mediate the impact of GLS1 reduction, we assessed regional GLS1 gene expression by determining mRNA content in adult and juvenile WT mice by RT-PCR. Samples were obtained from HIPP (adult n = 6, juvenile n = 6), FC (adult n = 6, juvenile n = 6), THAL (adult n = 4, juvenile n = 7), STR (adult n = 6, juvenile n = 7) and CBL (adult n = 6, juvenile n = 7). As can be seen in Fig 2A, GLS1 gene expression in the FC was higher in adult compared to juvenile WT mice, and was highest in HIPP and FC compared to other brain regions in both age groups. This was supported by a mixed-model ANOVA, which revealed a significant interaction of brain x age (F(4,10) = 9.0, p < 0.01,) as well as a main effect of brain (F(4,10)=99.9, p < 0.001). Pairwise comparisons for brain x age using Bonferroni adjustment showed a higher expression of GLS1 in the FC of adult compared to juvenile WT mice (p < 0.05), with no effect in other brain regions (all p values > 0.1). Pairwise comparisons with Bonferroni adjustment for age x brain comparisons showed that in both juveniles and adults, FC GLS1 gene expression did not differ from HIPP (p > 0.5), but was significantly higher in FC and HIPP than THAL (p < 0.05) and STR (p < 0.005), and CBL (p < 0.05). No differences between expression in the THAL, STR and CBL were found (all p values > 0.5).
Fig 2. GLS1 expression.
(A) WT GLS1 expression is reported as fold change relative to adult HIPP levels. Highest expression levels were observed in hippocampus (Hipp) and frontal cortex (FC). Differences between adults and juveniles were observed in FC. (B) GLS1 expression in GLS1 het samples is reported as fold change relative to matched WT samples. GLS1 expression was down regulated in the Hipp and FC of both juveniles and adults by 40–60%. n’s shown are for GLS1 hets; WT n’s are in panel A. Data represent fold change (2−ΔΔCt) ± s.e.m. compared to control, following GAPDH normalization. * p < 0.05 between age groups. ^ p < 0.05 relative to adult HIPP. # p < 0.05 relative to juvenile HIPP. ◇ t-test, p < 0.05 relative to WT values for the same region.
Genotypic differences in GLS1 gene expression
We then examined genotypic differences in GLS1 gene expression in adult and juvenile GLS1 hets (see previous section for WT n’s). Samples were obtained from HIPP (n = 4, juvenile n = 9), FC (adult n = 5, juvenile n = 9), THAL (adult n = 3, juvenile n = 6), STR (adult n = 5, juvenile n = 7), and CBL (adult n = 4, juvenile n = 7). As can be seen in Fig 2B, GLS1 gene expression was reduced in the HIPP of adult and juvenile GLS1 hets and the FC of adult GLS1 hets compared to their age-matched WT littermates (set at 100%). This was supported by a mixed-model ANOVA that revealed a main effect of genotype (F(1,22) = 16.0, p < 0.01), brain region (F(4,19) = 71.6, p < 0.001,) and a brain region x age x genotype interaction (F(13,21) = 4.4, p < 0.01). Pairwise comparisons with Bonferroni adjustment revealed significant differences between GLS1 hets and WT mice in the HIPP and FC of adults and juveniles, (all p values < 0.05). THAL, STR and CBL did not show any differences in GLS1 gene expression in adults and juveniles (all p values > 0.05)
NR1 gene expression in WT mice
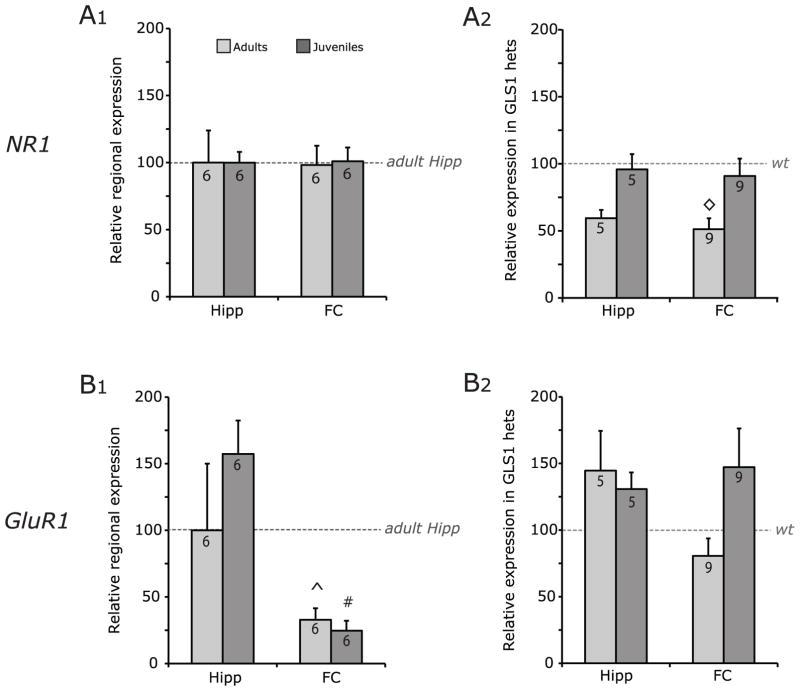
To address the potential synaptic impact of the GLS1 reduction, we examined the expression of key glutamate receptor subunits in WT mice in the regions that showed genotypic differences in GLS1 expression, namely the hippocampus and frontal cortex. As shown in Fig 3A1, there were no differences in NR1 gene expression between adults and juveniles in hippocampus (adult n = 6, juvenile n = 6) or frontal cortex (adult n = 6, juvenile n = 6). This was supported by a mixed-model ANOVA, which revealed no significant differences between adult and juvenile WT mice in hippocampus or frontal cortex (all F values < 0.1). Regional differences were not seen in either age group in pairwise comparisons of age x brain or brain x age (all p values > 0.1).
Fig 3. NR1 and GluR1 gene expression.
(A) NR1 expression. (A1) WT NR1 expression is reported as fold change relative to adult Hipp. No differences were found between Hipp and FC in adults or juveniles. (A2) NR1 expression in GLS1 het samples are reported as fold change relative to matched WT samples. NR1 expression was down regulated in Hipp (at trend level) and FC of adults, but not juveniles. (B) GluR1 expression. (B1) WT GluR1 expression is reported as fold change relative to adult Hipp levels. GluR1 expression was significantly lower in the FC than in the Hipp of both adult and juvenile samples. (B2), GluR1 expression levels in GLS1 hets are reported as fold change relative to matched WT mice. No genotypic differences were found. See Fig 2 legend for definition of significance symbols.
Genotypic differences in NR1 gene expression
We then examined NR1 gene expression in the hippocampus (adult n = 4, juvenile n = 9) and frontal cortex (adult n = 5, juvenile n = 9) of GLS1 het mice. As can be seen in Fig 3A2, NR1 gene expression trended towards a reduction in the hippocampus and was reduced in the frontal cortex of adults. No differences were detected in the hippocampus or frontal cortex of juveniles compared to their age-matched WT littermates (set at 100%). This was supported by a mixed-model ANOVA, which revealed a main effect of genotype (F(1,22) = 5.01, p < 0.05). Pairwise tests revealed genotypic differences between GLS1 hets and their WT controls trending towards significance in the hippocampus (p = 0.08) and significantly different in the frontal cortex of adult mice (p < 0.01), but no significant differences in either region in juvenile mice (p > 0.5).
GluR1 gene expression in WT mice
We examined GluR1 gene expression in hippocampus (adult n = 6, juvenile n = 6) and frontal cortex (adult n = 6, juvenile n = 6) of WT mice. As shown in Fig 3B1, GluR1 gene expression was higher in the hippocampus and there were no differences in GluR1 gene expression between adults and juveniles. A mixed-model ANOVA revealed a main effect of brain region (F(1,10) = 27.0, p < 0.001). Pairwise comparisons for age x brain showed that the expression of GluR1 in hippocampus was higher in frontal cortex in both adults (p < 0.05) and juveniles (p < 0.01). In pairwise comparisons of brain x age, there were no significant differences found between adults and juveniles (all p values > 0.1).
Genotypic differences in GluR1 gene expression
We then assessed GluR1 gene expression in hippocampus (adult n = 4, juvenile n = 9) and frontal cortex (adult n = 5, juvenile n = 9) of GLS1 het mice. As shown in Fig 3B2, GluR1 gene expression was not significantly altered in either brain region in GLS1 hets compared to their age-matched WT littermates (set at 100%). A mixed-model ANOVA revealed a main effect of brain region (F(1,22) = 96.9, p < 0.001). No other main effects or interactions were observed (all p values > 0.1). Pairwise comparisons revealed no significant differences between GLS1 hets and WT mice in the hippocampus and frontal cortex of adults and juveniles (all p values > 0.05).
Fraction analysis of genotypic differences in NR1 protein
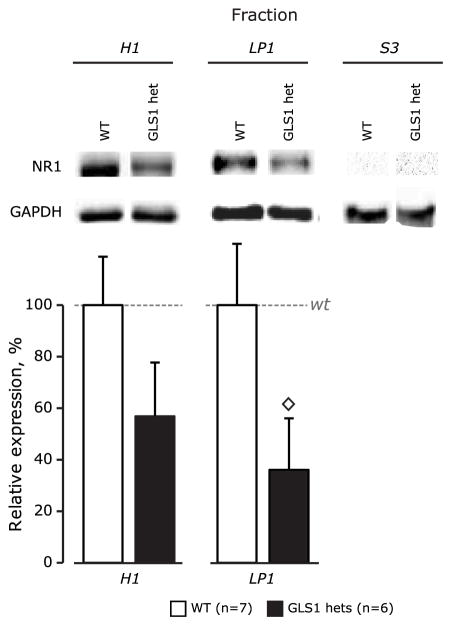
To determine whether diminution in NR1 gene expression would affect synaptic function and behavior in the hippocampus, we performed a Western analysis. In hippocampal homogenates (Fig 4), there was a trend to reduced NMDA receptor protein levels in GLS1 hets (p = 0.081). Fractionating the tissue revealed that there was a significant reduction in NMDA receptor levels in the light protein LP1 fraction (p = 0.036), which is enriched in postsynaptic density elements. The soluble S3 fraction, enriched in cytoplasmic elements, showed no detectable NR1 protein in either genotype.
Fig 4. NR1 protein levels.
NR1 protein was reduced in the Hipp of adult GLS1 het mice. Representative examples of NR1 (~120 kDa) and GAPDH at (~38 kDa) bands for total protein samples (H), synaptosomal membrane fractions (LP1) and cytosolic fractions (S3) were resolved by SDS-PAGE (shown above). The histograms depict relative mean levels of NR1 in WT and GLS1 het mice. Data were combined from two independent experiments. Significant genotypic differences were found in LP1. ◇ t-test, p < 0.05.
Fear conditioning to tone and context
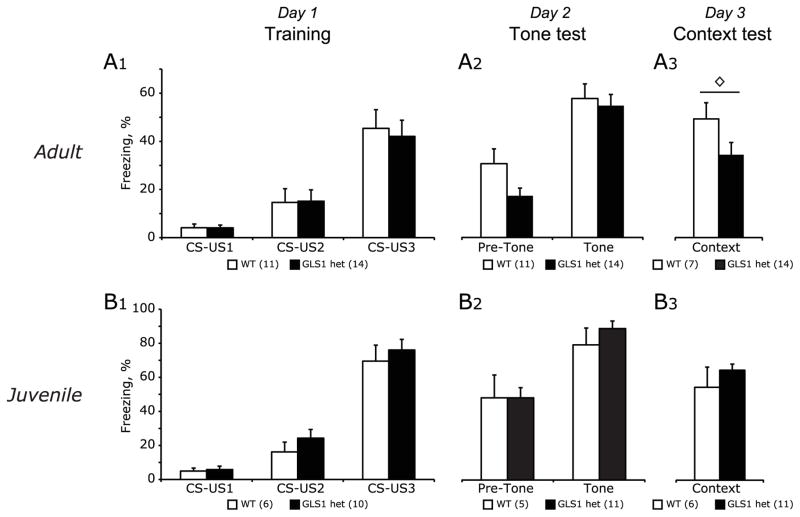
To see whether the adult-onset decrease in NR1 in hippocampus showed a relevant behavioral correlate, we measured cued and contextual fear conditioning in adult and juvenile GLS1 hets. Our previous study revealed a significant deficit in contextual learning in adult GLS1 hets (Gaisler-Salomon et al., 2009a). We confirmed this deficit in contextual fear conditioning in adult GLS1 hets, following the same paradigm. Data collected on Day 1 (Training) were analyzed by repeated-measures ANOVA with genotype as the between-subject factor and CS-US presentation as the within-subject factor. Both GLS1 hets (n = 14) and their age-matched WT littermates (n = 11) showed a significant increase in freezing with repeated tones (F(2,37) = 34, p < 0.001(Fig 5A1). On Day 2 (Tone test), results were analyzed by repeated measures ANOVA with genotype as the between-subject factor, and tone period as the within-subject factor. Both GLS1 hets and WT mice showed a significant increase in freezing during the tone period compared to the pre-tone period (F(1,23) = 46, p < 0.001; Fig 5A2). On Day 3 (Context test), results were analyzed using an independent t-test between genotypes. GLS1 hets (n = 14) froze significantly less than WT mice (n = 7; p < 0.05; Fig 5A3).
Fig 5. Fear conditioning in adult and juvenile mice.
(A) Fear conditioning in adult WT and GLS1 het mice. (A1) On Day 1 (Training), all animals showed increasing levels of freezing during tone presentation, indicating the acquisition of a fear response. (A2), On Day 2 (Tone Test), all animals showed enhanced freezing during the tone period compared to the pre-tone period. (A3) On Day 3 (Context Test), GLS1 hets showed significantly reduced contextual learning. (B) Fear conditioning in juvenile WT and GLS1 het mice. (B1) On Day 1, animals showed increasing levels of freezing during tone presentation, indicating the acquisition of a fear response. (B2) On Day 2, both genotypes showed increased freezing when presented with the tone in a different context. (B3) On day 3, no genotypic differences in contextual learning were seen. ◇ t-test, p < 0.05.
We then measured fear conditioning in juvenile WT (n=11) and GLS1 het (n=6) mice. Only in this experiment were both sexes used; ANOVAs testing genotype x sex x freezing response were done for each day and no sex differences were found (all p values > 0.05), so male and female data were combined. On Day 1 (Training), data from one WT mouse was excluded since its threshold for freezing was over two standard deviations from the mean. Both GLS1 hets (n = 10) and their age-matched WT littermates (n = 6) exhibited conditioned fear (main effect of CS-US pairing, F(1.7, 24) = 140.3, p < 0.0001; Fig 5B1). As Mauchly’s assumption of sphericity was violated, the Huyhn-Feldt correction was used. No other main effects or interactions were observed. On Day 2 (tone test), data from one GLS1 het mouse was excluded. Both GLS1 het (n = 11) and WT mice (n = 5) froze more during tone periods (main effect of tone period; F(1,14) = 25.3, p < 0.001; Fig 5B2). No other effects were observed (all F values < 1). On Day 3 (context test), percent freezing during the entire testing period was scored. Data were analyzed using an independent t-test between genotypes. No genotypic differences were observed in juveniles (GLS1 het n = 11; WT n = 6; p > 0.1; Fig 5B3).
Discussion
Our previous brain imaging data showed that mice globally haplodeficient in GLS1 have a selective reduction in CBV in the hippocampus, mainly in CA1, indicative of hypometabolism (Gaisler-Salomon et al., 2009a) that was the inverse of CBV imaging of patients with schizophrenia (Schobel et al., 2009); spontaneous activity in CA1 was diminished and synaptic strength measured at strong stimulus intensities was attenuated. Our present data extend the premise that GLS1 haplodeficiency results in an activity-dependent synaptic phenotype. We have found that baseline synaptic strength was unaffected in GLS1 hets, while LTP was attenuated. The hippocampus-specific impact was due in part to higher GLS1 gene expression in hippocampus compared to other regions in WT mice, although GLS1 is also highly expressed in frontal cortex. In GLS1 hets, GLS1 gene expression was similarly reduced in both hippocampus and frontal cortex. Analysis of glutamate receptor gene expression revealed that NR1 was selectively reduced in both regions. While GLS1 was reduced constitutively, the reduction in NR1 gene expression appeared only in adulthood. The reduction in NR1 was seen at the protein level, due mainly to reductions in the membrane fraction. Hippocampus-dependent contextual learning also showed a deficit that appeared only in adulthood. Although the link between NR1 and behavior is correlational, these observations suggest that global GLS1 haplodeficiency has its functional impact in adulthood, producing a subtle activity-dependent reduction in excitatory synaptic transmission in the hippocampus.
GLS1 hets show decreased CA1 LTP
We found no genotypic differences in evoked field potentials or in baseline strength of Schaffer collateral inputs to CA1, as measured by AMPA:NMDA ratios. AMPA receptors are typically inserted at postsynaptic sites marked by NMDA receptors when synapses undergo developmental or plastic changes (Shepherd and Huganir, 2007), so the ratio provides a normalized measure of synaptic strength. In contrast to these measures showing that baseline synaptic strength or properties were not affected, the crucial activity-dependent modification of these connections, namely LTP, was attenuated. Together with our previous data (Gaisler-Salomon et al., 2009a; Masson et al., 2006), the present data argue further that glutaminase function is involved in maintaining more intense synaptic activity, consistent with its role in replenishing glutamate via recycling through the glutamate-glutamine cycle.
In line with this, Kam and Nicoll (2007) showed that provision of extracellular glutamine — the substrate of glutaminase — has its most dramatic effects in enhancing excitatory transmission following strong K+ depolarization, and Tani et al. (2010) showed that epileptiform activity requires ongoing provision of glutamine. Similar to GLS1 hets, mice with haplodeficiency of vesicular glutamate transporter 1 (VGLUT1), a presynaptic molecule responsible for packaging glutamate into synaptic vesicles, show normal baseline activity but a lower fEPSP slope in the input-output curve and decreased LTP in CA1 (Balschun et al., 2009). Thus, attenuating glutamate production, packaging or release yields an activity-dependent synaptic phenotype.
Although baseline NMDA currents were normal in GLS1 het hippocampal neurons, we found that expression of NR1, an obligatory subunit of the NMDA receptor, was reduced. The involvement of NMDA receptors in CA1 LTP is well documented, and a decrease in NR1 gene expression has been associated with a decrease in LTP (Guilarte, 2003; Nihei et al., 2000). Thus, either reduced glutamate release during tetanic stimulation or reduced numbers of functional NMDA receptors could explain the reduction in LTP seen in the CA1 of GLS1 hets; however, the normal baseline NMDA currents and normal AMPA:NMDA ratios argue for a presynaptic effect. While CA1 is most implicated in our rCBV data, other hippocampal regions are also affected, and so it is possible that NMDA receptor levels are normal at the Schaffer collateral inputs to CA1, but altered at other synaptic connections.
This inference is supported by glutaminase in situ hybridization data in the Allen Brain Atlas (Lein et al., 2007) (http://mouse.brain-map.org/brain/gene/501/ExpressionGraph.html) showing that GLS1 is higher in CA3 than in CA1. Following the logic that the GLS1 deficiency will impact most where expression is highest, then CA3 neurons should be the most affected, possibly reducing their NR1 expression and their synaptic efficacy in their projections to CA1. A greater reduction in GLS1 expression in CA3 could explain the overall reduction in hippocampal NR1 expression. A comparatively lower reduction in GLS1 expression in CA1 would impact less on NR1 expression, accounting for normal NR1 expression in CA1, while at the same time accounting for the deficit in LTP due to reduced tetanic glutamate release.
Hippocampal and frontal cortex specificity of GLS1 gene expression
Our present results show that in both adult and juvenile WT mice, GLS1 gene expression in the hippocampus and frontal cortex is at least twice that in thalamus, striatum or cerebellum. This is consistent with data from the Allen Brain Atlas (Lein et al., 2007) (http://mouse.brain-map.org/brain/gene/501/ExpressionGraph.html) and GENSAT (Gong et al., 2003) (http://gensat.org/imagenavigator.jsp?imageID=53247#annotations), and is similar in rats (Najlerahim et al., 1990). Consistent with this, GLS1 haplodeficiency is associated with the most significant reductions in glutamate levels in the hippocampus and frontal cortex (Gaisler-Salomon et al., 2009a). The Allen Brain Atlas expression data show further that hippocampal expression is several-fold higher in hippocampus than cortex, indicating that a global reduction in GLS1 expression would have its greatest impact on glutamate-glutamine recycling in this region.
Glutamate-glutamine recycling is responsible for approximately 70% of neurotransmitter glutamate production (Hertz and Zielke, 2004; Sibson et al., 2001), so a deficit in GLS1 would be expected to affect synaptic function in the hippocampus and frontal cortex, and consequently hippocampal and frontal cortex-dependent physiology and behavior. Although our present findings show comparable NR1 expression in the hippocampus and frontal cortex, previous studies have shown that NR1 protein levels are higher in hippocampus (Petralia et al., 1994), making the hippocampus more vulnerable to activity-dependent measures of physiology and behavior. Furthermore, in a preliminary microarray expression study of the HIPP in GLS1 hets (Gaisler-Salomon et al., 2008), we found that while GLS1 expression was reduced (as expected), there were no compensatory changes in genes encoding glutamate-glutamine pathway proteins or other molecules involved in de novo glutamate synthesis. Taken together, these observations may provide an explanation for our previous findings (Gaisler-Salomon et al., 2009a) that clearly singled out the hippocampus as the brain region most functionally affected by GLS1 haplodeficiency.
We found that GLS1 expression in the frontal cortex increased with maturation. GLS1 expression in juvenile WT mice was approximately 60% of adult levels. In pups at postnatal day 4, GLS1 expression in frontal cortex was about 30% of adult levels (unpublished observations). In hippocampus GLS1 expression did not increase significantly with maturation (GLS1 expression in juvenile WT mice was approximately 80% of adult levels). In pups at postnatal day 4, GLS1 expression in hippocampus was about 30% of adult levels (unpublished observations). Our unpublished regional data for frontal cortex and hippocampus were consistent with our previous findings for whole brain (Masson et al., 2006). Low levels of GLS1 expression in pups are consistent with the postnatal onset of excitatory synaptic transmission in the rodent cortex in the first postnatal week (Frick et al., 2007).
Adult-onset NR1 reduction in GLS1 hets implies age-dependent regulatory mechanisms
NR1 is an essential subunit of NMDA receptors, and in human frontal cortex its expression reaches adult levels in adolescence (Henson et al., 2008) consistent with the present observations in WT Mice. In contrast, NR1 gene expression was about half of WT levels in the hippocampus and frontal cortex of GLS1 hets. A decrease in synaptic NMDA receptor levels, as confirmed in our Western analysis of hippocampal membrane fractions, may seem paradoxical in glutamate-deficient GLS1 hets, where receptor levels would be expected to increase with decreased neurotransmitter levels so as to maintain stable neural transmission. Contrary to this logic, manipulations that increase neuronal glutamate release chronically (Wang et al., 2010) or acutely (Kerdsan et al., 2009) up-regulate NMDA subunit receptor expression. Presynaptic NMDA receptors regulate glutamate release (Bardoni et al., 2004; McGuinness et al., 2010) and inhibition of presynaptic NMDA receptors can facilitate glutamate release (Yang et al., 2006). Decreased NR1 expression in GLS1 hets could thus reflect a change in the response of postsynaptic neurons to chronic down-regulation of glutamate release, or it could be a chronic response to a long-term decrease in activity-dependent glutamate release.
We found in GLS1 het mice that while GLS1 gene expression was decreased throughout life, the decrease in NR1 mRNA levels appeared only in adulthood, while no change in GluR1 expression was observed. Adolescence in mammals is characterized by significant changes in neuronal architecture and function; these changes are associated with dramatic changes in NMDA receptor numbers and subunit composition (Carpenter-Hyland and Chandler, 2007; Gore et al., 1996; Parent et al., 2008; Sisk and Foster, 2004). Thus, another plausible explanation for our observations is that less glutamate release is required to maintain NMDAR receptor gene expression during maturation than in adulthood. A lack of effect on GluR1 expression in either juvenile or adult GLS1 hets implies that GLS1 deficiency or adult NR1 deficiency does not impact GluR1. NR1 deficient mice also show no deficits in AMPA ligand binding (Duncan et al., 2002). In GLS1 hets, other AMPA receptor subunits may be affected. Alternatively, higher spatial resolution may be required to detect changes in AMPA receptor changes in specific neuronal subtypes (Abe et al., 2011). It is also possible that changes in glutamate release impact metabotropic glutamate receptors.
Adult-onset deficit in contextual learning in GLS1 hets
We found previously that adult GLS1 het mice display a deficit in contextual learning (Gaisler-Salomon et al., 2009a), which we have replicated. Contextual learning is a hippocampus-dependent task (Maren and Holt, 2000) that depends on NMDA receptor function (Harre et al., 2008; McHugh and Tonegawa, 2009), and has been associated with an increase in neuronal glutamate uptake in CA1 (Levenson et al., 2002). Reducing NMDA receptor expression interferes with learning contextually conditioned fear (Gao et al., 2010). Our present results support this confluence of mechanisms in showing a regional and temporal correlation between reduction in NR1 gene expression and reduced contextual learning.
Implications for the pharmacotherapy of schizophrenia
A crucial issue for any constitutive genetic knockdown is whether the adult phenotype arises during development or is due directly to the deficit in adulthood. GLS1 hets are deficient in glutaminase throughout life; however, mild reductions in NMDA receptor function do not arise until late in maturation, arguing that GLS1 haplodeficiency in adulthood is more important. While NMDA receptor hypomorphs engendered by NR1 deletion in early life (Belforte et al., 2010) or throughout development (Mohn et al., 1999) manifest a schizophreniform phenotype, GLS1 hets manifest a schizophrenia resilient phenotype despite their reduction in NMDA receptor function. Considering the constellation of behavioral and neurochemical phenotypes of GLS1 hets, it is likely that their resilient phenotype is due to the activity-dependent limit in synaptic glutamate release as a result of diminished glutamate-glutamine recycling, even in the face of reduced NMDA receptor function. Indeed, this argues further for the observation that schizophreniform symptoms arise directly from excessive glutamate release and indirectly from NMDA receptor hypofunction (Moghaddam, 2003). In GLS1 het mice with tempered activity-dependent glutamate release, the reduction in NMDA receptors is not sufficient to engender schizophreniform symptoms. We hypothesize that age-dependent NR1 reductions in GLS1 hets are responsible for their mild maturation-dependent fear conditioning deficits, point to the late maturational impact of GLS1 haplodeficiency, but do not account for the schizophrenia-resilient phenotype of the mice.
The implication is that a temporally limited genetic reduction in GLS1 expression in adulthood — or more practically GLS1 inhibition — would be therapeutic in schizophrenia, motivating the pursuit of GLS1 inhibiters for the pharmacotherapy of schizophrenia.
Acknowledgments
We thank Scott McKinney for conducting preliminary experiments and rtPCR software analysis development, Helene Bach-Mizrachi for advice on Westerns, Tali Rosenberg and Kobi Rosenblum for their help with protein fractions, and Celia Gellman and Susana Mingote for their critical reading of the manuscript.
Grant Support:
NIH T32 DA016224 and Israel Science Foundation Young Investigator grant 484/10 (IGS); NIH R01 NS049442 (OA); NIH K02 DA000356, R01 DA017978 and R01 MH087758 (SR).
References
- Abe Y, Namba H, Kato T, Iwakura Y, Nawa H. Neuregulin-1 signals from the periphery regulate AMPA receptor sensitivity and expression in GABAergic interneurons in developing neocortex. J Neurosci. 2011;31(15):5699–709. doi: 10.1523/JNEUROSCI.3477-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff M, Magalhaes P, Ram S. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57(3):270–6. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Balschun D, Moechars D, Callaerts-Vegh Z, Vermaercke B, Van Acker N, Andries L, D’Hooge R. Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cereb Cortex. 2009;20(3):684–693. doi: 10.1093/cercor/bhp133. [DOI] [PubMed] [Google Scholar]
- Barak S, Weiner I. The M1/M4 preferring agonist xanomeline reverses amphetamine-, MK801- and scopolamine-induced abnormalities of latent inhibition: putative efficacy against positive, negative and cognitive symptoms in schizophrenia. Int J Neuropsychopharmacol. 2011;14(9):1233–46. doi: 10.1017/S1461145710001549. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J Neurosci. 2004;24(11):2774–81. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, McCullumsmith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Increased expression of glutaminase and glutamine synthetase mRNA in the thalamus in schizophrenia. Schizophr Res. 2005;75(1):27–34. doi: 10.1016/j.schres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav. 2007;86(2):200–8. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–59. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65(2):154–64. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Duncan G, Miyamoto S, Gu H, Lieberman J, Koller B, Snouwaert J. Alterations in regional brain metabolism in genetic and pharmacological models of reduced NMDA receptor function. Brain Res. 2002;951(2):166–76. doi: 10.1016/s0006-8993(02)03156-6. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Inada K, Farrington JS, Koller BH, Moy SS. Neural activation deficits in a mouse genetic model of NMDA receptor hypofunction in tests of social aggression and swim stress. Brain Res. 2009;1265:186–95. doi: 10.1016/j.brainres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Feldmeyer D, Sakmann B. Postnatal development of synaptic transmission in local networks of L5A pyramidal neurons in rat somatosensory cortex. J Physiol. 2007;585(Pt 1):103–16. doi: 10.1113/jphysiol.2007.141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Miller GM, Chuhma N, Lee S, Zhang H, Ghoddoussi F, Lewandowski N, Fairhurst S, Wang Y, Conjard-Duplany A, et al. Glutaminase-deficient mice display hippocampal hypoactivity, insensitivity to pro-psychotic drugs and potentiated latent inhibition: relevance to schizophrenia. Neuropsychopharmacology. 2009a;34(10):2305–2322. doi: 10.1038/npp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Schobel SA, Small SA, Rayport S. How high-resolution basal-state functional imaging can guide the development of new pharmacotherapies for schizophrenia. Schizophr Bull. 2009b;35(6):1037–1044. doi: 10.1093/schbul/sbp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Wang Y, McKinney SM, Ramsey AJ, Sibille EL, Rayport S. Differential expression of AMPA and NMDA receptors in the hippocampus of glutaminase-deficient mice. Society for Neuroscience Abstracts. 2008:254.2. [Google Scholar]
- Gao C, Gill MB, Tronson NC, Guedea AL, Guzman YF, Huh KH, Corcoran KA, Swanson GT, Radulovic J. Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus. 2010;20(9):1072–82. doi: 10.1002/hipo.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar PA, Bustamante ML, Silva H, Aboitiz F. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem. 2009;111(4):891–900. doi: 10.1111/j.1471-4159.2009.06325.x. [DOI] [PubMed] [Google Scholar]
- Gluck MR, Thomas RG, Davis KL, Haroutunian V. Implications for altered glutamate and GABA metabolism in the dorsolateral prefrontal cortex of aged schizophrenic patients. Am J Psychiatry. 2002;159(7):1165–73. doi: 10.1176/appi.ajp.159.7.1165. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–25. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gore AC, Wu TJ, Rosenberg JJ, Roberts JL. Gonadotropin-releasing hormone and NMDA receptor gene expression and colocalization change during puberty in female rats. J Neurosci. 1996;16(17):5281–9. doi: 10.1523/JNEUROSCI.16-17-05281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16(6):2034–43. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte T. Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Mol Brain Res. 2003;113(1–2):37–43. doi: 10.1016/s0169-328x(03)00083-4. [DOI] [PubMed] [Google Scholar]
- Halene TB, Ehrlichman RS, Liang Y, Christian EP, Jonak GJ, Gur TL, Blendy JA, Dow HC, Brodkin ES, Schneider F, et al. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav. 2009;8(7):661–75. doi: 10.1111/j.1601-183X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ. Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain. Eur J Neurosci. 2008;27(3):644–53. doi: 10.1111/j.1460-9568.2008.06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Salimi K, Vadlamudi S, Hamer RM, Gilmore JH, Jarskog LF, Philpot BD. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb Cortex. 2008;18(11):2560–73. doi: 10.1093/cercor/bhn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27(12):735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Huwyler J, Kemp JA, Gill R. Evaluation of the NR2B-selective NMDA receptor antagonist Ro 63-1908 on rodent behaviour: evidence for an involvement of NR2B NMDA receptors in response inhibition. Neuropharmacology. 2003;44(3):324–41. doi: 10.1016/s0028-3908(02)00402-1. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol. 2005;93(4):1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods for Psychology. Belmont, CA: Cengage Wadsworth; 2010. p. 768. [Google Scholar]
- Inta D, Monyer H, Sprengel R, Meyer-Lindenberg A, Gass P. Mice with genetically altered glutamate receptors as models of schizophrenia: a comprehensive review. Neurosci Biobehav Rev. 2010;34(3):285–94. doi: 10.1016/j.neubiorev.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci. 2007;27(34):9192–200. doi: 10.1523/JNEUROSCI.1198-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdsan W, Thanoi S, Nudmamud-Thanoi S. Changes in glutamate/NMDA receptor subunit 1 expression in rat brain after acute and subacute exposure to methamphetamine. J Biomed Biotechnol. 2009;2009:329631. doi: 10.1155/2009/329631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvamme E, Torgner IA, Roberg BA. Kinetics and localization of brain phosphate activated glutaminase. J Neurosci Res. 2001;66(5):951–8. doi: 10.1002/jnr.10041. [DOI] [PubMed] [Google Scholar]
- Lang UE, Puls I, Muller DJ, Strutz-Seebohm N, Gallinat J. Molecular mechanisms of schizophrenia. Cell Physiol Biochem. 2007;20(6):687–702. doi: 10.1159/000110430. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5(2):155–61. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63(10):1372–6. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68(1):17–24. doi: 10.1016/j.biopsych.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: Wiley; 2002. p. 381. [Google Scholar]
- Maeshima H, Ohnuma T, Sakai Y, Shibata N, Baba H, Ihara H, Higashi M, Ohkubo T, Nozawa E, Abe S, et al. Increased plasma glutamate by antipsychotic medication and its relationship to glutaminase 1 and 2 genotypes in schizophrenia -- Juntendo University Schizophrenia Projects (JUSP) Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1410–8. doi: 10.1016/j.pnpbp.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110(1–2):97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Masson J, Darmon M, Conjard A, Chuhma N, Ropert N, Thoby-Brisson M, Foutz A, Parrot S, Miller GM, Jorisch R, et al. Mice lacking brain/kidney phosphate-activated glutaminase (GLS1) have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26(17):4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness L, Taylor C, Taylor RD, Yau C, Langenhan T, Hart ML, Christian H, Tynan PW, Donnelly P, Emptage NJ. Presynaptic NMDARs in the hippocampus facilitate transmitter release at theta frequency. Neuron. 2010;68(6):1109–27. doi: 10.1016/j.neuron.2010.11.023. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Tonegawa S. CA3 NMDA receptors are required for the rapid formation of a salient contextual representation. Hippocampus. 2009;19(12):1153–8. doi: 10.1002/hipo.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40(5):881–4. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349–52. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98(4):427–36. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Najlerahim A, Harrison PJ, Barton AJ, Heffernan J, Pearson RC. Distribution of messenger RNAs encoding the enzymes glutaminase, aspartate aminotransferase and glutamic acid decarboxylase in rat brain. Mol Brain Res. 1990;7(4):317–33. doi: 10.1016/0169-328x(90)90082-o. [DOI] [PubMed] [Google Scholar]
- Nihei MK, Desmond NL, McGlothan JL, Kuhlmann AC, Guilarte TR. N-methyl-D-aspartate receptor subunit changes are associated with lead-induced deficits of long-term potentiation and spatial learning. Neuroscience. 2000;99(2):233–42. doi: 10.1016/s0306-4522(00)00192-5. [DOI] [PubMed] [Google Scholar]
- Parent AS, Rasier G, Matagne V, Lomniczi A, Lebrethon MC, Gerard A, Ojeda SR, Bourguignon JP. Oxytocin facilitates female sexual maturation through a glia-to-neuron signaling pathway. Endocrinology. 2008;149(3):1358–65. doi: 10.1210/en.2007-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci. 1994;14(10):6102–20. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26(6):509–15. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30(6):284–91. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A. 2007;104(11):4642–6. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilström B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, et al. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006;26(33):8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadach E, Gaisler I, Schiller D, Weiner I. The latent inhibition model dissociates between clozapine, haloperidol, and ritanserin. Neuropsychopharmacology. 2000;23(2):151–61. doi: 10.1016/S0893-133X(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The Cell Biology of Synaptic Plasticity: AMPA Receptor Trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Sibille E, Su J, Leman S, Le Guisquet AM, Ibarguen-Vargas Y, Joeyen-Waldorf J, Glorioso C, Tseng GC, Pezzone M, Hen R, et al. Lack of serotonin1B receptor expression leads to age-related motor dysfunction, early onset of brain molecular aging and reduced longevity. Mol Psychiatry. 2007;12(11):1042–56. 975. doi: 10.1038/sj.mp.4001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG. In vivo 13C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during 2–13C glucose infusion. J Neurochem. 2001;76(4):975–89. doi: 10.1046/j.1471-4159.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Tani H, Dulla CG, Huguenard JR, Reimer RJ. Glutamine is required for persistent epileptiform activity in the disinhibited neocortical brain slice. J Neurosci. 2010;30(4):1288–300. doi: 10.1523/JNEUROSCI.0106-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bao X, Pal R, Agbas A, Michaelis EK. Transcriptomic responses in mouse brain exposed to chronic excess of the neurotransmitter glutamate. BMC Genomics. 2010;11:360. doi: 10.1186/1471-2164-11-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Woodhall GL, Jones RS. Tonic facilitation of glutamate release by presynaptic NR2B-containing NMDA receptors is increased in the entorhinal cortex of chronically epileptic rats. J Neurosci. 2006;26(2):406–10. doi: 10.1523/JNEUROSCI.4413-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Ninan I, Zhang H, Milner TA, Arancio O, Chao MV. BDNF-mediated neurotransmission relies upon a myosin VI motor complex. Nat Neurosci. 2006;9(8):1009–1018. doi: 10.1038/nn1730. [DOI] [PubMed] [Google Scholar]