Abstract
Psilocin (4-hydroxy-N,N-dimethyltryptamine) is a hallucinogen that acts as an agonist at 5-HT1A, 5-HT2A, and 5-HT2C receptors. Psilocin is the active metabolite of psilocybin, a hallucinogen that is currently being investigated clinically as a potential therapeutic agent. In the present investigation, we used a combination of genetic and pharmacological approaches to identify the serotonin (5-HT) receptor subtypes responsible for mediating the effects of psilocin on head twitch response (HTR) and the behavioral pattern monitor (BPM) in C57BL/6J mice. We also compared the effects of psilocin with those of the putative 5-HT2C receptor-selective agonist 1-methylpsilocin and the hallucinogen and non-selective serotonin receptor agonist 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT). Psilocin, 1-methylpsilocin, and 5-MeO-DMT induced the HTR, effects that were absent in mice lacking the 5-HT2A receptor gene. When tested in the BPM, psilocin decreased locomotor activity, holepoking, and time spent in the center of the chamber, effects that were blocked by the selective 5-HT1A antagonist WAY-100635 but were not altered by the selective 5-HT2C antagonist SB 242,084 or by 5-HT2A receptor gene deletion. 5-MeO-DMT produced similar effects when tested in the BPM, and the action of 5-MeO-DMT was significantly attenuated by WAY-100635. Psilocin and 5-MeO-DMT also decreased the linearity of locomotor paths, effects that were mediated by 5-HT2C and 5-HT1A receptors, respectively. In contrast to psilocin and 5-MeO-DMT, 1-methylpsilocin (0.6–9.6 mg/kg) was completely inactive in the BPM. These findings confirm that psilocin acts as an agonist at 5-HT1A, 5-HT2A, and 5-HT2C receptors in mice, whereas the behavioral effects of 1-methylpsilocin indicate that this compound is acting at 5-HT2A sites but is inactive at the 5-HT1A receptor. The fact that 1-methylpsilocin displays greater pharmacological selectivity than psilocin indicates that 1-methylpsilocin represents a potentially useful alternative to psilocybin for development as a potential therapeutic agent.
Keywords: Hallucinogen, head twitch response, knockout, locomotor activity, mice, obsessive–compulsive disorder, psilocin, psilocybin, serotonin
Introduction
Hallucinogenic drugs produce profound changes in thought and mood, depersonalization, and marked perceptual disturbances. Structurally, these drugs belong to two chemical classes: indoleamines and phenylalkylamines. Indoleamines, such as lysergic acid diethylamide (LSD), psilocin, N,N-dimethyltryptamine (DMT), and 5-methoxy-DMT (5-MeO-DMT) are non-selective serotonin (5-HT) receptor agonists that bind to 5-HT1A, 5-HT2A, and 5-HT2C receptors with moderate-to-high affinity (Blair et al., 2000; McKenna et al., 1990; Pierce and Peroutka, 1989). The phenylalkylamine class of hallucinogens includes phenethylamines such as mescaline and phenylisopropylamines such as 2,5-dimethoxy-4-iodoamphetamine (DOI); these compounds are highly selective for 5-HT2A and 5-HT2C receptors (Pierce and Peroutka, 1989). There is extensive evidence from both animals and humans that the characteristic effects of hallucinogens are mediated by interactions with the 5-HT2A receptor (reviewed by Halberstadt and Nichols, 2010). Other findings, however, indicate that the 5-HT1A receptor also plays a role in the behavioral effects of the indoleamines (Krebs-Thomson et al., 2006; Li et al., 2007; Winter et al., 2000).
A variety of behavioral assays have been used to characterize the acute effects of hallucinogens in rodents (Halberstadt and Geyer, 2010). The most common is the head twitch response (HTR), a paroxysmal rotational movement of the head that is induced by hallucinogens in rats and mice (Corne and Pickering, 1967; Yamamoto and Ueki, 1975). There is considerable evidence that the HTR is mediated by 5-HT2A receptor activation. Specifically, the HTR induced by hallucinogens is blocked by the highly selective 5-HT2A antagonist M100907 (Fantegrossi et al., 2005, 2006, 2008; Vickers et al., 2001), and is absent in 5-HT2A knockout mice (Gonzalez-Maeso et al., 2003, 2007). Nevertheless, it has been shown that activation of the 5-HT2C receptor attenuates 5-HT2A receptor-induced HTR in rats (Vickers et al., 2001). This finding raises the possibility that the interaction of hallucinogens with the 5-HT2C receptor may contribute to or modulate the behavioral effects of these agents.
The behavioral pattern monitor (BPM), which is designed to provide both quantitative and qualitative assessments of unconditioned locomotor and investigatory activity, has also been used extensively to examine the effects of hallucinogens in rats. When tested in a novel environment, phenylalkylamine and indoleamine hallucinogens reduce locomotor activity and investigatory responding (rearing and holepoking) and increase avoidance of central areas of the BPM chamber (Adams and Geyer, 1985; Geyer et al., 1979; Krebs-Thomson et al., 2006; Mittman and Geyer, 1991; Wing et al., 1990). The effects of DOI in the BPM are mediated by the 5-HT2A receptor (Krebs-Thomson et al., 1998), whereas the effects of LSD and 5-MeO-DMT are mediated by 5-HT1A and 5-HT2A receptors (Halberstadt et al., 2008; Krebs-Thomson and Geyer, 1996; Krebs-Thomson et al., 2006; Mittman and Geyer, 1991). A recent study examined the effects of DOI in a mouse version of the BPM. The effects of DOI on activity in the mouse BPM are dose related, with low doses increasing locomotor activity via the 5-HT2A receptor and higher doses decreasing locomotor activity via the 5-HT2C receptor (Halberstadt et al., 2009). Little is known, however, about the effects of indoleamine hallucinogens on locomotor activity in mice.
Psilocybin, the 4-phosphoryloxy derivative of DMT, is found in species of Psilocybe mushrooms that have a history of ceremonial use in Mexico. Psilocybin is rapidly dephosphorylated to psilocin in vitro (Eivindvik et al., 1989; Horita and Weber 1961a, 1961b) and in vivo (Hasler et al., 1997), and the latter drug is considered the pharmacologically active species. In recent years, a number of studies have examined the effects of psilocybin in human volunteers (Carter et al., 2004, 2005b; Gouzoulis-Mayfrank et al., 1998, 1999; Griffiths et al., 2006; Umbricht et al., 2003; Vollenweider et al., 1997, 2007; Wittmann et al., 2007). Indeed, preliminary clinical trials have assessed whether psilocybin is effective at reducing the symptoms of obsessive–compulsive disorder (OCD) (Moreno et al., 2006) and has efficacy as an anxiolytic agent in terminal cancer patients with anxiety (Grob et al., 2010). Other studies have examined how interactions with specific neurotransmitter receptors contribute to the effects of psilocybin in humans. Vollenweider et al. have reported that most of the subjective effects of psilocybin are blocked by pretreatment with the 5-HT2A antagonist ketanserin (Carter et al., 2005a, 2007; Vollenweider et al., 1998), indicating that the hallucinogenic effects of psilocybin are mediated primarily by actions at the 5-HT2A receptor. Other effects of psilocybin, however, such as reduction of arousal and vigilance, slowing of binocular rivalry, and impairment of multiple-object tracking, were not blocked by ketanserin (Carter et al., 2005a, 2007). The latter findings indicate that non-5-HT2A receptors are responsible for mediating some of the effects of psilocybin. Furthermore, it is also possible that the action of psilocybin at 5-HT2A receptors is modulated by interactions of the drug with other 5-HT receptor subtypes (e.g. Strassman 1996).
A few studies have examined the effects of hallucinogens in mice, but little is currently known about the acute behavioral effects of indoleamine hallucinogens in that species. Considering that psilocin activates several serotonin receptor subtypes (as reviewed above), further research into the specific receptor subtypes responsible for the behavioral effects of the drug is warranted. The objective of the present investigation was to assess the behavioral effects of psilocin in mice using the HTR assay and the BPM, two behavioral paradigms known to be sensitive to the effects of hallucinogens. We also used a combination of genetic and pharmacological approaches to identify the 5-HT receptor subtypes responsible for mediating the behavioral effects of psilocin. Finally, we compared the behavioral effects of psilocin with those of 1-methylpsilocin (a psilocin analog that is reported to act as a 5-HT2C receptor-selective agonist; Sard et al., 2005) and 5-MeO-DMT (a hallucinogen that is a prototypical 5-HT1A/2A/2C agonist). The goal of the studies with 1-methylpsilocin and 5-MeO-DMT was to determine whether other indoleamines produce psilocin-like behavioral effects in mice.
Materials and methods
Subjects
Mice were housed at a vivarium at the University of California San Diego (UCSD), USA, an AAALAC-approved animal facility that meets Federal and State requirements for care and treatment of laboratory animals. Male C57BL/6J mice were obtained from Jackson Labs (Bar Harbor, ME, USA); they were allowed to acclimate for approximately 1 week after arrival. The 5-HT2A wild-type (WT) and knockout (KO) mice were bred in-house; these animals, originally generated at Columbia University (New York, NY, USA) on a 129S6/SvEv background (Gonzalez-Maeso et al., 2003, 2007), were backcrossed (N10) onto the inbred C57BL/6 line. All breeding was conducted using heterozygous breeding pairs to remove the possibility of genetic drift between WT and KO mice and to ensure that all mice received equivalent embryonic environments and maternal care. The 5-HT2A WT and KO mice were weaned at 21–24 days of age, at which point a small portion of the tail (1.5 cm) was removed for subsequent genotyping by polymerase chain reaction (PCR). All mice were housed n = 4 per cage, separated by sex, in a climate-controlled room with a reversed light cycle (lights on at 20:00 hours, off at 08:00 hours). Food and water were provided ad libitum, except during behavioral testing. All testing occurred between 10:00 and 18:00 hours; animal testing was conducted in accord with the ‘Principles of Laboratory Animal Care’ NIH guidelines and were approved by the UCSD animal care committee. All efforts were made to minimize animal suffering and to reduce the total number of animals used.
Drugs
Drugs used were psilocin (National Institute on Drug Abuse, Rockville, MD, USA); 1-methylpsilocin, 6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride (SB 242,084; Tocris Bioscience, Ellisville, MO, USA); 5-methoxy-N,N-dimethyltryptamine oxalate (5-MeO-DMT), and N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclo-hexanecarboxamide maleate (WAY-100635; Sigma Chemical Co., St. Louis, MO, USA). Psilocin and 1-methylpsilocin were dissolved in 0.05– mM tartaric acid, brought up to pH 5–6 using 1 N NaOH. SB 242,084 was dissolved in sterile water containing 1% Tween 80. WAY-100635 was dissolved in sterile water. Doses of 5-MeO-DMT refer to the equivalent freebase weight. All drugs were administered subcutaneously at a volume of 5 mL/kg bodyweight.
Apparatus
Head twitch response
HTR was assessed in a transparent Plexiglas cage (18.4 × 29.2 × 12.7 cm) divided into four quadrants by plastic inserts. Four mice were tested simultaneously. The behavior of the mice was recorded by a CCD video camera located above the observation enclosure, digitized, and stored on a PC.
Mice were transferred to the testing room 1 h before testing. During the head twitch test session, the mice were placed in the test cage and monitored for 10 min. Subsequently, the number of head twitches (a rapid paroxysmal movement of the head with little or no involvement of the trunk) was counted by an observer blind to the treatment and genotype, where appropriate. The test cage was thoroughly cleaned between test sessions.
Mouse behavioral pattern monitor
Investigatory behavior and locomotor activity were measured in 10 mouse BPM chambers (San Diego Instruments, San Diego, CA, USA). The design of the mouse BPM system is based on the rat BPM (for a detailed description, see Risbrough et al., 2006). The mouse BPM chamber is a clear Plexiglas box containing a 30 × 60 cm holeboard floor. Each chamber is enclosed in a ventilated outer box to protect it from light and ambient noise from outside the chambers. The chamber contains 11 1.4-cm holes (three in the floor and eight in the walls), each provided with an infrared photobeam to detect investigatory nosepokes (holepokes). The location of the mouse is detected by a grid of 12 × 24 photobeams located 1 cm above the floor. Rearing is detected by an array of 16 photobeams placed 2.5 cm above the floor and aligned with the long axis of the chamber. The status of the photobeams is sampled every 55 ms. A change in the status of photobeams triggers the storage of the information in a binary data file together with the duration of the photobeam status. The raw data files are transformed into (x, y, t, event) ASCII data files comprised of the (x, y) location of the animal in the mouse BPM chamber with a resolution of 1.25 cm, the duration of each event (t), and whether a holepoke or rearing occurred (event). The animal’s position was defined across nine unequal regions (four corners, four walls, and center; Geyer et al., 1986). For these experiments, the measures assessed were distance traveled, center duration, total holepokes and rearings, and spatial scaling measures. The spatial scaling exponent d (Paulus and Geyer, 1991b) quantifies the geometrical structure of the locomotor path. A value of 1 represents a path in a straight line, 1.5 a meandering path, and 2 a highly circumscribed path. Mice were tested in the dark and during the dark phase of their light cycle.
The animals were brought into the testing room 1 h before testing. During testing, a white noise generator produced background noise at 65 dB. Injections were made under red lights in the testing room. Data were collected for 60 min. The chambers were cleaned thoroughly between testing sessions.
Procedure
Head twitch response
Psilocin, 1-methylpsilocin, and 5-MeO-DMT were administered immediately prior to testing. Details of the individual HTR experiments are listed in Table 1.
Table 1.
Details of individual head twitch response (HTR) experiments
| Experiment | Treatment | Animals |
|---|---|---|
| 1 | Vehicle or psilocin (0.3, 0.6, 1.2, 2.4, 4.8mg/kg) | n = 7–10 (51 total) |
| 2 | Vehicle or psilocin (0.6mg/kg) | WT and 5-HT2A KO mice, n = 7 (7 WT and 7 KO male mice, and 7 WT and 7 KO female mice) |
| 3 | Vehicle or 1-methylpsilocin (0.3, 0.6, 1.2, 2.4, 4.8, 9.6mg/kg) | n = 6–7 (48 total) |
| 4 | Vehicle or 1-methylpsilocin (9.6mg/kg) | WT and 5-HT2A KO mice, n = 5–7 (7 WT and 5 KO male mice, and 7 WT and 7 KO female mice) |
| 5 | Vehicle or 5-MeO-DMT (5, 10, 20 mg/kg) | n = 5–7 (24 total) |
| 6 | Vehicle or 5-MeO-DMT (20 mg/kg) | WT and 5-HT2A KO mice, n = 5–6 (12 WT and 11 KO male mice) |
Mouse behavioral pattern monitor
Psilocin, 1-methylpsilocin, and 5-MeO-DMT were administered 10 min prior to testing; SB 242,084 and WAY-100,635 were administered 30 min prior to testing. Details of the individual BPM experiments are listed in Table 2.
Table 2.
Details of individual Behavioral Pattern Monitor (BPM) experiments
| Experiment | Pretreatment | Treatment | Animals |
|---|---|---|---|
| 8 | — | Vehicle or psilocin (0.3, 0.6, 1.2, 2.4, 4.8 mg/kg) | n = 8–10 (54 total) |
| 9 | Vehicle or WAY-100635 (0.5 mg/kg) | Vehicle or psilocin (4.8 mg/kg) | n = 10 (40 total) |
| 10 | — | Vehicle or psilocin (4.8 mg/kg) | WT and 5-HT2A KO mice, n = 4–6 (12 WT and 9 KO male mice, and 8 WT and 10 KO female mice) |
| 11 | Vehicle or SB 242,084 (3 mg/kg) | Vehicle or psilocin (4.8 mg/kg) | n = 10 (40 total) |
| 12 | — | Vehicle or 1-methylpsilocin (0.6, 1.2, 2.4, 4.8, 9.6 mg/kg) | n = 9–10 (58 total) |
| 13 | — | Vehicle or 5-MeO-DMT (10, 20 mg/kg) | n = 11–12 (35 total) |
| 14 | Vehicle or WAY-100635 (0.5 mg/kg) | Vehicle or 5-MeO-DMT (10 mg/kg) | n = 7–12 (40 total) |
| 15 | Vehicle or SB 242,084 (3 mg/kg) | Vehicle or 5-MeO-DMT (10 mg/kg) | n = 10 (40 total) |
Data analysis
HTR was quantified as the number of occurrences during the 10-min observation period. Horizontal locomotor activity was quantified as distance traveled. Center duration was defined as the amount of time spent in the center region of the mouse BPM chamber. The number of holepokes and rearings were calculated as measures of investigatory behavior. Mouse BPM data were examined in 10-, 30-, and 60-min time resolutions. Data were analyzed by using one-, two-, or three-way analyses of variance (ANOVAs) with sex, genotype, pretreatment, and drug treatment as between-subject variables, and time as a repeated measure. Specific post-hoc comparisons between selected groups were done using Dunnett’s many-to-one test or Tukey’s studentized range method. Significance was demonstrated by surpassing an α-level of 0.05. Data are presented in figures using the time resolution that most clearly illustrates the time-course of drug effects or the interaction of pretreatment and treatment.
Results
Effect of psilocin on HTR
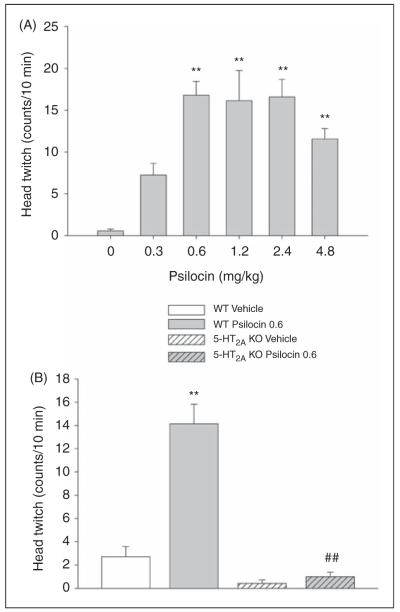
Psilocin produced an inverted U-shaped dose-response function on HTR (F(5,45) = 10.60, p < 0.0001). As shown in Figure 1A, the 0.6, 1.2, 2.4, and 4.8 mg/kg doses of psilocin significantly increased the number of head twitches (p < 0.01, Tukey’s test). The 0.6, 1.2, and 2.4 mg/kg doses of psilocin produced virtually identical effects on head twitch. The median effective dose of psilocin was approximately 0.3 mg/kg.
Figure 1.
Effect of psilocin on head twitch response. (A) Dose-response of psilocin. (B) Effect of 0.6 mg/kg psilocin in wild-type (WT) and 5-HT2A knockout (KO) mice. Data are presented as group means ± SEM. Drug doses are given in mg/kg. **p < 0.01, significant difference from vehicle control group; ##p < 0.01, significant difference from WT animals given psilocin.
To confirm that the HTR induced by psilocin is mediated by the 5-HT2A receptor, as reported previously (Gonzalez-Maeso et al., 2007), psilocin was tested in 5-HT2A KO mice. There was no main effect of sex on HTR, nor was there an interaction between sex and gene, or sex, gene, and drug, so data were collapsed across sex (the same was true for subsequent experiments using 5-HT2A KO mice). There was a significant main effect of psilocin on HTR (F(1,24) 37.80, p < 0.0001) and a significant interaction between gene = and drug (F(1,24) = 30.94, p < 0.0001). Post-hoc comparisons confirmed that the ability of 0.6 mg/kg psilocin to induce the HTR in WT mice was not seen in 5-HT2A KO mice (p < 0.01, Tukey’s test; Figure 1B).
Effect of 1-methylpsilocin on HTR
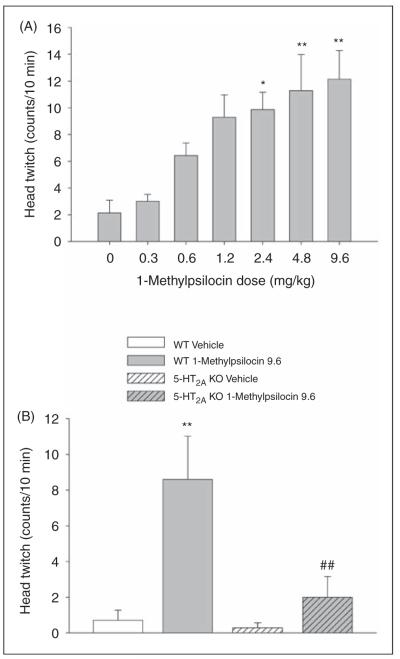
Administration of 1-methylpsilocin produced a dose-dependent increase in HTR (F(6,41) = 5.69, p=0.0002). Post-hoc analyses indicated that the 2.4, 4.8, and 9.6 mg/kg doses of psilocin significantly increased the number of head twitches (p < 0.05, 0.01, Tukey’s test; Figure 2A). The 9.6 mg/kg dose of 1-methylpsilocin was maximally effective, producing 12.1 ± 2.1 (mean ± SEM) head twitches during the 10-min observation period. The ED50 for 1-methylpsilocin was calculated as 0.7 mg/kg (95% confidence interval 0.2–1.2 mg/kg) by non-linear regression analysis.
Figure 2.
Effect of 1-methylpsilocin on head twitch response. (A) Dose-response of 1-methylpsilocin. (B) Effect of 9.6 mg/kg 1-methylpsilocin in wild-type (WT) and 5-HT2A knockout (KO) mice. Data are presented as group means ± SEM. Drug doses are given in mg/kg. *p < 0.05, **p < 0.01, significant difference from vehicle control group; ##p < 0.01, significant difference from WT animals given 1-methylpsilocin.
We compared the effect of 1-methylpsilocin in WT and 5-HT2A KO mice to determine whether the 1-methylpsilocin-induced HTR is sensitive to deletion of the 5-HT2A receptor gene. There was a significant main effect of 1-methylpsilocin (F(1,22) = 16.80, p=0.0005) and a significant interaction of gene and drug (F(1,22) = 6.94, p=0.015). Treatment with 9.6 mg/kg 1-methylpsilocin induced a significant HTR in WT mice and this effect was absent in 5-HT2A KO mice (p < 0.01, Tukey’s test; Figure 2B).
Effect of 5-MeO-DMT on HTR
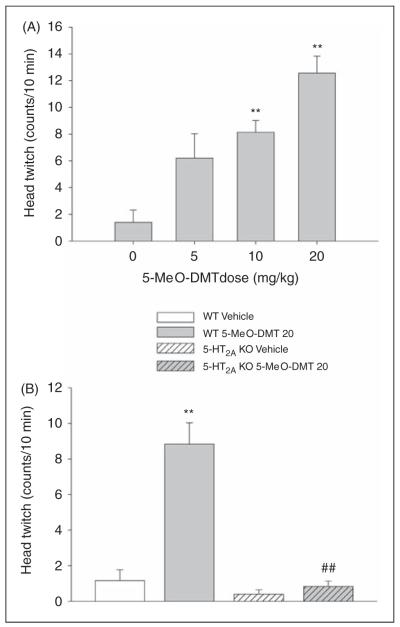
There was a dose-dependent increase in HTR after administration of 5-MeO-DMT (F(3,20) = 13.62, p < 0.0001). Specific comparisons demonstrated that the 10 and 20 mg/kg doses significantly increased the frequency of HTR (p < 0.01, Tukey’s test; Figure 3A).
Figure 3.
Effect of 5-MeO-DMT on head twitch response. (A) Dose-response of 5-MeO-DMT. (B) Effect of 20 mg/kg 5-MeO-DMT in wild-type (WT) and 5-HT2A knockout (KO) mice. Data are presented as group means ± SEM. Drug doses are given in mg/kg. **p < 0.01, significant difference from vehicle control group; ##p < 0.01, significant difference from WT animals given 5-MeO-DMT.
Administration of 5-MeO-DMT to WT and 5-HT2A KO mice confirmed that the effect on HTR is mediated by the 5-HT2A receptor, as evidenced by a main effect of drug (F(1,19) = 30.86, p < 0.0001) and an interaction of gene and drug (F(1,19) = 24.61, p = 0.0001). As shown in Figure 3B, the HTR induced by 20 mg/kg 5-MeO-DMT in WT mice is not seen in mice lacking the 5-HT2A receptor gene (p < 0.01, Tukey’s test).
Effect of psilocin in the BPM
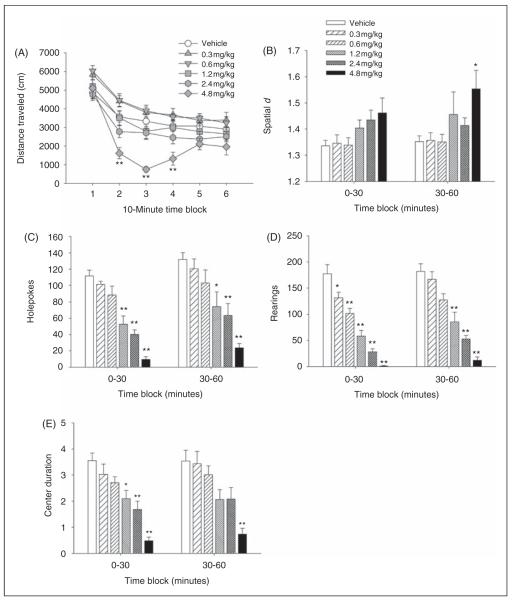
Psilocin reduced locomotor activity, as measured by distance traveled (F(5,48) = 9.96, p < 0.0001). The highest dose tested (4.8 mg/kg) was the most effective (see Figure 4A). There was also a drug × time interaction (F(25,240) = 2.98, p < 0.0001). Post-hoc analysis demonstrated that 4.8 mg/kg psilocin was the only dose that significantly reduced locomotor activity, and this effect occurred during the second, third, and fourth 10-min time blocks of the 60-min session (p < 0.01, Tukey’s test). The spatial scaling exponent, d, was altered by treatment with psilocin (F(5,48) = 2.74, p = 0.03). with vehicle-treated animals, mice Compared treated with 4.8 mg/kg psilocin exhibited more circumscribed patterns of locomotor activity, as indicated by increased spatial d (p < 0.05, Dunnett’s test; Figure 4B).
Figure 4.
Behavioral response to psilocin in mice in the Behavioral Pattern Monitor. Effect on (A) distance traveled (in cm), (B) spatial d, (C) number of holepokes, (D) number of rearings, and (E) time spent in the center (duration in min). Data are presented as group means±SEM for successive 10-min intervals (A) or group means±SEM (B)–(E). Drug doses are given in mg/kg. *p < 0.05, **p < 0.01, significant difference from vehicle control group.
There were main effects of psilocin on holepokes (F(5,48) = 17.90, p < 0.0001), rearings (F(5,48) = 37.12, p < 0.0001), and center duration (F(5,48) 11.43, p < 0.0001). Pair-wise comparisons demonstrated that = psilocin dose-dependently reduced holepoking (Figure 4C) and rearing behavior (Figure 4D), as well as center duration (Figure 4E), during the entire 60-min session (p < 0.05, 0.01, Tukey’s test). Notably, psilocin significantly reduced holepoking, rearing, and center duration when administered at doses ≤ 1.2 mg/kg, demonstrating that psilocin is capable of affecting those behavioral measures when administered at doses that are insufficient to alter locomotor activity or patterns to a significant degree.
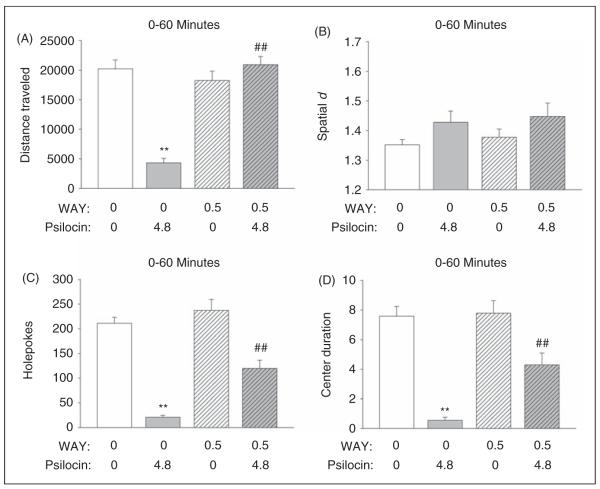
Effect of 5-HT1A receptor blockade on the response to psilocin in the BPM
Treatment with 4.8 mg/kg psilocin reduced distance traveled (F(1,36) = 24.36, p < 0.0001), and there was an interaction between psilocin treatment and time (F(5,180) = 8.06, p < 0.0001). Pretreatment with the 5-HT 1A antagonist WAY-100635 (0.5 mg/kg) attenuated the reduction of locomotor activity induced by psilocin, resulting in a significant interaction between psilocin treatment and pretreatment with WAY-100635 (F(1,36) = 47.57 p < 0.0001). Indeed, as shown in Figure 5A, WAY-100635 completely blocked the effect of psilocin on locomotor activity (p < 0.01, Tukey’s test). Although there was a significant main effect of pretreatment with WAY-100635 on distance traveled (F(1,36) 29.56, p < 0.0001), this was not confirmed by post-hoc analysis. There was a main effect of psilocin on spatial d (F(1,36) = 4.67, p = 0.02), but there was no interaction between treatment and pretreatment (Figure 5B).
Figure 5.
Effect of WAY-100635 on the behavioral response to psilocin. (A) Distance traveled (in cm). (B) Spatial d. (C) Number of holepokes. (D) Time spent in the center (duration in min). Data are presented as group means±SEM. Drug doses are given in mg/kg. **p < 0.01, significant difference from vehicle control group; ##p < 0.01, significant difference from animals given only psilocin.
As expected, 4.8 mg/kg psilocin reduced holepoking (F(1,36) = 100.67, p < 0.0001), rearing (F(1,36) = 70.36, p < 0.0001), and center duration (F(1,36) = 59.73, p < 0.0001). The ability of psilocin to reduce holepoking behavior was attenuated by WAY-100635 (pretreatment × treatment: F(1,36) = 5.63, p = 0.023), and this finding was supported by post-hoc analyses (p < 0.01, Tukey’s test; Figure 5C). There was also a pretreatment × treatment interaction for rearings (F(1,36) = 5.44, p < 0.03), but pair-wise comparisons demonstrated that WAY-100635 failed to significantly attenuate the effect of psilocin on this measure of investigatory behavior. Pretreatment with WAY-100635 also blocked the ability of psilocin to reduce time spent in the center of the chamber (F(1,36) = 6.68, p = 0.014), as confirmed by post-hoc analyses (p < 0.01, Tukey’s test; Figure 5D). There were main effects of pretreatment with WAY-100635 on holepoking (F(1,36) = 16.76, p = 0.0002) and center duration (F(1,36) = 8.32, p < 0.007), but there was no evidence from specific comparisons that pretreatment with WAY-100635 actually significantly altered holepoking or center duration in vehicle-treated animals.
Effect of 5-HT2A receptor gene deletion on the response to psilocin in the BPM
Although treatment with 4.8 mg/kg psilocin reduced distance traveled (main effect: F(1,35) = 5.34, p < 0.03; drug × time: F(5,175) = 12.34, p < 0.0001), deletion of the 5-HT2A receptor gene had no effect on the response to psilocin, as evidenced by the lack of a drug × gene interaction (F(1,35) = 0.21, NS). Likewise, there was no difference between the effects of psilocin on spatial d, rearings, holepokes, or center duration in 5-HT2A receptor KO mice compared with WT mice (data not shown). There was a main effect of gene on distance traveled that approached but did not reach significance (F(1,35) = 3.01, p < 0.1).
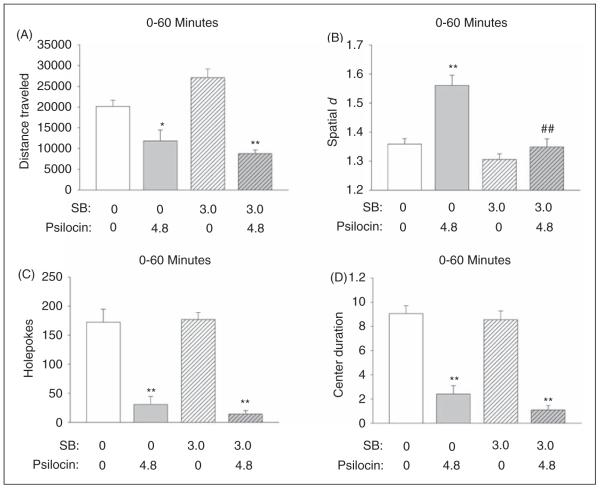
Effect of 5-HT2C receptor blockade on the response to psilocin in the BPM
There was a main effect of psilocin on distance traveled (F(1,36) = 49.46, p < 0.0001) and an interaction of treatment and pretreatment (F(1,36) = 6.92, p = 0.012). However, inspection of the data revealed that this interaction was not due to blockade of psilocin-induced hypoactivity by SB 242,084; rather, SB 242,084 modestly potentiated the effect of psilocin on locomotor activity (see Figure 6A). For spatial d, there was an interaction of pretreatment and treatment (F(1,36) = 9.29, p < 0.005) in addition to main effects of psilocin treatment (F(1,36) = 21.85, p < 0.0001) and SB 242,084 pretreatment (F(1,36)= 9.29, p < 0.0001). Psilocin significantly increased spatial d during the entire 60-min session (p < 0.01, Tukey’s test), and this effect was completely blocked by pretreatment with SB 242,084 (p < 0.01, Tukey’s test; Figure 6B). The effects of psilocin on other behavioral measures (rearings, holepokes, center duration) were not altered by pretreatment with SB 242,084 (Figure 6C, 6D).
Figure 6.
Effect of SB 242,084 on the behavioral response to psilocin. (A) Distance traveled (in cm). (B) Spatial d. (C) Number of holepokes. (D) Time spent in the center (duration in min). Data are presented as group means±SEM. Drug doses are given in mg/kg. *p < 0.05, **p < 0.01, significant difference from vehicle control group; ##p < 0.01, significant difference from animals given only psilocin.
Effect of 1-methylpsilocin in the BPM
1-methylpsilocin had no effect of locomotor activity, spatial d, rearings, holepokes, or center duration (data not shown). Importantly, 1-methylpsilocin was inactive even when tested at doses that had significant effects on HTR.
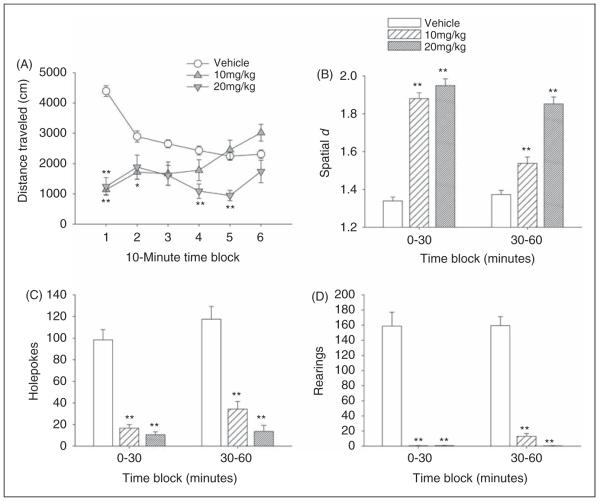
Effect of 5-MeO-DMT in the BPM
As was found with psilocin, administration of 5-MeO-DMT reduced distance traveled (main effect: F(2,32) = 17.55, p < 0.0001; drug × time: F(10,160) = 11.52, p < 0.0001). The lower dose of 5-MeO-DMT (10 mg/kg) reduced distance traveled significantly during the first 20 min of the test session (p < 0.05, 0.01, Tukey’s test), whereas 20 mg/kg 5-MeO-DMT reduced distance traveled for a longer period of time (p < 0.01, Tukey’s test; Figure 7A). Animals treated with 5-MeO-DMT made more circumscribed patterns of locomotor activity (main effect on spatial d: F(2,32) = 132.05, p < 0.0001; drug × time: F(2,32) = 28.28, p < 0.0001). Indeed, both 10 and 20 mg/kg 5-MeO-DMT increased spatial d during the entire 60-min test session (p < 0.01, Tukey’s test; Figure 7B).
Figure 7.
Behavioral response to 5-MeO-DMT in mice in the Behavioral Pattern Monitor. Effect on (A) distance traveled (in cm), (B) spatial d, (C) number of holepokes, and (D) number of rearings. Data are presented as group means±SEM for successive 10-min intervals (A) or group means±SEM (B)–(D). *p < 0.05, **p < 0.01, significant difference from vehicle control group.
There were main effects of 5-MeO-DMT on holepokes (F(2,32) = 65.75, p < 0.0001) and rearings (F(2,32) = 81.94, p < 0.0001). Pair-wise comparisons demonstrated that both doses of 5-MeO-DMT reduced holepokes (Figure 7C) and rearings (Figure 7D) significantly throughout the entire test session (p < 0.01, Tukey’s test). 5-MeO-DMT had no effect on center duration.
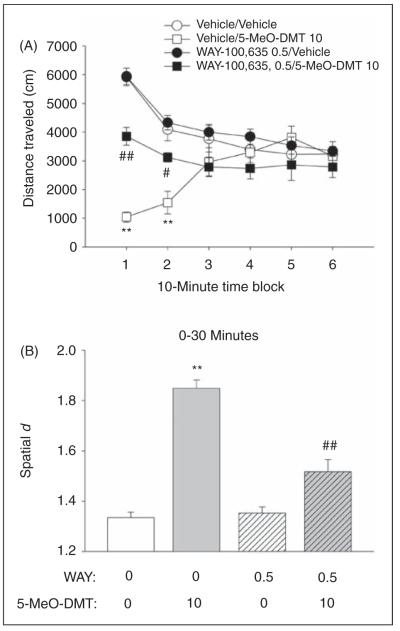
Effect of 5-HT1A receptor blockade on the response to 5-MeO-DMT in the BPM
Treatment with 10 mg/kg 5-MeO-DMT reduced distance traveled (treatment effect: F(1,36) = 17.63, p = 0.0002; treatment × time: F(5,180) = 24.25, p < 0.0001). There was an interaction of pretreatment, treatment, and time (F(5,180) = 9.18, p < 0.0001) on distance traveled. These interactions reflected a significant blockade of the effect of 5-MeO-DMT on distance traveled by 0.5 mg/kg WAY-100635 (p < 0.05, 0.01, Tukey’s test; Figure 8A). There was also an interaction of pretreatment and time (F(5,180) = 9.40, p < 0.0001), but this was not confirmed by post-hoc comparisons. For spatial d, there was an interaction of pretreatment and treatment (F(1,36) = 8.37, p < 0.007), as well as a three-way interaction between pretreatment, treatment, and time (F(1,36) = 47.30, p < 0.0001). In addition, there were main effects of 5-MeO-DMT treatment (F(1,36) = 36.56, p < 0.0001) and WAY-100635 pretreatment (F(1,36) = 7.51, p < 0.01) on spatial d, as well as interactions between 5-MeO-DMT and time (F(1,36) = 149.95, p < 0.0001) and WAY-100635 and time (F(1,36) = 33.44, p < 0.0001). As shown in Figure 8B, 5-MeO-DMT significantly increased spatial d during the first half of the 1 h session (p < 0.01, Tukey’s test), and this effect was blocked by pretreatment with WAY-100,635 (p < 0.01, Tukey’s test). Although there were main effects of 5-MeO-DMT treatment on rearings (F(1,36) = 47.71, p < 0.0001) and holepokes (F(1,36) = 57.55, p < 0.0001), there was no interaction between 5-MeO-DMT and WAY-100,635 pretreatment for those behavioral measures (data not shown).
Figure 8.
Effect of WAY-100635 on the behavioral response to 5-MeO-DMT. (A) Distance traveled (in cm). (B) Spatial d. Data are presented as group means±SEM for successive 10-min intervals (A) or group means±SEM (B) Drug doses are given in mg/kg. **p < 0.01, significant difference from vehicle control group; #p < 0.05, ##p < 0.01, significant difference from animals given only 5-MeO-DMT.
Effect of 5-HT2C receptor blockade on the response to 5-MeO-DMT in the BPM
Although there were interactions between SB 242,084 pretreatment and 5-MeO-DMT treatment for distance traveled (F(1,36) = 13.74, p = 0.0007) and holepokes (F(1,36) = 6.24, p < 0.02), inspection of the data revealed that this interaction reflected a potentiation of the effects of 5-MeO-DMT by SB 242,084, similar to the interaction with psilocin (data not shown). The effects of psilocin on other behavioral measures (rearings, spatial d) were not altered by pretreatment with SB 242,084 (data not shown).
Discussion
Indoleamine hallucinogens such as psilocin bind with high affinity to 5-HT recognition sites, including 5-HT1A, 5-HT2A, and 5-HT2C receptors (Blair et al., 2000; McKenna et al., 1990; Sard et al., 2005). In the present study, we examined the effect of treatment with the indoleamines psilocin, 1-methylpsilocin, and 5-MeO-DMT on HTR and locomotor activity and investigatory behavior in mice, and assessed the contributions of 5-HT receptors to these behavioral effects. Confirming the results of earlier studies (Corne and Pickering 1967; Darmani et al., 1990; Gonzalez-Maeso et al., 2003, 2007), psilocin and 5-MeO-DMT produced dose-dependent increases in HTR. 1-methylpsilocin also induced the HTR, although it was somewhat less potent than psilocin. Consistent with the fact that the HTR serves as a selective assay for 5-HT2A agonist activity in rodents, we found that the ability of psilocin, 1-methylpsilocin, and 5-MeO-DMT to induce the HTR is completely absent in 5-HT2A KO mice. It was previously reported that 5-HT2A KO mice do not display the HTR in response to psilocin (Gonzalez-Maeso et al., 2007), but the present investigation is the first to demonstrate that 5-HT2A KO mice are insensitive to the HTR-inducing effects of 5-MeO-DMT and 1-methylpsilocin. We used genetically modified mice to explore the involvement of 5-HT2A receptors because these animals are free from the problems of efficacy and selectivity that are often found with 5-HT2A receptor antagonists. We recently reported that the mouse BPM is sensitive to the behavioral effects of the phenylalkylamine hallucinogen DOI (Halberstadt et al., 2009), and thus we tested the effects of psilocin, 1-methylpsilocin, and 5-MeO-DMT in this behavioral paradigm (see Table 3). Psilocin reduced locomotor activity, investigatory behavior, and center duration. We have concluded that these effects are mediated by the 5-HT1A receptor because they were blocked by the selective 5-HT1A antagonist WAY-100635, but were not attenuated by pretreatment with the selective 5-HT2C receptor antagonist SB 242,084 or by 5-HT2A receptor gene deletion. 5-MeO-DMT produced psilocin-like behavioral effects in the mouse BPM, and the effects of 5-MeO-DMT on locomotor activity were also blocked by WAY-100635. In contrast to psilocin and 5-MeO-DMT, 1-methylpsilocin had no effect on any measure in the mouse BPM, even though the doses used had significant effects on HTR. Taken together, the results of these experiments indicate that psilocin and 5-MeO-DMT act as mixed 5-HT1A/5-HT2A agonists, whereas 1-methylpsilocin is acting more selectively as a 5-HT2A agonist.
Table 3.
Effects of psilocin,1-methylpsilocin, and 5-MeO-DMT in the Behavioral Pattern Monitor (BPM)
| Drug | Behavioral measure | Effect | Blocked by |
|---|---|---|---|
| Psilocin | Locomotor activity | ↓ | WAY-100635 |
| Spatial d | ↑ | SB 242,084 | |
| Holepoking | ↓ | WAY-100635 | |
| Rearing | ↓ | — | |
| Center duration | ↓ | WAY-100635 | |
| 1-Methylpsilocin | Locomotor activity | 0 | — |
| Spatial d | 0 | — | |
| Holepoking | 0 | — | |
| Rearing | 0 | — | |
| Center duration | 0 | — | |
| 5-MeO-DMT | Locomotor activity | ↓ | WAY-100635 |
| Spatial d | ↑ | WAY-100635 | |
| Holepoking | ↓ | — | |
| Rearing | ↓ | — | |
| Center duration | 0 | — |
Psilocin and 5-MeO-DMT increased the spatial scaling exponent spatial d, indicating that these compounds changed the pattern of the mouse’s locomotor movements. Notably, however, different receptor mechanisms were responsible for the effects of psilocin and 5-MeO-DMT on spatial d. The increase of spatial d induced by psilocin was blocked by SB 242,084 but not by WAY-100635. By contrast, the effect of 5-MeO-DMT on spatial d was completely blocked by WAY-100635 but was unaffected by SB 242,084. These data indicate that the effects of psilocin and 5-MeO-DMT on spatial d are mediated by 5-HT2C and 5-HT1A receptors, respectively. One of the more interesting findings with psilocin is that different 5-HT receptor mechanisms are responsible for the effects of the drug on locomotor activity and spatial d (i.e. 5-HT1A and 5-HT2C receptors, respectively), confirming that these behavioral measures are independent. Indeed, previous studies examining the effects of different classes of psychostimulants have also demonstrated that drug effects on spatial d are independent of changes in locomotor activity (Paulus and Geyer, 1991a).
In rats, 5-MeO-DMT produces decreases in locomotor activity in the BPM (Wing et al., 1990), an effect that is mediated by the 5-HT1A receptor (Krebs-Thomson et al., 2006). Importantly, we have now confirmed that 5-MeO-DMT reduces locomotor activity in mice through the same mechanism. Although it is generally accepted that most of the effects of hallucinogens are mediated by the 5-HT2A receptor (Halberstadt and Nichols, 2010; Nichols, 2004), these findings demonstrate that interactions with the 5-HT1A receptor also contribute to the behavioral effects of indoleamine hallucinogens. In fact, there is a large amount of evidence that the 5-HT1A receptor plays an especially prominent role in the mechanism of action of 5-MeO-DMT (Berendsen et al., 1989; Eison and Wright, 1992; Lucki et al., 1984; Sanchez et al., 1996; Smith and Peroutka, 1986; Spencer et al., 1987; Tricklebank et al., 1985; Winter et al., 2000). Other hallucinogenic indoleamines that have been shown to induce behavioral effects via the 5-HT1A receptor include LSD (Krebs and Geyer, 1994; Krebs-Thomson and Geyer, 1996) and N,N-dipropyltryptamine (DPT) (Fantegrossi et al., 2008; Li et al., 2007).
WAY-100635 completely blocked the effect of psilocin on locomotor activity, suggesting that this effect is mediated by the 5-HT1A receptor. As far as we are aware, besides the current report there is very little evidence that 5-HT1A interactions contribute to the behavioral effects of psilocin. Studies in human volunteers have shown that ketanserin almost completely blocked the subjective effects of psilocybin (Carter et al., 2007; Vollenweider et al., 1998), demonstrating that the hallucinogenic effects of psilocybin are mediated by actions at the 5-HT2A receptor. Likewise, it was previously reported that the discriminative stimulus induced by psilocybin in rats is attenuated by M100907 but not by WAY-100635 (Winter et al., 2007). This finding contrasts with 5-MeO-DMT-induced stimulus control, which is attenuated to varying degrees by WAY-100635 and the selective 5-HT2 antagonist pirenperone (Winter et al., 2000). Although differences between the pharmacology of psilocin and psilocybin could potentially explain the discrepant findings, the fact that psilocybin serves as a prodrug of psilocin argues that they are likely pharmacologically equivalent.
Given that psilocin markedly altered exploratory and investigatory behavior, it was somewhat surprising to find that the N1-methylated homologue 1-methylpsilocin was completely inactive in the mouse BPM. The fact that 1-methylpsilocin did not mimic the behavioral effects of psilocin and 5-MeO-DMT on locomotor activity indicates that this psilocin derivative does not act as a 5-HT1A agonist, at least within the dose range tested. Psilocin has been reported to bind to the 5-HT1A receptor with high affinity (Ki = 49 nM; Blair et al., 2000). According to experiments conducted by the NIMH Psychoactive Drug Screening Program (http://pdsp.med.unc.edu), 1-methylpsilocin displaces [3H]8-OH-DPAT binding to recombinant human 5-HT1A receptors with a Ki = 359 ± 38 nM (unpublished PDSP data). The fact that 1-methylpsilocin binds to the 5-HT1A receptor with approximately seven-fold lower affinity than psilocin may explain why the former agent had no effect when tested in the BPM. N1-alkyl substitution of tryptamines reduces agonist efficacy at the 5-HT2A receptor (Johnson et al., 1994). Although similar findings have not been reported for the 5-HT1A receptor, it is possible that N1-methylation may reduce the efficacy of psilocin at the 5-HT1A receptor.
Members of both the phenylalkylamine and indoleamine classes of hallucinogens produce remarkably consistent effects on investigatory and exploratory behavior in rats, including reductions of locomotor activity and investigatory responding and increased avoidance of the center of an open field (Adams and Geyer, 1985; Geyer et al., 1979; Krebs-Thomson et al., 2006; Mittman and Geyer, 1991; Wing et al., 1990). We recently reported that in mice the hallucinogen DOI can either increase or decrease locomotor activity depending on the dose administered, effects that are mediated by 5-HT2A and 5-HT2C receptors, respectively (Halberstadt et al., 2009). Here, we found that psilocin and 5-MeO-DMT reduce locomotor activity in mice via activation of 5-HT1A receptors. The fact that phenylalkylamine and indoleamine hallucinogens produce disparate behavioral effects in mice indicates that the mouse BPM may have utility in the detection of subtle behavioral differences between members of these two classes of hallucinogens. Further, given that activity in the mouse BPM is sensitive to stimulation of 5-HT1A, 5-HT2A, and 5-HT2C receptors, it is likely that this paradigm will prove useful in delineating the contributions of individual 5-HT receptor subtypes to the behavioral effects of hallucinogens. Since a similar BPM paradigm has been developed in humans (Perry et al., 2009), such studies could be extended across species, unlike the case for the HTR paradigm. These preclinical studies may inform the field on the mechanism of action of hallucinogens such as psilocybin that are currently being evaluated as potential therapeutic agents. Additional studies are currently in progress to characterize the effects and mechanism of action of a much larger series of phenylalkylamine and indoleamine hallucinogens on locomotor activity and investigatory responding in mice.
There is evidence that psilocybin and other hallucinogens can reduce OCD symptoms in humans (Moreno and Delgado, 1997; Moreno et al., 2006). The mechanism for this effect may involve activation of the 5-HT2C receptor (Rosenzweig-Lipson et al., 2007), and it has been reported that N1-methylation significantly enhances the selectivity of psilocin for the 5-HT2C receptor (Sard et al., 2005). Importantly, with regard to HTR, we found that 1-methylpsilocin evoked this behavior with lower potency than psilocin. Most 5-HT2A agonists that induce the HTR in rodents have hallucinogenic effects in humans; thus, it is very likely that 1-methylpsilocin would produce LSD-like effects if administered to humans at sufficient dosages. Although we did not directly compare the behavioral potencies of psilocin and 1-methylpsilocin for activation of the 5-HT2C receptor, 1-methylpsilocin is slightly more potent than psilocin as an agonist at human 5-HT2C receptors in vitro (Sard et al., 2005). Given these findings, it appears that 1-methylpsilocin has the potential to evoke psilocybin-like effects on OCD, but is less likely than psilocybin to provoke unwanted hallucinogenic effects if administered at equivalent doses. Furthermore, because 1-methylpsilocin is behaviorally inactive as a 5-HT1A agonist, it may be free from effects on arousal and vigilance that have been noted to occur in human volunteers after administration of psilocybin (Carter et al 2007). Although additional studies are needed to confirm the clinical efficacy of psilocybin, the present findings indicate that 1-methylpsilocin represents a potentially useful alternative to psilocybin for development as a therapeutic agent.
Acknowledgements
The authors thank Dr Bryan Roth and the NIMH-PDSP program for providing binding data for 1-methylpsilocin.
Funding
This work was supported by National Institute on Drug Abuse (awards R01DA002925 and F32DA025412) and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Adams LM, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:121–132. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Jenck F, Broekkamp CL. Selective activation of 5HT1A receptors induces lower lip retraction in the rat. Pharmacol Biochem Behav. 1989;33:821–827. doi: 10.1016/0091-3057(89)90477-2. [DOI] [PubMed] [Google Scholar]
- Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, et al. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem. 2000;43:4701–4710. doi: 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005a;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (Berl) 2007;195:415–424. doi: 10.1007/s00213-007-0930-9. [DOI] [PubMed] [Google Scholar]
- Carter OL, Pettigrew JD, Burr DC, Alais D, Hasler F, Vollenweider FX. Psilocybin impairs high-level but not low-level motion perception. NeuroReport. 2004;15:1947–1951. doi: 10.1097/00001756-200408260-00023. [DOI] [PubMed] [Google Scholar]
- Carter OL, Pettigrew JD, Hasler F, Wallis GM, Liu GB, Hell D, et al. Modulating the rate and rhythmicity of perceptual rivalry alternations with the mixed 5-HT2A and 5-HT1A agonist psilocybin. Neuropsychopharmacology. 2005b;30:1154–1162. doi: 10.1038/sj.npp.1300621. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Eison AS, Wright RN. 5-HT1A and 5-HT2 receptors mediate discrete behaviors in the Mongolian gerbil. Pharmacol Biochem Behav. 1992;43:131–137. doi: 10.1016/0091-3057(92)90649-z. [DOI] [PubMed] [Google Scholar]
- Eivindvik K, Rasmussen KE, Sund RB. Handling of psilocybin and psilocin by everted sacs of rat jejunum and colon. Acta Pharm Nord. 1989;1:295–302. [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, et al. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl) 2005;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, et al. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–129. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. Hallucinogen-like effects of N, N-dipropyltryptamine (DPT): Possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav. 2008;88:358–365. doi: 10.1016/j.pbb.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Light RK, Rose GJ, Petersen LR, Horwitt DD, Adams LM, et al. A characteristic effect of hallucinogens on investigatory responding in rats. Psychopharmacology (Berl) 1979;65:35–40. doi: 10.1007/BF00491975. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: Pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Thelen B, Lindenblatt H, Kovar KA, Sass H, et al. Effects of the hallucinogen psilocybin on habituation and prepulse inhibition of the startle reflex in humans. Behav Pharmacol. 1998;9:561–566. doi: 10.1097/00008877-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, et al. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylampheta-mine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology (Berl) 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187:268–283. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty MC, Halberstadt AL, McKay CR. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2010 doi: 10.1001/archgenpsychiatry.2010.116. doi:10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Hallucinogens. In: Koob G, Thompson R, Moal ML, editors. Encyclopedia of Behavioral Neuroscience. Academic Press; London: 2010. pp. 12–20. [Google Scholar]
- Halberstadt AL, Nichols DE. Serotonin and serotonin receptors in hallucinogen action. In: Muller C, Jacobs B, editors. Handbook of the Behavioral Neurobiology of Serotonin. Academic; London: 2010. pp. 621–636. [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N, N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology (Berl) 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, et al. 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler F, Bourquin D, Brenneisen R, Bar T, Vollenweider FX. Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm Acta Helv. 1997;72:175–184. doi: 10.1016/s0031-6865(97)00014-9. [DOI] [PubMed] [Google Scholar]
- Horita A, Weber LJ. Dephosphorylation of psilocybin to psilocin by alkaline phosphatase. Proc Soc Exp Biol Med. 1961a;106:32–34. doi: 10.3181/00379727-106-26228. [DOI] [PubMed] [Google Scholar]
- Horita A, Weber LJ. The enzymic dephosphorylation and oxidation of psilocybin and psilocin by mammalian tissue homogenates. Biochem Pharmacol. 1961b;7:47–54. doi: 10.1016/0006-2952(61)90124-1. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Loncharich RJ, Baez M, Nelson DL. Species variations in transmembrane region V of the 5-hydroxytryptamine type 2A receptor alter the structure–activity relationship of certain ergolines and tryptamines. Mol Pharmacol. 1994;45:277–286. [PubMed] [Google Scholar]
- Krebs KM, Geyer MA. Cross-tolerance studies of serotonin receptors involved in behavioral effects of LSD in rats. Psychopharmacology (Berl) 1994;113:429–437. doi: 10.1007/BF02245219. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. The role of 5-HT(1A) receptors in the locomotor-suppressant effects of LSD: WAY-100635 studies of 8-OH-DPAT, DOI and LSD in rats. Behav Pharmacol. 1996;7:551–559. [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: Influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology (Berl) 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. Behavioral effects of dipropyltryptamine in rats: Evidence for 5-HT1A and 5-HT2A agonist activity. Behav Pharmacol. 2007;18:283–288. doi: 10.1097/FBP.0b013e3281f19ca0. [DOI] [PubMed] [Google Scholar]
- Lucki I, Nobler MS, Frazer A. Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J Pharmacol Exp Ther. 1984;228:133–139. [PubMed] [Google Scholar]
- McKenna DJ, Repke DB, Lo L, Peroutka SJ. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology. 1990;29:193–198. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Dissociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology (Berl) 1991;105:69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Delgado PL. Hallucinogen-induced relief of obsessions and compulsions. Am J Psychiatry. 1997;154:1037–1038. doi: 10.1176/ajp.154.7.1037b. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. 2006;67:1735–1740. doi: 10.4088/jcp.v67n1110. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A scaling approach to find order parameters quantifying the effects of dopaminergic agents on unconditioned motor activity in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1991a;15:903–919. doi: 10.1016/0278-5846(91)90018-v. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991b;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus M, Young J, Kincaid M, Ferguson E, et al. A reverse-translational study of dysfunctional exploration in psychiatric disorders: From Mice to Men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce PA, Peroutka SJ. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology (Berl) 1989;97:118–122. doi: 10.1007/BF00443425. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, Lucki I, Malberg JE, et al. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology (Berl) 2007;192:159–170. doi: 10.1007/s00213-007-0710-6. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Arnt J, Moltzen E. Assessment of relative efficacies of 5-HT1A receptor ligands by means of in vivo animal models. Eur J Pharmacol. 1996;315:245–254. doi: 10.1016/s0014-2999(96)00621-8. [DOI] [PubMed] [Google Scholar]
- Sard H, Kumaran G, Morency C, Roth BL, Toth BA, He P, et al. SAR of psilocybin analogs: discovery of a selective 5-HT 2C agonist. Bioorg Med Chem Lett. 2005;15:4555–4559. doi: 10.1016/j.bmcl.2005.06.104. [DOI] [PubMed] [Google Scholar]
- Smith LM, Peroutka SJ. Differential effects of 5-hydroxy-tryptamine1a selective drugs on the 5-HT behavioral syndrome. Pharmacol Biochem Behav. 1986;24:1513–1519. doi: 10.1016/0091-3057(86)90477-6. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Jr, Glaser T, Traber J. Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N, N-dimethyltryptamine. Psychopharmacology (Berl) 1987;93:158–166. doi: 10.1007/BF00179927. [DOI] [PubMed] [Google Scholar]
- Strassman RJ. Human psychopharmacology of N,N-dimethyl-tryptamine. Behav Brain Res. 1996;73:121–124. doi: 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Forler C, Middlemiss DN, Fozard JR. Subtypes of the 5-HT receptor mediating the behavioural responses to 5-methoxy-N, N-dimethyltryptamine in the rat. Eur J Pharmacol. 1985;117:15–24. doi: 10.1016/0014-2999(85)90467-4. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vollenweider FX, Schmid L, Grübel C, Skrabo A, Huber T, et al. Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: Implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology. 2003;28:170–181. doi: 10.1038/sj.npp.1300005. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, et al. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology (Berl) 1990;100:417–425. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N, N-dimethyltryptamine: An indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA. Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav. 2007;87:472–480. doi: 10.1016/j.pbb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Carter O, Hasler F, Cahn BR, Grimberg U, Spring P, et al. Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol. 2007;21:50–64. doi: 10.1177/0269881106065859. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Behavioral effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rats and mice. Eur J Pharmacol. 1975;32:156–162. doi: 10.1016/0014-2999(75)90278-2. [DOI] [PubMed] [Google Scholar]