Abstract
Background
The hypermetabolic response to severe thermal injury is unlike any physiologic response seen in medicine. While some parallels can be drawn to shock and sepsis states, this response is typified by its intensity and duration. Our group has been interested in the myriad effects of estrogens after injury, specifically the ability of estrogens to reduce inflammatory responses. Given this, and the known link between severe inflammation and the hypermetabolic response, we examined the effects of a single dose of 17β estradiol administered after a severe thermal injury in rats.
Methods
Twelve male Sprague-Dawley rats were subject to either a sham burn or a 40% total body surface area burn, followed by fluid resuscitation. Burned animals were divided into a vehicle and treatment group, with injections given 15 minutes after the injury. Animals were monitored for a period of 45 days with markers of hypermetabolism (weight, fecal output, food intake, and serum insulin and glucose) measured daily.
Results
We identified a significant difference in daily measured weights between the burned groups. We observed a sparing of body mass during the acute phase lasting two weeks after the injury, and an improved recovery phase during the remainder of the study. Glucose and insulin levels during the first week of the study did not differ between the treatment groups.
Conclusion
Estrogen may have a role in preserving body mass after severe thermal injury. Further studies are required to determine if this spared body mass composition.
INTRODUCTION
Severe burns are a significant cause of death and disability, with children being particularly vulnerable to both the initial injury and sequelae (1). While initial resuscitation, wound care, and infection control have improved survival, severely burned patients continue to undergo profound metabolic changes associated with significant morbidity and mortality. Patients that sustain severe burns (i.e. greater than 20% total body surface area) predictably develop a hypermetabolic state that occurs in two distinct phases (2). The initial “ebb” phase is a period of reduced metabolism with lower cardiac output, decreased oxygen consumption, and reduced metabolism. At about five days after injury, the “ebb” state plateaus and gives way to the “flow” state, characterized by increased oxygen consumption and resting energy expenditure, reduced bone mass, hyperinsulinemia with peripheral insulin resistance, fat and protein catabolism, and growth retardation (3–8). Whole body catabolism can persist for more than two years after the initial injury (7).
Additional nutrition has been shown to be of limited benefit in severe burns thought to be associated with a persistent insulin resistance that lasts for up to three years after injury (9). Moreover, increased caloric intake leads to additional fat stores rather than the creation of lean muscle mass (10). Several therapies to slow or stop the burn hypermetabolic state have been investigated (2). Among the more promising agents are recombinant human growth hormone (11, 12), insulin (13), insulin-like growth factor and related ligands (14, 15), oxandrolone (16), and testosterone (17). In these patients, anticatabolic agents such as adrenergic antagonists are of some use (18).
Growing evidence implicates estrogen as a critical determinant of outcomes after severe stress (19–21) In the post-traumatic period, estrogen supports cardiovascular, immune, and neuronal functions and helps to regulate skin, bone, and lipid metabolism (19). At present it is unclear if estrogen has similar beneficial effects on the hypermetabolic state induced by severe thermal injury; however laboratory observations of animals sustaining severe burns suggest that those receiving single dose of estrogen after trauma have better outcomes than those that do not (20). The aim of this pilot study was to examine the effects of 17β-estradiol on multiple metabolic endpoints in a rodent model after being subjected to a severe thermal burn.
MATERIALS AND METHODS
Experimental model
Twelve male Sprague-Dawley rats, eight weeks of age and 300–325 grams, were obtained from Charles River Laboratories (Houston, TX) and housed in a UT-Southwestern Medical Center (UT Southwestern) animal care facilities for acclimation. Rats were maintained in a constant temperature environment with a 12-h light/dark cycle and had ad libitum access to commercial rat chow and tap water. All experiments were performed under a protocol approved by the UT Southwestern Institutional Animal Care and Research Advisory Committee, and the work conformed to all guidelines outlined in the Guide for the Care and Use of Laboratory Animals published by the American Physiological Society.
Injury Model and Treatment of Animals
Rats were randomly assigned to one of three groups: Sham burn, Scald burn with vehicle treatment, and scald burn with 17β-estradiol treatment. All animals received the same treatment with the exception of the scald or sham burn injury and subsequent study drug treatment. All rats were deeply anesthetized with isoflurane and secured in a template device that exposed 40% of the total body surface area (TBSA) over the back and flank of the animal. Hair was clipped to expose the skin over the area to be burned. In the scald burn groups, the area to be burned was immersed in 100°C water for 10 seconds while sham group animals were immersed in a room temperature water bath. The scald burn groups consequently received a full thickness dermal burn over 40% of the TBSA, which produces complete destruction of the underlying nerve tissue and sensory nerve endings while avoiding direct deep organ injury.
After water bath immersion, the rats were dried and resuscitated using the Parkland Burn Formula; 4 ml/kg per percent burn of lactated Ringer solution IP with 50% of the calculated volume being given immediately after completion of the burn and the remaining 50% administered over the next 8 hours after burn. All rats initially received 0.1 mg/kg of IP buprenorphine for possible discomfort. Following fluid resuscitation, each animal was housed in an individual metabolic cage, and warmed with a heating pad so that the animal could selfregulate temperature. No rats displayed signs of pain or discomfort and returned to normal movement and feeding within 15 minutes post anesthesia.
The scald burn groups received 0.5 mg/kg of 17 β-estradiol or vehicle (corn oil) subcutaneously at 15 minutes after injury. This subcutaneous delivery of 17β-estradiol has previously been shown to produce similar pharmacokinetics to intravenous dosing in previous rodent models, but with greater ease of delivery (21). Our group has utilized this technique of treating the animals at an interval following injury to provide a more clinically relevant model.
Metabolic endpoints
Body mass (in grams), food intake (in grams), and fecal output (in grams) were measured at the same time each day. All measurements were taken on electronic scales capable of measuring to one-tenth of a gram. Fasting blood samples (tail nicks) were drawn daily for the first 7 days after the water bath immersion. Glucose was measured using a glucometer and insulin was measured using an ELISA kit from ALPCO Immunoassays.
As an estimate of energy expenditure that was not accounted for by food intake or fecal mass, we utilized a formula first described by Izamis, et. al. (22) which was calculated by the formula:
[Weight gained/lost] − [food intake] − [fecal mass] = OE,
where OE is equal to the mass that could not be accounted for by these factors alone.
All areas of burned skin were treated with silvadene cream daily for wound care. Any animals that showed signs of possible burn wound infections were cultured, with plans for euthanizing that animal if a severe infection became apparent. This was done to minimize confounding factors that could affect the results of this pilot study with potentially septic animals.
Statistics
Repeated-Measures ANOVA was used to compare groups. A p value of < 0.05 was considered significant in all cases. Data that reached statistical significance were further subjected to Bonferroni’s post-test analysis to identify differences between treatment groups. Points or bars represent the mean value for each group and error bars indicate SEM (standard error of the mean). GraphPad Prism Software (San Diego, CA) was used to analyze all data and produce graphs.
RESULTS
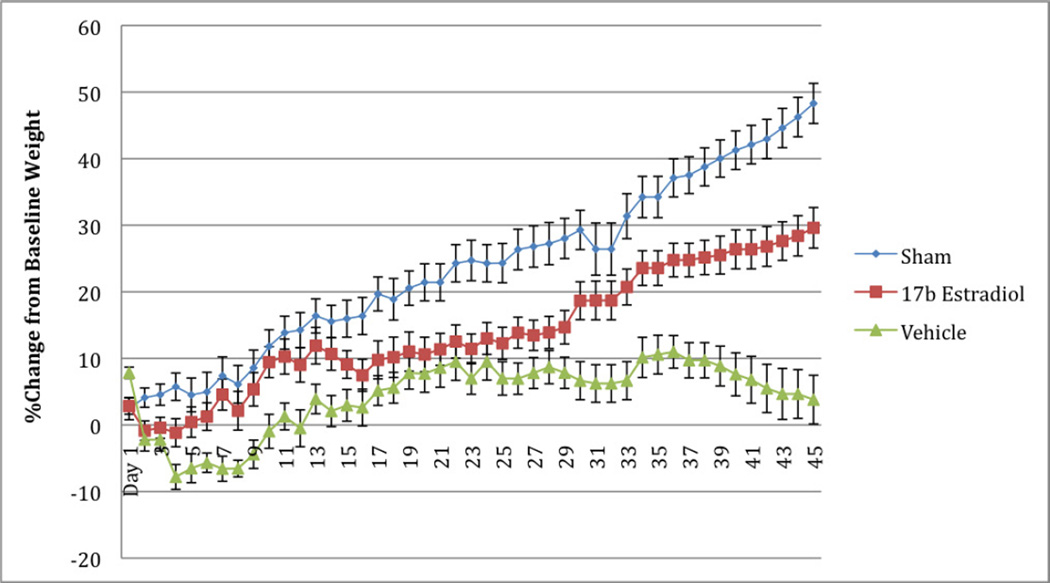
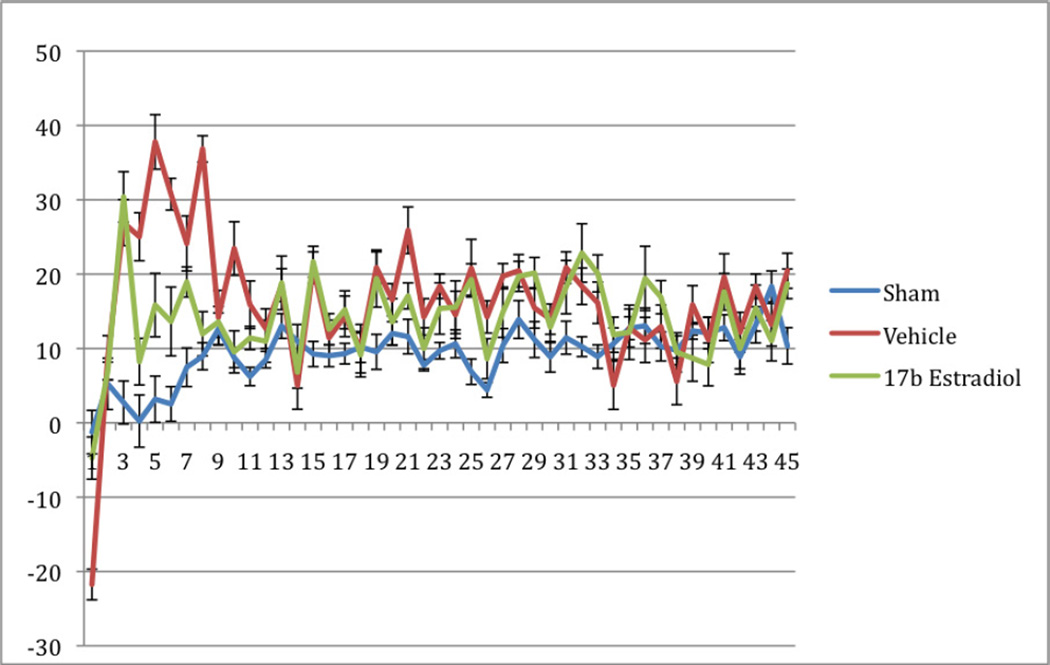
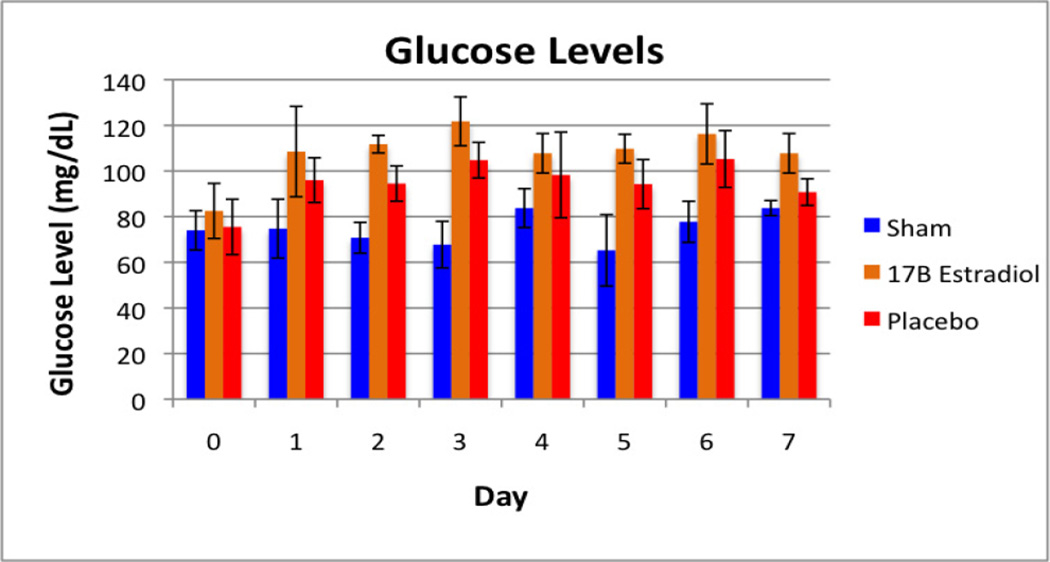
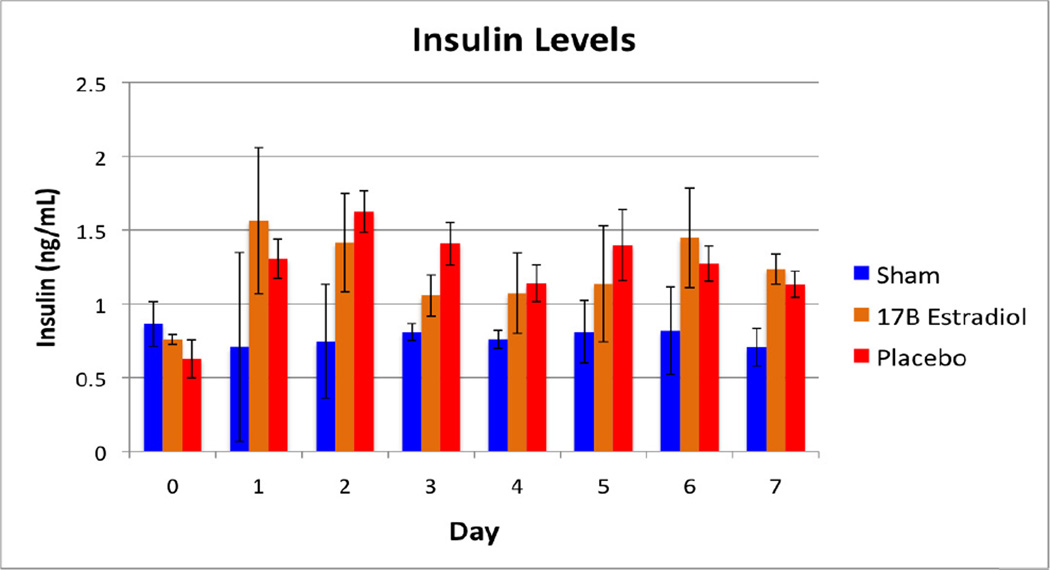
No animals naturally expired or underwent euthanasia during the 45-day study period. One animal was cultured twice during this period for possible wound infection, however no growth was obtained on these cultures. Daily food mass intake and daily fecal mass output did not differ significantly between the groups (Table 1). The body weight measurements are shown in Figure 1 as a percentage increase from baseline for each group. As expected, the sham group gained weight at a steady rate during the course of the study. The vehicle group, however, demonstrated a loss of body mass over the first week, recovering their baseline weights by day 12, and continuing to gain weight until approximately four weeks into the study, at which point weights began to decrease again. The 17β-estradiol group did not lose a significant amount of their baseline body weights, and after the second week of the study, continued to gain weight steadily. When weekly weight differences are compared, the differences between these groups were statistically significant during the first two weeks (Table 2). A statistically significant difference was observed again in the final week of the study. The mass lost to other energy values (OE) is demonstrated in Figure 2. Coinciding with the findings of the differences in weight loss, there was a statistically significant difference between the groups during the first ten days of the study, at which point the groups converged and no differences were seen. Figures 3 and 4 show the fasting glucose and insulin levels obtained during the first week of the study. No statistically significant differences were observed between the vehicle and treatment groups, although there was a significant elevation in glucose for both burned groups compared with the sham group (p<0.001). Similarly, insulin levels showed a statistically significant elevation in insulin in both the 17β estradiol and vehicle groups compared with the sham group at each day after day 1, a Bonferroni’s post-test did not show a difference between the two burned groups.
Table 1.
Average Daily Food Mass Intake and Fecal Mass Output.
| Group | Daily Food intake (g) | Daily Fecal output (g) |
|---|---|---|
| Sham | 29.75+2.6 | 14.0+4.8 |
| Vehicle | 31.0+4.5 | 13.8+2.3 |
| 17β Estradiol | 31.25+4.8 | 9.8+3.1 |
| p value | .22 | .09 |
Figure 1.
Rat Body Weight Changes from Baseline. Values are shown as a percent change from baseline weight for each day during the study period, with error bars representing the SEM.
Table 2.
Weight difference comparisons between the sham, vehicle, and treatment groups. Values are percentage body weight gained (or lost) compared to baseline weight.
| SHAM | VEHICLE | 17β-ESTRADIOL | p | |
|---|---|---|---|---|
| DAY 7 | 7.4±8.0 | −6.6±3.5 | 4.5±5.4 | 0.02 |
| DAY 14 | 15.5±5.8 | 2.1±5.4 | 10.7±5.9 | 0.03 |
| DAY 21 | 21.4±7.7 | 8.5±5.7 | 11.3±8.5 | 0.08 |
| DAY 28 | 27.2±10 | 8.6±6.2 | 13.9±5.8 | 0.02 |
| DAY 35 | 34.2±9.5 | 10.6±6.8* | 23.6±8.5 | 0.03 |
| DAY 45 | 48.2±9.0 | 3.8±13.4* | 29.6±9.3 | <0.01 |
Figure 2.
Mass Lost to Other Energy Processes (OE). Values are calculated using the formula [Body Mass Gained] − [Food Intake] − [Fecal Mass Output] = OE. Error bars represent SEM.
Figure 3.
Fasting glucose levels measured during the first week of the study period.
Figure 4.
Fasting Insulin Levels measured during the first week of the study period.
DISCUSSION
The hypermetabolic state that follows severe burns results in substantial, persistent morbidity, which in turn may contribute to mortality. Profound muscle catabolism leads to impaired strength and muscle function (23, 24) Reduced bone mass and linear bone growth is responsible for dramatically increased risk of fracture (3). These changes lead to retarded growth and development (5). The hypermetabolic state after burn is a period of altered homeostasis with altered levels of circulating catecholamines, prostaglandins, cytokines, sex hormones, glucagon and cortisol (9, 23). We report for the first time that a single pharmacologic large dose of 17β-estradiol attenuates the loss of mass that occurs after a scald burn injury in rat.
In support of this finding, while there are multiple factors that potentially influence the sex-related differences in outcomes after burn besides estrogens, elevated endogenous estrogen levels in animal models correlate strongly with favorable outcomes after various forms of acute illness and trauma, including cerebral ischemia (21), and trauma-hemorrhage (19). In children, while baseline estrogen levels abruptly decrease in both boys and girls after burn and remain significantly below normal for months (25), multiple studies have shown that the hypermetabolic response that follows serious thermal injury is more pronounced in post-pubescent boys than in girls, and this correlates with survival rates (26). Resting energy expenditures are greater in males than females and take longer to return to baseline after injury. Likewise, bone mineral content and percent fat content are more deranged in males than females (27). Catecholamines have profound catabolic effects on skeletal muscle. Males have demonstrated higher levels of urinary catecholamines than females throughout the post-burn period and these levels take 24 months to return baseline while females exhibit normal levels at 18 months post-burn (28). Previous work has shown that a single dose of 17β-estradiol blocks the rapid increase in various immunological markers seen after thermal injury, such as TNF-α, IL-1β, and the cytokine most closely correlated with post-burn outcomes, IL-6. Estrogen reduces inflammation and pro-apoptotic signaling that follow severe thermal injury (29). In these studies, we noticed that rats treated with estrogen appear to better tolerate the thermal injury and then recover faster from the insult. Given the favorable immunological effects of estrogen administration and the subsequent correlation with outcomes, we wanted to investigate the hormone’s effect on post-burn hypermetabolism. We chose to study male rats to avoid the potential confounds of the estrus cycle.
Further, it appears that exogenous estrogen administration partially prevents the lost mass due to excessive energy expenditures. Rats subjected to a room temperature water bath followed a relatively predictable increase in weight gain. Normal energy expenditures slightly exceeded food intake and fecal output. However, burn greatly increased the amount of energy expended, presumably due to hypermetabolic processes. We assume in this analysis that there is a direct correlation between daily food intake, fecal output, and body weight gain. One limitation of this study is that we did not quantify water homeostasis. While net loss/gain is negligible in normal rats, there may be insensible losses in rats subjected to thermal injury of roughly 40% of their TBSA. Estrogen administration partially, but not fully, attenuated the presumed hypermetabolic energy losses. We are encouraged by the results of this pilot study, yet readily acknowledge that larger test groups are required for more definitive and detailed conclusions. Nonetheless, these results, combined with more than 50 years of safety data of single-dose clinical intravenous estrogen, the ease of administration, and the relatively low cost of therapy do greatly strengthen the case for estrogen to be studied alongside other clinical post-burn treatments. Prior exogenous pharmacological interventions have shown promise in overcoming hypermetabolic processes. Growth hormone administration was a natural choice since it improves protein synthesis (30) but it does so at the expense of increased insulin resistance (31). Intensive insulin therapy, on the other hand, overcomes post-burn insulin resistance, and reduces catabolism, inflammation, sepsis, and mortality (32). However, insulin administration does not completely halt the hypermetabolic sequelae of severe burns. The testosterone analogue, oxandrolone, improves net muscle protein synthesis in healthy young men (16). While it only possesses a fraction of the virilizing androgenic effects of testosterone, there may be limitations of its use in women and prepubescent boys. This is especially true despite initial studies reporting a favorable safety profile, since the more effective regimens require repeated supraphysiological doses (0.1 mg/kg twice daily) (33). Other groups have already demonstrated that activation of ER-α by estrogens reduces food intake and increases body weight (34), and our findings here suggest that this mechanism may be relevant following severe burns.
In conclusion, we found that estrogen administration in rats following severe scald injury attenuates the hypermetabolic losses of body mass. Importantly, this rescue effect by 17β-estradiol administration after burn was independent of food intake and fecal output, and identifies a novel mechanism of estrogen’s protective effects following severe burn. These data provide additional support that single-dose parenteral estrogen administration may in the future be tested to decrease morbidity and mortality in this complex and difficult patient population, as a potentially safe, efficacious, easy to administer, and cost effective therapy.
Acknowledgments
Funding: This work is supported by the NIH T32 Grant T32GM08593, titled “Training in Trauma, Inflammation, Sepsis, and Critical Care” (Fiemu Nwariaku, M.D., PI) from the National Institute of General Medical Science
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Shah A, Suresh S, Thomas R, Smith S. Epidemiology and profile of pediatric burns in a large referral center. Clin Pediatr (Phila) 2011;50(5):391–395. doi: 10.1177/0009922810390677. [DOI] [PubMed] [Google Scholar]
- 2.Pereira CT, Murphy KD, Herndon DN. Altering metabolism. J Burn Care Rehabil. 2005;26(3):194–199. [PubMed] [Google Scholar]
- 3.Klein GL, Herndon DN, Langman CB, Rutan TC, Young WE, Pembleton G, et al. Long-term reduction in bone mass after severe burn injury in children. J Pediatr. 1995;126(2):252–256. doi: 10.1016/s0022-3476(95)70553-8. [DOI] [PubMed] [Google Scholar]
- 4.Milner EA, Cioffi WG, Mason AD, McManus WF, Pruitt BA., Jr A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma. 1994;37(2):167–170. doi: 10.1097/00005373-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125(3):392–395. doi: 10.1001/archsurg.1990.01410150114021. [DOI] [PubMed] [Google Scholar]
- 6.Tredget EE, Yu YM. The metabolic effects of thermal injury. World J Surg. 1992;16(1):68–79. doi: 10.1007/BF02067117. [DOI] [PubMed] [Google Scholar]
- 7.Przkora R, Barrow RE, Jeschke MG, Suman OE, Celis M, Sanford AP, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60(5):968–971. doi: 10.1097/01.ta.0000214580.27501.19. discussion 71. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe RR. Metabolic response to burn injury: nutritional implications. Keio J Med. 1993;42(1):1–8. doi: 10.2302/kjm.42.1. [DOI] [PubMed] [Google Scholar]
- 9.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ, 3rd, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94(5):1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart DW, Wolf SE, Herndon DN, Chinkes DL, Lal SO, Obeng MK, et al. Energy expenditure and caloric balance after burn: increased feeding leads to fat rather than lean mass accretion. Ann Surg. 2002;235(1):152–161. doi: 10.1097/00000658-200201000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aili Low JF, Barrow RE, Mittendorfer B, Jeschke MG, Chinkes DL, Herndon DN. The effect of short-term growth hormone treatment on growth and energy expenditure in burned children. Burns. 2001;27(5):447–452. doi: 10.1016/s0305-4179(00)00164-9. [DOI] [PubMed] [Google Scholar]
- 12.Hart DW, Herndon DN, Klein G, Lee SB, Celis M, Mohan S, et al. Attenuation of posttraumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233(6):827–834. doi: 10.1097/00000658-200106000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakurai Y, Aarsland A, Herndon DN, Chinkes DL, Pierre E, Nguyen TT, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222(3):283–294. 94, 97. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moller S, Jensen M, Svensson P, Skakkebaek NE. Insulin-like growth factor 1 (IGF-1) in burn patients. Burns. 1991;17(4):279–281. doi: 10.1016/0305-4179(91)90039-j. [DOI] [PubMed] [Google Scholar]
- 15.Herndon DN, Ramzy PI, DebRoy MA, Zheng M, Ferrando AA, Chinkes DL, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229(5):713–720. doi: 10.1097/00000658-199905000-00014. discussion 20-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheffield-Moore M, Urban RJ, Wolf SE, Jiang J, Catlin DH, Herndon DN, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999;84(8):2705–2711. doi: 10.1210/jcem.84.8.5923. [DOI] [PubMed] [Google Scholar]
- 17.Ferrando AA, Sheffield-Moore M, Wolf SE, Herndon DN, Wolfe RR. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001;29(10):1936–1942. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345(17):1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 19.Choudhry MA, Chaudry IH. 17beta-Estradiol: a novel hormone for improving immune and cardiovascular responses following trauma-hemorrhage. J Leukoc Biol. 2008;83(3):518–522. doi: 10.1189/jlb.0607369. [DOI] [PubMed] [Google Scholar]
- 20.Wigginton JG, Pepe PE, Idris AH. Rationale for routine and immediate administration of intravenous estrogen for all critically ill and injured patients. Crit Care Med. 2010;38(10 Suppl):S620–S629. doi: 10.1097/CCM.0b013e3181f243a9. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Wang X, Liu Q, Yang SH, Simpkins JW. Dose dependence and therapeutic window for the neuroprotective effects of 17beta-estradiol when administered after cerebral ischemia. Neurosci Lett. 2007;415(3):237–241. doi: 10.1016/j.neulet.2007.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izamis ML, Uygun K, Uygun B, Yarmush ML, Berthiaume F. Effects of burn injury on markers of hypermetabolism in rats. J Burn Care Res. 2009 Nov-Dec;30(6):993–1001. doi: 10.1097/BCR.0b013e3181bfb7b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248(3):387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 25.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelan HA, Shafi S, Parks J, Maxson RT, Ahmad N, Murphy JT, et al. Use of a pediatric cohort to examine gender and sex hormone influences on outcome after trauma. J Trauma. 2007;63(5):1127–1131. doi: 10.1097/TA.0b013e318154c1b8. [DOI] [PubMed] [Google Scholar]
- 27.Jeschke MG, Przkora R, Suman OE, Finnerty CC, Mlcak RP, Pereira CT, et al. Sex differences in the long-term outcome after a severe thermal injury. Shock. 2007;27(5):461–465. doi: 10.1097/01.shk.0000238071.74524.9a. [DOI] [PubMed] [Google Scholar]
- 28.Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock. 2010;33(4):369–374. doi: 10.1097/SHK.0b013e3181b92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gatson JW, Maass DL, Simpkins JW, Idris AH, Minei JP, Wigginton JG. Estrogen treatment following severe burn injury reduces brain inflammation and apoptotic signaling. J Neuroinflammation. 2009;6(30) doi: 10.1186/1742-2094-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gore DC, Honeycutt D, Jahoor F, Wolfe RR, Herndon DN. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991;126(1):38–43. doi: 10.1001/archsurg.1991.01410250042006. [DOI] [PubMed] [Google Scholar]
- 31.Gore DC, Honeycutt D, Jahoor F, Rutan T, Wolfe RR, Herndon DN. Effect of exogenous growth hormone on glucose utilization in burn patients. J Surg Res. 1991;51(6):518–523. doi: 10.1016/0022-4804(91)90175-l. [DOI] [PubMed] [Google Scholar]
- 32.Jeschke MG, Kulp GA, Kraft R, Finnerty CC, Mlcak R, Lee JO, et al. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med. 2010;182(3):351–359. doi: 10.1164/rccm.201002-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy KD, Thomas S, Mlcak RP, Chinkes DL, Klein GL, Herndon DN. Effects of long-tem oxandrolone administration in severely burned children. Surgery. 2004;136(2):219–224. doi: 10.1016/j.surg.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res. 2010 Sep 2;1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]