Abstract
The injection of bacteria (Staphylococcus aureus, Stenotrophomonas maltophilia) or true fungi (Candida albicans, Candida tropicalis) that are pathogenic to humans into the silkworm hemolymph leads to death of the larvae within 2 days. Antibiotics used for clinical purposes have therapeutic effects on silkworms infected with these pathogens. The 50% effective doses obtained by injection into the silkworm hemolymph are consistent with those reported for mice. Injection of vancomycin and kanamycin into the silkworm hemolymph was effective, but oral administration was not. Chloramphenicol, which is effective by oral administration, appeared in the silkworm hemolymph soon after injection into the midgut, whereas vancomycin did not. Isolated midgut membranes were impermeable to vancomycin. Thus, the ineffectiveness of oral administration of vancomycin to silkworms is due to a lack of intestinal absorption.
Mice have been used for evaluation of the therapeutic effects of antibiotics for decades. The use of mice for the development of antibiotics, however, creates a number of problems. One is cost; infection experiments in specific-pathogen-free facilities, which are essential for the maintenance of experimental mice, are expensive. Second are the ethical issues surrounding the use of mammalian animals for the development of medicine, which is regulated by laws in European countries (15). To overcome these problems, evaluation of the therapeutic effects of chemicals in an alternative animal model is desirable.
Invertebrates have been used as an animal model of infectious diseases. Infectious models using Caenorhabditis elegans with Pseudomonas aeruginosa (9), and the fruit fly, Drosophila melanogaster, with Escherichia coli (3, 7) have been reported. Although invertebrates do not have acquired immune systems, they have developed innate immunity, such as that expressed by Toll-like receptors (7), which is highly conserved and important for resistance to infection by microorganisms.
Silkworms are big enough to be used in injection experiments, big enough for preparations of the hemolymph to be made, and big enough for organs such as the midgut to be isolated; these are essential methods for studying the pharmacodynamics of drugs in individual bodies. The lower cost and smaller space required for the maintenance of silkworms compared to mice allow us to handle a larger number of animals in limited facilities. Because of the long history of the silk industry, the method for taking care of silkworms is well established. Silkworms have the potential to serve as a large-scale drug-screening system. It was previously reported that silkworms were killed by injection of Staphylococcus aureus or other human pathogenic bacteria and that clinically used antibiotics are effective in the silkworm system (6). Quantitative evaluation of therapeutic effects of drugs in the silkworm model and comparisons with mice and humans, however, remained to be done. In this study, we performed quantitative measurement of the therapeutic effects of antibiotics in silkworms. The results demonstrated that 50% effective doses (ED50) in silkworms are consistent with those in mice. The effectiveness of oral administration of various antibiotics to silkworms is also consistent with results for mammals.
MATERIALS AND METHODS
Microorganisms and antibiotics
Clinically isolated S. aureus strain MSSA1 (1) was used in this study. Stenotrophomonas maltophilia was isolated at the University of Tokyo Hospital. Candida tropicalis (16) was kindly provided by Mitsuyoshi Ueda at Kyoto University. Candida albicans (2) was kindly provided by the Microbial Chemistry Research Center.
Kanamycin, chloramphenicol, and tetracycline were purchased from Meiji Seika Kaisha, Ltd. (Tokyo, Japan), Sankyo Co., Ltd. (Tokyo, Japan), and Sigma Aldrich (St. Louis, Mo.), respectively. Reagent grade powders of arbekacin, teicoplanin, vancomycin, minocycline, flomoxef, linezolid, amphotericin B, and fluconazole were kindly provided by Meiji Seika Kaisha, Ltd., Fujisawa Pharmaceutical Co., Ltd. (Tokyo, Japan), Shionogi & Co., Ltd. (Osaka, Japan), Wyeth-Lederle Japan (Tokyo, Japan), Shionogi & Co., Ltd., Pharmacia K.K. (Tokyo, Japan), Bristol-Myers Squibb Company (New York, N.Y.). and Pfizer Inc. (New York, N.Y.), respectively.
Determination of MICs of antibiotics
For MIC determination, bacteria were visually examined in twofold serial dilutions of tested samples in LB10 liquid medium (12). Cultures were incubated at 37°C for 24 h. True fungi were incubated in RPMI 4210 medium at 37°C for 48 h, and MICs were determined as described previously (13). The MIC was defined as the lowest concentration of the drug that completely inhibited the growth of the strain, except for fluconazole. For fluconazole, the MIC was determined according to a scale of 0 to 4 and defined as the lowest concentration of drug that produced a prominent decrease in turbidity compared with that of the drug-free control (a score of <2) (13).
Determination of ED50 for antibiotics
Silkworm eggs (Hu·Yo × Tukuba·Ne) were purchased from Ehime Sansyu (Ehime, Japan) and fed Silkmate 2S (Nosan Corporation, Yokohama, Japan) until they developed to the fourth molted larva. On the first day of fifth-instar larvae, silkworms were fed for 1 day with an antibiotic-free artificial food, Silkmate (Katakura Industries Co., Ltd., Tokyo, Japan). Suspensions of S. aureus or S. maltophilia in 0.6% NaCl (3 × 107 cells in 0.05 ml) or of C. albicans or C. tropicalis in Sabouraud medium (each at 1 × 106 or 4 × 106 cells in 0.05 ml) were injected into the hemolymph through the dorsal surface of the silkworm. The injection was confirmed by localization of [32P]dCTP in the hemolymph. Antibiotics (0.05 ml in 0.6% NaCl) were injected into the hemolymph (i.h.) or by the intramidgut (i.m.) route immediately after injection of the pathogens. For i.m. administration, antibiotics were injected into the midgut from the abdomen of a living silkworm by using a 27-gauge needle. For oral (p.o.) administration, samples were mixed in food and fed to the silkworms overnight before injection of the pathogens. More than 10 silkworms were used for each dose of antibiotics. After injection, silkworms were placed in a safety cabinet (BHC-1303IIA;AirTech Japan, Ltd., Tokyo, Japan) at 27°C with 50% humidity. A single injection of antibiotics was performed to determine ED50. Silkworms were not fed after the injections. The ED50 was determined as the amount of drug per 1 gram of silkworm required for 50% survival under conditions in which more than 90% of silkworms were killed by the pathogenic microorganisms.
Assay of antibiotic transport through the midgut membrane into the hemolymph in living silkworms
Hemolymph (0.05 to 0.1 ml) was harvested after injections of chloramphenicol (100 μg) or vancomycin (500 μg) into the midgut by cutting off the rear legs of the animals with scissors. Concentrations of the antibiotics in the hemolymph were determined by high-performance liquid chromatography. The volume of the hemolymph of a silkworm with a body weight of 1.8 g was estimated to be 0.67 ml by injection into the hemolymph of [32P]dCTP, which is not absorbed from the midgut (H. Hamamoto et al., unpublished data). Hemolymph samples were stored with β-mercaptoethanol (1%) at −70°C until analysis.
Assay of antibiotic transport with isolated midguts
Fifth-instar larvae of silkworms were anesthetized by being placed on ice for 10 min. The heads of the animals were cut off with scissors, and the peritrophic membranes containing the digested food were removed. The remainders of the bodies were soaked in ice-cold saline containing 10 mM HEPES-NaOH (pH 7.6) and 0.6% NaCl, and the midguts were carefully removed. The anus side of the midgut was ligated with cotton thread, and 500 μl of the solution containing the antibiotics was enclosed in the midgut bag and then incubated in 10 ml of the saline at 25°C in a 50-ml disposable tube (Becton Dickinson Labware, Franklin Lakes, N.J.). The amounts of vancomycin and chloramphenicol transported from the inside of the midgut bag to the outside were determined by absorbance at 280 nm. The absorbances in the absence of antibiotics were subtracted as the background. The concentration of kanamycin was determined by quantitative measurement of growth inhibition of S. aureus, determined by absorbance at 600 nm (14).
RESULTS
Therapeutic effects of antibiotics against pathogenic bacteria in silkworms
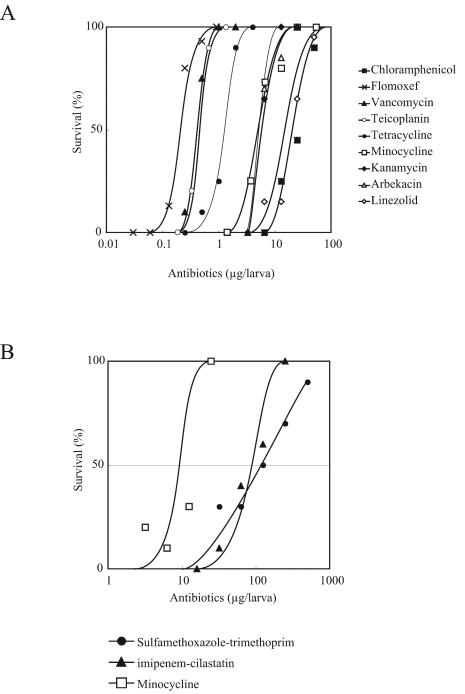
We examined whether antibiotics were effective in silkworms infected with S. aureus or S. maltophilia. The survival of silkworms was determined 2 days after injection of the bacterial suspension and antibiotics. Doses of S. aureus and S. maltophilia that killed 90% of the silkworms were used for control experiments without antibiotic administration. All tested antibiotics increased the number of surviving silkworms, depending on the dose (Fig. 1). The survival curves were used to determine the ED50, which was the dose of antibiotic that allowed for 50% survival of the silkworms (Table 1). The ED50-to-MIC ratio was less than 10 for all the samples tested. The ED50/MIC ratios obtained from silkworms were consistent with those obtained from mice in previous studies (4, 10).
FIG. 1.
Therapeutic effects of antibiotics in silkworms infected with S. aureus (A) or S. maltophilia (B). Data are representative of one of two identical experiments.
TABLE 1.
ED50 of antibiotics in a silkworm infection model with S. aureus or S. maltophilia
| Antibacterial agent | Bacteria | ED50 in silkworm (μg/g of larva) | MIC (μg/ml) | ED50/MIC ratio in:
|
|
|---|---|---|---|---|---|
| Silkworm | Mouse | ||||
| Kanamycin | S. aureus | 3 | 8 | 0.4 | |
| Arbekacin | S. aureus | 4 | 8 | 0.5 | |
| Teicoplanin | S. aureus | 0.3 | 0.5 | 0.6 | |
| Vancomycin | S. aureus | 0.3 | 1 | 0.3 | 1.1a |
| Tetracycline | S. aureus | 0.4 | 0.5 | 0.8 | |
| Minocycline | S. aureus | 3.9 | 0.4 | 9.8 | 3.5b |
| Chloramphenicol | S. aureus | 7 | 16 | 0.4 | |
| Flomoxef | S. aureus | 0.2 | 0.4 | 0.5 | 0.8b |
| Linezolid | S. aureus | 9 | 4 | 2.3 | 1.5a |
| Minocycline | S. maltophilia | 7.8 | 1 | 7.8 | |
| STc | S. maltophilia | 57 | 256 | 0.2 | |
| IPM/CSd | S. maltophilia | 50 | 256 | 0.2 | |
The number of bacteria (S. aureus) increased exponentially both in the hemolymph and in the tissue of silkworms after injection (6). This increase was repressed by injection of antibiotics in doses greater than the ED50 (chloramphenicol or vancomycin) into the hemolymph (data not shown).
Killing effects of pathogenic true fungi on silkworms and therapeutic effects of clinically used antifungal drugs
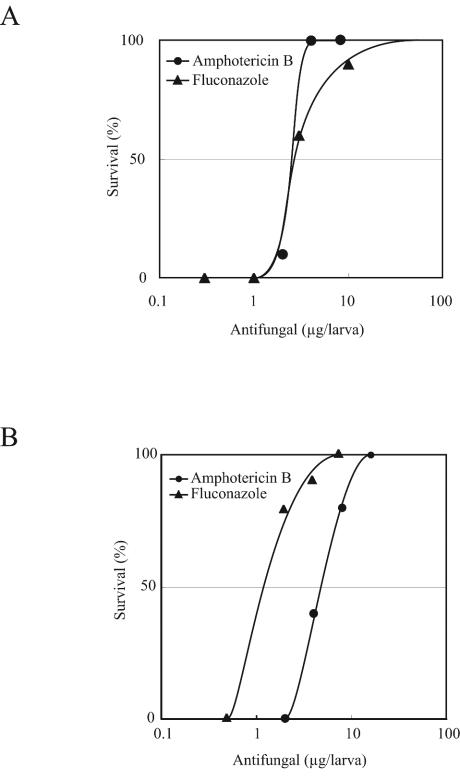
Injection of true fungi into the hemolymph resulted in silkworm death after 2 days. We tested whether amphotericin B and fluconazole were effective for silkworms infected with these true fungi. Results indicated that the number of surviving silkworms increased depending on the dose of the drugs (Fig. 2). ED50 were reproducibly determined from the survival curves. The ED50/MIC ratios for all of the antifungal drugs were less than 10 and were consistent with those from the mouse model (Table 2).
FIG. 2.
Therapeutic effects of antifungal drugs in silkworms infected with the true fungus C. tropicalis (A) or C. albicans (B). Data are representative of one of two identical experiments.
TABLE 2.
ED50 of antifungal agents in a silkworm model with C. tropicalis or C. albicans
| Antifungal agent | True fungus | ED50 in silkworm (μg/g of larva) | MIC (μg/ml) | ED50/MIC ratio in:
|
|
|---|---|---|---|---|---|
| Silkworm | Mousea | ||||
| Amphotericin B | C. tropicalis | 1.8 | 3.2 | 0.6 | 0.2 |
| C. albicans | 4.1 | 1.6 | 2.6 | 1.3 | |
| Fluconazole | C. tropicalis | 1.8 | 1.6 | 1.1 | 7.4 |
| C. albicans | 1.8 | 0.4 | 4.5 | 8.6 | |
ED50 and MIC were previously reported (5).
Oral administration of antibiotics to silkworms infected with pathogens
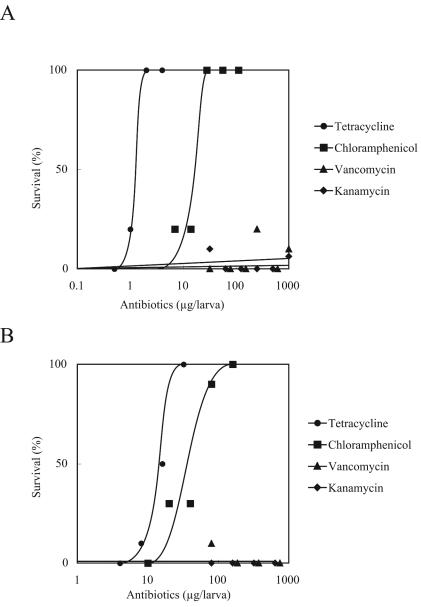
Injection of chloramphenicol and tetracycline i.m. (Fig. 3A) increased the survival of silkworms in a dose-dependent manner. The ED50 obtained for these antibiotics by i.m. administration were similar to those obtained by injection into the hemolymph (Table 3). On the other hand, vancomycin and kanamycin were ineffective when they were injected into the midgut (Fig. 3A). Chloramphenicol and tetracycline were effective even when mixed in food, whereas vancomycin and kanamycin were not (Fig. 3B; Table 1).
FIG. 3.
Therapeutic effects of antibiotics administered i.m. (A) or p.o. (B) on silkworms infected with S. aureus.
TABLE 3.
Oral administration of vancomycin or kanamycin does not have therapeutic effects in a silkworm model
| Antibiotic | ED50 (μg of antibiotic/g of larva) of drug administered by the following route:
|
||
|---|---|---|---|
| i.h. | i.m. | p.o. | |
| Chloramphenicol | 9 | 11 | 40 |
| Tetracycline | 0.4 | 1 | 8 |
| Vancomycin | 0.3 | >700 | >400 |
| Kanamycin | 3 | >700 | >500 |
Transport of antibiotics through midgut membranes in living silkworms
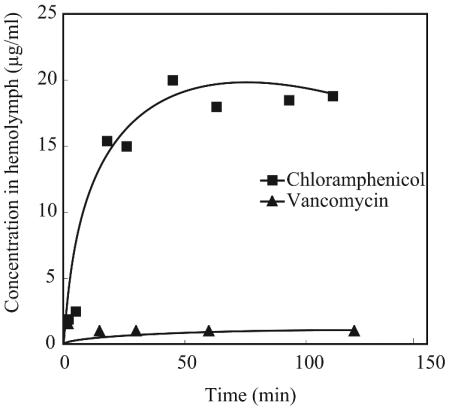
We next examined whether the ineffectiveness of vancomycin by the oral pathway in the silkworm model is due to the inability of this drug to penetrate midgut membranes. We injected vancomycin or chloramphenicol into the midguts of living silkworms and measured the concentrations of the drugs in the hemolymph. The results demonstrated that the concentration of vancomycin in the hemolymph did not increase when 500 μg of the drug was injected into the midgut. The concentration of chloramphenicol rapidly increased and was maintained at a high level when 100 μg of the drug was injected (Fig. 4).
FIG. 4.
Transport of chloramphenicol and vancomycin from the midgut to the hemolymph in living silkworms. All data points are averages of duplicate samples.
Assay of antibiotic transport through isolated midgut membranes
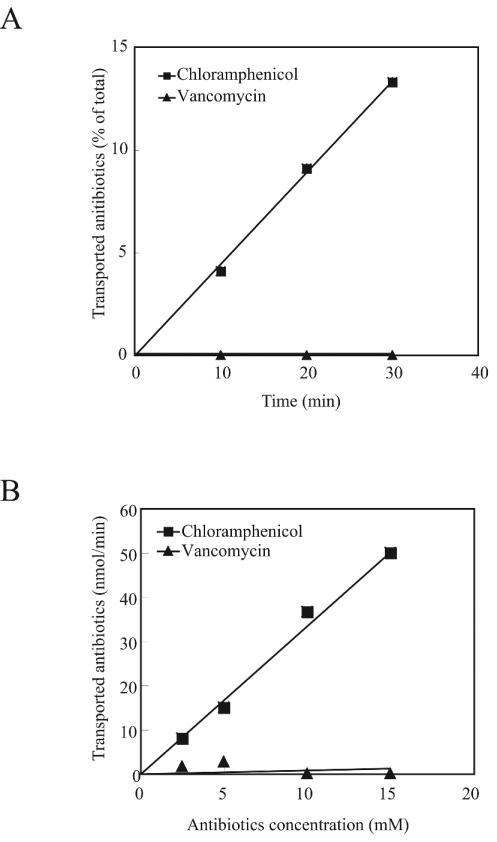
To further examine the inefficient absorption of vancomycin from the midgut, we performed an in vitro transport assay using isolated midguts. An antibiotic solution was enclosed in the midgut bag where both midgut ends were ligated with cotton thread, and the bag was incubated in saline. The silkworm midgut consists of a double membrane structure, the peritrophic membrane and the midgut epithelium (11). The peritrophic membrane is transparent and allows vancomycin and chloramphenicol to pass through (data not shown). Vancomycin did not pass through the midgut epithelial membrane (Fig. 5A), whereas the epithelial membrane was highly permeable to chloramphenicol (Fig. 5). The transport of vancomycin was negligible even when the concentration was increased to 15 mM. For chloramphenicol, the transport rate did not become saturated at 15 mM (Fig. 5B), suggesting that chloramphenicol is transported via passive diffusion and/or by a low-affinity pathway.
FIG. 5.
Transport of antibiotics from isolated midguts. (A) Time course of transport with 1 mM chloramphenicol or vancomycin. (B) Transport of chloramphenicol and vancomycin at various concentrations for 30 min. All data points are averages of duplicate samples.
DISCUSSION
In this study, we demonstrated that (i) silkworms are killed by injection of bacteria and true fungi that are pathogenic to humans, (ii) antibiotics clinically used for humans are effective for silkworms, and (iii) p.o. administration of vancomycin and kanamycin is ineffective in silkworms, as it is in humans. The results obtained with silkworms, in terms of sensitivity to pathogens and the effectiveness of antibiotics injected into blood vessels or administered p.o., are consistent with results obtained with mice. Silkworms have great advantages as an animal model: easy handling, low breeding costs, and no restrictions imposed by the ethical issues raised by the killing of mammalian animals. The silkworm model may be considered for antibiotic screening prior to the use of mice.
ED50/MIC ratios are useful for evaluating the pharmacodynamics of antibiotics. Smaller values indicate that the antibiotics functioned effectively in animal bodies, similar to their functioning in the culture medium in which the MICs were determined. This can be achieved if the drug concentrations are maintained in the body fluid for a long enough period without degradation or modification. The ED50/MIC ratios were less than 10 for all the antibiotics tested in this study. Therefore, we propose that a value lower than 10 in the silkworm model is a minimum requirement for antibiotics to merit further evaluation in mammalian systems. Another advantage of this model is that very small amounts of drugs can be tested. This is especially important when one is working with limited amounts of samples.
More pharmacokinetic data on antibiotics in silkworms are needed to evaluate the therapeutic effects. The distribution of hydrophilic solutes (e.g., β-lactam antibiotics) is greatly affected by protein binding in plasma (8). Characterization studies to determine the pharmacokinetics of compounds in silkworms are needed for further evaluation of the silkworm model.
Acknowledgments
We thank Kyoko Koinuma for technical assistance. We are grateful for Masayuki Igarashi (Microbial Chemistry Research Center) for helpful discussions. We also thank Mitsuyoshi Ueda (Kyoto University) and Atsushi Yoshida (The University of Tokyo hospital) for providing strains of C. tropicalis and S. maltophilia, respectively.
REFERENCES
- 1.Akimitsu, N., H. Hamamoto, R. Inoue, M. Shoji, A. Akamine, K. Takemori, N. Hamasaki, and K. Sekimizu. 1999. Increase in resistance of methicillin-resistant Staphylococcus aureus to beta-lactams caused by mutations conferring resistance to benzalkonium chloride, a disinfectant widely used in hospitals. Antimicrob. Agents Chemother. 43:3042-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ates, O., A. Gursoy, H. Altintas, G. Otuk, and S. Birteksoz. 2003. Synthesis and antimicrobial activity of {2-[2-(N,N-disubstituted thiocarbamoyl-sulfanyl)-acylamino] thiazol-4-yl} acetic acid ethyl esters. Arch. Pharm. (Weinheim) 336:39-46. [DOI] [PubMed] [Google Scholar]
- 3.Bernal, A., and D. A. Kimbrell. 2000. Drosophila Thor participates in host immune defense and connects a translational regulator with innate immunity. Proc. Natl. Acad. Sci. USA 97:6019-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford, C. W., J. C. Hamel, D. M. Wilson, J. K. Moerman, D. Stapert, R. J. Yancey, Jr., D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vivo activities of U-100592 and U-100766, novel oxazolidinone antimicrobial agents, against experimental bacterial infections. Antimicrob. Agents Chemother. 40:1508-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaito, C., N. Akimitsu, H. Watanabe, and K. Sekimizu. 2002. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 32:183-190. [DOI] [PubMed] [Google Scholar]
- 7.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 8.Levitt, D. G. 2003. The pharmacokinetics of the interstitial space in humans. BMC Clin. Pharmacol. 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahajan-Miklos, S., M. W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47-56. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda, H., K. Hori, Y. Kobayashi, T. Kikuyama, and H. Miwa. 1992. In vivo antibacterial activity of vancomycin. Chemotherapy (Tokyo) 40:451-458. (In Japanese with English abstract.) [Google Scholar]
- 11.Mitsuhashi, W., and K. Miyamoto. 2003. Disintegration of the peritrophic membrane of silkworm larvae due to spindles of an entomopoxvirus. J. Invertebr. Pathol. 82:34-40. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Okada, M., and S. Natori. 1985. Primary structure of sarcotoxin I, an antibacterial protein induced in the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J. Biol. Chem. 260:7174-7177. [PubMed] [Google Scholar]
- 15.Orlans, F. B., T. L. Beauchamp, R. Dresser, D. B. Morton, and J. P. Gluck. 1998. The human use of animals: case studies in ethical choice. Oxford University Press, New York, N.Y.
- 16.Tanaka, A., M. Yamamura, S. Kawamoto, and S. Fukui. 1977. Production of uricase by Candida tropicalis using n-alkane as a substrate. Appl. Environ. Microbiol. 34:342-346. [DOI] [PMC free article] [PubMed] [Google Scholar]