Abstract
In Enterobacter aerogenes and Klebsiella pneumoniae, efflux provides efficient extrusion of antibiotics and contributes to the multidrug resistance phenotype. One of the alkoxyquinoline derivatives studied here, 2,8-dimethyl-4-(2′-pyrrolidinoethyl)-oxyquinoline, restores noticeable drug susceptibility to resistant clinical strains. Analyses of energy-dependent chloramphenicol efflux indicate that this compound inhibits the efflux pump mechanism and improves the activity of structurally unrelated antibiotics on multidrug-resistant E. aerogenes and K. pneumoniae isolates.
Various multidrug resistance (MDR) phenotypes that confer active protection against environmental toxic compounds by efflux mechanisms have been described in Enterobacteriaceae (1, 9, 16, 27, 28). One of these drug ejection systems, the efflux detected in resistant gram-negative bacteria, depends on membrane energy and efficiently expels structurally unrelated antibiotic molecules across the bacterial envelope via a tripartite complex comprising an inner membrane pump, a periplasmic fusion protein, and an outer membrane channel (26, 31).
Enterobacter aerogenes and Klebsiella pneumoniae are frequently described in resistant nosocomial infections (2-4, 10, 12, 24). In these bacteria, the marRAB, acrAB-tolC, and ramA genes are involved in expression of the MDR phenotype (8, 29, 32). Moreover, various clinical isolates show alteration of nonspecific porins associated with the presence of active drug efflux; both processes maintain a very low intracellular concentration of drugs and contribute to a high resistance level for structurally unrelated molecules including β-lactam antibiotics, quinolones, tetracyclines, and chloramphenicol (5, 6, 21, 24). An important medicinal challenge is to find new compounds capable of circumventing the efflux machinery (7, 19, 20, 22, 30). The aim of this study was to analyze 4-alkoxy-substituted quinolines, termed efflux pump inhibitors (EPI), with respect to their ability to interfere with the efflux pump.
The strains used in this work were E. aerogenes EA3, EA27, and EA117 and K. pneumoniae KP55 clinical isolates exhibiting active efflux of norfloxacin or chloramphenicol (6, 15, 21) and TolC− and AcrA− E. aerogenes EA27 derivatives previously constructed (29). MICs, chloramphenicol uptake, potassium efflux, and β-lactamase activities were determined as previously described (13, 21).
Biological effect of alkoxyquinolines on a resistant E. aerogenes strain
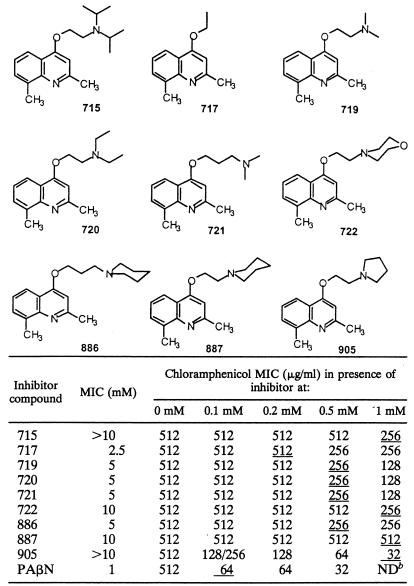
Documented clinical isolate EA27, overexpressing the AcrAB complex owing to a frameshift mutation in acrR (21, 29), was used to determine the activity of nine alkoxyquinolines. The alkoxyquinoline compounds and phenylalanine-arginine-β-naphthylamide (PAβN), a previously characterized EPI (20, 22), showed poor intrinsic antimicrobial activities with high MICs (Table 1). These low intrinsic activities allowed us to analyze the restoring effect of the molecules on the antibiotic susceptibility of several MDR strains. The various compounds were assayed for the ability to induce a decrease in the chloramphenicol resistance of E. aerogenes EA27 (Table 1). Compound 905 was effective as a reverse chemosensitizer of chloramphenicol susceptibility, with a 16-fold decrease in the MIC. This effect was observed at an alkoxyquinoline concentration corresponding to 1/10 of its proper MIC (Table 1).
TABLE 1.
Antibacterial and chemical properties of various alkoxyquinolinesa
Syntheses of the compounds have been previously described (17). MICs were determinated in Mueller-Hinton broth as previously described on clinical isolate EA27 (21). The chloramphenicol susceptibility of E. aerogenes EA27 was tested in the presence of various concentrations of alkoxyquinolines or PAβN (0 to 1 mM). The underlined values correspond to chloramphenicol MICs obtained for a compound concentration corresponding to 1/10 of its proper MIC. Values are means of three independent determinations.
ND, not determined (corresponding to the MIC of PAβN).
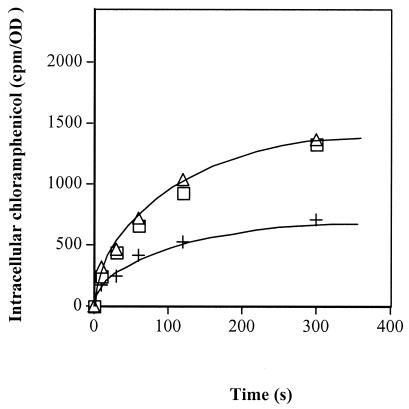
The effects of compound 905 and PAβN on intracellular chloramphenicol accumulation were evaluated in strain EA27. Addition of compound 905 induced a twofold increase in the intracellular antibiotic concentration (Fig. 1), and we observed a similar accumulation in the presence of PAβN. These results suggest that compound 905 induces inhibition of chloramphenicol pump activity, the AcrAB/TolC system, which is overexpressed in this strain (29).
FIG. 1.
Effect of compound 905 on chloramphenicol accumulation in E. aerogenes EA27. Exponential-phase bacteria in Luria-Bertani broth were removed, resuspended in sodium phosphate buffer, and incubated with radiolabeled chloramphenicol for various times (15, 22). Intracellular accumulations were followed in the absence (+) or presence of compound 905 (□) or PAβN (▵) at 0.1 mM. Values (expressed as counts per minute/optical density [cpm/OD]) were obtained from independent duplicate measurements.
Effects of compound 905 and PAβN on E. aerogenes EA27 membrane
A major concern with the chemosensitizer is a possible permeabilizing effect on the membrane. To clarify this point, we analyzed membrane integrity in two independent ways. We measured potassium leakage (13) following addition of the alkoxyquinoline molecule. No significant K+ release was observed after the addition of compound 905, even at a high concentration, whereas noticeable potassium release was obtained under the same conditions with polymyxin B, a well-known inducer of membrane permeabilization (data not shown). In addition, we tested the effects of compound 905 and PAβN on β-lactamase localization in EA27 cells. The products did not induce significant detection of periplasmic activity in the medium (Table 2), suggesting that compound 905 had no permeabilizing effect on E. aerogenes membrane under the conditions restoring antibiotic susceptibility.
TABLE 2.
β-Lactamase localization and compound 905 or PAβN treatmenta
| Compound | β-Lactamase activity (mU) in:
|
β-Lactamase in medium (%) | |
|---|---|---|---|
| Cell suspension | Lysate of cell suspension | ||
| None | 24 | 640 | 3.8 |
| 905 | 30 | 670 | 4.5 |
| PAβN | 60 | 690 | 8.7 |
The rate of nitrocefin hydrolysis was investigated in strain EA27 incubated in the absence or presence of PAβN or compound 905 (0.2 and 1 mM, respectively). Enzymatic activity was measured in equivalent amounts of intact cell suspension and the resulting total cell lysate obtained after sonication as previously described (21). The ratio of detected β-lactamase activity in cell suspension to that in lysate of cell suspension corresponds to the percentage of periplasmic activity detected in cell medium without cell disruption. One milliunit of β-lactamase was defined as the amount of enzyme that hydrolyzed 1 mmol of nitrocefin min−1 mg of protein−1 at 25°C (21). Values are means of two independent determinations.
Effect of compound 905 on the drug susceptibility of various efflux pump producers
We tested the activity of compound 905 in restoring susceptibility to structurally unrelated antibiotic classes (Table 3). This compound increased the susceptibility of four clinical isolates, three resistant E. aerogenes strains and one resistant K. pneumoniae strain, for norfloxacin, tetracycline, and chloramphenicol (Table 3), which are efflux pump substrates (6, 15, 22). In contrast, we observed no significant variation in the MICs of cefepime. It is important to note that a severe alteration of porin, which is involved in the uptake of hydrophilic solutes, has been previously reported in the different isolates tested: EA3 synthesizes a channel-altered porin, while EA27, EA117, and KP55 produce very small porin amounts (6, 11, 15, 21). Mutations in the antibiotic target, e.g., substitutions in the QRDR domain of GyrA, have been reported in isolate EA117 (15).
TABLE 3.
Effects of compound 905 on susceptibilities to structurally unrelated antibiotics in E. aerogenes EA3, EA27, and EA117 and K. pneumoniae KP55
| Strain | Presence of compound 905 (1 mM) | MIC (μg/ml)a
|
|||
|---|---|---|---|---|---|
| CM | NFX | TC | CEF | ||
| EA27 | − | 512 | 256 | 16 | 32 |
| EA27 | + | 32 | 64 | 4 | 64 |
| EA3 | − | 512 | 128 | 8 | 64 |
| EA3 | + | 32 | 32 | 0.5 | ND |
| EA117 | − | 512 | 256 | 16 | 64 |
| EA117 | + | 32 | 64 | 0.5 | 128 |
| KP55 | − | 32 | 16 | 512 | 128 |
| KP55 | + | 4 | 2 | 64 | 128 |
Drugs were tested alone (−) or in the presence of alkoxyquinoline (+). MICs are means of three independent determinations. CM, chloramphenicol; NFX, norfloxacin; TC, tetracycline; CEF, cefepime; ND, not determined.
To evaluate the contribution of compound 905 as a putative AcrAB/TolC pump inhibitor, we investigated the effect of compound 905 on EA27 and AcrA− and TolC− derivatives previously characterized (29). The chloramphenicol and norfloxacin MICs for AcrA− and TolC− strains were not modified by the addition of compound 905 (Table 4). In addition, the AcrA or TolC knockout generated an increase in chloramphenicol and norfloxacin susceptibility (29) slightly greater than that obtained with the inhibitors in the parental strains (Table 4).
TABLE 4.
Effects of compound 905 on the chloramphenicol and norfloxacin susceptibilities of EA27 and AcrA− and TolC− derivatives
| Strain | Presence of inhibitor compound 905 | MIC (μg/ml)a
|
|
|---|---|---|---|
| CM | NFX | ||
| EA27 | − | 512 | 256 |
| + | 32 | 64 | |
| TolC− | − | 32 | 16 |
| + | 16 | 8 | |
| AcrA− | − | 32 | 32 |
| + | 16 | 32 | |
Chloramphenicol (CM) and norfloxacin (NFX) were tested alone or in the presence of compound 905. MIC are means of three independent determinations.
Identification of inhibitors of the efflux pump mechanism is of particular interest as regards the restoring intracellular antibiotic concentration. In this study, nine alkoxyquinolines were assayed on MDR clinical E. aerogenes strains as potential inhibitors of the efflux mechanism. Of these derivatives, one molecule, compound 905, induced an efficient increase in chloramphenicol, tetracycline, and fluoroquinolone susceptibility in various strains expressing the drug efflux process. The partial recovery of antibiotic susceptibility obtained with compound 905 is related to the other resistance mechanisms, mutation or modifying enzymes, reported in clinical isolates. Moreover, when compound 905 is added during incubation with chloramphenicol, an increase in intracellular antibiotic accumulation is observed in an MDR strain which overexpressed the AcrAB efflux pump (29). These results provide clear evidence that this alkoxyquinoline blocks antibiotic ejection in E. aerogenes and in K. pneumoniae clinical isolates. Although this response may be associated with an interfering effect that occurs during active pumping out of the antibiotic molecule, the efficiency of susceptibility restoration depends on the respective affinity of the transported drug and that of the competitor for the pump system involved in the efflux mechanism. A strong effect of compound 905 was observed on the drug susceptibility of strain EA27, which overexpresses the AcrAB pump, while no significant effect was obtained on the AcrA− and TolC− derivatives, for which the MICs of the corresponding antibiotics are lower.
Interestingly, we recently reported that 4-[2′-(piperidino)ethyl]-thioquinoline and 7-nitro-8-methyl-4-[2′-(piperidino)propyl]-thioquinoline at a high concentration are able to induce a slight increase in chloramphenicol susceptibility in strain EA27 (14). Similarly, 7-nitro-8-methyl-4-[2′-(piperidino)ethyl]-aminoquinoline blocks chloramphenicol efflux (23). Consequently, structure-activity relationship studies concerning homologous derivatives may be fruitfully undertaken to find more active molecules belonging to the series described here. Owing to the resolution of the three-dimensional structure of pump components (18, 25), the development of efficient responses to MDR bacterial pathogens with this family of inhibitors is open.
Acknowledgments
We thank C. Bollet, E. Pradel, and A. Davin-Regli for discussions. Labeled chloramphenicol was a generous gift from Aventis Hoechst Marion Roussel (Romainville, France).
This study was supported by the Université de la Méditerranée, the Centre National de la Recherche Scientifique, and Astra-Zeneca (ESCMID grant to J.-M. P.).
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1999. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7:410-413. [DOI] [PubMed] [Google Scholar]
- 2.Arpin, C., C. Coze, A. M. Rogues, J. P. Gachie, C. Bebear, and C. Quentin. 1996. Epidemiological study of an outbreak due to multidrug-resistant Enterobacter aerogenes in a medical intensive care unit. J. Clin. Microbiol. 34:2163-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornet, C., A. Davin-Regli, C. Bosi, J.-M. Pagès, and C. Bollet. 2000. Imipenem resistance of Enterobacter aerogenes mediated by outer membrane permeability. J. Clin. Microbiol. 38:1048-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, P. A., C. Urban, N. Mariano, S. J. Projan, J. J. Rahal, and K. Bush. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charrel, R. N., J.-M. Pagès, P. De Mico, and M. Malléa. 1996. Prevalence of outer membrane porin alteration in β-lactam-antibiotic-resistant Enterobacter aerogenes. Antimicrob. Agents Chemother. 40:2854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier, J., J.-M. Pagès, A. Eyraud, and M. Malléa. 2000. Membrane permeability modifications are involved in antibiotic resistance in Klebsiella pneumoniae. Biochem. Biophys. Res. Commun. 274:496-499. [DOI] [PubMed] [Google Scholar]
- 7.Chevalier, J., S. Atifi, A. Eyraud, A. Mahamoud, J. Barbe, and J.-M. Pagès. 2001. New pyridoquinoline derivatives as potential inhibitors of the fluoroquinolone efflux pump in resistant Enterobacter aerogenes strains. J. Med. Chem. 44:4023-4026. [DOI] [PubMed] [Google Scholar]
- 8.Chollet, R., C. Bollet, J. Chevalier, M. Malléa, J.-M. Pagès, and A. Davin-Regli. 2002. mar operon involved in multidrug resistance of Enterobacter aerogenes. Antimicrob. Agents Chemother. 46:1093-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, S. P., H. Hächler, and S. B. Levy. 1993. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J. Bacteriol. 175:1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davin-Regli, A., D. Monnet, P. Saux, C. Bosi, R. Charrel, A. Barthelemy, and C. Bollet. 1996. Molecular epidemiology of Enterobacter aerogenes acquisition: one-year prospective study in two intensive care units. J. Clin. Microbiol. 34:1474-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dé, E., A. Baslé, M. Jaquinod, N. Saint, M. Malléa, G. Molle, and J.-M. Pagès. 2001. A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin. Mol. Microbiol. 41:189-198. [DOI] [PubMed] [Google Scholar]
- 12.De Gheldre, Y., M. J. Struelens, Y. Glupczynski, P. De Mol, N. Maes, C. Nonhoff, H. Chetoui, C. Sion, O. Ronveaux, M. Vaneechoutte, and le Groupement pur le Dépistage, l'Etude et la Prevention des Infections Hospitalières (GDEPIH-GOSP1Z). 2001. National epidemiologic surveys of Enterobacter aerogenes in Belgian hospitals from 1996 to 1998. J. Clin. Microbiol. 39:889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Kouhen, R., and J.-M. Pagès. 1996. Dynamic aspects of colicin N translocation through the Escherichia coli outer membrane. J. Bacteriol. 178:5316-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo S., J. Chevalier, A. Mahamoud, A. Eyraud, J.-M. Pagès, and J. Barbe. 2003. 4-Alkoxy and 4-thioalkoxyquinoline derivatives as chemosensitizers for the chloramphenicol resistant clinical Enterobacter aerogenes 27 strain. Int. J. Antimicrob. Agents 22:270-273. [DOI] [PubMed] [Google Scholar]
- 15.Gayet, S., R. Chollet, G. Molle, J.-M. Pagès, and J. Chevalier. 2003. Modification of outer membrane protein profile and evidence suggesting an active drug pump in Enterobacter aerogenes clinical strains. Antimicrob. Agents Chemother. 47:1555-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George, A. M. 1996. Multidrug resistance in enteric and other Gram-negative bacteria. FEMS Microbiol. Lett. 139:1-10. [DOI] [PubMed] [Google Scholar]
- 17.Kayirere, M. G., A. Mahamoud, J. Chevalier, J. C. Soyfer, A. Crémieux, and J. Barbe. 1998. Synthesis and antibacterial activity of new 4-alkoxy, 4-aminoalkyl and 4-alkylthioquinoline derivatives. Eur. J. Med. Chem. 33:55-63. [Google Scholar]
- 18.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 19.Lewis, K. 2001. In search of natural substrates and inhibitors of MDR pumps. J. Mol. Microbiol. Biotechnol. 3:247-254. [PubMed] [Google Scholar]
- 20.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malléa, M., J. Chevalier, C. Bornet, A. Eyraud, A. Davin-Regli, C. Bollet, and J.-M. Pagès. 1998. Porin alteration and active efflux: two in vivo drug resistance strategies used by Enterobacter aerogenes. Microbiology 144:3003-3009. [DOI] [PubMed] [Google Scholar]
- 22.Malléa, M., J. Chevalier, A. Eyraud, and J.-M. Pagès. 2002. Inhibitors of antibiotic efflux pump in resistant Enterobacter aerogenes strains. Biochem. Biophys. Res. Commun. 293:1370-1373. [DOI] [PubMed] [Google Scholar]
- 23.Malléa, M., A. Mahamoud, J. Chevalier, S. Alibert-Franco, P. Brouant, J. Barbe, and J.-M. Pagès. 2003. Alkylaminoquinolines inhibit the bacterial antibiotic efflux pump in multidrug-resistant clinical isolates. Biochem. J. 376:801-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Martinez, L., A. Pascual, M. C. Conejo, I. Garcia, P. Joyanes, A. Domenech-Sanchez, and V. J. Benedi. 2002. Energy-dependent accumulation of norfloxacin and porin expression in clinical isolates of Klebsiella pneumoniae and relationship to extended-spectrum β-lactamase production. Antimicrob. Agents Chemother. 46:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami, S., R. Nakashima, E. Yamashita, and A. Yamaguchi. 2002. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419:587-593. [DOI] [PubMed] [Google Scholar]
- 26.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 27.Nikaido, H. 2001. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin. Cell Dev. Biol. 12:215-223. [DOI] [PubMed] [Google Scholar]
- 28.Poole, K. 2001. Multidrug resistance in Gram-negative bacteria. Curr. Opin. Microbiol. 4:500-508. [DOI] [PubMed] [Google Scholar]
- 29.Pradel, E., and J.-M. Pagès. 2002. The AcrAB-TolC efflux pump contributes to multidrug resistance in the nosocomial pathogen Enterobacter aerogenes. Antimicrob. Agents Chemother. 46:2640-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renau, T. E., R. Leger, E. M. Flamme, J. Sangalang, M. W. She, R. Yen, C. L. Gannon, D. Griffith, S. Chamberland, O. Lomovskya, S. J. Hecker, V. J. Lee, T. Ohta, and K. Nakayama. 1999. Inhibitors of efflux pumps in P. aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J. Med. Chem. 42:4928-4931. [DOI] [PubMed] [Google Scholar]
- 31.Saier, M. H., and I. T. Paulsen. 2001. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 12:205-213. [DOI] [PubMed] [Google Scholar]
- 32.Schneiders, T., S. G. B. Amyes, and S. B. Levy. 2003. Role of AcrR and RamA in fluoroquinolone resistance in clinical Klebsiella pneumoniae isolates from Singapore. Antimicrob. Agents Chemother. 47:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]