Abstract
This study of healthy volunteers shows that the relative bioavailability of albendazole formulations that use arachis oil-polysorbate 80 or hydroxypropyl-β-cyclodextrin as an excipient was enhanced 4.3- and 9.7-fold compared to the results seen with commercial tablets. Administration of macrogol suppositories did not result in measurable plasma concentrations of albendazole sulfoxide.
The therapeutic response of albendazole (20 to 50%) in cases of echinococcosis is variable and difficult to predict (2, 8). Albendazole's poor intestinal absorption (<5%) (due to its low aqueous solubility) is probably a major determinant of the variable response rate (9). In animal studies, the main strategy to enhance albendazole bioavailability was by that of increasing water and lipid solubility by the use of cosolvents or surfactants or of incorporating albendazole into particles. The relative bioavailability of albendazole in mice increased 1.8-fold by combining it with the cosolvent Transcutol (diethylene glycol monoethylether) (18). A formulation of a solid dispersion enhanced the relative bioavailability in rabbits threefold (11). Albendazole coadministered with the surfactant sodium taurocholate or polysorbate enhanced the area under the concentration curve (AUC) value for rats by 55 or 88%, respectively (3, 4, 5), whereas incorporation into liposomes increased the uptake in rats by more than twofold (19) and incorporation into a cyclodextrin increased the AUC for sheep by 37% (6, 16).
For humans, several strategies were studied to enhance albendazole bioavailability. When combined with a fatty meal, administration of albendazole sulfoxide (ABZSX) increased the maximum concentration of drug in serum (Cmax) 4.5- to 9-fold (1, 7, 12, 15). To inhibit CYP3A4 isozyme-related ABZSX degradation, albendazole was coadministered with cimetidine. However, this caused a 52% decrease in ABZSX Cmax values (probably due to inhibition of gastric acid secretion) (15, 17), suggesting that absorption is pH dependent. When coadministered with grapefruit juice, the ABZSX Cmax was enhanced 3.2-fold, probably due to inhibition of the intraluminal degradation of albendazole by CYP3A4 enzymes (15). In one human study, a soybean oil emulsion was used to enhance albendazole solubility and showed a 1.6-fold enhancement of relative bioavailability compared with tablets (14).
The present report presents findings for a human study comparing the relative levels of bioavailability of three new pharmaceutical albendazole formulations with that of the commercially available tablet. These three new formulations include an oral suspension with arachis oil and the surfactant polysorbate 80 (aimed at increasing lipid solubility), an oral solution of albendazole incorporated into hydroxypropyl-β-cyclodextrin (aimed at increasing water solubility), and a macrogol suppository (aimed at avoiding the intestinal degradation of albendazole).
The suspension was prepared by mixing 9.0 g of albendazole (Duchefa, Haarlem, The Netherlands) with 36.0 g of arachis oil, 2.0 g of anise oil, 112.5 g of polysorbate 80, 2.16 g of citric acid, and 2.7 g of potassium sorbate dissolved in 315 ml of water. This emulsion was mixed with 453 g of suspension vehicle (1,068 g = 1,000 ml of suspension vehicle containing 263 g of syrup [63% g/g of sucrose], 0.75 g of methylparahydroxybenzoate, 0.75 g of citric acid, 10 g of sodium carboxymethylcellulose, 10 g of aluminum magnesium silicate, and 783.5 g of water); 80 ml of the suspension contained 800 mg of albendazole. The oral solution was prepared with 40% (g/vol) hydroxypropyl-β-cyclodextrin (Wacker Chemie, Munich, Germany) in water containing 3 mg of albendazole/ml, 50 mM citric acid, and 0.15% methylparahydroxybenzoate. The suppositories contained 400 mg of albendazole each in macrogol 1500 and macrogol 4000 (3:1). The actual albendazole contents were checked by high-performance liquid chromatography and were all between 95 to 105% of the nominal content. The reference formulation was the tablet (Eskazole; Glaxo-Smith-Kline, Zeist, The Netherlands).
A total of 10 volunteers (four males and six females 18 to 41 years of age, 55 to 110 kg in body weight, and 19 to 34 kg/m2 in body mass) participated on four subsequent occasions, with a washout period of 1 week between study days. Adverse events were registered when reported by the volunteers. After the subjects fasted overnight, 125 ml of Coca Cola Classic (pH = 2.5) was administered (to reduce possible intersubject variability of gastric pH) followed by albendazole administration. Food was withheld for 4 h after the drug was taken, but noncaffeine, nonalcoholic beverages were allowed freely. Then, a light meal (30 g of fat, 49 g of carbohydrates, 25 g of protein, 575 kcal) was offered and dietary restrictions were removed.
Immediately prior to and 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 24 h after drug administration, 7.5-ml venous blood samples were taken. All volunteers gave written informed consent; the study was approved by the Institutional Review Board.
ABZSX plasma concentrations were measured by high-performance liquid chromatography with UV detection (290 nm) using a modification of the method of Kitzman et al. (10). The calibration curve was linear (50 to 5,000 μg/liter; r = 0.99998; n = 6). Control specimens (50, 100, and 1,000 μg of ABZSX/liter) were injected during every analytical run. The quantification limit was 20 μg of ABZSX/liter, the within-day coefficients of variation of the calibration controls (20, 500, and 5,000 μg/liter) were 6.9, 4.1, and 4.8%, respectively, and the interday coefficients were 8.5, 3.8, and 3.9%.
For each subject, the Cmax, AUC0-∞ (the area under the plasma concentration-versus-time curve to infinity), Tmax (the time to reach Cmax), and T1/2 (elimination half-life) were estimated by applying a two-compartment pharmacokinetic model and Bayesian curve fitting (MW\Pharm 3.50; Mediware, Zuidlaren, The Netherlands). Relative bioavailability values were calculated according to the ratio of the AUC0-∞ of a solution, suspension, or suppository relative to that of the tablets. Cmax and Tmax values were read from the data.
One-way analyses of variance and Wilcoxon signed ranks nonparametric tests were performed (SPSS 10.1 for Windows; SPSS, Chicago, Ill.).
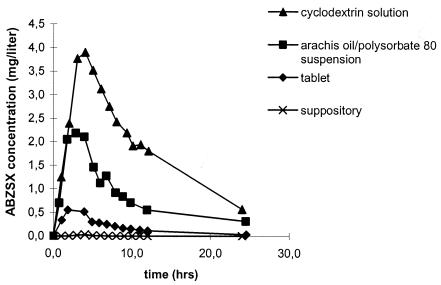
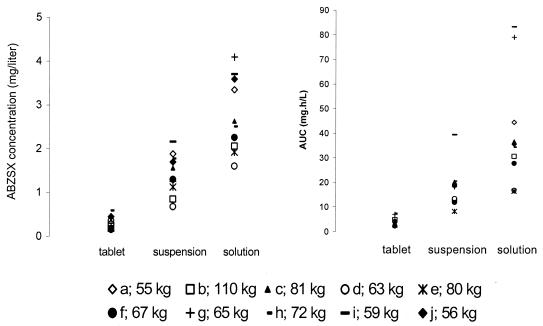
ABZSX concentration-time curves for subject J are shown in Fig. 1, and Cmax and AUC0-∞ values for all 10 individuals are shown in Fig. 2. No ABZSX was detected in plasma following administration of the suppositories. The parent drug albendazole was not detected in plasma from any of our subjects.
FIG. 1.
ABZSX concentration-versus-time curves for subject J after administration of a single dose of 800 mg of albendazole (given as a cyclodextrin solution, an arachis oil-polysorbate 80 suspension, a tablet, and a suppository).
FIG. 2.
Cmax and AUC0- 52 values for ABZX in 10 healthy subjects (subjects a to j) after administration of a single dose of 800 mg of albendazole (given as a tablet, an arachis oil-polysorbate 80 suspension, and a cyclodextrin solution).
Bayesian fits were satisfactory, with mean r2 values of 0.999, 0.991, and 0.999 (n = 10) for tablets, the suspension, and the cyclodextrin solution, respectively.
In comparison to the results seen with tablets, the mean Cmax was enhanced 4.8-fold by the arachis oil-polysorbate 80 suspension (P = 0.002) and 9.2-fold by the cyclodextrin solution (P = 0.002) (Table 1). The coefficients of variation were high (47% for tablets, 33% for the suspension, and 31% for the solution).
TABLE 1.
Cmax, Tmax, T1/2, AUC0-∞, and relative bioavailability of three different albendazole formulations (tablet, arachis oil-polysorbate 80 suspension, and cyclodextrin solution) for 10 healthy volunteers
| Dosage form | Cmax (mg/liter)a | Tmax (h)a | AUC0-∞ (mg · h/liter)a (95% CI)b | Fc |
|---|---|---|---|---|
| Tablet | 0.30 ± 0.14 | 3.18 ± 0.96 | 4.20 ± 1.85 (2.87-5.52) | 1 |
| Suspension | 1.43 ± 0.47 | 3.37 ± 1.62 | 18.22 ± 8.53 (12.13-24.33) | 4.34 |
| Solution | 2.77 ± 0.86 | 4.36 ± 1.80 | 40.56 ± 23.15 (24.01-57.12) | 9.66 |
Results represent means ± standard deviations.
CI, confidence interval.
F, relative bioavailability. Results represent means.
In comparison to the results seen with tablets, the relative bioavailability of the suspension was enhanced 4.3-fold (P = 0.002) and that of the cyclodextrin solution was enhanced 9.7-fold (P = 0.002; Table 1). Tmax values for the tablets, the suspension, and the solution were similar (P > 0.05), as were T1/2 values (P > 0.05).
Seven subjects had diarrhea 4 to 6 hours following administration of the solution with hydroxypropyl-β-cyclodextrin. No other adverse events were observed.
Improving the bioavailability of albendazole may result in better outcome of treatment. Our study focused on enhancing albendazole absorption by improving lipid solubility and water solubility and by avoiding intestinal metabolic breakdown. The possible effect of the interindividual variable gastric pH on absorption was minimized by administering Coca-Cola Classic (pH = 2.5) immediately before administering albendazole.
It is well known that combining albendazole with a fatty meal increases bioavailability 4.5- to 9-fold (1, 7, 12, 15). Whether the surfactant effect of the bile salts which are secreted after a fatty meal also contributes to albendazole absorption in humans is not clear. In animal studies, however, surfactants have been shown to improve albendazole absorption (3, 4, 5). These observations were the rationale for developing a formulation which contains a combination of arachis oil and the surfactant polysorbate 80. With this formulation, enhanced Cmax and relative bioavailability results were indeed seen. However, the degree of enhancement is in the lower range of what was reported respecting the effect of combining albendazole with a fatty meal. Thus, the additional effect of combining arachis oil with the surfactant polysorbate 80 was not evident.
Water solubility of albendazole was improved by incorporating the drug into hydroxypropyl-β-cyclodextrin, and the inclusion complex seems to be sufficiently labile to release free albendazole appropriately. The observed enhancement of Cmax and the relative bioavailability results with this formulation were in the upper range of what was reported respecting the effect of combining albendazole with a fatty meal. In this respect, our reference cohort (i.e. the subjects who were administered a tablet after an overnight fast) does not represent the ideal clinically applicable control, which would have consisted of subjects administered a tablet taken with a fatty meal. Nevertheless, we think our data provide insight into new formulations of albendazole in which absorption is enhanced.
Unfortunately, the cyclodextrin solution was not tolerated well. When administered in the current dose and volume (800 mg in 267 ml), the cyclodextrin solution caused diarrhea (probably due to the osmotic effect of cyclodextrin) with almost all subjects. Nevertheless, the relative bioavailability was high and a three- to fivefold-lower dose may be sufficient to achieve high plasma ABZSX concentrations without diarrhea.
The aim of administering albendazole as a suppository was that of avoiding the metabolic breakdown of albendazole by CYP3A4 enzymes located in the small-intestinal mucosa. Macrogol (which readily dissolves in body fluids and thereby releases albendazole in the rectum) (13) was used as a vehicle. However, the administration of macrogol suppositories did not result in any detectable ABZSX concentrations. The failure of the macrogol suppositories is not well understood, but it might be related to the poor water solubility of albendazole, to the neutral pH (pH ± 7.5) (which decreases albendazole absorption) in the rectum, or possibly to intraluminal degradation. A fatty suppository vehicle might be more effective.
Intestinal absorption was not delayed by administering albendazole as an oil-surfactant suspension or as a cyclodextrin solution, as was evident from results showing similar Tmax values. Also, ABZSX elimination half-life values following administration of the oil-surfactant suspension and the cyclodextrin solution were comparable to the results seen with the tablets. The observed data with respect to the main parameters (Cmax, Tmax, and T1/2) and the absence of any detectable ABZ concentrations indicate that the absorption rate of albendazole, the rapid metabolism into ABZSX, and the subsequent breakdown were not affected by the administration of albendazole as an oil-surfactant suspension or as a cyclodextrin solution.
We conclude that in comparison to the results seen with the tablet form, bioavailability can be significantly enhanced by the administration of albendazole as an oil-surfactant suspension or as a cyclodextrin solution. Rectal administration of albendazole-containing macrogol suppositories failed. Further studies investigating methods of improving tolerability and clinical efficacy of the oil-surfactant suspension and the cyclodextrin solution are warranted.
REFERENCES
- 1.Awadzi, K., M. Hero, N. O. Opoku, D. W. Büttner, P. A. Coventry, M. A. Prime, M. L. E. Orme, and G. Edwards. 1994. The chemotherapy of onchocerciasis XVII. A clinical evaluation of albendazole in patients with onchocerciasis; effects of food and pretreatment with ivermectin on drug response and pharmacokinetics. Trop. Med. Parasitol. 45:203-208. [PubMed] [Google Scholar]
- 2.Davis, A., H. Dixon, and Z. S. Pawlowski. 1989. Multicentre clinical trials of benzimidazole-carbamates in human cystic echinococcosis (phase 2). Bull. W. H. O. 67:503-508. [PMC free article] [PubMed] [Google Scholar]
- 3.del Estal, J. L., A. I. Alvarez, C. Villaverde, P. Coronel, S. Fabra, and J. G. Prieto. 1991. Effect of surfactants on albendazole absorption. J. Pharm. Biomed. Anal. 9:1161-1164. [DOI] [PubMed] [Google Scholar]
- 4.del Estal, J. L., A. I. Alvarez, C. Villaverde, A. Justel, and J. G. Prieto. 1994. Increased systemic bioavailability of albendazol when administered with surfactants in rats. Int. J. Pharm. 102:257-260. [Google Scholar]
- 5.del Estal, J. L., A. I. Alvarez, C. Villaverde, and J. G. Prieto. 1993. Comparative effects of anionic, natural bile acid surfactants and mixed micelles on the intestinal absorption of the anthelmintic albendazole. Int. J. Pharm. 91:105-109. [Google Scholar]
- 6.Evrard, B., P. Chiap, P. DeTullio, F. Ghalmi, G. Piel, T. van Hees, J. Crommen, B. Losson, and L. DaLattre. 2002. Oral bioavailability in sheep of albendazole from a suspension and from a solution containing hydroxypropyl-beta-cyclodextrin. J. Control. Release 85:45-50. [DOI] [PubMed] [Google Scholar]
- 7.Homeida, M., W. Leahy, S. Copeland, M. M. Ali, and D. W. Harron. 1994. Pharmacokinetic interaction between praziquantel and albendazole in Sudanese men. Ann. Trop. Med. Parasitol. 88:551-559. [DOI] [PubMed] [Google Scholar]
- 8.Horton, R. J. 1997. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 64:79-93. [DOI] [PubMed] [Google Scholar]
- 9.Jung, H., L. Medina, L. Garcia, I. Fuentes, and R. Moreno-Esparza. 1998. Absorption studies of albendazole and some physicochemical properties of the drug and its metabolite albendazole sulphoxide. J. Pharm. Pharmacol. 50:43-48. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman, D., K. J. Cheng, and L. Fleckenstein. 2002. HPLC assay for albendazole and metabolites in human plasma for clinical pharmacokinetic studies. J. Pharm. Biomed. Anal. 30:801-813. [DOI] [PubMed] [Google Scholar]
- 11.Kohri, N., Y. Yamayoshi, H. Xin, K. Iseki, N. Sato, S. Todo, and K. Miyazaki. 1999. Improving the oral bioavailability of albendazole in rabbits by the solid dispersion technique. J. Pharm. Pharmacol. 51:159-164. [DOI] [PubMed] [Google Scholar]
- 12.Lange, H., R. Eggers, and J. Bircher. 1988. Increased systemic availability of albendazole when taken with a fatty meal. Eur. J. Clin. Pharmacol. 34:315-317. [DOI] [PubMed] [Google Scholar]
- 13.Lund, W. 1994. The pharmaceutical codex: principles and practice of pharmaceutics, 12th ed. The Pharmaceutical Press, London, United Kingdom.
- 14.Mingjie, W., X. Shuhua, C. Junjie, L. Bin, F. Cheng, S. Weixia, and P. Hotez. 2002. Albendazole-soybean oil emulsion for the treatment of human cystic echinococcosis: evaluation of bioavailability and bioequivalence. Acta Trop. 83:177-181. [DOI] [PubMed] [Google Scholar]
- 15.Nagy, J., H. G. Schipper, R. P. Koopmans, J. J. Butter, C. J. van Boxtel, and P. A. Kager. 2002. Effect of grapefruit juice or cimetidine coadministration on albendazole bioavailability. Am. J. Trop. Med. Hyg. 66:260-263. [DOI] [PubMed] [Google Scholar]
- 16.Piel, G., B. Evrard, T. Van Hees, G. Llabres, and L. Delattre. 1999. Development of a parenteral and of an oral formulation of albendazole with cyclodextrins. Stp. Pharma. Sci. 9:257-260. [Google Scholar]
- 17.Schipper, H. G., R. P. Koopmans, J. Nagy, J. J. Butter, P. A. Kager, and C. J. van Boxtel. 2000. Effect of dose increase or cimetidine co-administration on albendazole bioavailability. Am. J. Trop. Med. Hyg. 63:270-273. [PubMed] [Google Scholar]
- 18.Torrado, S., M. L. Lopez, G. Torrado, F. Bolas, G. Torrado, and R. A. Cadorniga. 1997. Novel formulation of albendazole solution: oral bioavailability and efficacy evaluation. Int. J. Pharm. 156:181-187. [Google Scholar]
- 19.Wen, H., R. R. C. New, M. Muhmut, J. H. Wang, Y. H. Wang, J. H. Zhang, Y. M. Shao, and P. S. Craig. 1996. Pharmacology and efficacy of liposome-entrapped albendazole in experimental secondary alveolar echinococcosis and effect of co-administration with cimetidine. Parasitology 13:111-121. [DOI] [PubMed] [Google Scholar]