Abstract
Purpose
The aim of this study was to evaluate the efficacy and safety of hexaminolevulinate fluorescence cystoscopy in the diagnosis of bladder cancer.
Materials and Methods
In a prospective design, we included patients who had a bladder lesion suggesting bladder cancer. Patients with massive hematuria, urethral Foley catheter insertion, chronic retention state, or urinary tract infection were excluded. After the bladder was emptied, hexaminolevulinate was gently administered into the bladder. One hour later, cystoscopy under white light and blue light was performed. After marking the lesions confirmed with white light or blue light, transurethral resection of the bladder lesion and pathologic confirmation were done. Transurethral resection of the lesions that were negative in both white and blue light was also performed.
Results
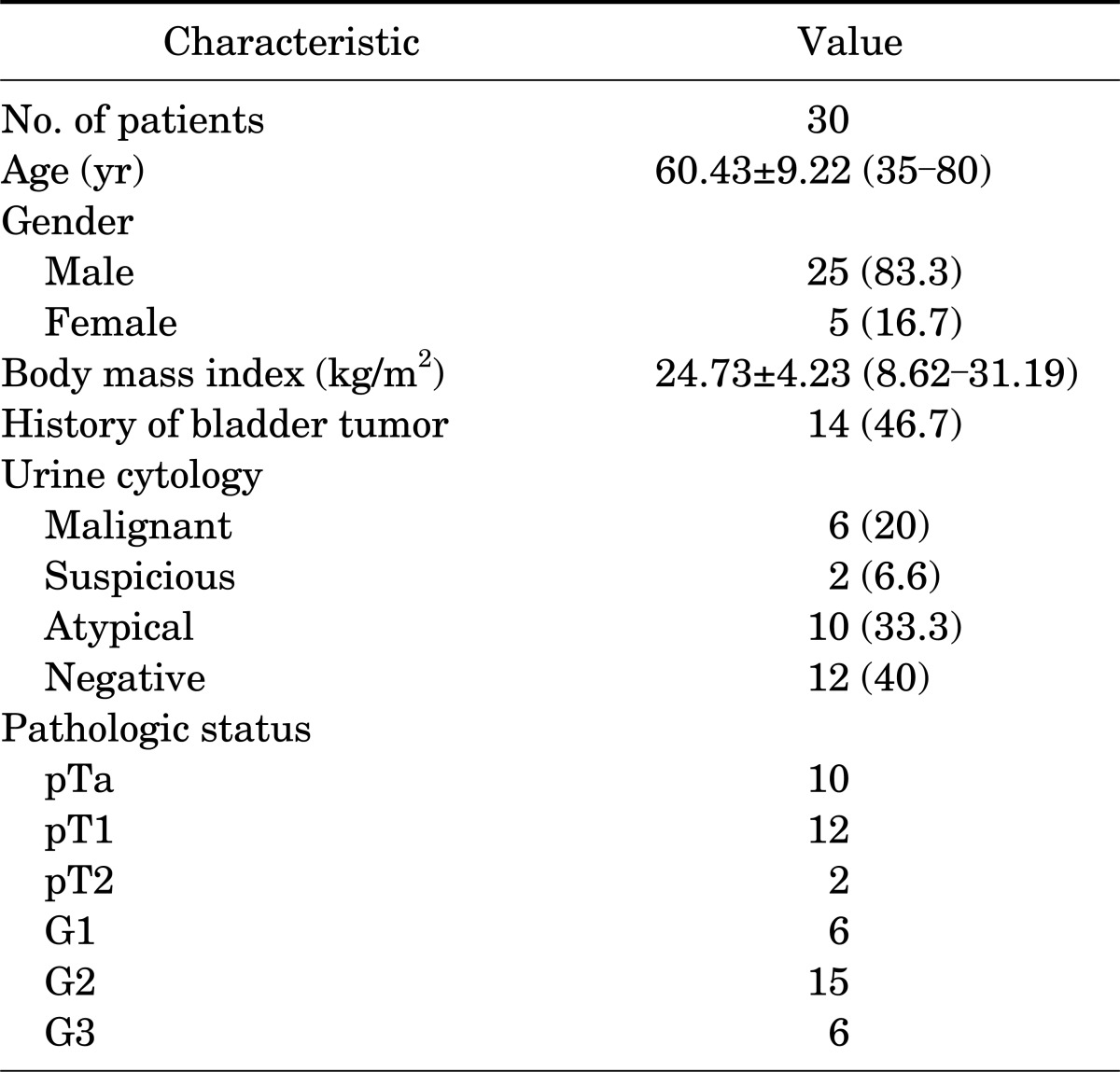
From April 2010 to September 2010, 30 patients were enrolled. From the total of 30 patients (25 men and 5 women; mean age, 60.4±9.22 years), 134 specimens were extracted. Among these, 101 specimens showed positive results by blue light cystoscopy (BLC). The sensitivity of BLC and white light cystoscopy (WLC) was 92.3% and 80.8%, respectively (p=0.021). The specificity of BLC and WLC was 48% and 49.1%, respectively (p>0.05). The positive and negative predictive values of BLC were 71.2% and 81.8%, respectively, whereas those of WLC were 72.0% and 68.6%, respectively. With WLC, 48 specimens showed negative findings, but of that group, 15 specimens (31.2%) were revealed to be malignant with BLC. There were no significant side effects in the 24 hours after the instillation of hexaminolevulinate.
Conclusions
Photodynamic diagnosis with hexaminolevulinate helps to find tumors that could be missed by use of WLC only. Photodynamic diagnosis might be valuable in complete resection as well as for more accurate diagnosis of bladder tumor.
Keywords: Bladder cancer, Diagnostic imaging, Hemainolevulinate
INTRODUCTION
Urothelial carcinoma of the bladder is the sixth most frequent malignant disease in industrial countries [1] and the second common cancer of the urogenital system in Korea. Approximately 70% of all patients with newly diagnosed urothelial cancer have nonmuscle-invasive bladder cancer [2]. Initial treatment of such tumors consists of transurethral resection (TUR), but at the first follow-up, the recurrence rate is between 3.4% and 45.8% [3,4]. The probabilities of recurrence and progression for Ta and T1 bladder cancer at 1 year range from 15 to 61% and from <1 to 17%, respectively. At 5 years, the probabilities of recurrence and progression range from 31 to 78% and from <1 to 45%, respectively [5].
Although white light cystoscopy (WLC) is still regarded as the gold standard for the diagnosis of bladder cancer, its sensitivity and specificity are not entirely satisfactory [6,7]. Small bladder cancers or flat lesions such as carcinoma in situ (CIS) can be easily overlooked. It has been estimated that approximately 10 to 20% of tumors are not discovered by conventional WLC [8]. Such remnant tumors could lead to a significant increase in incomplete resection of tumors, which in turn would lead to a high risk of recurrence of bladder cancer. Resection or complete destruction of a bladder cancer is considered the main factor preventing the recurrence of bladder cancer.
To resolve these problems, fluorescence cystoscopy has been studied and, more recently, photodynamic diagnosis (PDD) has been viewed as a valuable aid to TUR of bladder cancer [9].
To date, two PDD agents have been studied in larger trials: delta-aminolevulinic acid (ALA) and its derivative, hexaminolevulinate (HAL; Hexvix, PhotoCure, Norway) [10-12]. ALA was the first product used for PDD. After the improved ester HAL was introduced, PDD came into broad practical use. The basis of PDD is the preferential accumulation of the photoactive porphyrin in neoplastic tissue, resulting in red fluorescence-emitting tumors. HAL and 5-ALA have been studied in many large trials. Compared with 5-ALA, HAL provides the benefits of increased selectivity, brighter fluorescence, and a shorter instillation time. HAL generates up to four times the levels of the fluorescent molecule, protoporphyrin IX, in half the time.
The European Association of Urology Guidelines state that fluorescence cystoscopy, performed in blue light and using a porphyrin-based photosensitizer, may help in discovering suspicious areas not visible in white light [9]. However, there were previously no data for HAL cystoscopy in Korean patients.
The aim of this study was therefore to evaluate the efficacy of HAL fluorescence cystoscopy in the diagnosis and management of bladder cancer and to compare it with that of standard cystoscopy.
MATERIALS AND METHODS
We conducted a prospective study at our hospital between April 2010 and September 2010. Our Institutional Review Board approved this study, and written informed consent was obtained from all participants before enrollment.
Thirty patients were diagnosed as having bladder cancer by use of cystoscopy or urine cytology. Cytology-positive results meant that malignancy or suspicious malignancy was observed on two consecutive urine cytology tests.
Patients were excluded if they had massive hematuria (blue light is highly absorbed by blood and clots), moderate to severe leukocyturia, urethral Foley catheter insertion, chronic retention state, urinary tract infection, history of bladder cancer, or imaging evidence of upper urinary tract disease, pregnancy, hypersensitivity to porphyrins, or renal or hepatic impairment.
A solution of HAL hydrochloride (50 ml, 1.7 mg/ml, 8 mM, Hexvix, PhotoCure, Norway) in buffered saline solution was instilled via a 14-Fr Foley catheter inserted into the bladder and retained for 60 minutes before cystoscopy. The Storz D-light-C system with a xenon arc lamp as a source was used in all cases. One hour later, the bladder was first inspected under WLC, and the number and location of cancers were noted, followed by blue light cystoscopy (BLC) with all suspected lesions documented before TUR. After marking the lesions confirmed with WLC or BLC, TUR of the lesion and pathologic confirmation were followed. A total of 134 biopsy procedures were done. TUR of the lesions that were negative under both white and blue light was also performed randomly to calculate sensitivity and specificity.
The primary endpoint was the sensitivity and specificity of HAL in the diagnosis and treatment of bladder cancer. In addition, we compared sensitivity and specificity between WLC and BLC, positive and negative predictive values, and side effects of the drug.
RESULTS
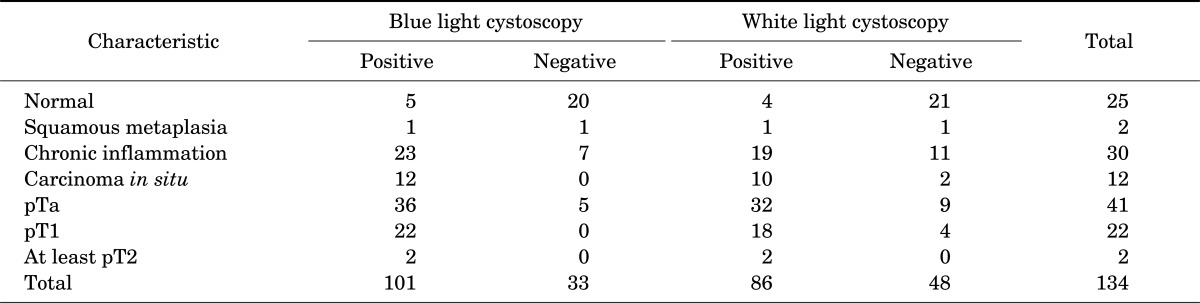
Between April 2010 and September 2010, 30 patients who had a bladder lesion suggestive of bladder cancer were enrolled. The patients' characteristics are shown in Table 1. A total of 25 males and 5 females were included; the mean age of the study sample was 60.4±9.22 years. Of the patients, 53.4% (16) had recurrent disease and 46.6% of the patients (14) had newly diagnosed cancer. From the total of 30 patients, 134 specimens were extracted and more informations were in Table 2, 3. Bladder cancer was diagnosed in 77 specimens, and nonbladder cancer was diagnosed in 57 specimens. In normal tissue, 25 were normal tissue, 2 were squamous metaplasia, and 30 were chronic inflammation. Of the chronic inflammation specimens, 23 were positive in BLC, compared with 19 positive in WLC.
TABLE 1.
Baseline characteristics of the patients
Values are presented as mean±SD (range) or number (%).
TABLE 2.
Fluorescence and pathologic characteristics of 134 specimens
Values are presented as no. of patients.
TABLE 3.
Fluorescence findings and pathologic diagnosis for BLC specimens
BLC, blue light cystoscopy; WLC, white light cystoscopy.
There were 12 CIS lesions. With BLC, all CIS lesions were detected, whereas only 10 lesions (83%) were detected with WLC.
1. White light cystoscopy
A total of 86 specimens showed positive results with WLC. Among these cases, 62 cases were confirmed bladder cancer, whereas 24 specimens showed negative findings with WLC. Therefore, the positive and negative predictive value of WLC were 72.1% and 68.8%, respectively. The sensitivity and specificity of WLC were 80.5% (62/77) and 57.8% (33/57), respectively.
2. Blue light cystoscopy
A total of 101 specimens showed positive results with BLC. Among these cases, 72 cases were confirmed bladder cancer. Thus, the positive predictive value of BLC in the diagnosis of bladder cancer was 71.2%. Thirty-three specimens showed negative results with BLC. After examination of these lesions, 27 cases had not shown bladder cancer. Thus, the negative predictive value of BLC in the diagnosis of bladder cancer was 81.8%.
The sensitivity of BLC in the diagnosis of bladder tumor was 92.3% (72/78) and the specificity was 49.1% (27/56). With WLC, 48 specimens were negative, but 23 of them were positive with BLC (47.91%). Within this group, 15 specimens were revealed to be bladder cancer. The sensitivity of BLC was higher than that of WLC (p=0.021), whereas the specificity of WLC and BLC showed no significant difference.
3. Side effects
Side effects of HAL are rarely reported. We checked urinary retention, nausea, vomiting, constipation, pain, fever, skin lesions, elevated liver enzymes or bilirubin, and sepsis. However, the examination was well tolerated and there were no significant side effects in the 24 hours after the instillation of HAL.
DISCUSSION
PDD has recently been viewed as a valuable aid to TUR of bladder cancer as reflected in current guidelines. The original substance used for fluorescence endoscopy was ALA (5-aminolevulinic acid). Improved tumor detection by ALA-induced fluorescence endoscopy has been demonstrated by several investigators and averages 20% compared with conventional WLC.
Kriegmair et al. [13] showed that sensitivity of the PDD group was 97% and the specificity was 67%, with 73% and 69% in the WLC group. Filbeck et al. [14] showed similar results of 96% and 67% in the PDD group for sensitivity and specificity versus 68% and 66% in the WLC group.
In addition, Daniltchenko et al. [15] studied the long-term benefits of 5-ALA in TUR of superficial bladder cancer in 102 patients with a median follow-up of 42 months for the PDD group and 39 months for the WLC group. The recurrence-free survival rate was higher in the PDD group (41%) than in the WLC group (25%).
By comparison with ALA, HAL provides deeper tissue penetration and improved accumulation in neoplastic cells. Lange et al. [16] showed a high level of demarcation between red fluorescence malignant cells and blue light normal tissues.
Schmidbauer et al. [17] studied 52 patients with bladder cancer and calculated that HAL imaging has 96% sensitivity compared with 73% for WLC. After several studies indicated that HAL cystoscopy has the potential for improved detection in superficial bladder cancer, open, large randomized studies comparing the effectiveness of PDD using HAL with WLC showed similar results.
Jichlinski et al. [18] reported that among 45 patients, a total of 43 were diagnosed by fluorescence cystoscopy compared with 33 diagnosed by white light for sensitivity of 96% and 73%. HAL cystoscopy was found to be particularly useful for finding CIS in 13 patients, because all except 1 were diagnosed or confirmed by HAL cystoscopy. Jocham et al. [12] reported that 96% of all tumors were detected with HAL imaging compared with 77% by use of standard cystoscopy. This difference was particularly noticeable for dysplasia (93% vs. 48%), CIS (95% vs. 68%), and superficial papillary tumors (96% vs. 85%). Overall, 17% of patients received more appropriate treatment.
The diagnostic accuracy in our series was significantly improved for BLC (92.3%) by comparison with WLC (80.5%). We demonstrated that 29.5% of our patients received a diagnosis of bladder cancer by BLC, whereas they were diagnosed as having no cancer on WLC. These additional findings of cancers have a significant impact on more complete treatment.
CIS is an emphasized target of PDD. CIS is highly predictive of progression to invasive cancer and requires prompt treatment but is not easily detected under WLC. The results obtained by Geavlete et al. [19] described a CIS detection rate of 95.7% for BLC, superior to WLC, which diagnosed only 51% of the CIS lesions. Other studies showed similar results of a CIS detection rate of 97% for BLC and 58% for WLC. Our study also showed that 100% (12/12) of CIS lesions were detected by BLC.
No local or systemic adverse events were reported, including urinary retention, nausea, vomiting, constipation, pain, fever, skin lesion, elevated liver enzyme or bilirubin, or sepsis. In our study, a total of 30 patients were enrolled and our mean follow-up period was 21.1 months and the bladder cancer recurrence rate was 20% (6 patients). Among those patients, 3 patients showed T1 stage bladder cancer and among them, 2 patients experienced recurrence with T2 stage bladder cancer and were treated by radical cystectomy. Another 3 patients showed Ta stage bladder cancer and recurrence of the same stage bladder cancer. No recurrence was found in the patients with CIS lesions.
This study had some limitations. Whereas an increased bladder cancer detection rate has an impact on tumor detection and more precise treatment of bladder cancer, there are insufficient data about the relation between increased diagnostic accuracy and the bladder cancer recurrence rate or survival benefit. Lately, some studies have shown that PDD leads to improved treatment in 1 of 5 patients and reduces residual cancer rates and increases cancer-free survival significantly. Additional studies about PDD and the bladder cancer survival rate are needed.
CONCLUSIONS
PDD with HAL helps to detect cancer lesions that could be missed with WLC only, especially CIS lesions. PDD might be valuable in complete resection as well as for more accurate diagnosis of bladder cancer.
Footnotes
The authors have nothing to disclose.
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Holmang S, Hedelin H, Anderstrom C, Holmberg E, Johansson SL. Prospective registration of all patients in a geographical region with newly diagnosed bladder carcinomas during a two-year period. Scand J Urol Nephrol. 2000;34:95–101. doi: 10.1080/003655900750016698. [DOI] [PubMed] [Google Scholar]
- 3.Brausi M, Collette L, Kurth K, van der Meijden AP, Oosterlinck W, Witjes JA, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002;41:523–531. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 4.Witjes JW, Kiemeney LA, Verbeek AL, Heijbroek RP, Debruyne EM the members of the Dutch South East Cooperative Urological Group. Random bladder biopsies and the risk of recurrent superficial bladder cancer: a prospective study in 1026 patients. World J Urol. 1992;10:231–234. [Google Scholar]
- 5.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–477. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Denzinger S, Burger M, Walter B, Knuechel R, Roessler W, Wieland WF, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology. 2007;69:675–679. doi: 10.1016/j.urology.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Jakse G, Algaba F, Malmstrom PU, Oosterlinck W. A second-look TUR in T1 transitional cell carcinoma: why? Eur Urol. 2004;45:539–546. doi: 10.1016/j.eururo.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Jichlinski P, Leisinger HJ. Fluorescence cystoscopy in the management of bladder cancer: a help for the urologist. Urol Int. 2005;74:97–101. doi: 10.1159/000083277. [DOI] [PubMed] [Google Scholar]
- 9.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Jocham D, Stepp H, Waidelich R. Photodynamic diagnosis in urology: state-of-the-art. Eur Urol. 2008;53:1138–1148. doi: 10.1016/j.eururo.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 11.Witjes JA, Moonen PM, van der Heijden AG. Comparison of hexaminolevulinate based flexible and rigid fluorescence cystoscopy with rigid white light cystoscopy in bladder cancer: results of a prospective Phase II study. Eur Urol. 2005;47:319–322. doi: 10.1016/j.eururo.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Jocham D, Witjes F, Wagner S, Zeylemaker B, van Moorselaar J, Grimm MO, et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol. 2005;174:862–866. doi: 10.1097/01.ju.0000169257.19841.2a. [DOI] [PubMed] [Google Scholar]
- 13.Kriegmair M, Baumgartner R, Knuchel R, Stepp H, Hofstadter F, Hofstetter A. Detection of early bladder cancer by 5-aminolevulinic acid induced porphyrin fluorescence. J Urol. 1996;155:105–109. [PubMed] [Google Scholar]
- 14.Filbeck T, Roessler W, Knuechel R, Straub M, Kiel HJ, Wieland WF. Clinical results of the transurethreal resection and evaluation of superficial bladder carcinomas by means of fluorescence diagnosis after intravesical instillation of 5-aminolevulinic acid. J Endourol. 1999;13:117–121. doi: 10.1089/end.1999.13.117. [DOI] [PubMed] [Google Scholar]
- 15.Daniltchenko DI, Riedl CR, Sachs MD, Koenig F, Daha KL, Pflueger H, et al. Long-term benefit of 5-aminolevulinic acid fluorescence assisted transurethral resection of superficial bladder cancer: 5-year results of a prospective randomized study. J Urol. 2005;174:2129–2133. doi: 10.1097/01.ju.0000181814.73466.14. [DOI] [PubMed] [Google Scholar]
- 16.Lange N, Jichlinski P, Zellweger M, Forrer M, Marti A, Guillou L, et al. Photodetection of early human bladder cancer based on the fluorescence of 5-aminolaevulinic acid hexylester-induced protoporphyrin IX: a pilot study. Br J Cancer. 1999;80:185–193. doi: 10.1038/sj.bjc.6690338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidbauer J, Witjes F, Schmeller N, Donat R, Susani M, Marberger M, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004;171:135–138. doi: 10.1097/01.ju.0000100480.70769.0e. [DOI] [PubMed] [Google Scholar]
- 18.Jichlinski P, Guillou L, Karlsen SJ, Malmstrom PU, Jocham D, Brennhovd B, et al. Hexyl aminolevulinate fluorescence cystoscopy: new diagnostic tool for photodiagnosis of superficial bladder cancer: a multicenter study. J Urol. 2003;170:226–229. doi: 10.1097/01.ju.0000060782.52358.04. [DOI] [PubMed] [Google Scholar]
- 19.Geavlete B, Mulţescu R, Georgescu D, Jecu M, Geavlete P. HAL fluorescence cystoscopy and TURB one year of Romanian experience. J Med Life. 2009;2:185–190. [PMC free article] [PubMed] [Google Scholar]