Abstract
Purpose
We evaluated the value of a combined approach of T1-weighted (T1W) imaging, T2-weighted (T2W) imaging, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), and diffusion-weighted imaging (DWI) for the detection of prostate cancer and extracapsular extension (ECE) in patients with prostate cancer by using pathologic data after radical prostatectomy.
Materials and Methods
From April 2009 to December 2011, 126 patients who underwent radical prostatectomy and prostate MRI for prostate cancer were analyzed retrospectively. The MRI findings were compared with the pathologic findings of the radical prostatectomy specimens in each patient. The sensitivity, specificity, and accuracy of the detection of prostate cancer and extracapsular extension were analyzed.
Results
The prostate cancer detection rate by use of T1W and T2W imaging, DCE-MRI, and their combination was 65.1%, 69.0%, and 80.2%, respectively (p=0.023). The detection rate using T1W and T2W imaging, DCE-MRI, DWI, and their combination was 57.7%, 65.4%, 67.3%, and 80.8%, respectively (p=0.086). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of combination MRI (T1W, T2W, and DCE-MRI) for ECE were 46.4%, 91.4%, 83.9%, and 68.1%, respectively. The sensitivity of combination MRI (T1W, T2W, and DCE-MRI) for ECE tended to increase as the prostate-specific antigen level rose (p=0.010). The sensitivity, specificity, PPV, and NPV of combination MRI (T1W, T2W, DCE-MRI, and DWI) for ECE were 65.0%, 87.5%, 76.5%, and 80.0%, respectively.
Conclusions
A combined approach of T1W, T2W, and DCE-MRI with DWI demonstrated an accurate detection rate of prostate cancer. Also, combination approaches showed a high specificity for predicting ECE, although sensitivity was relatively lower. Therefore, these methods are reliable for predicting prostate cancer. However, a new protocol is necessary to enhance the sensitivity for predicting ECE.
Keywords: Diagnosis, Magnetic resonance imaging, Prostatic neoplasms
INTRODUCTION
Prostate cancer is the most commonly diagnosed malignancy of the male genitourinary tract [1]. Since the prostate-specific antigen (PSA) test was introduced in 1986, detection of early-stage prostate cancer has been increasing with simple blood screening. Generally, radical prostatectomy is known as a best management in patients with localized prostate cancer who have at least a 10-year life expectancy. Furthermore, a number of well-designed studies have reported that radical prostatectomy shows excellent results in long-term oncologic control; thus, it is regarded as an established option for managing locally advanced prostate cancer [2,3]. In particular, comprehension of the anatomy and advanced surgical techniques can improve the quality of life in patients who undergo radical prostatectomy by preserving sexual potency and urinary incontinence. The decision of whether a nerve-sparing procedure will be chosen is made by information gained from the preoperative PSA level, digital rectal examination (DRE), imaging, and biopsy findings, such as number, location, grade, and Gleason score. However, each of these parameters has a limitation for predicting the final pathological results.
Transrectal ultrasound (TRUS), computed tomography (CT), and magnetic resonance imaging (MRI) are commonly used tools for imaging the prostate. TRUS can measure the volume of the prostate accurately, but is inappropriate for determining local staging of cancer owing to low specificity and low diagnostic accuracy [4]. Although CT can help to identify nodal invasion, it has insufficient roles in detection of localized prostate cancer. Thus, MRI has been recommended for precise imaging for the detection and staging of prostate cancer [5]. Recently, several reports have shown that endorectal MRI and dynamic contrast-enhanced (DCE) MRI can contribute to prediction of extracapsular extension (ECE) [6,7]. However, only a few studies exist to predict ECE by use of a combined approach of DCE-MRI and diffusion-weighted imaging (DWI).
In this study, therefore, we evaluated the value of a combined approach of T1-weighted (T1W) imaging, T2-weighted (T2W) imaging, DCE-MRI, and DWI for detection of prostate cancer and ECE in patients with prostate cancer by use of pathologic data after radical prostatectomy.
MATERIALS AND METHODS
1. Subjects
From April 2009 to December 2011, 143 patients who underwent radical prostatectomy for prostate cancer were analyzed retrospectively. The collection and analysis of all samples was approved by the Institutional Review Board of our institution. Adenocarcinoma of the prostate was diagnosed in all patients by means of TRUS-guided prostate needle biopsies. For staging work-up, all patients underwent 3-Tesla (3-T) MRI (T1W, T2W, and DCE-MRI with and without DWI) before surgery. Owing to changing MRI protocols (adding DWI), 52 patients underwent DWI including T1W, T2W, and DCE-MRI. We excluded the patients who had undergone MRI in other hospitals to minimize the bias according to different MRI protocols. Finally, 126 patients were enrolled in the study. The MRI findings were compared with the pathologic findings of the radical prostatectomy specimens in each patient.
2. Protocol for prostate MRI and imaging diagnostic criteria
All prostate MRI was performed on a 3-T system (Intera Achieva 3.0T, Philips Medical Systems, Best, The Netherlands). Sequences acquired included thin-section high-spatial-resolution sagittal, axial, and coronal T2W spin-echo images of the prostate with the following parameters: the Repetition Time (TR) 3,000-4,100 ms, the echo time (TE) 80-90 ms, slice thickness 5.0 mm, gap 0.5 mm. Axial T1W images were TR 585 ms, TE 10 ms, slice thickness 5.0 mm, gap 0.5 mm. Axial DWI were TR 6500 ms, TE 55 ms, slice thickness 3.0 mm, gap 0.3 mm, 106×77 matrix, and acquisition time 5 minutes 29 seconds, with free breath. DCE-MRI was TR 4.6-7.7 ms, TE 2-4 ms, slice thickness 2.0 mm.
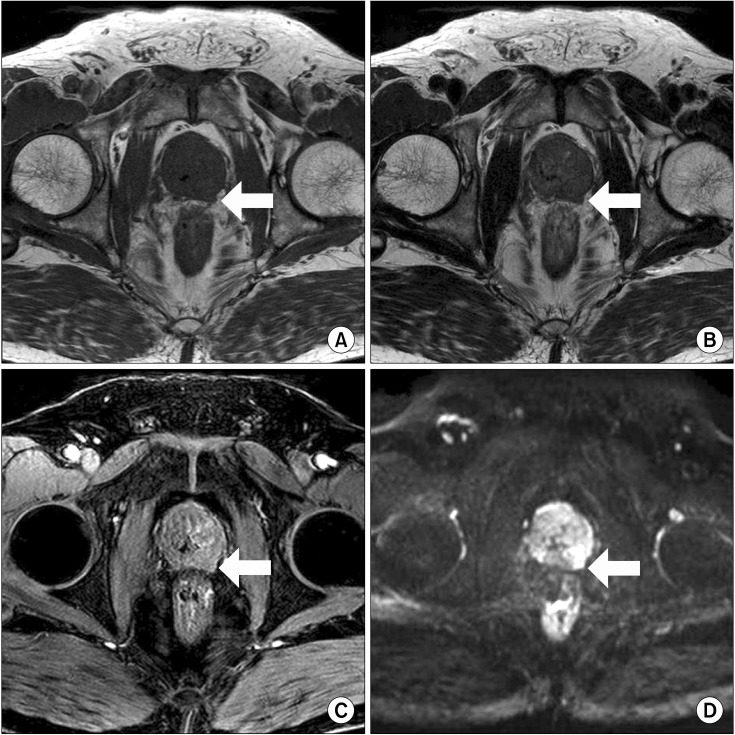
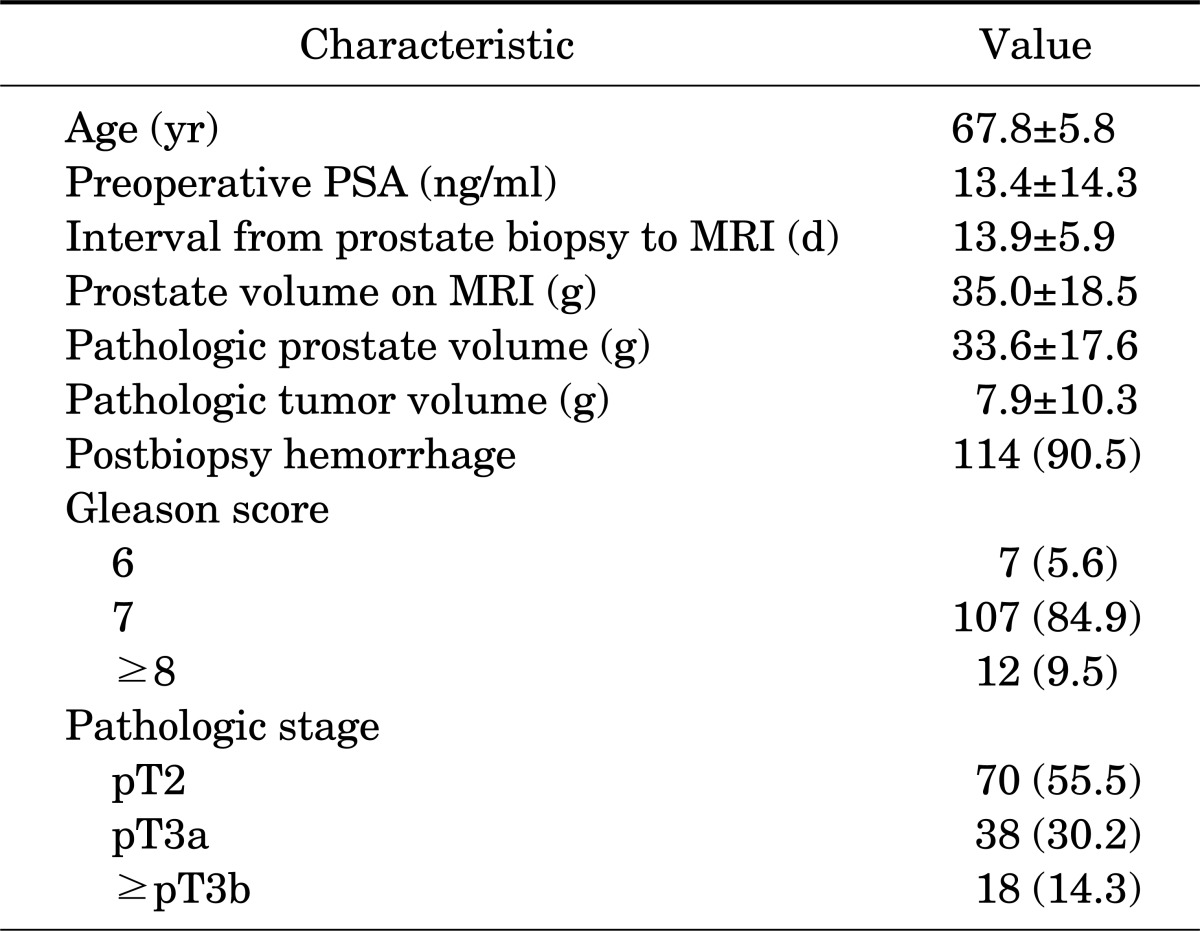
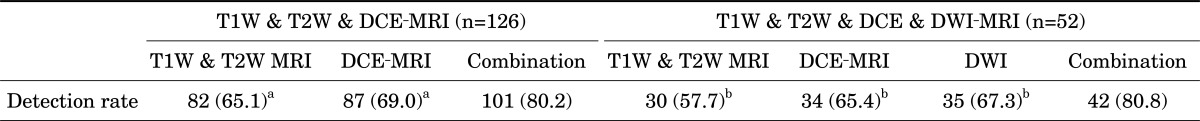
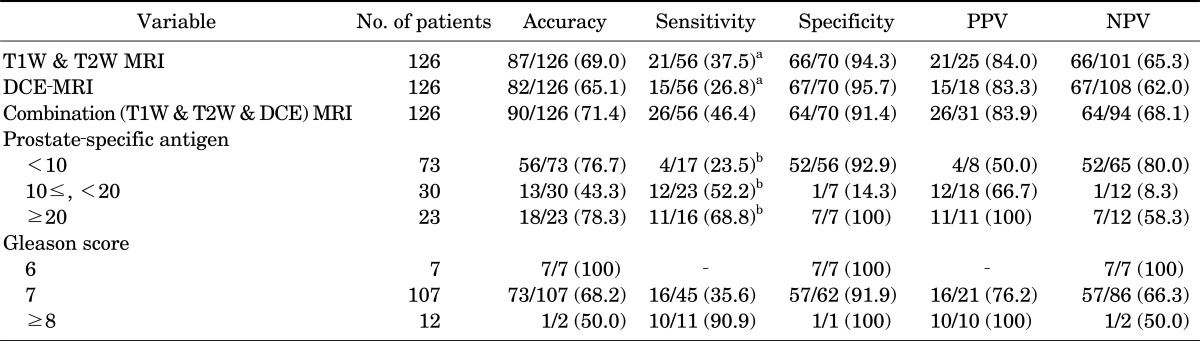
The MRI images were assessed by single experienced radiologist. The following criteria were regarded as ECE on MRI: asymmetry of the neurovascular bundle, angulated contour of the prostate gland, irregularity of the margin, and tumor envelopment of the neurovascular bundle [8] (Fig. 1).
FIG. 1.
Extracapsular extension of prostate cancer on various sequences. (A) T1-weighted imaging, (B) T2-weighted imaging, (C) dynamic contrast-enhanced magnetic resonance imaging, and (D) diffusion-weighted image.
3. Pathological specimens
After radical prostatectomy, all specimens were classified according to stage by using the tumor-node-metastasis 2002 staging classification. The pathological evaluation was performed for cancer location, Gleason score, histologic type, tumor size, extracapsular involvement, seminal vesicle invasion, lymphatic/vascular invasion, lymph node metastasis, perineural invasion, and surgical margin involvement.
4. Statistical analysis
Statistical analysis was performed by using the McNemar test and the chi-square trend test to determine a statistically significant linear trend in sensitivity for detecting ECE according to PSA and Gleason score. IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) with p<0.05 considered statistically significant.
RESULTS
1. Baseline characteristics
The mean age of the patients was 67.8±5.8 years and the mean preoperative PSA value was 13.4±14.3 ng/ml. The time interval from prostate biopsy to MRI was 13.9±5.9 days. There were 114 patients (90.5%) with postbiopsy hemorrhage shown on the MRI. Other baseline characteristics of the patients are presented in Table 1.
TABLE 1.
Baseline characteristics of the participants (n=126)
Values are presented as mean±SD or number (%).
PSA, prostate-specific antigen; MRI, magnetic resonance imaging.
2. Detection rate of prostate cancer using MRI (T1W, T2W, and DCE-MRI vs. T1W, T2W, DCE-MRI, and DWI)
The prostate cancer detection rate using T1W and T2W imaging, DCE-MRI, and their combination was 65.1%, 69.0%, and 80.2%, respectively (p<0.01 each compared with the combination, McNemar test). The detection rate using T1W and T2W imaging, DCE-MRI, DWI, and their combination was 57.7%, 65.4%, 67.3%, and 80.8%, respectively (p<0.05 each compared with the combination, McNemar test). The detection rate of prostate cancer is presented in Table 2.
TABLE 2.
Detection rates of prostate cancer of T1-weighted, T2-weighted, and dynamic contrast-enhanced MRI versus T1W, T2W, dynamic contrast-enhanced, and diffusion-weighted imaging in prostate cancer patients
Values are presented as number (%).
T1W, T1-weighted; T2W, T2-weighted; DCE, dynamic contrast-enhanced; DWI. diffusion-weighted imaging; MRI, magnetic resonance imaging.
a:McNemar test: p<0.01, each, b:McNemar test: p<0.05, each.
3. Accuracy of MRI in predicting extracapsular extension (T1W, T2W, and DCE-MRI)
The sensitivity of T1W and T2W imaging, DCE-MRI, and combination MRI for the detection of ECE was 37.5%, 26.8%, and 46.4%, respectively (p<0.05 each compared with the combination, McNemar test). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of combination MRI for ECE were 46.4%, 91.4%, 83.9%, and 68.1%, respectively. The sensitivity of combination MRI (T1W, T2W, and DCE-MRI) for ECE tended to increase as PSA rose (p=0.010) (Table 3).
TABLE 3.
Accuracy of T1-weighted, T2-weighted, and dynamic contrast-enhanced imaging in predicting extracapsular extension
Values are presented as number (%).
T1W, T1-weighted; T2W, T2-weighted; DCE, dynamic contrast-enhanced; MRI, magnetic resonance imaging; PPV, positive predictive value; NPV, negative predictive value.
a:McNemar test: p<0.05, each, b:Chi-square test: linear by linear association, p=0.010.
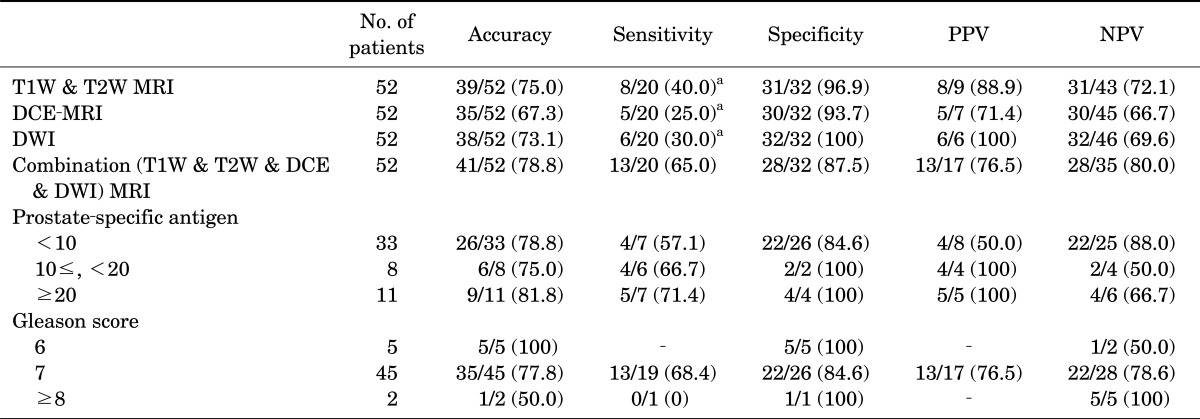
4. Accuracy of MRI in predicting extracapsular extension (T1W, T2W, DCE-MRI, and DWI)
The sensitivity of T1W and T2W imaging, DCE-MRI, DWI, and combination MRI for detection of ECE was 40.0%, 25.0%, 30.0%, and 65.0%, respectively (p<0.01 each compared with the combination, McNemar test). The sensitivity, specificity, PPV, and NPV of combination MRI for ECE were 65.0%, 87.5%, 76.5%, and 80.0%, respectively (Table 4).
TABLE 4.
Accuracy of T1-weighted, T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging in predicting extracapsular extension
Values are presented as number (%).
PPV, positive predictive value; NPV, negative predictive value; T1W, T1-weighted; T2W, T2-weighted; MRI, magnetic resonance imaging; DCE, dynamic contrast-enhanced; DWI, diffusion-weighted imaging.
a:McNemar test: p<0.01, each.
DISCUSSION
In this study, the combined approach of T1W, T2W, DCE, and DWI-MRI could demonstrate accurate detection of prostate cancer and moderate sensitivity of prediction of ECE. The detection rate of prostate cancer according to the combined approach with and without DWI-MRI was about 80%. These results are very similar to those of other previous studies (which only performed DCE-MRI) [9,10]. However, only a few studies have assessed the prediction of ECE with DCE-MRI and DWI-MRI simultaneously.
Prediction of ECE is considered to be very important to the clinician's decision of whether to perform nerve-sparing prostatectomy in patients with localized prostate cancer. Of course, it is possible for an experienced operator to predict ECE in the operation field. However, many operators usually predict ECE according to the combined results of MRI, PSA, Gleason score, and number of positive prostate biopsy results preoperatively. Moreover, PSA, Gleason score, and number of positive prostate biopsy results without MRI cannot accurately predict ECE.
Generally, prostate cancer has a lower signal intensity than the normal peripheral zone of the prostate on conventional T2W MRI [11]. To predict tumor stage, one of the most important roles of MRI in prostate cancer has been the diagnosis of ECE. ECE on MRI has been described as a localized bulge of the prostatic contour, a thickening or disruption of the prostatic capsule, an infiltrative strand in the periprostatic fat, or asymmetry of the neurovascular bundle [12]. In several studies, MRI has had good specificity but low sensitivity in the detection of prostate cancer [13,14]. Our combined approach of T1W, T2W, and DCE-MRI showed similar results.
Because of the need for more accurate detection of prostate cancer and ECE, MRI has improved greatly, and advanced MRI techniques such as DWI, DCE-MRI, and MR spectroscopy have been introduced. DWI is based on the movement of water molecules within the intracellular and extracellular spaces, and in prostate cancer this movement is decreased. DCE-MRI is based on changes in vascular characteristics; thus, DCE-MRI gives more information about the perfusion of the prostate and the tumor. In prostate cancer, cancer tissues have more and earlier contrast enhancement owing to larger feeding vessels [15]. Bloch et al. [16] reported that DCE-MRI improved the accuracy of T2-weighted MRI compared with T2-weighted MRI alone in the detection of ECE. However, only a few studies have been conducted on the combined approach of T1W, T2W, DCE-MRI, and DWI in prostate cancer. In the present study, we evaluated the value of a combined approach of T1W, T2W, DCE-MRI, and DWI for the detection of prostate cancer and ECE in prostate cancer. In both subgroups (T1W, T2W, and DCE-MRI vs. T1W, T2W, DCE-MRI, and DWI), the combined approach increased the accuracy of the detection rate of prostate cancer and the accuracy, sensitivity, and specificity of predicting ECE. Additionally, the sensitivity for the detection of ECE tended to increase as PSA levels rose.
Recently, Bloch et al. [6] reported that the overall sensitivity, specificity, PPV, and NPV for ECE with high spatial resolution DCE 3T-MRI were 75%, 92%, 79%, and 91%, respectively. These results demonstrated a higher sensitivity than in with our results. This difference may be because more than 90% of our data included postbiopsy hemorrhage, which could have interfered with the reading by the radiologist. In our institute, MRI is usually performed when prostate cancer is diagnosed after biopsy to evaluate the clinical stage. Therefore, owing to hemorrhage within prostate tissues, it may be difficult to assess the location of cancer lesions and to determine the range and border of the tumor [17]. However, Lee et al. [18] recently reported that hemorrhage had no significant association with the interval from biopsy to MRI. It is possible that because the technique of MRI in prostate cancer is evolving, its sensitivity and specificity are continuously being improved.
In summary, the combined approaches of T1W, T2W, DCE-MRI, and DWI had relatively reasonable accuracy, sensitivity, and specificity for the detection of prostate cancer and for predicting ECE. However, additional studies are necessary to consolidate these results.
CONCLUSIONS
The combined approach of T1W, T2W, and DCE-MRI with DWI demonstrated an accurate detection rate of prostate cancer. Also, the combination approaches showed a high specificity for predicting ECE, although sensitivity was relatively lower. Therefore, these methods are reliable for predicting prostate cancer. However, a new protocol is necessary to enhance sensitivity for predicting ECE.
ACKNOWLEDGEMENTS
This work was supported by a 2011 research grant from Chungbuk National University.
Footnotes
The authors have nothing to disclose.
References
- 1.Levi F, Lucchini F, Negri E, Boyle P, La Vecchia C. Leveling of prostate cancer mortality in Western Europe. Prostate. 2004;60:46–52. doi: 10.1002/pros.20058. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance WT, Elkin EB, Yee DS, Feifer A, Ehdaie B, Jacks LM, et al. Locally advanced prostate cancer: a population-based study of treatment patterns. BJU Int. 2012;109:1309–1314. doi: 10.1111/j.1464-410X.2011.10760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joniau SG, Van Baelen AA, Hsu CY, Van Poppel HP. Complications and functional results of surgery for locally advanced prostate cancer. Adv Urol. 2012;2012:706309. doi: 10.1155/2012/706309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beerlage HP, Aarnink RG, Ruijter ET, Witjes JA, Wijkstra H, Van De Kaa CA, et al. Correlation of transrectal ultrasound, computer analysis of transrectal ultrasound and histopathology of radical prostatectomy specimen. Prostate Cancer Prostatic Dis. 2001;4:56–62. doi: 10.1038/sj.pcan.4500495. [DOI] [PubMed] [Google Scholar]
- 5.Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243:28–53. doi: 10.1148/radiol.2431030580. [DOI] [PubMed] [Google Scholar]
- 6.Bloch BN, Genega EM, Costa DN, Pedrosa I, Smith MP, Kressel HY, et al. Prediction of prostate cancer extracapsular extension with high spatial resolution dynamic contrast-enhanced 3-T MRI. Eur Radiol. 2012;22:2201–2210. doi: 10.1007/s00330-012-2475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SY, Kim JJ, Kim TH, Lim SH, Han DH, Park BK, et al. The role of endorectal magnetic resonance imaging in predicting extraprostatic extension and seminal vesicle invasion in clinically localized prostate cancer. Korean J Urol. 2010;51:308–312. doi: 10.4111/kju.2010.51.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claus FG, Hricak H, Hattery RR. Pretreatment evaluation of prostate cancer: role of MR imaging and 1H MR spectroscopy. Radiographics. 2004;24(Suppl 1):S167–S180. doi: 10.1148/24si045516. [DOI] [PubMed] [Google Scholar]
- 9.Perdona S, Di Lorenzo G, Autorino R, Buonerba C, De Sio M, Setola SV, et al. Combined magnetic resonance spectroscopy and dynamic contrast-enhanced imaging for prostate cancer detection. Urol Oncol. 2011 Sep 07; doi: 10.1016/j.urolonc.2011.07.010. [Epub]. http://dx.doi.org/10.1016/j.urolonc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Puech P, Potiron E, Lemaitre L, Leroy X, Haber GP, Crouzet S, et al. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology. 2009;74:1094–1099. doi: 10.1016/j.urology.2009.04.102. [DOI] [PubMed] [Google Scholar]
- 11.Schiebler ML, Tomaszewski JE, Bezzi M, Pollack HM, Kressel HY, Cohen EK, et al. Prostatic carcinoma and benign prostatic hyperplasia: correlation of high-resolution MR and histopathologic findings. Radiology. 1989;172:131–137. doi: 10.1148/radiology.172.1.2472644. [DOI] [PubMed] [Google Scholar]
- 12.Outwater EK, Petersen RO, Siegelman ES, Gomella LG, Chernesky CE, Mitchell DG. Prostate carcinoma: assessment of diagnostic criteria for capsular penetration on endorectal coil MR images. Radiology. 1994;193:333–339. doi: 10.1148/radiology.193.2.7972739. [DOI] [PubMed] [Google Scholar]
- 13.Schiebler ML, Schnall MD, Pollack HM, Lenkinski RE, Tomaszewski JE, Wein AJ, et al. Current role of MR imaging in the staging of adenocarcinoma of the prostate. Radiology. 1993;189:339–352. doi: 10.1148/radiology.189.2.8210358. [DOI] [PubMed] [Google Scholar]
- 14.Westphalen AC, Coakley FV, Qayyum A, Swanson M, Simko JP, Lu Y, et al. Peripheral zone prostate cancer: accuracy of different interpretative approaches with MR and MR spectroscopic imaging. Radiology. 2008;246:177–184. doi: 10.1148/radiol.2453062042. [DOI] [PubMed] [Google Scholar]
- 15.Engelbrecht MR, Huisman HJ, Laheij RJ, Jager GJ, van Leenders GJ, Hulsbergen-Van De Kaa CA, et al. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology. 2003;229:248–254. doi: 10.1148/radiol.2291020200. [DOI] [PubMed] [Google Scholar]
- 16.Bloch BN, Furman-Haran E, Helbich TH, Lenkinski RE, Degani H, Kratzik C, et al. Prostate cancer: accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging: initial results. Radiology. 2007;245:176–185. doi: 10.1148/radiol.2451061502. [DOI] [PubMed] [Google Scholar]
- 17.White S, Hricak H, Forstner R, Kurhanewicz J, Vigneron DB, Zaloudek CJ, et al. Prostate cancer: effect of postbiopsy hemorrhage on interpretation of MR images. Radiology. 1995;195:385–390. doi: 10.1148/radiology.195.2.7724756. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Chang IH, Moon YT, Kim KD, Myung SC, Kim TH, et al. Effect of prostate biopsy hemorrhage on MRDW and MRS imaging. Korean J Urol. 2011;52:674–680. doi: 10.4111/kju.2011.52.10.674. [DOI] [PMC free article] [PubMed] [Google Scholar]