Abstract
Purpose
We present our initial experience and surgical outcomes for the most recent refinement of bilateral intrafascial nerve-sparing extraperitoneal laparoscopic radical prostatectomy (nsELRP).
Materials and Methods
Among 62 patients who underwent laparoscopic radical prostatectomy, 50 patients underwent intrafascial nsELRP by a single surgeon at Pusan National University Hospital from November 2011 to April 2012. As part of the intrafascial technique, the dissection plane is directly on the prostatic capsule to preserve most of the periprostatic fascia containing small vessels and nerves, endopelvic fascia, neurovascular bundle, and puboprostatic ligament. Postoperative continence recovery was established by daily consumption of pads. Follow-up was done at 2 weeks, 6 weeks, and 3 months after surgery.
Results
The patients' mean age was 66.5±6.2 years. The mean operation time and mean blood loss were 149.3±28.1 minutes and 155.4±168.1 ml, respectively. The mean hospitalization time and mean catheterization time were 6.3±5.1 days and 5.5±4.7 days, respectively. Two weeks after the operation, a total of 14 patients (28.0%) were pad-free but the other incontinent patient group used on average 2.3 pads per day. After 6 weeks, 35 patients (70.0%) achieved pad-free status and 7 patients (14.0%) required more than 2 pads per day. At 3 months after surgery, a total of 31 patients were available for follow-up, and 26 patients (83.9%) were pad-free.
Conclusions
Compared with conventional laparoscopic prostatectomy, the intrafascial nsELRP procedure enables the preservation of periprostatic structures that are essential to the recovery of surgical structures related to continence. As a result, early postoperative continence can be achieved.
Keywords: Anatomy, Laparoscopy, Prostatectomy
INTRODUCTION
Laparoscopic radical prostatectomy (LRP) and retropubic radical prostatectomy (RRP) are well-established procedures for the management of localized prostate cancer [1,2]. Recently, cancer treatment strategies have focused more on patient quality of life after surgery, thus requiring minimally invasive techniques. In 2002, an initial experience with extraperitoneal laparoscopic radical prostatectomy (ELRP) showed similar oncological outcomes to intraperitoneal prostatectomy without intraperitoneal complications [3]. Due to the low perioperative and postoperative mortality rate, postradical prostatectomy incontinence, which interferes with the patient's quality of life, has become an issue. One-year continence rates are excellent after RRP and LRP in larger series [4]. However, the early return of continence remains a challenge and has a major impact on the postoperative patient's health-related quality of life.
Further understanding of the anatomy of adjacent structures of the prostate, such as the bladder neck, urethra, fascia, ligaments, and neurovascular bundle (NBV), led to the development of nerve-sparing ELRP (nsELRP) and the most recent intrafascial nsELRP, which shows a positive surgical outcome of early continence [5,6].
In this study, we report our experience with 50 consecutive patients who underwent intrafascial nsELRP by a single surgeon.
MATERIALS AND METHODS
1. Patient selection criteria and evaluation
Among 62 patients, 50 patients with clinically localized prostate cancer at the time of preoperative magnetic resonance image (MRI) with PSA levels less than 10 ng/ml and Gleason scores less than 7 (3+4) were included for intrafascial nsELRP from November 2011 to April 2012. If there were any suspicious lesions with extracapsular extension of tumors or high Gleason scores (7 [4+3] to 10), conventional radical prostatectomy and lymph node dissection were performed. A small group of patients underwent intrafascial nsELRP despite their higher PSA levels of more than 10 ng/ml because they had low Gleason scores (≤6).
2. The surgical techniques
Patient positioning and trocar placement
In short, the patient was placed in the supine position with mild head-down tilt. The preparation of the space of Retzius began with a 2-cm sized incision in the infraumbilical crease laterally to the midline, which was then carried down to the posterior rectus sheath where a balloon trocar was inserted and the preperitoneal space was developed. Finally, the camera trocar was placed and further trocars were inserted with attention to the course of the epigastric vessels and peritoneum. Next, the 12-mm assistant port, 12-mm operator port, and 5-mm operator port were placed sequentially.
Retroperitoneal preparation
The fatty tissue surrounding the prostate and pelvic space was removed to expose the landmark structures such as the pubic arch, symphysis, endopelvic fascia, bladder, and prostate. The anterior surfaces of the bladder and prostate as well as the endopelvic fascia became visible to the operator. The removal of preprostatic fibro-fatty tissues facilitates more definite differentiation between the prostate and the bladder.
Initial approach and bladder neck dissection
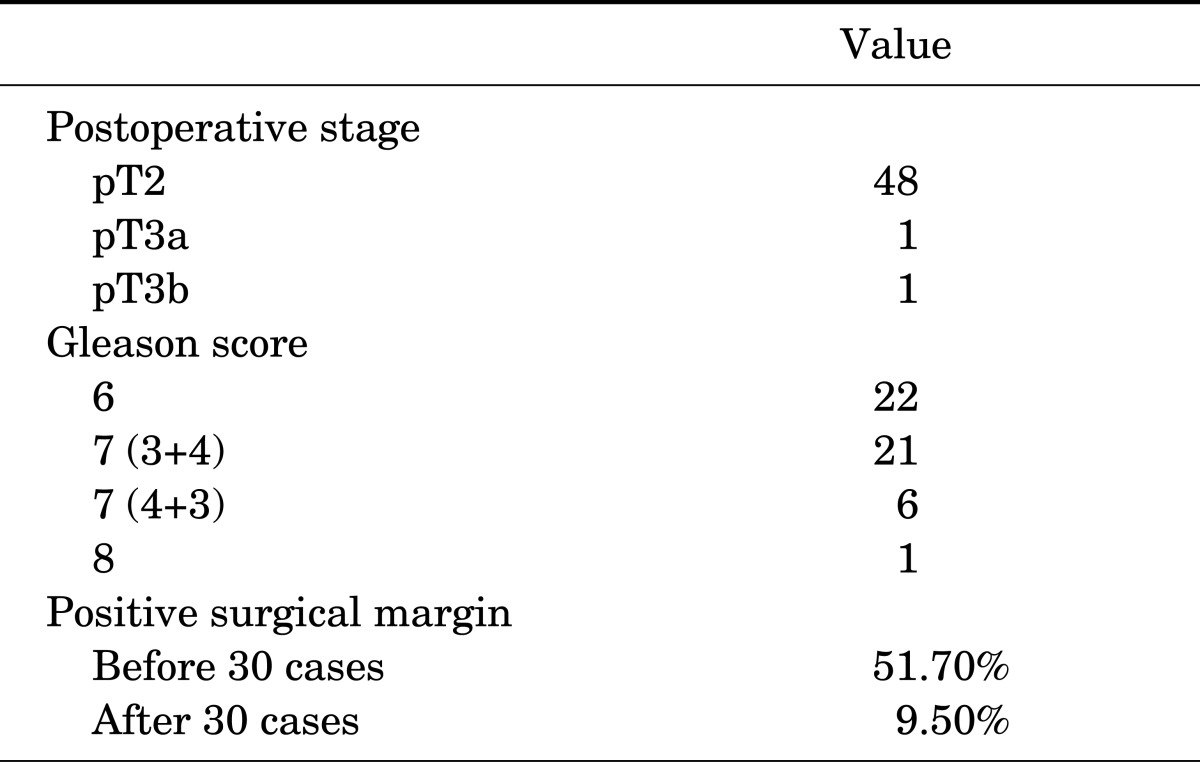
The first step of the intrafascial procedure was different from conventional ELRP. The endopelvic fascia and pubo-prostatic ligament were not incised and the deep dorsal venous complex was not ligated at the beginning of the procedure. A bilateral incision of the periprostatic fascia was made medial to the puboprostatic ligament (PPL) and directed to the base of the prostate. The right plane of dissection was recognized when the surface of the prostate was completely smooth. As a result, the development of a plane between the prostate and overlaying fascia was possible and the operator could detach the prostate from its enveloping fascia. All lateral periprostatic fascia, endopelvic fascia, and puboprostatic fascia remained intact (Fig. 1).
FIG. 1.
Initial approach for intrafascial nerve-sparing extraperitoneal laparoscopic radical prostatectomy. Incision is made medial to the puboprostatic ligament. PPL, puboprostatic ligament; P, prostate; Bl, bladder; Ef, endopelvic fascia.
Preparation of the seminal vesicles
Careful coagulation-free dissection is necessary during bilateral seminal vesicle dissection because the pelvic plexus and NVB run in close proximity to the tip of the seminal vesicle. Five-millimeter titanium clips (Ligaclip, Ethicon Inc., Somerville, NJ, USA) were used to ligate the seminal vesicular artery.
When the vas deferens and seminal vesicle were both fully dissected, the Denonvilliers fascia was visualized. The Denonvilliers fascia was stripped down to find the correct plan for the intrafascial dissection of the prostate. After identification of the prostate capsule, the dissection was gradually directed toward the apex of the prostate in the midline to avoid injury of the NBVs.
Dissection of the prostatic pedicle and NBV
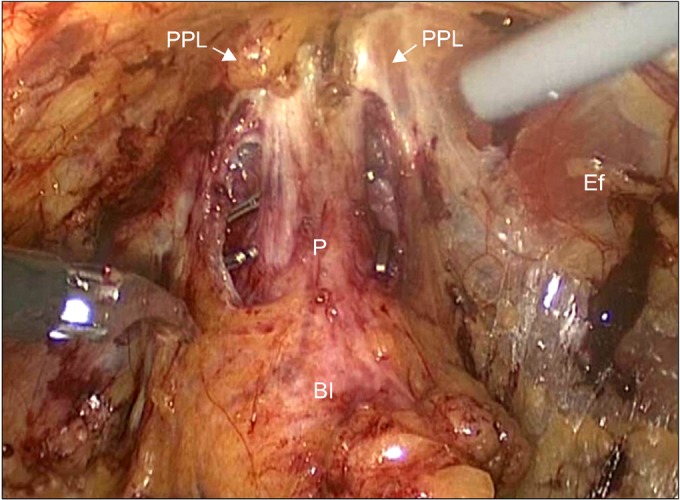
The prostate was fully detached from its surrounding fascias but was still attached by the pedicles and the apex. The surface of the prostatic capsule was clearly seen medially and laterally. The assistant took the seminal vesicle and vas deferens and gently elevated them ventrally to allow clear sight of the prostatic pedicle. The prostatic pedicles were then clipped and cut in a step-by-step manner directly on the surface of the prostatic capsule without excessive traction or electrocautery. When dissecting the NBV, care must be taken to avoid damage to the NVB. After the correct plane was opened, the dissection was performed by using cold scissors in an essentially avascular plane. Traction and electrocautery on the prostate and the NVB were also avoided. Meticulous dissection is needed between the lateral side of the prostate and the lateral remnant of the periprostatic fascia toward the apex of the prostate to preserve the accessory NVB (Fig. 2). The assistant retracted the partially mobilized prostate to the opposite side. The dissection was performed on the opposite side of the prostate pedicle and the NVB in the same fashion as that of the primary side. Finally, the prostate was completely detached from the surrounding fascias, bladder, prostatic pedicles, and NVBs.
FIG. 2.
Prostatic pedicle dissection and sparing of the neurovascular bundles. Both anterior and posterior neurovascular bundles were preserved by using cold scissors. P, prostate; aNVB, accessory neurovascular bundle; pNVB, predominant neurovascular bundle.
Dissection of the urethra
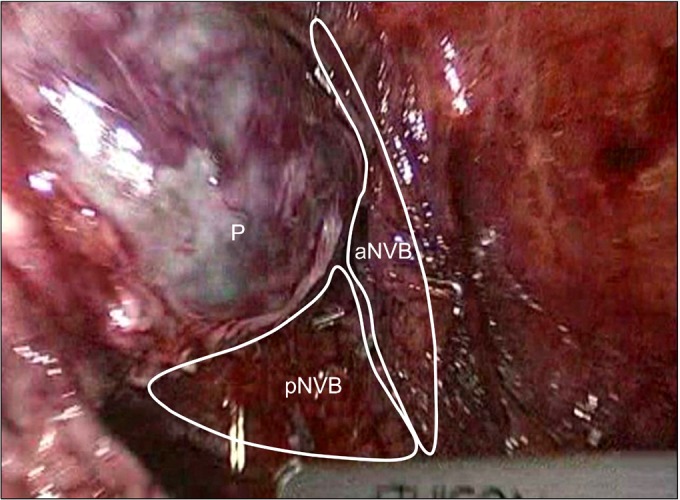
Sharp dissection of the prostate from the external sphincter and urethra at the site of the apex was performed. We did not ligate the deep dorsal venous plexus (Santorini plexus). The assistant retracted the prostate to secure a clear view of the apex margin of the prostate. Dissection and division of the external urethral sphincter and prostate were carefully made (Fig. 3). Dissection of the urethra was performed proximally very close to the prostate to preserve the urethral length as much as possible. Vertical dissection was performed with cold scissors alone to achieve complete division of the prostate from the urethra. An endoscopic retrieval bag was used to remove the prostate.
FIG. 3.
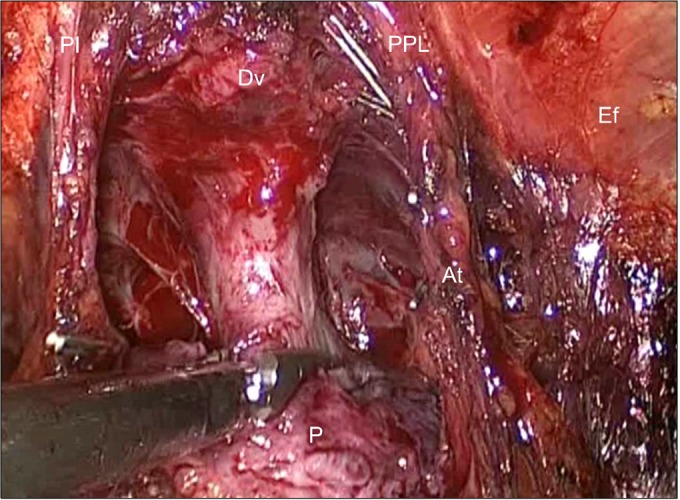
Dissection of the prostate apex and urethra. Urethral dissection was performed proximally very close to the prostate to preserve urethral length. We did not ligate the deep dorsal venous plexus. The endopelvic fascia, which envelops the levator ani muscle, was preserved during surgery and the longer urethra was preserved. Both puboprostatic ligaments and arcuous tendinosus were also preserved. Dv, deep dorsal venous plexus; U, urethra; P, prostate; Ef, endopelvic fascia; PPL, puboprostatic ligament; At, arcuous tendinosus.
3. Postoperative evaluation
All patients underwent cystography on the 4th postoperative day. After identification of no contrast leakage at the anastomosis site, the Foley catheter was removed. Postoperative evaluation of continence was performed by evaluation of the number of pads used per day. Postoperative follow-up was defined at 2 weeks, 6 weeks, and 3 months. Postoperative interviews were also conducted. Complete continence was defined as usage of no pads and patient's report of no urinary leakage.
We also evaluated erectile function and oncologic outcome after nsELRP.
4. Statistical analysis
Statistical analysis was performed by using PASW ver. 18.0 (IBM Co., Armonk, NY, USA), with statistical significance considered at p<0.05. Spearman's correlation test was used to evaluate the factors related to early continence recovery after nsELRP.
RESULTS
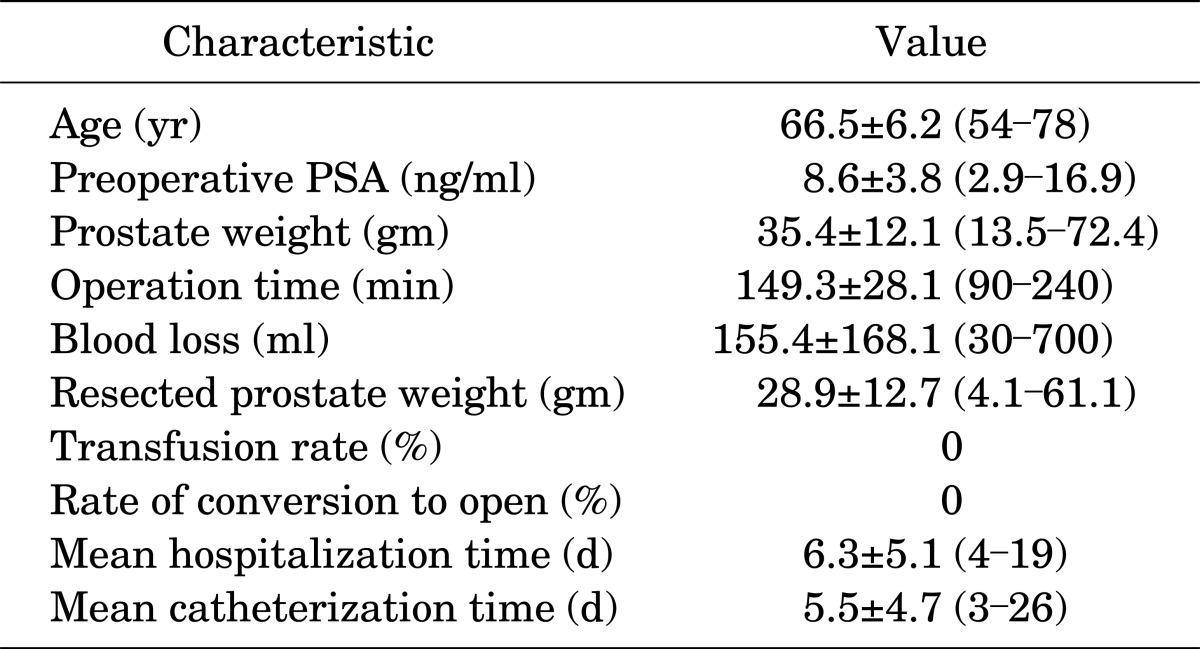
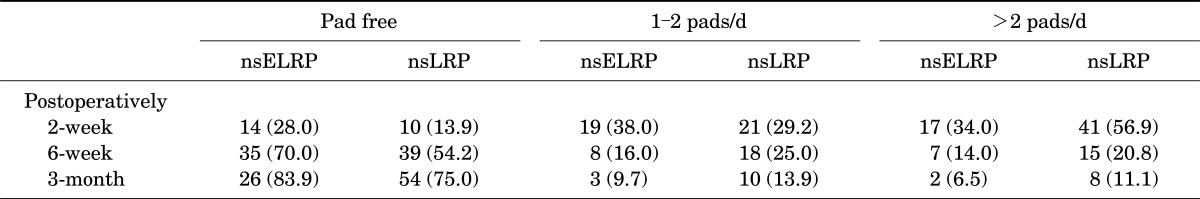
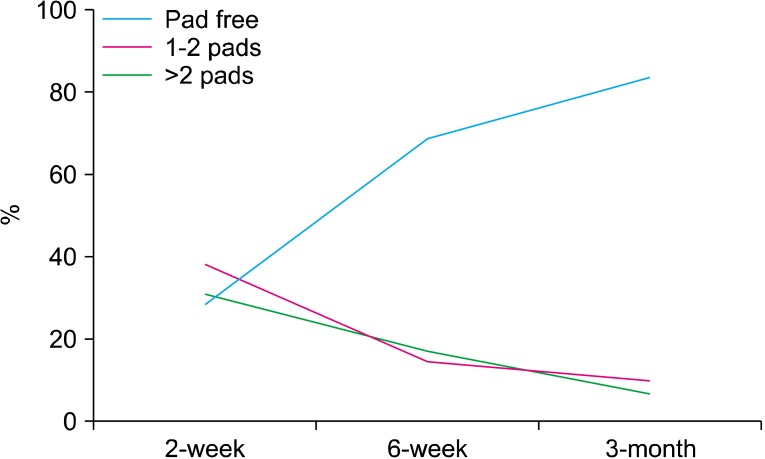
A total of 50 consecutive patients underwent intrafascial nsELRP by one surgeon. Preoperative and perioperative data are summarized in Table 1. The rate of conversion to open surgery and the transfusion rate were both 0%. The mean operation time was 149.3 minutes (range, 90 to 240 minutes). The mean hospitalization time was 6.3 days (range, 4 to 19 days), and the mean catheterization time was 5.5 days (range, 3 to 26 days). Postoperative continence results are shown in Table 2. At the 2-week follow-up after surgery, 14 patients (28.0%) achieved total continence and on average 2.3 pads were required for the other incontinent patients. A total of 35 patients (70.0%) were continent at 6 weeks after surgery, 8 patients (16.0%) had mild stress incontinence (1 to 2 pads), and 7 patients (14.0%) required >2 pads per day (Fig. 4). A total of 31 patients were available for follow-up at 3 months after surgery, and 26 patients (83.9%) were pad-free. Compared with conventional nerve-sparing LRP, intrafascial nsELRP showed early continence recovery (Table 2).
TABLE 1.
Preoperative and perioperative patient characteristics (n=50)
Values are presented as mean±SD (range).
PSA, prostate-specific antigen.
TABLE 2.
Continence after intrafascial nsELRP and conventional nsLRP
Values are presented as number (%).
nsELRP, nerve-sparing extraperitoneal laparoscopic radical prostatectomy; nsLRP, nerve-sparing laparoscopic radical prostatectomy.
FIG. 4.
Continence rates after intrafascial nerve-sparing extraperitoneal laparoscopic radical prostatectomy.
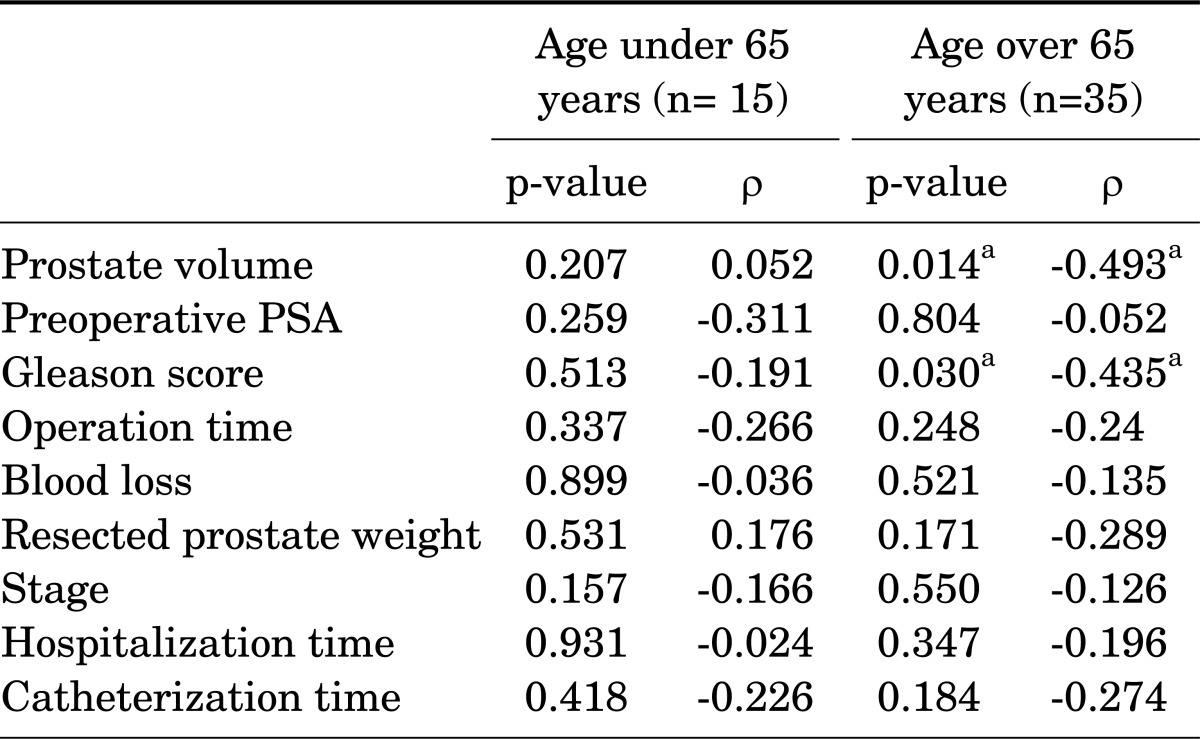
In patients with a relatively old age of above 65 years, preoperative low prostate volume and low Gleason score were related with early continence at 6 weeks after surgery (Table 3).
TABLE 3.
Factors related to early continence at 6 weeks after surgery
PSA, prostate-specific antigen.
a:Statistical significance.
Signs of early recovery of potency (morning erection or erection sensation) were reported by 19 patients (38.0%) at 6 weeks after surgery and the rate increased to 54.8% at 3 months after surgery. The average positive surgical margin rate was 34.0%, respectively. But from the 30 cases, the positive surgical margin rate decreased to 9.5% (Table 4). Most positive surgical margins were seen at the apex of the prostate.
TABLE 4.
Oncologic outcomes (n=50)
Early postoperative complications were encountered. Two patients (4.0%) with anastomosis site leakage were treated with prolonged catheterization for an additional 1 week. Two patients (4.0%) with acute urinary retention after catheter removal were treated with re-catheterization for 1 week.
DISCUSSION
Urinary incontinence is potentially the most debilitating complication of radical prostatectomy. The risk of urinary incontinence is not small and is variable among surgeons. It has been shown that the incidence of postoperative incontinence depends on the urologist's experience, patient's age (increased frequency after 70 years), and whether the operative technique includes minimal distal incision of the endopelvic fascia, preservation of the bladder neck, bilateral nerve-sparing surgery, or preservation of the PPL [7,8]. In the American Urological Association guidelines, the reported risk of urinary incontinence ranges from 3 to 74% for radical prostatectomy [9]. Continence mechanisms involve many structures, including the PPL, Denonvilliers' fascia, levator muscle, endopelvic fascia, and internal and external sphincters. Ventrally, the proximal prostate is covered by muscle fibers originating from the outer longitudinal bladder muscle and extending over the gland. These fibers constitute a detrusor apron [10,11].
The pubovesical/PPLs (PV/PPLs) are paired fibrous bands originating from visceral endopelvic fascia. They insert on the distal third of the posterior surface of the pubic bone adjacent and anterior to the urethral sphincter [11]. The visceral component of the endopelvic fascia covers the pelvic organs including the prostate, bladder, and rectum, and it is fused with the anterior fibromuscular stroma of the prostate at the upper ventral aspect of the gland [12-14]. Along the pelvic sidewall at the lateral aspect of the prostate and bladder, the parietal and the visceral components of the endopelvic fascia are fused. As a fascial condensation, this fusion is often recognizable as a whitish line and is named the fascial tendinous arch of the pelvis. It stretches from the PV/PPLs to the ischial spine. During surgery, access to the lateral prostate may be gained by incision of the endopelvic fascia either medial or lateral to this fusion [10]. The PV/PPLs stabilize the prostate, urethra, and bladder to the pubic bone and are considered an important part of the "suspensory system" of the continence mechanism [15-19]. Some authors have suggested that preservation of these ligaments during radical prostatectomy may improve early recovery of urinary continence, but no definitive evidence has yet been established [17,18]. Preservation of the PV/PPLs is facilitated by using the perineal and laparoscopic approach, whereas during open retropubic prostatectomy, the PV/PPLs are more difficult to preserve [20,21]. Some authors have suggested that avoiding incision of the endopelvic fascia during radical prostatectomy, often combined with an intrafascial nerve-sparing procedure, might improve early recovery of urinary continence as well as improve postoperative erectile function, but definitive evidence has yet to be established [12,22]. The parietal endopelvic fascia includes fascia of the levator ani muscle. The incision of this fascia immediately lateral to the fascial tendinous arch incises the levator ani fascia (LAF) and leaves the muscle fibers of the levator ani bare and the LAF adherent to the prostate [10,19]. An incision of the visceral endopelvic fascia medial to the fascial tendinous arch results in a dissection plane that leaves the levator ani muscle covered with its fascia without exposure of its fibers [12,22]. The result is a prostate covered only by prostatic fascia (PF), when present, and not by a layer of LAF [12,13]. This fascia is not a discrete single-layered structure stretching over the lateral surface of the prostate. Laparoscopic surgery may offer an improved identification of these structures, resulting in less damage to the structures around the prostate. We could also identify these structures during the operation, which is related to recovery of postoperative urinary continence.
In addition, others have showed that continence rates correlate with differences in the mean functional urethral length and the existing differences in the maximal urethral closure pressure postprostatectomy [11,15,16,23]. Poore et al. [17] reported an earlier return of continence with a PPL-sparing technique versus a nonsparing technique, but the final outcomes were equivalent. Other authors also advocate the latter technique and have reported encouraging results [11,15,16,23]. We believe that during mobilization of the prostate and especially during apical dissection, the intactness of the urethral supporting structures are of paramount importance because this avoids shear stress to the urethra as well as possible denervation. In our study, we observed an earlier return to continence in patients who underwent intrafascial nsELRP compared with previous conventional nerve-sparing LRP. Complete continence was achieved by 70.0% of patients who underwent nsELRP at 6 weeks after surgery and by 83.9% at 3 months after surgery. In the control group, however, 55.2% of patients achieved complete continence at 6 weeks after surgery and 75.0% at 3 months after surgery.
Reconstruction methods such as periurethral suspension stitch, bladder neck reconstruction, and posterior reconstruction have also been shown to provide improved early continence recovery, but the results are still controversial [24,25]. Therefore, we focused on the preservation of normal structures rather than performing reconstruction after destruction of these structures.
Studies have reported that older age may be the only increasing risk factor for postprostatectomy incontinence [26], but in our study, increased prostate volume and Gleason score were related with post-prostatectomy incontinence in the older aged group (age over 65 years). We speculate that large prostate volume and old age may be related to preoperative bladder and external urethral sphincter dysfunction leading to interference with postprostatectomy recovery of continence. A preoperative urodynamic study may be helpful in proving the relationship between prostate volume and old age with postoperative incontinence.
Anatomical studies [27,28] have illustrated the prostatic neuroanatomy in detail, detecting additional neural tissues to the nerve bundles on the anterior midpart and posterior surface of the prostate. Costello et al. [29] recently showed that most of the NVB descends posteriorly to the seminal vesicle. The nerves pass anteriorly and converge at the midprostatic level, and when they approach the apex, they diverge again. The anterior and posterior nerves of the NVB are separated by 3 cm at the level of the base of the prostate. At this anatomic site, the cavernosal nerves are not easily distinguished from the surrounding tissues and care should be taken during urethra-vesical anastomosis. Walsh [30] proposed that the NVB is enclosed within the two layers of the lateral pelvic fascia composed of the lateral layer of the levator fascia and the medial layer of the PF. Kiyoshima et al. [14] proved that the NVB was located on the posterolateral region of the prostate in 48% of their patients. In the remaining patients (52%), the NVB was widely distributed on the entire lateral aspect of the prostate without any specific localization. Thus, the authors proposed performing wide dissection of the lateral aspect of the prostate during radical prostatectomy to preserve the NVB. Therefore, meticulous dissection and disuse of electro-cauterization at the lateral and apex sides of the prostate are required to preserve the NVB by intrafascial nsELRP.
Anastasiadis et al. [1] reported potency rates of 30% and 41% at 12 months after LRP (n=230) and open retropubic prostatectomy (n=70), respectively. After preservation of one or both NVBs, the potency rates increased from 37 to 44% with the retropubic approach and from 46 to 53% with LRP, respectively. Patients younger than 60 years who underwent bilateral NVB preservation were reported to be potent in 72% and 81% of cases, respectively. Graefen et al. [20] reported rates of erections of 96.5%, 90.7%, and 84.3% and rates of intercourse of 69.0%, 52.8%, and 37.3% at 12 months after bilateral RRP in men <55 years, 55 to 65 years, and >65 years, respectively. In our study, the patients were generally old aged and were not sexually active; thus, we could not obtain potency recovery data. However, 19 patients (38.0%) reported subjective signs of potency recovery (morning erection or erection sensation) at 6 weeks after surgery, and at 3 months after surgery, the percentage was increased to 54.8% of patients.
The oncologic data of 1,000 LRPs at the Montsouris Institute revealed positive surgical margin rates of 6.9% for pT2a and 34% for pT3b tumors [2]. In our study, the overall positive surgical margin rate was 34.0%. We consider this high positive margin rate as a "learning curve." In fact, from 30 cases, positive surgical margin rates decreased compared with the first 30 cases (51.7% vs. 9.5%). Most positive margins were seen at the apex of the prostate.
CONCLUSIONS
Intrafascial nsELRP is a further evolution of the current nsELRP. The initial results are promising, providing favorable functional outcomes and oncologic results similar to other RP techniques.
Footnotes
The authors have nothing to disclose.
References
- 1.Anastasiadis AG, Salomon L, Katz R, Hoznek A, Chopin D, Abbou CC. Radical retropubic versus laparoscopic prostatectomy: a prospective comparison of functional outcome. Urology. 2003;62:292–297. doi: 10.1016/s0090-4295(03)00352-2. [DOI] [PubMed] [Google Scholar]
- 2.Guillonneau B, el-Fettouh H, Baumert H, Cathelineau X, Doublet JD, Fromont G, et al. Laparoscopic radical prostatectomy: oncological evaluation after 1,000 cases a Montsouris Institute. J Urol. 2003;169:1261–1266. doi: 10.1097/01.ju.0000055141.36916.be. [DOI] [PubMed] [Google Scholar]
- 3.Stolzenburg JU, Do M, Pfeiffer H, Konig F, Aedtner B, Dorschner W. The endoscopic extraperitoneal radical prostatectomy (EERPE): technique and initial experience. World J Urol. 2002;20:48–55. doi: 10.1007/s00345-002-0265-4. [DOI] [PubMed] [Google Scholar]
- 4.Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 5.Stolzenburg JU, Liatsikos EN, Rabenalt R, Do M, Sakelaropoulos G, Horn LC, et al. Nerve sparing endoscopic extraperitoneal radical prostatectomy: effect of puboprostatic ligament preservation on early continence and positive margins. Eur Urol. 2006;49:103–111. doi: 10.1016/j.eururo.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Stolzenburg JU, Rabenalt R, Tannapfel A, Liatsikos EN. Intrafascial nerve-sparing endoscopic extraperitoneal radical prostatectomy. Urology. 2006;67:17–21. doi: 10.1016/j.urology.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 7.Salomon L, Sebe P, De la Taille A, Vordos D, Hoznek A, Yiou R, et al. Open versus laparoscopic radical prostatectomy: part I. BJU Int. 2004;94:238–243. doi: 10.1111/j.1464-410X.2004.04950.x. [DOI] [PubMed] [Google Scholar]
- 8.Salomon L, Sebe P, De la Taille A, Vordos D, Hoznek A, Yiou R, et al. Open versus laparoscopic radical prostatectomy: Part II. BJU Int. 2004;94:244–250. doi: 10.1111/j.1464-410X.2004.04951.x. [DOI] [PubMed] [Google Scholar]
- 9.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Myers RP. Practical surgical anatomy for radical prostatectomy. Urol Clin North Am. 2001;28:473–490. doi: 10.1016/s0094-0143(05)70156-7. [DOI] [PubMed] [Google Scholar]
- 11.Myers RP. Detrusor apron, associated vascular plexus, and avascular plane: relevance to radical retropubic prostatectomy--anatomic and surgical commentary. Urology. 2002;59:472–479. doi: 10.1016/s0090-4295(02)01500-5. [DOI] [PubMed] [Google Scholar]
- 12.Takenaka A, Hara R, Soga H, Murakami G, Fujisawa M. A novel technique for approaching the endopelvic fascia in retropubic radical prostatectomy, based on an anatomical study of fixed and fresh cadavers. BJU Int. 2005;95:766–771. doi: 10.1111/j.1464-410X.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- 13.Savera AT, Kaul S, Badani K, Stark AT, Shah NL, Menon M. Robotic radical prostatectomy with the "Veil of Aphrodite" technique: histologic evidence of enhanced nerve sparing. Eur Urol. 2006;49:1065–1073. doi: 10.1016/j.eururo.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 14.Kiyoshima K, Yokomizo A, Yoshida T, Tomita K, Yonemasu H, Nakamura M, et al. Anatomical features of periprostatic tissue and its surroundings: a histological analysis of 79 radical retropubic prostatectomy specimens. Jpn J Clin Oncol. 2004;34:463–468. doi: 10.1093/jjco/hyh078. [DOI] [PubMed] [Google Scholar]
- 15.Steiner MS. The puboprostatic ligament and the male urethral suspensory mechanism: an anatomic study. Urology. 1994;44:530–534. doi: 10.1016/s0090-4295(94)80052-9. [DOI] [PubMed] [Google Scholar]
- 16.Presti JC, Jr, Schmidt RA, Narayan PA, Carroll PR, Tanagho EA. Pathophysiology of urinary incontinence after radical prostatectomy. J Urol. 1990;143:975–978. doi: 10.1016/s0022-5347(17)40155-8. [DOI] [PubMed] [Google Scholar]
- 17.Poore RE, McCullough DL, Jarow JP. Puboprostatic ligament sparing improves urinary continence after radical retropubic prostatectomy. Urology. 1998;51:67–72. doi: 10.1016/s0090-4295(97)00479-2. [DOI] [PubMed] [Google Scholar]
- 18.Deliveliotis C, Protogerou V, Alargof E, Varkarakis J. Radical prostatectomy: bladder neck preservation and puboprostatic ligament sparing: effects on continence and positive margins. Urology. 2002;60:855–858. doi: 10.1016/s0090-4295(02)01956-8. [DOI] [PubMed] [Google Scholar]
- 19.Burnett AL, Mostwin JL. In situ anatomical study of the male urethral sphincteric complex: relevance to continence preservation following major pelvic surgery. J Urol. 1998;160:1301–1306. [PubMed] [Google Scholar]
- 20.Graefen M, Walz J, Huland H. Open retropubic nerve-sparing radical prostatectomy. Eur Urol. 2006;49:38–48. doi: 10.1016/j.eururo.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Tewari AK, Bigelow K, Rao S, Takenaka A, El-Tabi N, Te A, et al. Anatomic restoration technique of continence mechanism and preservation of puboprostatic collar: a novel modification to achieve early urinary continence in men undergoing robotic prostatectomy. Urology. 2007;69:726–731. doi: 10.1016/j.urology.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Stolzenburg JU, Rabenalt R, Do M, Schwalenberg T, Winkler M, Dietel A, et al. Intrafascial nerve-sparing endoscopic extraperitoneal radical prostatectomy. Eur Urol. 2008;53:931–940. doi: 10.1016/j.eururo.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 23.Albers DD, Faulkner KK, Cheatham WN, Elledge EF, Coalson RE. Surgical anatomy of the pubovesical (puboprostatic) ligaments. J Urol. 1973;109:388–392. doi: 10.1016/s0022-5347(17)60432-4. [DOI] [PubMed] [Google Scholar]
- 24.Rocco B, Cozzi G, Spinelli MG, Coelho RF, Patel VR, Tewari A, et al. Posterior musculofascial reconstruction after radical prostatectomy: a systematic review of the literature. Eur Urol. 2012;62:779–790. doi: 10.1016/j.eururo.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Hurtes X, Roupret M, Vaessen C, Pereira H, Faivre d'Arcier B, Cormier L, et al. Anterior suspension combined with posterior reconstruction during robot-assisted laparoscopic prostatectomy improves early return of urinary continence: a prospective randomized multicentre trial. BJU Int. 2012;110:875–883. doi: 10.1111/j.1464-410X.2011.10849.x. [DOI] [PubMed] [Google Scholar]
- 26.Cambio AJ, Evans CP. Minimising postoperative incontinence following radical prostatectomy: considerations and evidence. Eur Urol. 2006;50:903–913. doi: 10.1016/j.eururo.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Sievert KD, Hennenlotter J, Laible I, Amend B, Schilling D, Anastasiadis A, et al. The periprostatic autonomic nerves: bundle or layer? Eur Urol. 2008;54:1109–1116. doi: 10.1016/j.eururo.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Ganzer R, Blana A, Gaumann A, Stolzenburg JU, Rabenalt R, Bach T, et al. Topographical anatomy of periprostatic and capsular nerves: quantification and computerised planimetry. Eur Urol. 2008;54:353–360. doi: 10.1016/j.eururo.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004;94:1071–1076. doi: 10.1111/j.1464-410X.2004.05106.x. [DOI] [PubMed] [Google Scholar]
- 30.Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160(6 Pt 2):2418–2424. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]