Abstract
Schwannomas are benign tumors that arise from the neural sheath of Schwann cells. Renal schwannomas are extremely rare and are commonly misdiagnosed as renal cell carcinoma, which typically results in a radical nephrectomy. We present a case of a renal schwannoma that mimics a renal pelvis tumor.
Keywords: Kidney, Neurilemmoma
Schwannomas are tumors of the nerve sheath that are usually benign. The tumor is commonly found in the head, neck, and extremities. Although 3% of schwannomas occur in the retroperitoneum, involvement of the kidney is extremely uncommon [1]. A preoperative diagnosis is difficult; therefore, renal schwannomas are often diagnosed incidentally during pathologic examination.
The renal hilum can be a site of renal schwannoma development because parasympathetic nerve fibers of the kidney accompany the renal artery, which enters into the renal hilum. However, many renal schwannomas are located in the renal parenchyma and mimic a renal cell carcinoma rather than a transitional cell carcinoma [2]. Herein, we report a case of a renal schwannoma that mimics a transitional cell carcinoma and that was treated by laparoscopic nephroureterectomy.
CASE REPORT
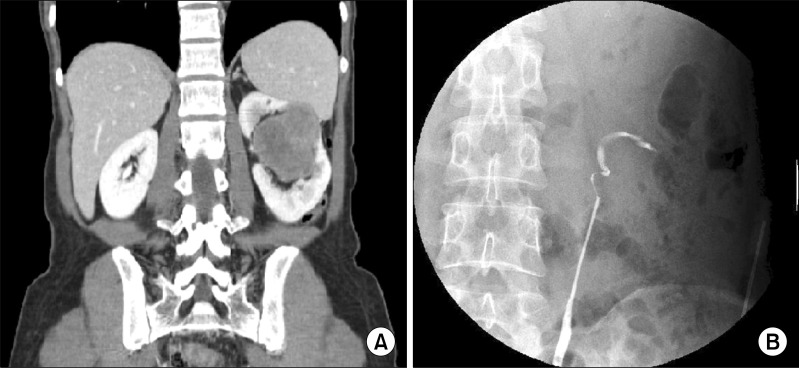
A 40-year-old woman presented with left flank pain of 2 weeks duration. She had no other urologic symptoms and no history of gross hematuria. A physical examination indicated a palpable hard mass over the left upper quadrant, but no flank tenderness. The results of laboratory studies, which included a complete blood count, a blood chemistry analysis, urinalysis, and a urine culture, were within normal limits. The results of a urine cytologic examination were also negative. Computed tomography (CT) revealed a renal tumor approximately 7.1 cm in size in the left renal pelvis area. The mass was low-attenuated, lobulated, and minimally enhanced. The mass was primarily located in the renal hilum, and the renal pelvo-calyceal system was not identified. In addition, the renal tumor was well demarcated from the renal parenchyma, and the mass was abutting the posterior aspect of the pancreas body and the inferior aspect of the spleen via the renal parenchyma (Fig. 1). A retrograde pyelography (RGP) was performed to identify the renal pelvis tumor. On RGP, the left ureteropelvic junction was kinked, the upper calyces were obliterated, and the calyx was filled with an irregular collection of contrast (Fig. 1).
FIG. 1.
(A) A computed tomography scan shows a centrally located renal mass abutting the spleen via the renal parenchyma. (B) A retrograde pyelograph shows a kinked left ureteropelvic junction, totally collapsed upper calyces, and an irregularly contrast-filled lower calyx.
We decided to perform a nephroureterectomy because the renal pelvis tumor could not be excluded completely. The left kidney was removed by retroperitoneal laparoscopic surgery, and then the lower ureter and bladder cuff were excised by using an open maneuver. At the time of surgery, the mass was well dissected from the adjacent tissues and was not adherent to the spleen or pancreas.
Grossly, the tumor was well-circumscribed, 6.8×6.5×3.5 cm in size, and located in the renal pelvis, compressed adjacent to the renal parenchyma. The tumor was oval shaped and lobulated, and the cut surface was light yellow to gray-white in appearance (Fig. 2).
FIG. 2.
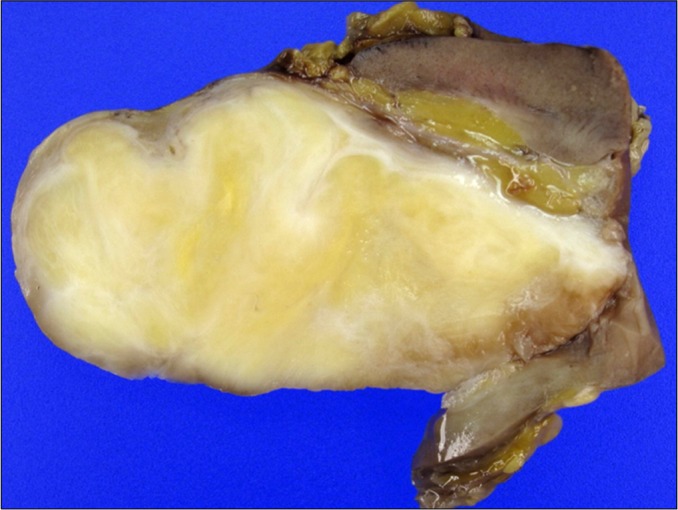
Gross photograph of the resected schwannoma. The lobulated mass is well-circumscribed and light yellow to gray-white in appearance and was located in the renal pelvis and compressed adjacent to the renal parenchyma.
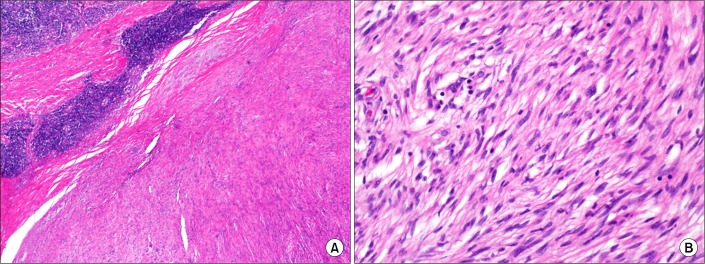
Histologic examination showed that the tumor consisted of a solid proliferation of spindle-shaped cells of relatively uniform appearance with small amounts of indistinct cytoplasm and oval dark-stained nuclei. The tumor cells were indistinguishable from those of an angiomyolipoma (which has scanty adipose tissue and blood vessels), gastrointestinal stromal tumor (GIST), or leiomyoma. The characteristic histologic findings of a schwannoma, i.e., a highly ordered cellular component (Antoni A) that palisades (Verocay bodies) with a plus myxoid component (Antoni B), were not identified. However, a peripheral cuff of lymphoid aggregates, suggestive of schwannoma rather than GIST, was observed (Fig. 3A). Interlacing bundles of spindle cells showed neither nuclear atypia nor mitotic figures (Fig. 3B).
FIG. 3.
Histologic features of the resected schwannoma. (A) Most areas consisted of interlacing bundles of spindle cells and collagen and a peripheral cuff of lymphoid aggregates (H&E, ×100). (B) No nuclear atypia or mitotic figures were evident in the spindle cells (H&E, ×400).
Immunohistochemical staining with HMB45, S-100, smooth muscle actin (SMA), calponin, desmin, C-kit, and Ki-67 was performed. The tumor cells showed diffuse strong positivity for S-100 protein (Fig. 4). The tumor cells were negative for HMB45, SMA, calponin, desmin, and C-kit. Our final pathologic diagnosis was primary renal pelvic schwannoma.
FIG. 4.
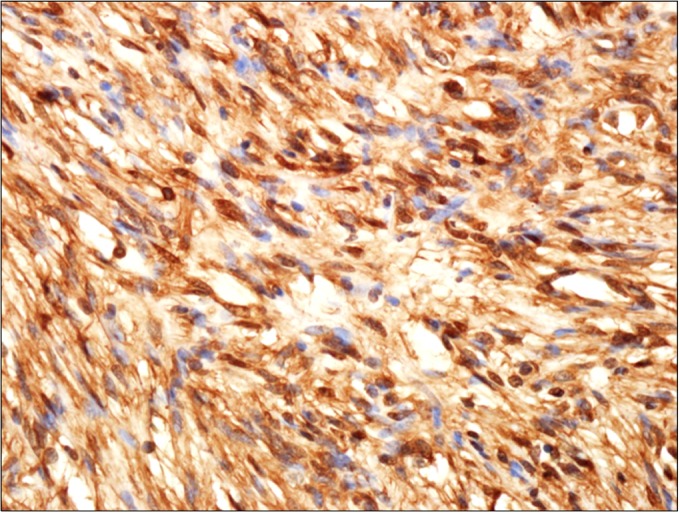
Immunohistochemical findings. Immunostaining showed diffuse strong positivity for S-100 (schwann cells) (×400).
DISCUSSION
A schwannoma is a benign nerve sheath tumor that arises along the course of any myelinated nerve. Although most commonly found in the head and extremities, schwannomas can occur in virtually any part of the body. Retroperitoneal schwannomas are rare, and they account for only 1 to 3% of all schwannomas and only 1% of all retroperitoneal tumors [1]. Renal schwannomas occur most commonly in patients between 40 and 60 years of age, predominantly in males (male:female ratio, 5:3). Renal hilum involvement was identified in only 2 of the 16 previously reported cases of renal schwannoma [2].
Because they are usually slow growing and asymptomatic, renal schwannomas are often found incidentally in patients presenting with vague and nonspecific symptoms. The most common symptoms of a renal schwannoma are flank pain (43%) and a palpable mass (37%). The most common location of these tumors is the renal parenchyma (50%), and the average size of these tumors is 10 cm.
Differential diagnostic considerations include renal cell carcinoma, renal angiomylipoma, sarcomatoid carcinoma, solitary fibrous tumor, leiomyoma, rhabdomyosarcoma, and angiosarcoma [3]. Renal schwannomas are difficult to diagnose preoperatively from radiographic findings. In 1989, Ghiatas and Faleski [4] described the CT features of a benign schwannoma as a well-circumscribed homogeneous or inhomogeneous soft tissue mass. The inhomogenicity of the schwannoma on CT has been ascribed to the areas of hypocellularity adjacent to more cellular areas, cystic degeneration due to vascular thrombosis, xanthoma formation, and hypercellular regions adjacent to dense bundles of collagen [2,4]. However, these findings are not disease specific. Findings from magnetic resonance imaging (MRI) might provide some clues, including iso-intensity on T1-weighted images and a high signal intensity on T2-weighted images. Gadolinium-enhanced T1-weighted images indicate a strong and homogeneous enhancement in the solid part of the tumor [5]. In our case, we assumed that the tumor was a transitional cell carcinoma; therefore, we did not perform an MRI. Fine-needle aspiration biopsy may be useful if Schwann cells are found in the sample, but the tissues obtained in this manner are often inadequate for diagnosis and may confound the diagnosis because of cellular pleomorphism in degenerated areas that can be interpreted as malignancy [6].
Malignant degeneration is extremely rare. Malignant schwannomas act as high-grade sarcomas and have a high likelihood of producing local recurrence and distant metastasis. The diagnosis of a malignant peripheral nerve sheath tumor lacks standardized diagnostic criteria, but rather features dense fascicles in a "marble-like" pattern consisting of asymmetrically tapered spindle cells [6].
Histologically, schwannomas consist of compact cellular lesions (Antoni A tissue) and loose, hypocellular, myxoid lesions with microcystic spaces (Antoni B tissue). S-100 immunostaining was useful in the differential diagnosis and confirmed the neuroectodermal origin of the tumor. The current case had typical patterns of a schwannoma and was positive for S-100 on immunostaining [7].
Surgical excision is the treatment of choice for these tumors. Because these tumors are usually thought to be renal cell carcinoma before surgery, a radical nephrectomy is commonly performed. If a schwannoma is suspected, laparoscopic resection may be useful because a retroperitoneal schwannoma, which is commonly localized and hypovascular, can easily be dissected from the adjacent tissues. However, local recurrence and malignant changes are possible with benign schwannomas despite a prior benign diagnosis [8]. Therefore, complete resection of the tumor is very important. In the current case, the tumor was completely resected en-bloc, and its pathologic nature was confirmed to be benign.
Footnotes
The authors have nothing to disclose.
References
- 1.Gubbay AD, Moschilla G, Gray BN, Thompson I. Retroperitoneal schwannoma: a case series and review. Aust N Z J Surg. 1995;65:197–200. doi: 10.1111/j.1445-2197.1995.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 2.Hung SF, Chung SD, Lai MK, Chueh SC, Yu HJ. Renal Schwannoma: case report and literature review. Urology. 2008;72:716.e3–716.e6. doi: 10.1016/j.urology.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 3.Gobbo S, Eble JN, Huang J, Grignon DJ, Wang M, Martignoni G, et al. Schwannoma of the kidney. Mod Pathol. 2008;21:779–783. doi: 10.1038/modpathol.2008.52. [DOI] [PubMed] [Google Scholar]
- 4.Ghiatas AA, Faleski EJ. Benign solitary schwannoma of the retroperitoneum: CT features. South Med J. 1989;82:801–802. doi: 10.1097/00007611-198906000-00037. [DOI] [PubMed] [Google Scholar]
- 5.Kitagawa K, Yamahana T, Hirano S, Kawaguchi S, Mikawa I, Masuda S, et al. MR imaging of neurilemoma arising from the renal hilus. J Comput Assist Tomogr. 1990;14:830–832. doi: 10.1097/00004728-199009000-00034. [DOI] [PubMed] [Google Scholar]
- 6.Daneshmand S, Youssefzadeh D, Chamie K, Boswell W, Wu N, Stein JP, et al. Benign retroperitoneal schwannoma: a case series and review of the literature. Urology. 2003;62:993–997. doi: 10.1016/s0090-4295(03)00792-1. [DOI] [PubMed] [Google Scholar]
- 7.Alvarado-Cabrero I, Folpe AL, Srigley JR, Gaudin P, Philip AT, Reuter VE, et al. Intrarenal schwannoma: a report of four cases including three cellular variants. Mod Pathol. 2000;13:851–856. doi: 10.1038/modpathol.3880150. [DOI] [PubMed] [Google Scholar]
- 8.Nishio A, Adachi W, Igarashi J, Koide N, Kajikawa S, Amano J. Laparoscopic resection of a retroperitoneal schwannoma. Surg Laparosc Endosc Percutan Tech. 1999;9:306–309. [PubMed] [Google Scholar]