Summary
Background and objectives
It is unclear if hemodiafiltration leads to a better quality of life compared with hemodialysis. It was, therefore, the aim of this study to assess the effect of hemodiafiltration on quality of life compared with hemodialysis in patients with ESRD.
Design, setting, participants, & measurements
This study analyzed the data of 714 patients with a median follow-up of 2 years from the Convective Transport Study. The patients were enrolled between June of 2004 and December of 2009. The Convective Transport Study is a randomized controlled trial on the effect of online hemodiafiltration versus low-flux hemodialysis on all-cause mortality. Quality of life was assessed with the Kidney Disease Quality of Life—Short Form. This questionnaire provides data for a physical and mental composite score and describes kidney disease-specific quality of life in 12 domains. The domains have scales from 0 to 100.
Results
There were no significant differences in changes in health-related quality of life over time between patients treated with hemodialysis (n=358) or hemodiafiltration (n=356). The quality of life domain patient satisfaction declined over time in both dialysis modalities (hemodialysis: −2.5/yr, −3.4 to −1.5, P<0.001; hemodiafiltration: −1.4/yr, −2.4 to −0.5, P=0.004).
Conclusions
Compared with hemodialysis, hemodiafiltration had no significant effect on quality of life over time.
Introduction
Health-related quality of life (HRQOL) is a multidimensional concept. It comprises a patients’ perspective on physical, mental, and social domains of health (1). In a chronic condition like ESRD, improving patients’ HRQOL is considered a relevant treatment goal (2). The HRQOL of patients with ESRD has been described as worse than the HRQOL of patients with congestive heart failure, chronic lung disease, or cancer (3). Besides an important outcome in itself, reduced HRQOL has been related to hospitalization and mortality (4,5).
Of the various renal replacement therapies, observational studies have suggested that kidney transplantation offers the most preferable health status in general (6). However, because the availability of donor kidneys is limited, the effect of contemporary renal replacement therapies on HRQOL is an important issue. Hemodiafiltration (HDF) is a relatively new therapy (7). It combines diffusion with convection to clear middle molecular weight substances more effectively compared with hemodialysis (HD) (7,8). Recently, it was shown by our group that treatment with HDF did not result in reduced mortality or cardiovascular events compared with HD (9). The effect of an improved uremic environment on HRQOL, however, remains unclear: earlier studies on the effect of HDF on HRQOL were inconclusive and based on findings from small (n=44–111) or nonrandomized studies (10–15). Therefore, the aim of this study was to assess the effect of HDF compared with HD on HRQOL in a large randomized clinical trial.
Materials and Methods
Patients and Study Design
This randomized analysis is based on data obtained from 714 HD patients who participated in the Convective Transport Study (CONTRAST). Between June of 2004 and December of 2009, they were recruited from dialysis centers in The Netherlands (n=26), Canada (n=2), and Norway (n=1). As described previously, CONTRAST is a randomized, controlled trial (ISRCTN38365125) that compares the effects of low-flux HD and online HDF on all-cause mortality and cardiovascular events (8). The analysis of HRQOL was prespecified as a secondary outcome measure. Online HDF was performed in the postdilution mode with a target convection volume of 6 L/h. This amount was based on a targeted filtration rate between 25% and 33% of an extracorporeal blood flow rate between 300 and 400 ml/min (so-called filtration fraction), which seemed achievable in clinical practice. Blood flow rates could be increased in the HDF arm to improve convection volumes. For HDF, synthetic high-flux dialyzers were used (FX80, 27%; FX100, 11%; Optiflux F200NR, 8%; Fresenius Medical Care, Bad Homburg, Germany; Polyflux 170H, 25%; Polyflux 210H, 27%; Gambro AB, Stockholm, Sweden; other dialyzers, 2%). HD patients were treated with synthetic low-flux dialyzers (F6HPS, 5%; F8HPS, 45%; Optiflux 18NR, 9%; Fresenius; Polyflux 14L, 2%; Polyflux 17L, 30%; Gambro; other, 9%).
Patients were randomized centrally into a 1:1 ratio and stratified per participating center. Patients were eligible for inclusion if they were treated two or three times per week with HD for at least 2 months, with a minimum dialysis urea Kt/V (i.e., urea clearance divided by volume of distribution) of ≥1.2. Exclusion criteria were age below 18 years, treatment with HD, HDF, or high-flux HD in the 6 months before randomization, a life expectancy less than 3 months because of nonrenal disease, participation in another clinical intervention trial evaluating cardiovascular outcomes, and severe incompliance regarding frequency and duration of dialysis treatment (8). The study was conducted in accordance with the Declaration of Helsinki and approved by the medical ethics review boards of all participating hospitals. Written informed consent was obtained from all patients before enrolment.
Measurements
At baseline, standardized forms were used to collect demographic, clinical, and laboratory data. Demographic data included age, sex, race, educational level, and employment status. Clinical characteristics recorded were medical history, including the cause of kidney disease, presence of diabetes mellitus, previous cardiovascular disease, HD dose (single-pool Kt/V urea), time on dialysis, treatment time in hours, and estimated residual GFR. Laboratory values were measured in the different participating hospitals using standard techniques. Study visits were performed at 3-month intervals. Interdialytic urinary samples were collected in patients with a urinary production of 100 ml/d or more. Estimated residual GFR was calculated as the mean of creatinine and urea clearances and adjusted for body surface area (milliliters per minute per 1.73 meter2) (16). Additional information regarding the measured characteristics has been reported previously (8). Because of the nature of the intervention, it was not possible to blind the patients, the local study nurses, or the investigators for the treatment assignment.
Kidney Disease Quality of Life—Short Form
HRQOL was assessed at baseline and every subsequent year of follow-up with the validated Kidney Disease Quality of Life—Short Form (KDQOL-SF) version 1.3 (http://gim.med.ucla.edu/kdqol/downloads/-download.html) (17,18). This questionnaire covers different domains to face the multidimensional nature of HRQOL. It was distributed by local study nurses who helped the patients to fill out the forms, if necessary. The KDQOL-SF can be split up in a generic part and a disease-specific part. First, the generic part is formed by the Short Form with 36 questions (SF-36) version 1 (19). The eight domains of the SF-36 can be summarized in two summary scores, one for physical functioning (the physical composite score [PCS]) and one for mental functioning (the mental composite score [MCS]). These summaries are constructed in a way that a score of 50 represents the mean of the general US population with an SD of 10 (20). Because the PCS and MCS may be easier to understand than the eight individual domains of the SF-36, we focused on these composite scores with regard to generic HRQOL. Second, the disease-specific part of the KDQOL-SF consists of 44 kidney disease-targeted questions. The responses to these items are condensed in 12 domains. The HRQOL domains have a score from 0 to 100, with higher scores indicating the absence of problems. A difference of five points has been proposed to be clinically relevant with regard to individual domains, and a difference of three points has been proposed to be clinically relevant with regard to the composite scores (19,20).
Statistical Analyses
Data were analyzed according to an intention-to-treat principle (i.e., according to assigned instead of received treatment). All variables were reported as proportions, means with SD, or medians with interquartile range when appropriate. Differences in changes in HRQOL domains over time were assessed with multilevel linear regression. Because HRQOL scores from the same patient are likely to be dependent, there is a two-level hierarchy with HRQOL scores over time (level 1) nested within patients (level 2). For every HRQOL domain, we evaluated which model had the best fit. We expanded the model step by step and assessed for every step if this expansion led to a significantly lower log-likelihood using the chi-squared likelihood ratio test. We started off with fixed coefficients and then a random intercept for HRQOL and a random slope for HRQOL, and we evaluated different covariance structures and growth curves. In the end, the vast majority of models had a random intercept and slope for HRQOL over time, an unstructured covariance structure, and a first-order polynomial. The residuals of all models had a normal distribution. Differences in subgroups were evaluated using interaction terms. Eight subgroups were evaluated: age, sex, residual renal function (diuresis≥100 m/24 h), dialysis vintage, diabetes, albumin, previous cardiovascular disease, and convection volume. The Benjamini and Hochberg False Discovery Rate was used to correct for multiple comparisons (21). Missing data were handled with multilevel models (22). Results were considered statistically significant when P<0.05 (two-tailed comparison). Statistical analyses were performed with SPSS 18 (SPSS Inc., Chicago, IL).
Results
Patient Characteristics
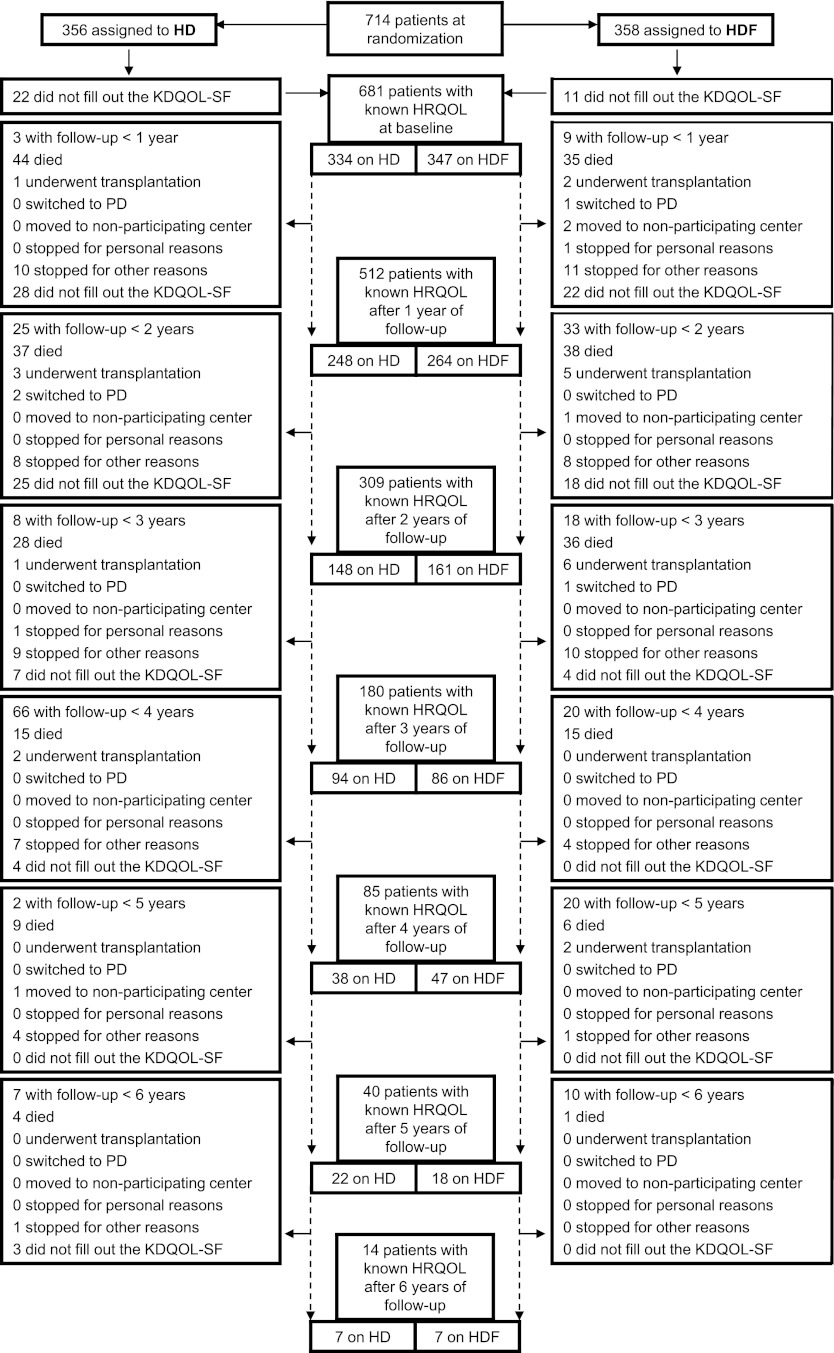
The baseline characteristics of the patients on HD (n=356) and HDF (n=358) are summarized in Table 1. The mean age was 64 years in both groups (SD HD±13, SD HDF±14); 65% of patients on HD were male, and 60% of patients on HDF were male. Figure 1 shows the patient flow over time. Of 714 patients enrolled, 14 patients did not fill out a single KDQOL-SF (11 patients assigned to HD and 3 patients assigned to HDF). Over time, 93% of the KDQOL-SFs were filled out. The HRQOL domain sexual function was not analyzed, because most patients (88%) did not fill out its questions. The median follow-up of these patients was 2.0 years (interquartile range=1.1–3.0). The average convection volume over time in patients on HDF was 19±6 (SD) L/session.
Table 1.
Patient characteristics at baseline
| HD (n=356) | HDF (n=358) | |
|---|---|---|
| Demographic | ||
| Age (years) | 64±13 | 64±14 |
| Sex (% male) | 65 | 60 |
| Caucasian (%) | 83 | 85 |
| Socioeconomic status | ||
| Employed (%) | 10 | 11 |
| High educational statusa (%) | 21 | 20 |
| Clinical parameters | ||
| Cause of kidney failure (%) | ||
| Vascular | 27 | 29 |
| Diabetes mellitus | 17 | 21 |
| Nephritis | 24 | 20 |
| Cystic kidney disease | 7 | 7 |
| Other/unknown | 25 | 23 |
| Dialysis vintage (yr) | 2.2 (1.0–4.0) | 1.8 (1.0–3.8) |
| Dialysis frequency (% 3×/wk) | 95 | 93 |
| Session time (h) | 4.0 (3.5–4.0) | 4.0 (3.5–4.0) |
| spKt/V urea | 1.4±0.2 | 1.4±0.2 |
| eGFR (ml/min per 1.73 m2)b | 3.2 (1.2–5.4) | 3.1 (1.7–6.2) |
| BMI (kg/m2) | 26±5 | 25±5 |
| Diabetes (%) | 23 | 26 |
| History of cardiovascular disease (%) | 46 | 42 |
| Hemoglobin (g/dl) | 12±1 | 12±1 |
| Albumin (g/dl) | 3.7±0.5 | 3.7±0.5 |
| Phosphorus (mg/dl) | 5.1±1.5 | 5.1±1.6 |
Mean ± SD or median (interquartile range). To convert hemoglobin in grams per deciliter to millimoles per liter, multiply by 0.62. To convert albumin in grams per deciliter to grams per liter, multiply by 10. To convert phosphorus in milligrams per deciliter to millimoles per liter, multiply by 0.323. HD, hemodialysis; HDF, hemodiafiltration; spKt/V, urea clearance divided by volume of distribution, single pool; eGFR: estimated residual GFR; BMI, body mass index.
High educational status is college or university level.
In 377 patients with diuresis≥100 ml/24 h (53%).
Figure 1.
Patient flow diagram. Because patients were included at different points in time, potential follow-up time varies per patient. The median follow-up of these patients was 2.0 years (interquartile range=1.1–3.0). The line between consecutive years of follow-up is dashed to point out that a measurement was sometimes skipped (e.g., 17 patients did not fill out the KDQOL-SF at baseline but did fill it out after 1 year of follow-up). HD, hemodialysis; HDF, hemodiafiltration; HRQOL, health-related quality of life; KDQOL-SF, Kidney Disease Quality of Life—Short Form; PD, peritoneal dialysis.
Quality of Life at Baseline and over Time
Table 2 shows the HRQOL at baseline. The mean PCS was 40±10 in HD patients and 39±11 in HDF patients. The mean MCS was 50±12 in both groups.
Table 2.
Health-related quality of life at baseline
| HD (n=356) | HDF (n=358) | |
|---|---|---|
| Generic domains (SF-36) | ||
| Physical summary (PCS) | 40±10 | 39±11 |
| Mental summary (MCS) | 50±12 | 50±12 |
| Kidney disease-specific domains | ||
| Symptom/problem list | 80±12 | 79±14 |
| Effects of kidney disease on daily life | 72±19 | 71±19 |
| Burden of kidney disease | 46±26 | 45±25 |
| Work status | 0 (0−50) | 0 (0−50) |
| Cognitive function | 78±20 | 80±18 |
| Quality of social interaction | 83±16 | 81±17 |
| Sleep | 62±21 | 62±20 |
| Social support | 83 (67−100) | 83 (67−100) |
| Dialysis staff encouragement | 75 (63−100) | 75 (63−100) |
| Overall health | 55±24 | 54±21 |
| Patient satisfaction | 70±23 | 70±24 |
Mean ± SD or median (interquartile range). The domains have a range from 0 to 100, with higher scores indicating a preferable health status or a relative absence of problems. HD, hemodialysis; HDF, hemodiafiltration; SF-36, Short Form with 36 questions version 1; PCS, the physical composite score; MCS, the mental composite score.
In patients on both HD and HDF, multiple HRQOL domain scores declined significantly over time (Table 3). The HRQOL domain patient satisfaction declined in both dialysis modalities (HD: −2.5 points/yr, 95% confidence interval=−3.4 to −1.5, P<0.001; HDF: −1.4 points/yr, 95% confidence interval=−2.4 to −0.5, P=0.004). One HRQOL domain improved, namely overall health, in patients on HDF (+0.9, 0.1–1.7, P=0.03). Over time, there were no significant differences in HRQOL between patients on HDF and HD, although a trend was observed to a worse MCS (−0.5, −1.1 to 0.0, P=0.06) and an improved effects of kidney disease on daily life (+1.1, −0.1 to 2.4, P=0.06) in patients on HDF.
Table 3.
Health-related quality of life over time in patients treated with hemodialysis and hemodiafiltration
| HD (n=356) | HDF (n=358) | HDF versus HD | ||||
|---|---|---|---|---|---|---|
| Δ | P Value | Δ | P Value | Δ | P Value | |
| Generic domains (SF-36) | ||||||
| Physical summary (PCS) | −0.8 (−1.1 to −0.5) | <0.001a | −0.2 (−0.6 to 0.1) | 0.13 | 0.4 (−0.3 to 1.1) | 0.29 |
| Mental summary (MCS) | 0.1 (−0.3 to 0.5) | 0.78 | −0.5 (−0.8 to −0.1) | 0.01a | −0.5 (−1.1 to 0.0) | 0.06 |
| Kidney disease-specific domains | ||||||
| Symptom/problem list | −1.1 (−1.8 to −0.4) | 0.003a | −0.6 (−1.2 to 0.0) | 0.07 | 0.4 (−0.5 to 1.4) | 0.33 |
| Effects of kidney disease on daily life | −1.3 (−2.3 to −0.4) | 0.01a | −0.3 (−1.1 to 0.4) | 0.38 | 1.1 (−0.1 to 2.4) | 0.06 |
| Burden of kidney disease | −0.2 (−1.0 to 0.6) | 0.69 | −0.9 (−1.7 to −0.1) | 0.02a | −0.7 (−1.7 to 2.4) | 0.21 |
| Work status | −0.4 (−1.4 to 0.6) | 0.42 | −1.1 (−2.0 to −0.1) | 0.03a | −0.6 (−2.0 to 0.8) | 0.41 |
| Cognitive function | −0.4 (−0.9 to 0.2) | 0.22 | −1.1 (−1.7 to −0.4) | 0.001a | −0.7 (−2.0 to 0.7) | 0.35 |
| Quality of social interaction | −0.1 (−0.9 to 0.6) | 0.71 | −0.4 (−1.2 to 0.4) | 0.36 | −0.2 (−1.3 to 0.9) | 0.68 |
| Sleep | 0.3 (−0.3 to 0.9) | 0.35 | 0.4 (−0.2 to 1.1) | 0.17 | 0.1 (−0.8 to 1.0) | 0.76 |
| Social support | −0.2 (−1.1 to 0.7) | 0.65 | −0.4 (−1.2 to 1.1) | 0.37 | −0.2 (−1.4 to 1.1) | 0.81 |
| Dialysis staff encouragement | −1.0 (−1.8 to −0.1) | 0.02 | 0.0 (−0.7 to 0.8) | 0.96 | 1.0 (−0.1 to 2.1) | 0.09 |
| Overall health | 0.2 (−0.8 to 1.2) | 0.69 | 0.9 (0.1 to 1.7) | 0.03a | 0.7 (−0.5 to 2.0) | 0.25 |
| Patient satisfaction | −2.5 (−3.4 to −1.5) | <0.001a | −1.4 (−2.4 to −0.5) | 0.004a | 0.0 (0.0 to 0.0) | 0.29 |
The median follow-up was 2.0 years (interquartile range=1.1–3.0). HD, hemodialysis; HDF, hemodiafiltration; Δ, change in health-related quality of life over time per year; SF-36, Short Form with 36 questions version 1; PCS, the physical composite score; MCS, the mental composite score.
Statistically significant P value.
Subgroup analyses did not identify patient groups in which HDF may have had a clinical relevant benefit on HRQOL.
Discussion
This study showed that HDF had no significant effect on patients’ HRQOL compared with HD. Although there were changes over time in HRQOL for patients on HDF and HD, these changes did not result in significant differences over time between both dialysis modalities. There was a trend to a worse MCS and an improved effects of kidney disease on daily life in patients on HDF versus HD. Because their effect sizes were small and opposite, they implicate no clear benefit for either therapy.
To our knowledge, this study is the first large randomized study to analyze the effect of HDF on HRQOL. The results of earlier studies were inconclusive (Table 4): two studies showed an increase in physical HRQOL in patients on HDF (13,15), and three studies found no significant differences between dialysis modalities (11,12,14). Furthermore, no clinical meaningful differences in HRQOL have been identified in patients on low- versus high-flux HD (23) or hemofiltration versus HD (24,25). It should be noted that these studies used different questionnaires to assess HRQOL. Perhaps, this difference might explain why two studies did find a positive effect of HDF on HRQOL, whereas others did not. However, although the work by Lin et al. (13) used a self-developed form, the work by Schiffl (15) applied the Kidney Disease Questionnaire, which was also used in the work by Ward et al. (12). The works by Lin et al. (13) and Schiffl (15) found a positive effect of HDF on physical HRQOL, but the work by Ward et al. (12) did not.
Table 4.
Previous studies on hemodiafiltration and health-related quality of life
| Ref | Design | Intervention | Number of Patients | Follow-Up (mo) | HRQOL Instrument | Effect on HRQOL |
|---|---|---|---|---|---|---|
| 11 | Cross-sectional | HDF ⇔ HD ⇔ PD | 1013 (71 on HDF) | N/A | KS and SIP | No difference |
| 12 | RCT | HDF ⇔ high-flux HD | 44 (24 on HDF) | ±12 | KDQ | No difference |
| 13 | RCT | HDF ⇔ high-flux HD | 111a | Unclear | Self-developed | Better physical wellbeing in HDF (32%) |
| 15 | Crossover | HDF ⇔ high-flux HD | 76 | 2×24 | KDQ | Better perception of physical symptoms in HDF (26%) |
| 14 | Observational | HDF ⇔ high-flux HD ⇔ low-flux HD | 2165 (253 on HDF) | ±21 (HDF) | SF-36 | No difference |
HRQOL, health-related quality of life; HDF, hemodiafiltration; HD, hemodialysis; PD, peritoneal dialysis; KS, Karnofsky Performance Scale (34); SIP, sickness impact profile (35); RCT, randomized clinical trial; KDQ, Kidney Disease Questionnaire (36); SF-36, Short Form 36 (19).
Randomization into four groups: 3×/wk HD, 3×/wk HDF, and two intermediate versions with a 2×/wk versus 1×/wk distribution of HD or HDF.
When we put the baseline HRQOL of our population in the context of other large studies, the previous finding that dialysis patients have a severely impaired physical functioning but mental functioning comparable with the general population normal scores was supported (present study: PCS=39±11, MCS=50±12; Hemodialysis [HEMO] Study: PCS=36±10, MCS=50±11 [26]; Dialysis Outcomes and Practice Patterns Study: PCS=35±11, MCS=45±12 [27]).
A difference in HRQOL of five points or more has been proposed as being clinically relevant (19). Such a notion would mean that, in our study, only the decline in patient satisfaction is likely to have clinical relevance over several years of time. This decline was shown in both dialysis modalities. It is in agreement with previous findings that showed a negative relation with dialysis vintage (28,29). Patient satisfaction has been related to various factors (28–31), including a positive association with compliance (30) and a negative association with comorbidity (28). An increase in age seems less relevant, because age has been both positively (32) and negatively (29) related to patient satisfaction.
Between dialysis modalities, there was no clinical relevant or statistical significant difference in HRQOL. Apparently, the effect of HDF on the removal of middle molecules did not impact patients’ health status. A limitation of our study is the favorable patient selection within the context of a randomized clinical trial. This limitation might affect changes in HRQOL over time within groups, but it is not likely to affect differences in HRQOL between HDF and HD. The external validity of our study is supported by the fact that the variables age, sex, and primary kidney disease of our patients are similar to the variables in all Dutch patients registered in the RENINE database (www.renine.nl). Also, it was not registered if patients needed assistance to fill out the KDQOL-SF (e.g., from a nurse). Differences in the number of patients needing assistance in completing the questionnaire could have introduced bias. However, there is no reason to assume differences between HD and HDF populations in this respect. A third limitation is that the variability in HRQOL might be underestimated because of missing data (7%). One could, furthermore, speculate that HRQOL differences might arise during a prolonged follow-up period. However, because there was no statistically significant difference between HDF and HD in multiple HRQOL domains, it is unlikely that a more pronounced, clinically relevant difference would arise over time, especially when taking the high mortality rate of dialysis patients into account (33).
In conclusion, the results from this first large randomized, controlled trial comparing the effect of HDF with HD on HRQOL show that HDF had no effect on this important outcome. Apparently, alterations in the dialysis prescription, which can be made within the context of three dialysis sessions (4 h/wk) as studied herein and in the HEMO study, are unlikely to significantly impact HRQOL.
Disclosures
A.H.A.M, G.A.d.W., M.P.C.G., E.L.P., N.C.v.d.W., C.H.d.H., R.L., M.A.v.d.D., and M.L.B. have no disclosures. M.J.N. received funding for research from Baxter and Fresenius. P.M.t.W. received funding for research from Abbott, Baxter, Gambro, Fresenius, and Roche and honoraria for lectures from Amgen, Roche, Genzyme, and Fresenius. P.J.B. received funding for research from Fresenius and Gambro and honoraria for lectures from Fresenius, Gambro, Solvay, and Novartis.
Acknowledgments
We are grateful to all patients and technical and medical staff who participated in this project.
This work was made possible by The Netherlands Organization for Health Research and Development (ZonMw) Grant 170882802. The Convective Transport Study is financially supported by Dutch Kidney Foundation (Nierstichting Nederland) Grant C02.2019 and unrestricted grants from Fresenius Medical Care Netherlands and Gambro Lundia AB, Sweden. Additional support was received from the Dr. E.E. Twiss Fund, Roche Netherlands, and the International Society of Nephrology/Baxter Extramural Grant Program.
The funding sources had no role in the design, data collection, analysis, manuscript preparation, interpretation, or decision to submit the manuscript for publication.
Convective Transport Study investigators. Canada: M. Dorval, Georges-L Dumont Regional Hospital, Moncton; R. Lévesque, CHUM St Luc Hospital, Montreal. The Netherlands: M.G. Koopman, Academic Medical Center, Amsterdam; C.J.A.M. Konings, Catharina Hospital, Eindhoven; W.P. Haanstra, Dialysis Clinic Noord, Beilen; M. Kooistra, Dianet Dialysis Centers, Utrecht; T. Noordzij, Fransiscus Hospital, Roosendaal; G.W. Feith, Gelderse Vallei Hospital, Ede; M. van Buren, Haga Hospital, The Hague; J.J.G. Offerman, Isala Clinics, Zwolle; E.K. Hoogeveen, Jeroen Bosch Hospital, Hertogenbosch; F. de Heer, Maasland Hospital, Sittard; P.J. van de Ven, Maasstad Hospital, Rotterdam; T.K. Kremer Hovinga, Martini Hospital, Groningen; W.A. Bax, Medical Center Alkmaar, Alkmaar; J.O. Groeneveld, Onze Lieve Vrouwe Gasthuis, Amsterdam; A.T.J. Lavrijssen, Oosterschelde Hospital, Goes; A.M. Schrander-Van der Meer, Rijnland Hospital, Leiderdorp; L.J.M. Reichert, Rijnstate Hospital, Arnhem; J. Huussen, Slingeland Hospital, Doetinchem; P.L. Rensma, St. Elisabeth Hospital, Tilburg; Y. Schrama, St. Fransiscus Gasthuis, Rotterdam; H.W. van Hamersvelt, University Medical Center St. Radboud, Nijmegen; W.H. Boer, University Medical Center Utrecht, Utrecht; W.H. van Kuijk, VieCuri Medical Center, Venlo; M.G. Vervloet, VU Medical Center, Amsterdam; I.M.P.M.J. Wauters, Zeeuws-Vlaanderen Hospital, Terneuzen. Norway: I. Sekse, Haukeland University Hospital, Bergen.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editoral, “Can We Improve Quality of Life of Patients on Dialysis?” on pages 1–4.
References
- 1.Testa MA, Simonson DC: Assesment of quality-of-life outcomes. N Engl J Med 334: 835–840, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Hemodialysis Adequacy 2006 Work Group : Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Mittal SK, Ahern L, Flaster E, Maesaka JK, Fishbane S: Self-assessed physical and mental function of haemodialysis patients. Nephrol Dial Transplant 16: 1387–1394, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol 12: 2797–2806, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Knight EL, Ofsthun N, Teng M, Lazarus JM, Curhan GC: The association between mental health, physical function, and hemodialysis mortality. Kidney Int 63: 1843–1851, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink MG: Quality of life assessed with the Medical Outcomes Study Short Form 36-Item Health Survey of patients on renal replacement therapy: A systematic review and meta-analysis. Value Health 10: 390–397, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Blankestijn PJ, Ledebo I, Canaud B: Hemodiafiltration: Clinical evidence and remaining questions. Kidney Int 77: 581–587, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Penne EL, Blankestijn PJ, Bots ML, van den Dorpel MA, Grooteman MP, Nube MJ, van der Tweel I, Ter Wee PM, the CONTRAST study group : Effect of increased convective clearance by on-line hemodiafiltration on all cause and cardiovascular mortality in chronic hemodialysis patients—the Dutch CONvective TRAnsport STudy (CONTRAST): Rationale and design of a randomised controlled trial [ISRCTN38365125]. Curr Control Trials Cardiovasc Med 6: 8, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grooteman MP, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, den Hoedt CH, van der Tweel I, Lévesque R, Nubé MJ, ter Wee PM, Blankestijn PJ, CONTRAST Investigators : Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol 23: 1087–1096, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Hoedt CH, Mazairac AHA, Dorpel MA, Grooteman MP, Blankestijn PJ: The effect of hemodiafiltration on mortality, inflammation and quality of life. In: Hemodiafiltration—A New Era, edited by Kawanishi H, Yamashita AC, Basel, Switzerland, Karger, 2010, pp 39–52 [Google Scholar]
- 11.Moreno F, López Gomez JM, Sanz-Guajardo D, Jofre R, Valderrábano F, Spanish Cooperative Renal Patients Quality of Life Study Group : Quality of life in dialysis patients. A Spanish multicentre study. Nephrol Dial Transplant 11[Suppl 2]: 125–129, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Ward RA, Schmidt B, Hullin J, Hillebrand GF, Samtleben W: A comparison of on-line hemodiafiltration and high-flux hemodialysis: A prospective clinical study. J Am Soc Nephrol 11: 2344–2350, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Lin CL, Huang CC, Chang CT, Wu MS, Hung CC, Chien CC, Yang CW: Clinical improvement by increased frequency of on-line hemodialfiltration. Ren Fail 23: 193–206, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Canaud B, Bragg-Gresham JL, Marshall MR, Desmeules S, Gillespie BW, Depner T, Klassen P, Port FK: Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int 69: 2087–2093, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Schiffl H: Prospective randomized cross-over long-term comparison of online haemodiafiltration and ultrapure high-flux haemodialysis. Eur J Med Res 12: 26–33, 2007 [PubMed] [Google Scholar]
- 16.Fouque D, Vennegoor M, ter Wee P, Wanner C, Basci A, Canaud B, Haage P, Konner K, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Tordoir J, Vanholder R: EBPG guideline on nutrition. Nephrol Dial Transplant 22[Suppl 2]: ii45–ii87, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB: Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 3: 329–338, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Korevaar JC, Merkus MP, Jansen MA, Dekker FW, Boeschoten EW, Krediet RT, NECOSAD-study group : Validation of the KDQOL-SF: A dialysis-targeted health measure. Qual Life Res 11: 437–447, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Snow KK, Kosinski M, Gandek B: SF-36 Health Survey-Manual and Interpretation Guide, Boston, The Health Institute, New England Medical Center, 1993 [Google Scholar]
- 20.Ware JE, Kosinski M, Keller SD: SF-36 Physical and Mental Health Summary Scales: A User's Manual, 2nd Ed., Boston, The Health Institute, New England Medical Center, 1994 [Google Scholar]
- 21.Hochberg Y, Benjamini Y: More powerful procedures for multiple significance testing. Stat Med 9: 811–818, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Peters SA, Bots ML, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR, 3rd, O’Leary DH, Evans GW, Raichlen JS, Moons KG, Koffijberg H, METEOR study group : Multiple imputation of missing repeated outcome measurements did not add to linear mixed-effects models. J Clin Epidemiol 65: 686–695, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Unruh M, Benz R, Greene T, Yan G, Beddhu S, DeVita M, Dwyer JT, Kimmel PL, Kusek JW, Martin A, Rehm-McGillicuddy J, Teehan BP, Meyer KB, HEMO Study Group : Effects of hemodialysis dose and membrane flux on health-related quality of life in the HEMO Study. Kidney Int 66: 355–366, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Altieri P, Sorba G, Bolasco P, Asproni E, Ledebo I, Cossu M, Ferrara R, Ganadu M, Cadinu F, Serra G, Cabiddu G, Sau G, Casu D, Passaghe M, Bolasco F, Pistis R, Ghisu T, Second Sardinian Multicentre Study : Predilution haemofiltration—the Second Sardinian Multicentre Study: Comparisons between haemofiltration and haemodialysis during identical Kt/V and session times in a long-term cross-over study. Nephrol Dial Transplant 16: 1207–1213, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Beerenhout CH, Luik AJ, Jeuken-Mertens SG, Bekers O, Menheere P, Hover L, Klaassen L, van der Sande FM, Cheriex EC, Meert N, Leunissen KM, Kooman JP: Pre-dilution on-line haemofiltration vs low-flux haemodialysis: A randomized prospective study. Nephrol Dial Transplant 20: 1155–1163, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Allen KL, Miskulin D, Yan G, Dwyer JT, Frydrych A, Leung J, Poole D, Hemodialysis (HEMO) Study Group : Association of nutritional markers with physical and mental health status in prevalent hemodialysis patients from the HEMO study. J Ren Nutr 12: 160–169, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK: Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 64: 339–349, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Rubin HR, Fink NE, Plantinga LC, Sadler JH, Kliger AS, Powe NR: Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA 291: 697–703, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Wasserfallen JB, Moinat M, Halabi G, Saudan P, Perneger T, Feldman HI, Martin PY, Wauters JP: Satisfaction of patients on chronic haemodialysis and peritoneal dialysis. Swiss Med Wkly 136: 210–217, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kovac JA, Patel SS, Peterson RA, Kimmel PL: Patient satisfaction with care and behavioral compliance in end-stage renal disease patients treated with hemodialysis. Am J Kidney Dis 39: 1236–1244, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Barendse SM, Speight J, Bradley C: The Renal Treatment Satisfaction Questionnaire (RTSQ): A measure of satisfaction with treatment for chronic kidney failure. Am J Kidney Dis 45: 572–579, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Lopes AA, Bragg-Gresham JL, Goodkin DA, Fukuhara S, Mapes DL, Young EW, Gillespie BW, Akizawa T, Greenwood RN, Andreucci VE, Akiba T, Held PJ, Port FK: Factors associated with health-related quality of life among hemodialysis patients in the DOPPS. Qual Life Res 16: 545–557, 2007 [DOI] [PubMed] [Google Scholar]
- 33.US Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 34.Karnofsky DA, Burchenal JH: The clinical evaluation of chemotherapeutic agents in cancer. In: Evaluation of Chemotherapeutic Agents, edited by MacLeod CM, New York, Columbia University Press, 1949, pp 191–205 [Google Scholar]
- 35.Bergner M, Bobbitt RA, Carter WB, Gilson BS: The Sickness Impact Profile: Development and final revision of a health status measure. Med Care 19: 787–805, 1981 [DOI] [PubMed] [Google Scholar]
- 36.Laupacis A, Muirhead N, Keown P, Wong C: A disease-specific questionnaire for assessing quality of life in patients on hemodialysis. Nephron 60: 302–306, 1992 [DOI] [PubMed] [Google Scholar]