Summary
Background and objectives
High serum levels of fibroblast growth factor-23 (FGF-23) are associated with mortality in patients with ESRD, but whether it still acts as a phosphaturic factor is unknown. This study aimed to explore the role of circulating FGF-23 on urinary phosphate excretion and phosphate balance in maintenance hemodialysis (MHD) patients with residual renal function (RRF).
Design, setting, participants, & measurements
There were 134 MHD patients enrolled in this cross-sectional study from June to July 2010. Demographics, laboratory data, and excretion capacity of phosphate were recorded. Multivariable linear regression was used to analyze the relationship of serum phosphate and the tubular reabsorption rate of phosphate with other factors.
Results
The median age of the patients was 61.0 years and 47.8% were male. Thirty percent of the patients had high urinary output (>200 ml/d) accompanied by lower serum levels of phosphate, calcium, intact parathyroid hormone, and FGF-23 compared with those with low urine output (≤200 ml/d). The independent predictors of serum phosphate were normalized protein nitrogen appearance, intact parathyroid hormone, and FGF-23 in the low urine output group and female sex and GFR in the high urine output group. The tubular reabsorption rate of phosphate decreased to 50% of the normal level in patients with RRF. Elevated circulating FGF-23 was significantly associated with lower tubular phosphate reabsorption after adjusting for GFR.
Conclusions
RRF is associated with significant capacity to excrete phosphate in MHD patients and high levels of serum FGF-23 may promote phosphate excretion by remnant nephrons.
Introduction
Hyperphosphatemia is a common complication in patients undergoing maintenance hemodialysis (MHD) (1). Previous studies have reported that the residual GFR was negatively associated with serum phosphate in uremic patients undergoing peritoneal dialysis or hemodialysis (2,3). The beneficial role of residual renal function (RRF) on phosphate control may be partially explained by increased phosphaturia. However, the mechanism and capacity of phosphate excretion by functional nephrons in MHD patients have not been fully elucidated.
Under normal physiologic conditions, renal excretion of phosphate is mainly determined by expression of type II Na-Pi cotransporters (NaPi-IIa and NaPi-IIc) at the apical membrane of tubular epithelial cells (4). The regulators of the expression of NaPi-IIa include dietary phosphate, parathyroid hormone (PTH), vitamin D, insulin, phosphatonins, and so forth (4). Fibroblast growth factor-23 (FGF-23) is one of the regulators primarily produced by osteoblasts (5). Previous clinical studies of patients with GFRs >15 ml/min per 1.73 m2 have suggested that FGF-23 increases with decreasing GFR and is associated with augmented phosphaturia (6).
Dialysis patients have serum FGF-23 levels that are 100–1000 times higher than that in the general population (7), and FGF-23 is positively associated with serum phosphate level (8). It is unknown whether elevated serum FGF-23 regulates urinary phosphate excretion in dialysis patients. On the contrary, recent data have established a relationship between high levels of FGF-23 and increased mortality and vascular calcifications in hemodialysis patients (9,10). However, the mechanism is unclear. FGF-23 may merely be a marker of disturbed calcium-phosphate metabolism, or it may be related to certain unknown toxins that induce cardiovascular diseases (5).
FGF-23 acts as a phosphaturic factor in participants with normal kidneys and in those with renal disease. The RRF of hemodialysis patients helps to control the level of serum phosphate. Therefore, we speculate that FGF-23 also plays a physiologic role in the regulation of phosphate excretion in these patients. In this cross-sectional study, we assessed the role of RRF in phosphate homeostasis and the association of high circulating levels of FGF-23 with urinary phosphate excretion in MHD patients.
Materials and Methods
Patients
This study recruited 134 patients who were on MHD three times per week for at least 3 months in our dialysis center (Fudan University, Shanghai, China) from June to July 2010. Exclusion criteria included severe malnutrition, hepatic insufficiency, acute or chronic infection, heart failure, and malignancy. The Ethics Committee on Human Research at Huashan Hospital approved this study and all patients provided written informed consent. Patients were treated with a Dialog+ dialysis machine (B. Braun, Germany) and low-flux dialyzers made of polysulfone (1.2 m2) or triacetate (1.3 m2). Same agents of phosphate binder (CaCO3) and active vitamin D (calcitriol) were prescribed to the patients.
Because urinary output was markedly different on dialysis day and nondialysis day, two-day urine (from the beginning of the second treatment to the beginning of the third treatment) were collected from patients with urine for grouping, biochemical detection, and GFR calculation. There is no research that shows how much daily urine may affect the phosphate balance in hemodialysis patients. Therefore, we first divided the patients into the following three groups based on average daily urine volume: group I, 0 ml/d; group II, 0–200 ml/d; and group III, >200 ml/d. Group I and group II were then combined according to serum phosphate level and phosphate excretion of remnant kidney. Demographic and clinical data were recorded at the time of enrollment.
Fifteen healthy participants with normal serum electrolytes and renal function were randomly selected from a cohort of healthy volunteers to evaluate the renal tubular reabsorption rate of electrolytes. Participants with acquired disorders known to affect the serum electrolyte levels were excluded.
Biochemical Determinations
Predialysis blood samples were obtained on the mid-week dialysis day for routine laboratory assessment by standard techniques. The serum levels of intact PTH (iPTH) (Santa Cruz, CA), intact FGF-23 (Immutopics, San Clemente, CA), soluble α-Klotho (Immuno-Biologic Laboratories Co. Ltd. Japan), and 1,25(OH)2D3 (Imnunodiagnostic Systems, Boldon, UK) were measured by ELISA according to the manufacturer’s protocol.
Two daily urine collections were pooled for the creatinine and urea clearance calculations. The GFR was estimated as the mean of creatinine and urea clearance adjusted for body surface area (ml/min per 1.73 m2) using the geometric mean of postdialysis and predialysis plasma concentration of creatinine and urea to estimate the mean creatinine and urea concentration during the 48 hours (3,11). GFR was considered zero in anuric patients. The single-pool Kt/V urea delivered by hemodialysis (sp-d Kt/V urea) was estimated by the second-generation Daugirdas equation (12).
Estimation of Phosphate Removal by One Session of Hemodialysis Treatment
Thirteen dialysate samples were collected from each patient during the mid-week dialysis treatment (at 5 minutes and 15 minutes, and then every 15 minutes for the first 2 hours, and every 30 minutes for the last 2 hours) and sent for measurement of phosphate. The phosphate removal by a 4-hour hemodialysis dialysis session was calculated as the area under the dialysate phosphate concentration-time curve, using the “trapezoidal rule” (13). This value was multiplied by 3 to calculate the phosphate removal in 1 week.
Calculation of Urinary Phosphate Excretion and Renal Tubular Reabsorption Rate
Two daily urine collections were pooled for renal electrolyte clearance calculation but used separately for quantifying urinary phosphate excretion per week, which was calculated as four times the value of the non-dialysis day plus three times the value of the dialysis day. The renal tubular reabsorption rate of electrolyte was calculated as: T = 1 − (C/GFR), where T was the tubular transport rate of the electrolyte and C was the renal clearance rate for electrolyte. Serum ionized calcium was calculated using the following formula: serum ionized Ca (mg/dl) = serum total Ca (mg/dl) × (100% – %serum protein-bound Ca), where %serum protein-bound Ca = 0.8 × albumin (g/L) + 0.2 × globulin (g/L) + 3 (14).
Assessment of Phosphate Balance in MHD Patients
Considering the wide variety of Chinese food as well as poor accuracy of diet diaries recorded by patients, we used an alternative method instead of dietary interview to estimate phosphate intake (mg/d), which was equal to the protein intake (g/d) multiplied by 15 (mean 15 mg Pi/g protein) (15,16). The normalized protein equivalent nitrogen appearance (nPNA) was chosen as a surrogate of dietary protein intake (17,18). With the reported theoretical value of phosphate binding capacity of CaCO3 and gastrointestinal tract absorption rate (60%) (15,19,20), phosphate balance of patients was expressed as follows: (dietary phosphate − binding phosphate) × 60% − urinary phosphate excretion − dialysate phosphate removal.
Statistical Analyses
All statistical analyses were performed using STATA 10.0 software (STATA Corporation, College Station, TX). The demographic characteristics of enrolled patients were initially described using descriptive statistics. The significance of trends across the three groups was assessed by the nonparametric test for trend across ordered groups developed by Cuzick (21). Differences in two groups of patients were assessed by the Wilcoxon rank-sum test. The relationship of serum phosphate level and other factors were analyzed using multivariate linear regression and the explanatory variables were sex, age, dialysis vintage, body mass index, serum albumin, nPNA, Kt/V, residual GFR, serum iPTH, serum FGF-23, serum α-Klotho, serum 1,25(OH)2D3, dose of calcitriol, and dose of CaCO3. First, a univariate linear regression model was used to test for significance without adjusting the other covariates. A stepwise procedure was then performed on variables with P values ≤0.2 from the univariate analysis due to the concern of the overfitting to identify factors independently associated with serum phosphate level. A two-tailed P value <0.05 was considered significant.
Two separate models were used to identify factors significantly associated with serum phosphate, one for patients with urine >200 ml/d and one for patients with urine ≤200 ml/d. Finally, Spearman rank correlation analysis was used to identify associations and another model was developed by the same procedures to investigate the relationship between tubular reabsorption rate of phosphate and other explanatory variables (GFR, Log-transformed FGF-23, Log-transformed iPTH, 1,25(OH)2D3, Log-transformed α-Klotho, and serum phosphate).
Results
Patient Characteristics
Table 1 shows the baseline demographic and clinical characteristics of the 134 MHD patients. There were no significant differences among the three groups with regard to sex, age, diabetes, systolic BP, dialyzer, and dialysis Kt/V. However, there were significant trends for longer dialysis vintage, lower diastolic BP, and lower GFR in patients with low urinary output.
Table 1.
Demographic and biochemical indices of nutritional status and mineral metabolism of enrolled MHD patients (n=134)
| Group I | Group II | Group III | P for Trenda | |
|---|---|---|---|---|
| Urine = 0 ml/d (n=50) | Urine ≤200 ml/d (n=44) | Urine >200 ml/d (n=40) | ||
| Demographic characteristics | ||||
| Sex (% male) | 40.0 | 54.6 | 50.0 | 0.31 |
| Age (yr) | 62.5±10.8 | 61.1±12.4 | 60.3±12.5 | 0.50 |
| Dialysis vintage (yr) | 10.1 (5.4, 12.9) | 3.5 (1.7, 7.3) | 1.5 (0.7, 2.0) | <0.001 |
| Diabetes (%) | 6.0 | 18.2 | 17.5 | 0.10 |
| Systolic BP (mmHg) | 119.9±21.2 | 130.1±19.5 | 126.0±14.4 | 0.14 |
| Diastolic BP (mmHg) | 68.8±12.7 | 73.4±11.6 | 74.9±11.0 | 0.03 |
| Triacetate/polysulfone (n:n) | 10:40 | 9:35 | 9:31 | 0.78 |
| Kt/V-dialysis | 1.5±0.3 | 1.4±0.2 | 1.4±0.3 | 0.27 |
| GFR (ml/min per 1.73 m2) | 0 | 0.4 (0.2, 0.5) | 2.8 (1.8, 5.4) | <0.001 |
| Urinary output (ml/d) | 0 | 100 (50, 200) | 563 (343, 908) | <0.001 |
| Nutritional status | ||||
| BMI (kg/m2) | 20.7±3.1 | 21.7±2.9 | 21.4±3.0 | 0.24 |
| nPNA (g/kg per day) | 1.1±0.2 | 1.1±0.2 | 1.1±0.2 | 0.69 |
| Serum creatinine (mg/dl) | 10.6±1.9 | 11.0±2.1 | 8.4±2.6 | <0.001 |
| BUN (mg/dl) | 72.3±12.3 | 72.5±11.5 | 61.9±12.0 | <0.001 |
| Hemoglobin (g/dl) | 11.2±1.3 | 10.7±2.0 | 10.7±1.6 | 0.16 |
| Hematocrit (%) | 34.4±3.7 | 33.6±3.7 | 32.7±4.7 | 0.14 |
| Albumin (g/dl) | 3.9±0.2 | 3.9±0.2 | 3.9±0.3 | 0.43 |
| Mineral metabolism | ||||
| Phosphate (mg/dl) | 5.9±1.5 | 5.9±1.6 | 4.8±1.4 | 0.002 |
| Calcium (mg/dl) | 9.8±0.8 | 9.3±1.1 | 9.0±0.8 | <0.001 |
| iPTH (pg/ml) | 471.1±477.1 | 293.7±229.2 | 248.8±210.3 | 0.02 |
| FGF-23 (pg/ml) | 470.2±377.5 | 435.5±381.5 | 134.6±213.4 | <0.001 |
| α-Klotho (pg/ml) | 316.6±111.0 | 239.8±42.7 | 341.0±197.8 | 0.90 |
| 1,25(OH)2D3 (pg/ml) | 3.5±2.9 | 4.6±4.3 | 4.3±2.9 | 0.19 |
| Clinic medications | ||||
| Dosage of calcitriol (μg/w) | 1.1±2.2 | 0.8±1.3 | 1.0±1.6 | 0.50 |
| Dosage of CaCO3 (g/d) | 3.6±2.2 | 3.0±1.9 | 2.2±2.2 | 0.003 |
| Low calcium dialysis (%) | 30.0 | 15.9 | 7.5 | 0.01 |
Values indicate means ± SDs, medians (interquartile ranges), or proportions. MHD, maintenance hemodialysis; BMI, body mass index; nPNA, normalized protein nitrogen appearance; iPTH, intact parathyroid hormone; FGF-23, fibroblast growth factor-23.
Significance for trend was tested across all three groups. All patients were treated with the same agents of active vitamin D and phosphate binder. Low calcium dialysis was defined by the concentration of calcium in the dialysate, which was 2.5 mEq/L (1.25 mmol/L).
Nutritional Status and Mineral Metabolism
Table 1 also shows no significant differences in mean body mass index, nPNA, hemoglobin, hematocrit, and albumin, suggesting that nutritional status was similar in three groups. However, trends for higher levels of serum creatinine and BUN in patients with low urinary output were observed, due to the limited urinary excretion of these substances.
Analysis of mineral metabolism indicated significant trends for higher serum phosphate, calcium, iPTH, and FGF-23, greater dose of CaCO3 and usage of low calcium dialysis in patients with low urinary output, but no significant differences in serum α-Klotho and 1,25(OH)2D3 concentrations, weekly calcitriol intake. Taken together, patients with daily urinary volume >200 ml were less likely to suffer from metabolic disturbances and altered mineral status.
RRF and Serum Phosphate Control
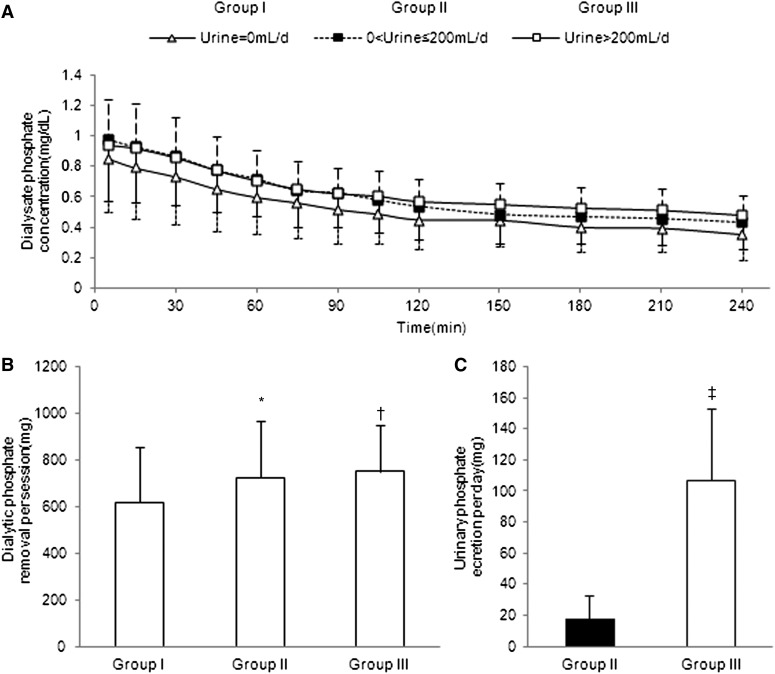
Figure 1A shows the change of dialysate phosphate concentration during dialysis of each group. The total amount of phosphate removal by a 4-hour hemodialysis session was significantly less in anuric patients than in the other two groups (group I, 617±235 mg; group II, 724±245 mg; group III, 752±199 mg) (Figure 1B). Urinary phosphate elimination per day (average elimination on dialysis day and nondialysis day) was significantly greater in group III than in group II (group II, 17±15 mg; group III, 107±46 mg) (Figure 1C).
Figure 1.
Removal of phosphate in MHD patients from groups I (anuric), II (urine output ≤200 ml/d), and III (urine output >200 ml/d). (A) Changes of dialysate phosphate concentration during a 4-hour hemodialysis treatment. (B) Total amount of phosphate removal by a 4-hour hemodialysis. *P<0.05 group I versus group II. †P<0.01 group I versus group III. (C) Daily urinary phosphate excretion (group II versus group III). ‡P<0.001. Values show means ± SDs.
Because urinary phosphate elimination was very limited in group II and serum phosphate level was very similar in group II and group I (P=0.91), we compared the estimated phosphate metabolic balance of group I+II with group III (Table 2). Compared with patients of group III, patients in group I+II had equivalent weekly dietary phosphate intake and dialysis phosphate removal, but more calcium-salt binding phosphate as well as less urinary phosphate excretion (as expected). Thus, patients in group I+II had a greater net accumulation of phosphate (△P).
Table 2.
Estimated weekly phosphate metabolic balance in MHD patients
| Group I+II | Group III | P | |
|---|---|---|---|
| Urine ≤200 ml/d (n=94) | Urine >200 ml/d (n=40) | ||
| Dietary phosphate (mg/w) | 6514±1297 | 6724±1512 | 0.35 |
| Removal by dialysis (mg/w) | 1983±728 | 2124±672 | 0.18 |
| Removal by urine (mg/w) | 122±106 | 769±318 | <0.001 |
| Removal by CaCO3 (mg/w) | 397±252 | 266±263 | 0.01 |
| △P (mg/w) | 1641±832 | 982±1014 | <0.001 |
Values are means ± SD. Dietary phosphate was estimated as follows: nPNA × 15 × dry body weight. △P was calculated as: (dietary phosphate − CaCO3 binding phosphate) × 60% − phosphate elimination by dialysis and urine. MHD, maintenance hemodialysis; nPNA, normalized protein nitrogen appearance.
In the multivariable regression model (Tables 3 and 4), nPNA, iPTH, and FGF-23 were independently associated with serum phosphate in patients with low urinary output (group I+II) (Table 3), whereas female sex and GFR were independently related to serum phosphate in patients with high urinary output (group III) (Table 4).
Table 3.
Multiple regression analysis of factors associated with serum phosphate in MHD patients whose daily urinary output was ≤200 ml
| Variable | Group I+II: Urine ≤200 ml/d (n=94) | |||
|---|---|---|---|---|
| Univariable Model | Multivariable Model | |||
| Regression Coefficient | P | Regression Coefficient | Pa | |
| Sex (female) | 0.46 | 0.15 | ||
| Age (yr) | −0.04 | 0.01 | ||
| Dialysis vintage (yr) | 0.02 | 0.53 | ||
| GFR (ml/min per 1.73 m2) | −0.46 | 0.44 | ||
| BMI (kg/m2) | 0 0.004 | 0.93 | ||
| Albumin (g/dl) | 0.95 | 0.18 | ||
| nPNA (g/kg per day) | 2.55 | 0.003 | 1.70 | 0.02 |
| Kt/V (one dialysis session) | −0.21 | 0.73 | ||
| iPTH (pg/ml) | 0.002 | <0.001 | 0.001 | 0.001 |
| FGF-23 (pg/ml) | 0.002 | <0.001 | 0.002 | <0.001 |
| α-Klotho (pg/ml) | −0.001 | 0.44 | ||
| 1,25(OH)2D3 (pg/ml) | 0.01 | 0.80 | ||
| Dosage of calcitriol (μg/w) | −0.04 | 0.64 | ||
| Dosage of CaCO3 (g/d) | 0.24 | 0.002 | ||
MHD, maintenance hemodialysis; BMI, body mass index; nPNA, normalized protein nitrogen appearance; iPTH, intact parathyroid hormone; FGF-23, fibroblast growth factor-23.
P from multiple regression adjusted for nPNA, iPTH, and FGF-23. The adjusted R2 was 0.41.
Table 4.
Multiple regression analysis of factors associated with serum phosphate in MHD patients whose daily urinary output was >200 ml
| Variable | Group III: Urine >200 ml/d (n=40) | |||
|---|---|---|---|---|
| Univariable Model | Multivariable Model | |||
| Regression Coefficient | P | Regression Coefficient | Pa | |
| Sex (female) | 0.86 | 0.05 | 1.13 | 0.01 |
| Age (yr) | −0.06 | 0.001 | ||
| Dialysis vintage (yr) | −0.15 | 0.22 | ||
| GFR (ml/min per 1.73 m2) | −0.19 | 0.003 | −0.22 | 0.001 |
| BMI (kg/m2) | 0.07 | 0.39 | ||
| Albumin (g/dl) | 0.71 | 0.36 | ||
| nPNA (g/kg per day) | −1.46 | 0.27 | ||
| Kt/V (one dialysis session) | −0.49 | 0.59 | ||
| iPTH (pg/ml) | 0.002 | 0.04 | ||
| FGF-23 (pg/ml) | 0.002 | 0.05 | ||
| α-Klotho (pg/ml) | −0.002 | 0.17 | ||
| 1,25(OH)2D3 (pg/ml) | 0.03 | 0.71 | ||
| Dosage of calcitriol (μg/w) | 0.12 | 0.41 | ||
| Dosage of CaCO3 (g/d) | 0.32 | 0.001 | ||
MHD, maintenance hemodialysis; BMI, body mass index; nPNA, normalized protein nitrogen appearance; iPTH, intact parathyroid hormone; FGF-23, fibroblast growth factor-23.
P from multiple regression adjusted for sex and GFR. The adjusted R2 was 0.3.
Circulating FGF-23 and Renal Phosphate Elimination in Dialysis Patients with RRF
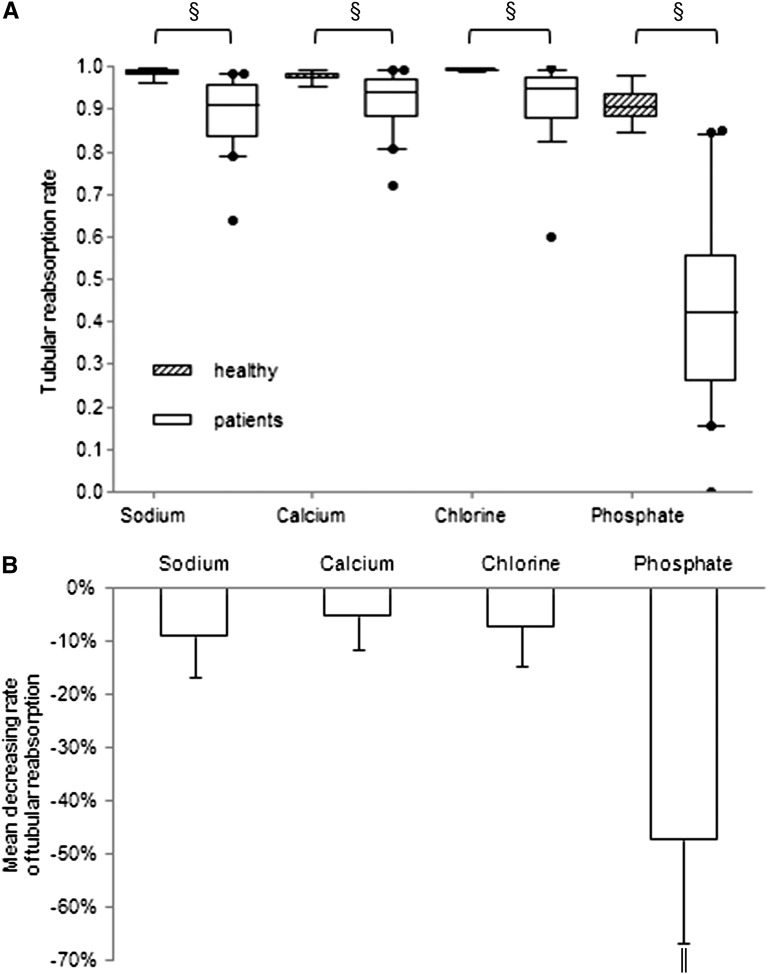
The weekly urinary excretion of phosphate in group III patients ranged from 300 to 1500 mg. Because urinary phosphate excretion is determined by tubular reabsorption, we compared the renal tubular reabsorption rate of sodium, calcium, chlorine, and phosphate of group III patients and 15 healthy volunteers (Figure 2). The mean (±SD) age of healthy volunteers was 49.4±21.8 years and 73% were male. The mean GFR was 112±17 ml/min per 1.73 m2. The mean serum levels of sodium, calcium, phosphate, and chlorine were 139.5±7.5 mEq/L, 9.0±0.9 mg/dl, 3.2±0.9 mg/dl, and 104.2±4.9 mEq/L, respectively. The tubular reabsorption rates of all four electrolytes were significantly lower in patients than those in healthy volunteers (Na: 89%±7% versus 99%±1%, P<0.001; Ca: 92%±6% versus 98%±1%, P<0.001; Cl: 92%±7% versus 99%±0.3%, P<0.001; and PO4: 44%±19% versus 91%±4%, P<0.001) (Figure 2A). The tubular phosphate reabsorption rate was even approximately 50% lower in patients, much greater than the decrease of other electrolytes (P<0.001) (Figure 2B).
Figure 2.
Tubular reabsorption of electrolytes in MHD patients with high urinary output. (A) Renal tubular reabsorption rates of sodium, calcium, chlorine, and phosphate in MHD patients and healthy controls. Boxes represent the interquartile range, with the upper and lower edges representing the 75th and 25th percentiles, respectively. The central horizontal lines represent the median levels. The vertical whiskers above and below the boxes represent the range of 5%–95% percentiles. Circles beyond the whiskers represent severe outliers. §P<0.001 versus control. (B) Percentage decrease in the rate of electrolytes in MHD patients. ∥P<0.001 comparison of phosphate with sodium, calcium, or chlorine.
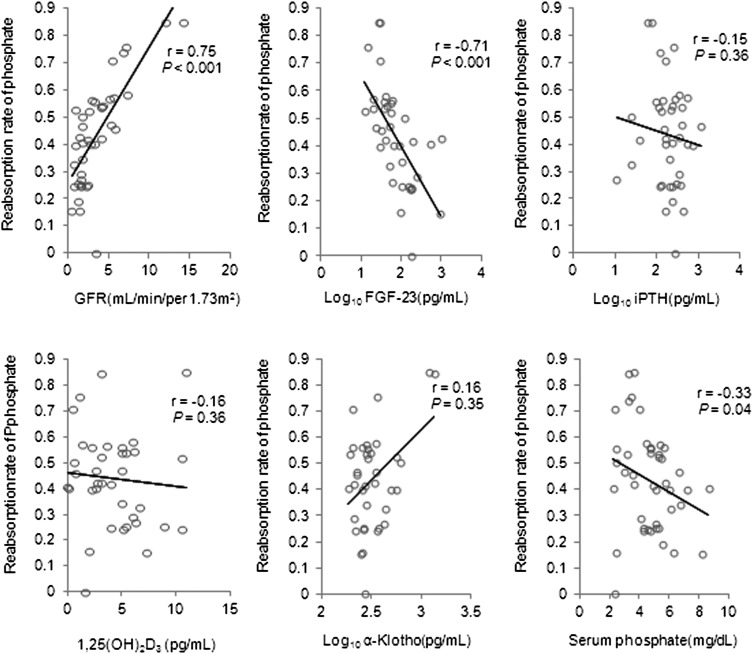
Spearman rank correlation analysis indicated that tubular phosphate reabsorption was correlated with GFR, Log10 FGF-23 (transformed to normalize the data), and serum phosphate (Figure 3). Finally, multivariate regression analysis showed that Log10 FGF-23 and GFR had an independent association with tubular phosphate reabsorption (Table 5).
Figure 3.
Correlation of tubular reabsorption rate of phosphate and GFR, Log10 FGF-23, Log10 iPTH, 1,25(OH)2D3, Log10 α-Klotho, and serum phosphate in 40 patients whose urinary output was >200 ml/d.
Table 5.
Multiple regression analysis of factors affecting tubular reabsorption rate of phosphate in patients with urine >200 ml/d
| Independent Variable | Dependent Variable: Phosphate Reabsorption Rate | |||
|---|---|---|---|---|
| Univariable Model | Multivariable Model | |||
| Regression Coefficient | 95% CI | Regression Coefficient | 95% CI | |
| GFR (ml/min per 1.73 m2) | 0.05 | (0.04 to 0.06) | 0.04 | (0.02 to 0.05) |
| Log10 FGF-23 (pg/ml) | −0.26 | (−0.36 to −0.15) | −0.15 | (−0.24 to −0.06) |
| Log10 iPTH (pg/ml) | −0.05 | (−0.21 to 0.10) | ||
| 1,25(OH)2D3 (pg/ml) | −0.005 | (−0.03 to 0.02) | ||
| Log10 α-Klotho (pg/ml) | 0.51 | (0.16 to 0.86) | ||
| Serum phosphate (mg/dl) | −0.04 | (−0.08 to 0.002) | ||
The adjusted R2 was 0.65. 95% CI, 95% confidence interval; FGF-23, fibroblast growth factor-23; iPTH, intact parathyroid hormone.
Discussion
Hyperphosphatemia is a frequent complication in ESRD patients (1). A previous study of peritoneal dialysis patients demonstrated that a small amount of residual GFR (average: <2 ml/min per 1.73 m2; maximum: 8 ml/min per 1.73 m2) contributed up to half of the total creatinine clearance and was independently associated with phosphate control (2). Another study reported that MHD patients with GFRs >4.13 ml/min per 1.73 m2 were more likely to have phosphate levels of 3.5–5.5 mg/dl (3). Current data further suggested that many indicators of mineral metabolism (serum phosphate, calcium, iPTH, and FGF-23) were closer to normal levels in MHD patients with RRF (urinary output >200 ml/d; GFR interquartile range, 1.8–5.4 ml/min per 1.73 m2) than patients without RRF (urinary output ≤200 ml/d). In addition, the circulating levels of α-Klotho in MHD patients were lower than the reported normal level (22), which was concordant with a previous study (23). Shimamura et al. reported that α-Klotho was positively associated with estimated GFR in patients with stage 1–5 CKD (24). We also found that the α-Klotho level was slightly higher in patients with high urinary output, although no significant trend was observed in the three groups. Recently, several studies showed that increased phosphate, FGF-23, or decreased Klotho is positively associated with mortality in both CKD and healthy adults (9,25,26). Therefore, the protection of RRF may help to reduce mortality by improving the mineral metabolism in MHD patients (27).
In this study, we made a rough assessment of ΔP in MHD patients and ignored phosphate releasing from bone or intracellular spaces, which was difficult to measure in vivo. Surprisingly, we found that anuric patients with higher serum phosphate concentration had lower phosphate removed by one hemodialysis session than patients with RRF, suggesting that the predialysis serum phosphate was not the only factor affecting the phosphate removal (28,29).The underlying mechanisms may partly be explained by higher body weight accompanied by greater quantity of phosphate distributed in phosphate pools in patients with RRF (13). Or the in vivo environment of patients with RRF may be more conductive to phosphate transfer from the intracellular compartment to the circulation (30). In addition, the anuric patients may have accumulated a higher level of nondialyzable molecules binding with phosphate and impeding phosphate removal. The kinetics of phosphate during hemodialysis need to be further explored.
Another interesting finding was that the weekly phosphate excretion by urine in patients with RRF ranged from 300 to 1500 mg, equivalent to the total removal by a single 4-hour hemodialysis session. It is important to note that phosphate clearance by the remnant kidney is a continuous process, which has a protective effect in preserving the stability of serum solutes (31). Therefore, phosphate retention in patients with RRF was much lower, which led to fewer requirements for phosphate binding agents, consistent with a previous report (3).
Multiple regression analysis indicated that the factors influencing serum phosphate were completely different in MHD patients with or without RRF. As expected, serum iPTH, FGF-23, and nPNA were independent determinants in patients without RRF. It is known that in early stage CKD, phosphate balance is maintained by a compensatory decrease in renal tubular reabsorption of phosphate that is mediated by elevated PTH and FGF-23 (32). As CKD progresses, total phosphate excretion cannot keep pace with phosphate increasing. In turn, hyperphosphatemia induces an increase in serum FGF-23 level and promotes parathyroid hyperplasia (33,34). The extremely elevated FGF-23 possibly inhibits bone mineralization, and the secondary hyperparathyroidism may further aggravate hyperphosphatemia by bone resorption (35,36). Therefore, breaking this vicious cycle is the key to treatment of mineral disorders in MHD patients without RRF, whereas the restriction of dietary phosphate intake should be given adequate attention. Our data confirmed that the excessive daily protein intake was one of the important reasons for high incidence of hyperphosphatemia in such a group of patients. Li et al. recently showed that 2-month restriction of dietary protein intake (0.8 g/kg per day) supplemented with keto acids significantly decreased serum phosphate levels in anuric MHD patients with refractory hyperphosphatemia, and did not induce malnutrition (37). Although protein intake is recommended to be 1.2 g/kg per day in MHD patients by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines (38), the optimal protein intake may also need to be individualized in different patients, especially for patients without RRF and treated with a low dose of dialysis (39).
In MHD patients with RRF, the determinants of serum phosphate were female sex and GFR. The reasons why female sex is an independent factor may be that 85% of female patients were postmenopausal in this study. The low circulating concentrations of estradiol were reported to be associated with increased serum phosphate (40) because estrogen was known as an inhibitor of bone resorption and acted directly to suppress sodium-dependent phosphate reabsorption in the renal proximal tubules (41,42). The negative correlation of GFR and serum phosphate is consistent with reports by Wang and Penne and is complementary to studies showing a significant contribution of RRF to phosphate homeostasis (2,3).
It must be noted that the phosphate elimination by residual kidney was not only due to glomerular filtration but also tubular transport. The notable findings were that the tubular reabsorption rates of sodium, calcium, and chloride were close to normal levels, which excluded the possibility of general tubular damage, seemed to support the “intact nephron hypothesis” proposed by Bricker et al. 50 years ago (43). However, the tubular reabsorption rate of phosphate in these patients declined by approximately 50%. We speculate that the remaining nephrons retain a sensitive control mechanism(s) underlying the tubular phosphate excretion. The index of the renal threshold for phosphate (TmP/GFR; normal range: 2.8–4.4 mg/dl) (44) ranged from 0 to 3.5 mg/dl in this study, which excluded the possibility of increasing in the filtered load of phosphate produced by hyperphosphatemia or by an increase in GFR per nephron. At the same time, multiple regression analysis showed that circulating FGF-23 was independently associated with tubular phosphate reabsorption, but serum PTH and soluble α-Klotho were not. PTH and FGF-23 are known to regulate the expression of NaPi-II in proximal tubules in normal condition and early CKD (6,32). Although a growing body of evidence suggests that elevated FGF-23 contributes to morbidity and mortality in dialysis patients, a cause-effect relationship has not yet been established (9,10). In fact, therapy with active vitamin D sterols would be expected to increase circulating levels of FGF-23 but this treatment actually may improve the survival rate of dialysis patients (45). Thus, the clinical relevance of elevated FGF-23 in dialysis patients remains unclear. On the basis of the results of this study, we believe that FGF-23 instead of PTH may still promote renal phosphate excretion in MHD patients with RRF.
Experimental studies have shown that FGF-23 acting on Klotho/FGFR reduces the expression of NaPi-IIa (46). In this study, the circulating α-Klotho was significantly decreased in MHD patients and was not associated with either tubular phosphate reabsorption rate or serum phosphate, although intravenous administration of secreted Klotho was reported to induce phosphaturia in normal and FGF-23−/−mice (47). It may be explained by underuremic conditions in which there is a higher circulating concentration of FGF-23, and FGF-23 may bind to its receptor with low affinity without Klotho (48). The biologic role of circulating Klotho needs further investigation (23).
We acknowledge several limitations of this study. First, we cannot infer the causality of the associations identified in our analysis. Second, our sample size was rather small and all patients were from a single institution, so there may have been some selection bias. A third limitation is that the use of nPNA, the theoretical value of phosphate binding capacity of CaCO3 and GI tract absorption rate to estimate phosphate balance, was not the most accurate way. Fourth, we were unable to exclude the possible influence of dietary factors. Gut-renal interactions participate in control of serum phosphate by a substance with phosphatonin-like actions and this phosphaturic response is not accompanied by increases in FGF-23, serum phosphate, or PTH (49).
In conclusion, RRF contributes significantly to phosphate homeostasis and upregulation of FGF-23 may be a compensatory response that promotes phosphate excretion in MHD patients with RRF.
Disclosures
None.
Acknowledgments
We are grateful to all patients and medical staff who participated in this project.
This work was supported in part by the Major State Basic Research Development Program of China (973 program, No. 2012CB517700) and the China Natural Science Foundation (81170684/30971373 to J.C.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Tentori F: Mineral and bone disorder and outcomes in hemodialysis patients: Results from the DOPPS. Semin Dial 23: 10–14, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Wang AY, Woo J, Sea MM, Law MC, Lui SF, Li PK: Hyperphosphatemia in Chinese peritoneal dialysis patients with and without residual kidney function: What are the implications? Am J Kidney Dis 43: 712–720, 2004 [PubMed] [Google Scholar]
- 3.Penne EL, van der Weerd NC, Grooteman MP, Mazairac AH, van den Dorpel MA, Nubé MJ, Bots ML, Lévesque R, ter Wee PM, Blankestijn PJ, CONTRAST investigators : Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol 6: 281–289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murer H, Hernando N, Forster I, Biber J: Proximal tubular phosphate reabsorption: Molecular mechanisms. Physiol Rev 80: 1373–1409, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Russo D, Battaglia Y: Clinical significance of FGF-23 in patients with CKD. Int J Nephrol 2011: 364890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Viaene L, Bammens B, Meijers BK, Vanrenterghem Y, Vanderschueren D, Evenepoel P: Residual renal function is an independent determinant of serum FGF-23 levels in dialysis patients. Nephrol Dial Transplant 27: 2017–2022, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y: FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int 65: 1943–1946, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasrallah MM, El-Shehaby AR, Salem MM, Osman NA, El Sheikh E, Sharaf El Din UA: Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant 25: 2679–2685, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT, NECOSAD Study Group : Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 62: 1046–1053, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Daugirdas JT: Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J Am Soc Nephrol 4: 1205–1213, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Li H, Liao H, Yu Y, You L, Zhu J, Huang B, Yuan L, Hao C, Chen J: Phosphate removal model: An observational study of low-flux dialyzers in conventional hemodialysis therapy. Hemodial Int 16: 363–376, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Beloosesky Y, Grinblat J, Weiss A, Grosman B, Gafter U, Chagnac A: Electrolyte disorders following oral sodium phosphate administration for bowel cleansing in elderly patients. Arch Intern Med 163: 803–808, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kuhlmann MK: Management of hyperphosphatemia. Hemodial Int 10: 338–345, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Fouque D, Pelletier S, Mafra D, Chauveau P: Nutrition and chronic kidney disease. Kidney Int 80: 348–357, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT, NECOSAD Study Group : Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 15: 1061–1070, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27–39, 1980 [DOI] [PubMed] [Google Scholar]
- 19.Schucker JJ, Ward KE: Hyperphosphatemia and phosphate binders. Am J Health Syst Pharm 62: 2355–2361, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ramirez JA, Emmett M, White MG, Fathi N, Santa Ana CA, Morawski SG, Fordtran JS: The absorption of dietary phosphorus and calcium in hemodialysis patients. Kidney Int 30: 753–759, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Cuzick J: A Wilcoxon-type test for trend. Stat Med 4: 87–90, 1985 [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y: Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun 398: 513–518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama K, Imura A, Ohkido I, Maruyama Y, Yamazaki Y, Hasegawa H, Urae J, Sekino H, Nabeshima YI, Hosoya T: Serum soluble α-klotho in hemodialysis patients. Clin Nephrol 77: 347–351, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis [published online ahead of print March 29, 2012]. Clin Exp Nephrol doi:10.1007/s10157-012-0621-7 [DOI] [PubMed] [Google Scholar]
- 25.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L: Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci 66: 794–800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR: Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20: 381–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brener ZZ, Kotanko P, Thijssen S, Winchester JF, Bergman M: Clinical benefit of preserving residual renal function in dialysis patients: An update for clinicians. Am J Med Sci 339: 453–456, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Bevington A, Brough D, Baker FE, Hattersley J, Walls J: Metabolic acidosis is a potent stimulus for cellular inorganic phosphate generation in uraemia. Clin Sci (Lond) 88: 405–412, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Albalate M, de la Piedra C, Fernández C, Lefort M, Santana H, Hernando P, Hernández J, Caramelo C: Association between phosphate removal and markers of bone turnover in haemodialysis patients. Nephrol Dial Transplant 21: 1626–1632, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Pohlmeier R, Vienken J: Phosphate removal and hemodialysis conditions. Kidney Int Suppl 78: S190–S194, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR: Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 375: 895–905, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Prié D, Ureña Torres P, Friedlander G: Latest findings in phosphate homeostasis. Kidney Int 75: 882–889, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Ramon I, Kleynen P, Body JJ, Karmali R: Fibroblast growth factor 23 and its role in phosphate homeostasis. Eur J Endocrinol 162: 1–10, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q, Qiu J, Li H, Lu Y, Wang X, Yang J, Wang S, Zhang L, Gu Y, Hao CM, Chen J: Cyclooxygenase 2 promotes parathyroid hyperplasia in ESRD. J Am Soc Nephrol 22: 664–672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Feng JQ: FGF23 in skeletal modeling and remodeling. Curr Osteoporos Rep 9: 103–108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llach F: Parathyroidectomy in chronic renal failure: Indications, surgical approach and the use of calcitriol. Kidney Int Suppl 29: S62–S68, 1990 [PubMed] [Google Scholar]
- 37.Li H, Long Q, Shao C, Fan H, Yuan L, Huang B, Gu Y, Lin S, Hao C, Chen J: Effect of short-term low-protein diet supplemented with keto acids on hyperphosphatemia in maintenance hemodialysis patients. Blood Purif 31: 33–40, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Kopple JD: National Kidney Foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 37[Suppl 2]: S66–S70, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Nakao T, Matsumoto H, Okada T, Kanazawa Y, Yoshino M, Nagaoka Y, Takeguchi F: Nutritional management of dialysis patients: Balancing among nutrient intake, dialysis dose, and nutritional status. Am J Kidney Dis 41[Suppl 1]: S133–S136, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Lindsay R, Coutts JR, Hart DM: The effect of endogenous oestrogen on plasma and urinary calcium and phosphate in oophorectomized women. Clin Endocrinol (Oxf) 6: 87–93, 1977 [DOI] [PubMed] [Google Scholar]
- 41.Krassas GE, Papadopoulou P: Oestrogen action on bone cells. J Musculoskelet Neuronal Interact 2: 143–151, 2001 [PubMed] [Google Scholar]
- 42.Uemura H, Irahara M, Yoneda N, Yasui T, Genjida K, Miyamoto KI, Aono T, Takeda E: Close correlation between estrogen treatment and renal phosphate reabsorption capacity. J Clin Endocrinol Metab 85: 1215–1219, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Slatopolsky E: The intact nephron hypothesis: The concept and its implications for phosphate management in CKD-related mineral and bone disorder. Kidney Int Suppl 79: S3–S8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagga A, Bajpai A, Menon S: Approach to renal tubular disorders. Indian J Pediatr 72: 771–776, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Wolf M, Betancourt J, Chang Y, Shah A, Teng M, Tamez H, Gutierrez O, Camargo CA, Jr, Melamed M, Norris K, Stampfer MJ, Powe NR, Thadhani R: Impact of activated vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol 19: 1379–1388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuro-o M: Phosphate and Klotho. Kidney Int 79[Suppl 121]: S20–S23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW: Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Razzaque MS: FGF23-mediated regulation of systemic phosphate homeostasis: Is Klotho an essential player? Am J Physiol Renal Physiol 296: F470–F476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marks J, Debnam ES, Unwin RJ: Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Renal Physiol 299: F285–F296, 2010 [DOI] [PubMed] [Google Scholar]