Summary
Background and objectives
Double-chamber peritoneal dialysis fluids exert less toxicity by their neutral pH and reduced glucose degradation product content. The role of the buffer compound (lactate and bicarbonate) has not been defined in humans.
Design, setting, participants, & measurements
A multicenter randomized controlled trial in 37 children on automated peritoneal dialysis was performed. After a 2-month run-in period with conventional peritoneal dialysis fluids, patients were randomized to neutral-pH, low-glucose degradation product peritoneal dialysis fluids with 35 mM lactate or 34 mM bicarbonate content. Clinical and biochemical monitoring was performed monthly, and peritoneal equilibration tests and 24-hour clearance studies were performed at 0, 3, 6, and 10 months.
Results
No statistically significant difference in capillary blood pH, serum bicarbonate, or oral buffer supplementation emerged during the study. At baseline, peritoneal solute equilibration and clearance rates were similar. During the study, 4-hour dialysis to plasma ratio of creatinine tended to increase, and 24-hour dialytic creatinine and phosphate clearance increased with lactate peritoneal dialysis fluid but not with bicarbonate peritoneal dialysis fluid. Daily net ultrafiltration, which was similar at baseline (lactate fluid=5.4±2.6 ml/g glucose exposure, bicarbonate fluid=4.9±1.9 ml/g glucose exposure), decreased to 4.6±1.0 ml/g glucose exposure in the lactate peritoneal dialysis fluid group, whereas it increased to 5.1±1.7 ml/g glucose exposure in the bicarbonate content peritoneal dialysis fluid group (P=0.006 for interaction).
Conclusions
When using biocompatible peritoneal dialysis fluids, equally good acidosis control is achieved with lactate and bicarbonate buffers. Improved long-term preservation of peritoneal membrane function may, however, be achieved with bicarbonate-based peritoneal dialysis fluids.
Introduction
Multichamber peritoneal dialysis (PD) solutions store the glucose separate from the buffer at a very low pH, thereby reducing the formation of glucose degradation products (GDPs) during heat sterilization and storage. The buffer agent can be lactate, bicarbonate, or a mixture of both. The final fluid instilled after mixing is buffered at a neutral pH in contrast to the acidic milieu of conventional solutions. Numerous in vitro and animal studies showed superior biocompatibility of multichamber PD solutions (1–5). Recent randomized controlled trials suggest improved preservation of residual renal function (6,7) and ultrafiltration capacity (8) and lowering of systemic concentrations of advanced glycation end products (9,10), which contribute to accelerated vasculopathy and amyloidosis. Registry findings suggest an improved patient survival with low-GDP fluids (11,12).
While the toxicity of GDP has been investigated in detail (13), the clinical impact of the buffer compound is as yet unknown. Lactate, particularly at low pH, has detrimental effects on peritoneal mesothelial and leukocyte function (14,15). At neutral pH, lactate still impairs human peritoneal mesothelial cell viability and function, whereas cellular functions are preserved with bicarbonate buffer (16–19). Thus, the PD buffer choice may impact on peritoneal membrane morphology and function.
Thus far, clinical trials have been limited to the comparison of conventional, lactate-based, acidic PD solutions with solutions with variably lower concentrations of GDP (20,21) and varying buffer composition with final pH ranging from 5.5 to 7.4 (22–26). A single small trial in adults compared two low-GDP solutions (a pH-neutral, 10 mM bicarbonate, 30 mM lactate solution) with an acidic, 40 mM lactate solution. At 2 weeks, mean daily ultrafiltration was slightly higher with the pH-neutral, bicarbonate-containing solution (27). To determine whether bicarbonate or lactate better preserves peritoneal solute and water transport capacity, we now treated children in a randomized prospective trial with dual-chamber PD solutions only differing with respect to the buffer compound and not pH or GDP composition.
Materials and Methods
Patients
The study was performed in eight pediatric dialysis units in Germany (four units), Austria (one unit), Finland (one unit), France (one unit), and Italy (one unit) in full compliance with the Declaration of Helsinki and the current European Union Good Clinical Practice guidelines for clinical trials. The study was registered at ClinicalTrails.gov (NCT01632046). Ethical committee approval for the study protocol was obtained in each center. Written informed consent was obtained from all parents and young adult patients, and assent was obtained from the patients below 18 years of age.
Patients were eligible for the trial if they were 1 month to 21 years of age, received chronic automated PD treatment with an average peritoneal fill volume close to 1100 ml/m2 body surface area, had no severe chronic pulmonary, cardiac, liver, or malignant disease, had no history of peritonitis within the last 3 weeks, and had no clinical evidence of major peritoneal adhesions. The 2-year recruitment period started in March of 2004.
Of 42 children recruited for the trial, 37 children (15 girls) were available for central randomization with a blocking factor of four at the end of the 2-month run-in period. Five children were excluded before entering the study phase because of renal transplantation (n=2), noncompliance (n=2), and switch to hemodialysis (n=1); 24 children completed the study: 13 children in the lactate group and 11 children in the bicarbonate group. At randomization, median patient age was 12.8 (0.3–21.7) years in the lactate PD fluid (L-PDF; Balance) group and 10.3 (0.2–19.0) years in the bicarbonate content PD fluid (B-PDF; BicaVera) group. The 21.7-year-old patient was retarded, and exclusion from the analysis did not result in different outcome. Mean duration of PD at the time of entering the run-in period was 2.2 (0.1–89.2) and 1.3 (0.2–36.2) months, respectively. Three patients had experienced at least one episode of peritonitis before entering the study. The underlying diseases were renal hypo-/dysplasia (n=10), obstructive uropathy (n=6), focal segmental glomerulosclerosis (n=4), nephronophthisis (n=4), hemolytic uremic syndrome (n=3), nephrotic syndrome (n=2), autosomal recessive polycystic kidney disease (n=2), postasphyxia ESRD (n=2), Alport syndrome (n=1), nephritis (n=1), Henoch–Schönlein nephritis (n=1), and unknown in (n=1).
Study Design
This investigator-initiated study was performed as an international open prospective randomized multicenter trial with parallel design. Stable dialysis conditions were assured by an 8-week run-in period, during which time the patients were treated with conventional, single-chamber PD fluid, pH 5.5, containing 35 mM lactate (C-PDF; CAPD 1.5%/2.3%/4.2%; Fresenius Medical Care, Bad Homburg, Germany). Oral sodium bicarbonate supplementation was discontinued. Subsequently, the patients were randomized to a 10-month treatment period with either a 35 mM lactate-based double-chamber solution, pH 7.0 (Balance 1.5%, 2.3%, 4.25%; Fresenius Medical Care, Bad Homburg, Germany) or a 34 mM bicarbonate-based double-chamber solution, pH 7.4 (BicaVera 1.5%, 2.3%, 4.25%; Fresenius Medical Care, Bad Homburg, Germany). Both solutions contained identical concentrations of sodium (134 mM), calcium (1.75 mM), and magnesium (0.5 mM) and either 15, 23, or 42.5 g/L dextrose.
During the study, all patients maintained their previous automated PD prescription, which was adapted according to clinical needs and adequacy targets (28). The families documented the PD regimen and daily ultrafiltration rates at home; mean weekly ultrafiltration was recorded for analysis. In case of peritonitis, the run-in period was prolonged until 3 weeks after completion of the antibiotic therapy. Oral bicarbonate medication was only given in patients with blood bicarbonate levels below 17 mmol/L, despite sufficient dialysis efficacy. The recommended dosage was 0.5 mmol/kg per day divided into three doses. Every 4 weeks, a physical examination and a laboratory workup, including a capillary blood gas analysis and a bioimpedance measurement at 50 kHz for assessment of hydration status, were performed. Standardized Peritoneal Equilibration Test (PET) using 1100 ml fill volume/m2 BSA and 2.3% glucose according to standardized pediatric procedural guidelines (29) and dialytic and urine 24-hour clearance studies were obtained at 0, 3, 6, and 10 months. Intraperitoneal pressure was measured at the end of the run-in period with C-PDF and after 3 months of PD with L-PDF and B-PDF. Episodes of peritonitis were diagnosed and treated according to a standardized protocol (30). In patients with peritonitis episodes in the last month before a PET (one patient in each group), the study period was prolonged, and the PET postponed by 4 weeks.
Laboratory Analysis
Capillary blood pH, pCO2, and pO2 were measured immediately using a blood gas analyzer, and actual bicarbonate concentrations were calculated from the Henderson–Hasselbach equation. The blood and dialysate concentrations of glucose, creatinine, urea, lactate, electrolytes, albumin, and β2-microglobulin and the serum levels of liver enzymes, alkaline phosphatase, total protein, triglyceride, cholesterol, and C-reactive protein were measured at each center using standard analytical methods. Dialysate creatinine measurements were corrected for the presence of glucose as described previously (29).
Statistical Analyses
Unpaired t tests were applied to evaluate baseline characteristics between the two groups, and a Mann–Whitney rank sum test was used in case of non-normal data distribution. In case of binary data, a chi-squared test was used. The primary endpoint was the PET 4-hour dialysis to plasma ratio creatinine. Two-way repeated-measures ANOVA was applied for time- and treatment-related effects using the SAS software (SAS, Cary, NC). Results were reported using adjusted P values according to Huynh-Feldt to take different variance structures into account. Longitudinal data modeling using the MIXED procedure applying unstructured covariance matrices for correlation between time points was performed for sensitivity analysis. With this complementary approach, information from patients with partially missing data over time points could be taken into account. Changes over time in clinical, dialytic, and biochemical parameters measured at four weekly and three to four monthly intervals, respectively, were analyzed using the ANOVA approach, and P values were interpreted descriptively. Stepwise linear regression analysis was used to identify independent predictors of the change in the lactate and bicarbonate treatment groups. Data are given as mean ± SD if not indicated otherwise.
Results
Baseline Findings
The biochemical profile and PD treatment modalities were comparable in both patient groups at randomization (Table 1). Likewise, 2- and 4-hour small-, middle-, and large-molecule transport kinetics, 4-hour clearance rates, and ultrafiltration did not differ at baseline (Table 2).
Table 1.
Anthropometric, biochemical, and peritoneal dialysis prescription characteristics of all patients at the time of randomization
| L-PDF | B-PDF | P Value | |
|---|---|---|---|
| Male/femalea | 14/6 | 8/9 | 0.16 |
| Height (cm) | 134.5±34.7 | 129.4±31.4 | 0.47 |
| Weight (kg) | 36.5±23.0 | 31.5±15.0 | 0.50 |
| BMI (kg/m2) | 18.4±4.6 | 17.5±2.7 | 0.64 |
| Capillary pH | 7.38±0.08 | 7.37±0.03 | 0.86 |
| Serum bicarbonate (mmol/L) | 22.7±3.2 | 22.1±2.0 | 0.73 |
| Base excess (mmol/L)b | −2.0 (−5.4, 0.1) | −2.5 (−3.8, −1.6) | 0.57 |
| Hemoglobin (g/L) | 12.0±1.4 | 11.2±1.7 | 0.15 |
| Serum creatinine (mg/ml) | 7.1±0.9 | 6.6±0.6 | 1.00 |
| Serum urea (mg/ml) | 89.0±4.6 | 97.3±8.5 | 0.24 |
| Serum albumin (g/L) | 38.2±1.4 | 36.4±1.3 | 0.45 |
| Serum β2-microglobulin (g/L) | 23.8±2.3 | 21.5±2.6 | 0.46 |
| Serum inorganic phosphorous (mmol/L) | 1.7±0.1 | 1.7±0.1 | 0.93 |
| Number of cycles per nightb | 6 (5, 7) | 7 (5, 7) | 0.16 |
| Time on cycler (h) | 7.8±1.7 | 7.8±1.3 | 0.99 |
| Fill volume (ml/m2) | 1007±132 | 993±164 | 0.20 |
| Glucose exposure (g/m2 per day) | 113±46 | 99±25 | 0.64 |
All numbers are presented as mean ± SD. P values are from unpaired t tests; exceptions are indicated. L-PDF, lactate peritoneal dialysis fluids; B-PDF, bicarbonate content peritoneal dialysis fluids; BMI, body mass index.
P value results from a chi-squared test.
Values are expressed as median (interquartile range), and P values resulted from the median test.
Table 2.
Peritoneal transport characteristics during baseline visit with conventional single-chamber peritoneal dialysis solution and after 3, 6, and 10 months with lactate peritoneal dialysis solution or bicarbonate-buffered peritoneal dialysis solution
| Visit 0 | Visit 3 | Visit 6 | Visit 10 | P Valuesa (Group, Time, and Interaction) | |||||
|---|---|---|---|---|---|---|---|---|---|
| C-PDF, L-PDF Group | C-PDF, B-PDF Group | L-PDF | B-PDF | L-PDF | B-PDF | L-PDF | B-PDF | ||
| 4 h D/P creatinine | 0.62±0.15 | 0.65±0.12 | 0.67±0.13 | 0.68±0.13 | 0.67±0.12 | 0.64±0.12 | 0.72±0.12 | 0.66±0.13 | 0.83, 0.41, 0.18 |
| 4 h Kt/V | 0.45±0.11 | 0.46±0.08 | 0.43±0.09 | 0.48±0.10 | 0.41±0.06 | 0.48±0.09 | 0.44±0.07 | 0.47±0.08 | 0.07, 0.53, 0.66 |
| 4 h D/P phosphate | 0.56±0.14 | 0.56±0.15 | 0.63±0.12 | 0.59±0.19 | 0.60±0.14 | 0.56±0.20 | 0.62±0.13 | 0.55±0.14 | 0.78, 0.78, 0.44 |
| 4 h D/P albumin | 0.011±0.007 | 0.013±0.010 | 0.012±0.009 | 0.015±0.011 | 0.012±0.011 | 0.011±0.008 | 0.012±0.009 | 0.014±0.007 | 0.19, 0.06, 0.18 |
| 4 h D/P B2M | 0.10±0.05 | 0.10±0.04 | 0.12±0.07 | 0.15±0.11 | 0.13±0.08 | 0.10±0.06 | 0.11±0.07 | 0.11±0.05 | 0.92, 0.36, 0.84 |
| 4 h D/D0 glucose | 0.32±0.07 | 0.33±0.08 | 0.32±0.09 | 0.30±0.08 | 0.34±0.09 | 0.36±0.09 | 0.32±0.09 | 0.33±0.09 | 0.72, 0.04, 0.19 |
| Glucose resorption (g/4 h) | 16.9±7.8 | 15.2±7.5 | 18.0±7.0 | 16.7±7.1 | 17.2±5.6 | 15.6±7.7 | 19.8±5.0 | 15.9±7.0 | 0.22, 0.61, 0.21 |
| UF/g glucose (ml) | 5.44±2.58 | 4.89±1.92 | 5.27±1.64 | 4.75±1.91 | 4.49±1.03 | 4.48±1.48 | 4.57±1.05 | 5.13±1.71 | 0.16, 0.31, 0.006 |
All numbers are presented as mean ± SD. C-DPF, conventional single-chamber peritoneal dialysis solution; L-PDF, lactate peritoneal dialysis solution; B-PDF, bicarbonate-buffered peritoneal dialysis solution; D/P, dialysis to plasma ratio; B2M, β2-microglobulin; UF, ultrafiltration.
P values resulting from repeated-measures ANOVA (group, time, and group × time interaction included as categorical variables) using all available observations over time.
Biochemistry and Dialysis Prescription during the Study
There was neither consistent change in serum electrolytes, urea, phosphate, albumin, and β2-microglobulin from baseline with single-chamber solution to the subsequent treatment periods with low-GDP PD nor was there any change over time within or between the two treatment groups. Serum creatinine increased from 7.1±0.9 to 8.7±1.0 mg/dl in the L-PDF group and from 6.6±0.6 to 9.2±0.7 mg/dl in the B-PDF group (P=0.93 for group, P<0.001 for time, P=0.85 for interaction).
Dialytic glucose exposure increased from 113±46 to 126±50 g/m2 per day in the L-PDF group and from 100±26 to 116±28 g/m2 per day in the B-PDF group (P=0.25 for group, P<0.001 for time, P=0.27 for interaction), whereas the number of cycles remained constant. Likewise, there were no statistically significant differences regarding total daily dwell time (7.8±1.7 and 7.8±1.3 hours at visit 0 and 7.0±0.7 and 8.0±1.1 hours at visit 10) and dwell volume (1007±131 and 993±164 ml/m2 at visit 0 and 1036±166 and 1007±157 ml/m2 at visit 10 with L-PDF and B-PDF, respectively).
Residual renal function declined during the study. Baseline renal clearance of creatinine was 5.5±1.4 and 5.4±1.3 ml/min per 1.73 m2 in the L-PDF and B-PDF groups, respectively, and decreased to 2.3±0.9 and 1.8±0.8 ml/min per 1.73 m2 at the end of the study (P=0.68 for group, P=0.006 for time, P=0.50 for interaction). Daily urine output also declined, but it did not differ between the L-PDF and B-PDF groups at any time point.
Acid–Base Status
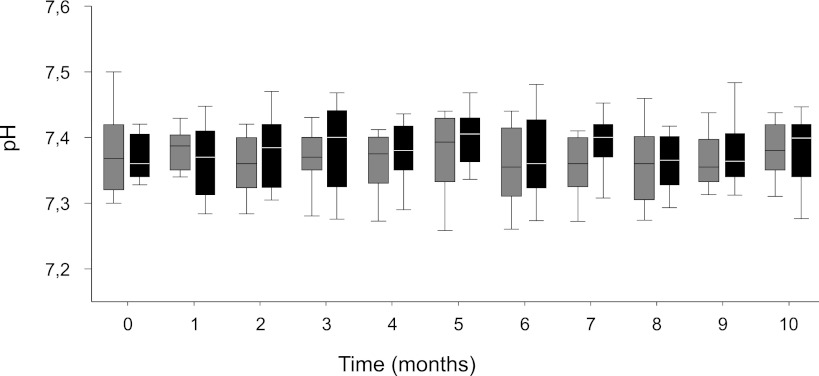
Capillary blood pH, HCO3, and CO2 remained largely unchanged throughout the study with L-PDF and B-PDF (Figure 1). At baseline, oral buffer intake was 0.9±0.4 mmol/kg per day in four patients with L-PDF and 0.6±0.2 mmol/kg per day in five patients with B-PDF. After 10 months, oral buffer intake was 0.5±0.3 mmol/kg per day in two patients in the L-PDF and 0.5 mmol/kg per day in one patient in the B-PDF group.
Figure 1.
Metabolic acidosis control. Blood pH in patients treated with lactate peritoneal dialysis fluid (L-PDF; gray box plots) and bicarbonate content peritoneal dialysis fluid (B-PDF; black box plots). Capillary blood samples obtained monthly showed no differences between the groups. Data are median and 25th and 75th percentiles; whiskers represent 10th and 90th percentiles.
Peritoneal Solute and Water Transport
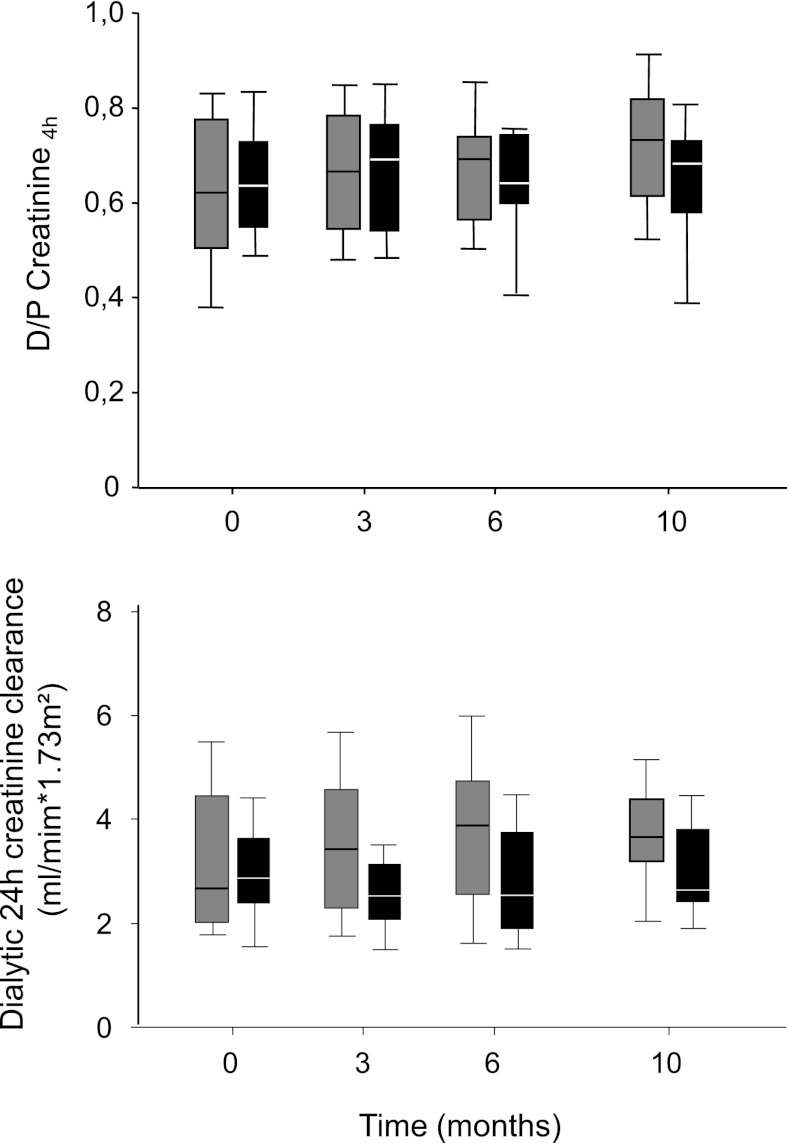
PETs were performed at the end of the 2-month run-in period with C-PDF and after 3, 6, and 10 months with L-PDF and B-PDF treatment. During the 10-month treatment period, the 4-hour dialysis to plasma ratio of creatinine tended to increase with L-PDF (Table 2) but remained stable with B-PDF (P=0.83 for group, P=0.41 for time, P=0.18 for interaction) (Figure 2). Longitudinal modeling yielded similar results (P=0.59 for group, P=0.32 for time, P=0.09 for interaction); 24-hour dialytic creatinine clearance at baseline and after 10 months was 3.3±0.3 and 3.7±0.3 ml/min per 1.73 m2 with L-PDF and 3.0±0.2 and 3.0±0.3 ml/min per 1.73 m2 with B-PDF (P=0.04 for group, P=0.47 for time, P=0.64 for interaction) (Figure 2), and 24-hour dialytic phosphate clearance increased with L-PDF from 3.2±1.4 at baseline to 3.6±1.3 ml/min per 1.73 m2 at visit 10 and declined with B-PDF from 2.9±1.2 to 2.2±0.8 ml/min per 1.73 m2 (P=0.02 for group, P=0.55 for time, P=0.68 for interaction). Twenty-four–hour dialytic creatinine and phosphate clearance increased in children treated with L-PDF compared with children treated with B-PDF. Neither age nor time on PD were independent predictors of the outcome of solute transport as determined by the repeated PET.
Figure 2.
Peritoneal creatinine transport. Dialysate over plasma creatinine ratio (upper) and 24-hour dialytic creatinine clearance (lower) in patients treated with L-PDF (gray box plots) and B-PDF (black box plots) for 10 months. Data are median and 25th and 75th percentiles; whiskers represent 10th and 90th percentiles (P=0.83 and P=0.04 for group effect, respectively).
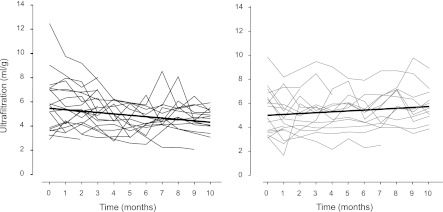
Glucose resorption and the 4-hour D/D0 glucose ratio remained largely constant in both treatment groups throughout the study (Table 2). Mean daily ultrafiltration per glucose was similar at baseline, decreased significantly with L-PDF, and tended to increase with B-PDF (Table 2), resulting in significant treatment-related changes in ultrafiltration capacity over time (P=0.16 for group, P=0.31 for time, P=0.006 for interaction) (Figure 3). Longitudinal modeling yielded similar results (P=0.35 for group, P=0.18 for time, P<0.001 for interaction). Neither initial ultrafiltration capacity nor the changes over time were correlated with age, the time spent on PD, or glucose exposure as calculated by univariate and multivariate approach. Intraperitoneal pressure was similar at baseline with C-PDF in both groups with 600, 1000, and 1400 ml/m2 fill volume (10.1±2.9 versus 8.4±4.2 cm H2O and 1000 ml/m2 fill volume,) and after 3 months with L-PDF and B-PDF (11.0±3.1 versus 9.9±2.0 cm per 1000 ml/m2 fill volume).
Figure 3.
Peritoneal ultrafiltration capacity over time. Ultrafiltration per 1 g glucose administered in patients on L-PDF (left) and B-PDF (right). Based on the daily ultrafiltration rates documented by the parents, monthly averages are given from each patient (P=0.006 for interaction).
Safety
Of 42 children entering the study, 37 children were randomized to L-PDF or B-PDF; 24 children completed the study: 13 children in the lactate group and 11 children in the bicarbonate group. Seven patients discontinued the study during L-PDF, and six patients discontinued the study during B-PDF treatment. Drop-out reasons were transplantation (n=7, two patients on L-PDF and five patients on B-PDF), switch to hemodialysis (n=4; three patients on L-PDF and one patient on B-PDF treatment), icodextrin use (n=1 on L-PDF), and recurrent hernia (n=1 on B-PDF).
Seven patients developed 11 episodes of peritonitis: four episodes with C-PDF (1/15 treatment months), four episodes with L-PDF, and three episodes with B-PDF (1/35 and 1/37 treatment months, respectively). Two of three patients who developed more than one episode of peritonitis switched to hemodialysis. Exit site and tunnel infections developed in three patients: one patient during C-PDF, one patient during L-PDF, and one patient during B-PDF treatment. Five patients developed hernia: two patients during C-PDF, two patients during L-PDF, and one patient during B-PDF. Dialysate leakage developed in three patients: two patients were on C-PDF and one patient was on B-PDF. BP was in the age-specific normal range; three patients required antihypertensive medications: two patients in the L-PDF group and one patient in the B-PDF group. Bioimpedance measurements at 50 kHz were similar at the start of the study (695±87 Ω with L-PDF and 752±98 Ω with B-PDF) and did not change significantly in either treatment arm.
Discussion
This trial is the first clinical trial comparing two PD solutions differing only with respect to the buffer compound (i.e., lactate and bicarbonate) but not with respect to pH, electrolyte, or GDP composition (20,21). Whereas metabolic acidosis was equally well controlled with both PD solutions, better preservation of ultrafiltration capacity was observed with bicarbonate-based PD fluid. Thus, the choice of the buffer may have an impact on long-term peritoneal membrane function.
Peritoneal base fluxes differ substantially when bicarbonate- and lactate-based PD fluids are applied. Large amounts of bicarbonate are lost into the peritoneum, and high amounts of lactate are absorbed with lactate-based PDF. In contrast, with bicarbonate-based PDF, the loss of lactate into the dialysate is negligible. A previous randomized trial in children showed improved correction of metabolic acidosis with a bicarbonate PDF compared with a conventional, lactate-based fluid (24). Improved acid–base status was also observed when a lactate-based, low-GDP fluid was compared with a conventional, lactate-based PDF (6), suggesting that the GDP content and/ or the pH of the fluid, rather than the buffer type per se, determines acid–base balance. This notion is supported by the present study, with similar long-term control of acid–base status with the lactate- and bicarbonate-based low-GDP, pH-neutral PD fluid.
The observed differences in solute transport and ultrafiltration capacity emerging over time between low-GDP fluids only differing in buffer type are less straightforward to explain. Distinct effects of lactate and bicarbonate on peritoneal cells have been described in vitro. Lactate, particularly combined with a low pH, alters peritoneal mesothelial cell and leukocyte functions (14,15). Even when buffered at neutral pH, lactate still impairs human peritoneal mesothelial cell viability, reduces ATP content (16), suppresses mitochondrial activity (17), and stimulates release of the angiogenic cytokines vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (18). These cellular functions remain largely unaltered with bicarbonate. Superfusion of rat peritoneum with L-PDF caused a transient increase in arteriolar flow and capillary recruitment, whereas B-PDF did not affect the hemodynamic parameters (31). In rats, two time per day intraperitoneal injections of L-PDF over 5 weeks induced more peritoneal neoangiogenesis than B-PDF (19). These experimental findings suggest mechanisms for the observed differences in ultrafiltration capacity and creatinine and phosphate transport over time with B-PDF and L-PDF in our children. Interestingly, tumors generate considerable amounts of lactate from glycolysis and glutaminolysis, leading to marked induction of angiogenesis through VEGF/VEGFR2 (32). Lactate enters into endothelial cells through a monocarboxylate transporter, activates an autocrine NF-кB/IL-8 pathway, and drives endothelial cell migration and tube formation (33). In a variety of human cancers, lactate tissue levels correlate with outcome, with high levels inducing more neoangiogenesis and reflecting a poor prognosis (34–36). It is interesting to speculate that, in PD, the repeated exposure of the peritoneum to supraphysiological lactate concentrations might promote local neoangiogenesis and thereby, trigger ultrafiltration failure.
Incubation of peritoneal mesothelial cells with B-PDF increases, whereas L-PDF decreases aquaporin-1 abundance and aquaporin-1–dependent cell migration (37). Thus, B-PDF may promote the adaptive capacity of the mesothelial cell layer in vivo, this promotion, however, does not yet explain the observed differences in ultrafiltration capacity in our children.
Most of the clinical studies thus far were limited to the comparison of conventional with low-GDP PD fluids and various buffer compositions. Randomized cross-over comparison of L-PDF with C-PDF in 71 adult PD patients showed higher dialysis to plasma ratio creatinine ratios, lower ultrafiltration rates, and higher residual renal urine output with L-PDF; a subgroup analysis in 18 anuric patients, however, did not reconfirm the differences in peritoneal solute and water transport (26). The work by Raaijmakers et al. (38) showed a higher sodium-free water transport rate with a 25 mM bicarbonate and 10–15 mM lactate containing low-GDP solution compared with a conventional, purely lactate-based PDF. Although the difference in GDP content was assumed to be causative of the observed differences, an independent role of the buffer type and/or fluid pH could not be excluded. In a short-term cross-over comparison of two low-GDP solutions containing either 40 mM lactate buffered at acidic pH or 10 mM bicarbonate and 30 mM lactate at neutral pH, higher daily ultrafiltration was observed with the latter (27). A retrospective analysis of a large PD patient cohort suggests improved survival with the use of a low-GDP, pH-neutral, bicarbonate/ lactate-based PDF compared with PDF (12). Complementing these findings, our study provides the first direct evidence that the buffer type impacts on the long-term evolution of ultrafiltration capacity independent of pH and GDP content. We believe that our study design involving continuous monitoring of daily ultrafiltration over 10 months combined with repeated PET studies as well as the fact that the study was conducted in children, in whom peritoneal transport assessment is much less confounded by preexisting vascular (39) and peritoneal tissue pathology (40) than adult patients, constituted a highly sensitive approach for the detection of PD fluid-related changes in peritoneal membrane transport function. Thus, despite a relatively small number of patients studied, we were able to detect significant time-dependent differences of ultrafiltration capacity and small solute transport rates.
Our study has some limitations related to study design and sample size. Because repetitive peritoneal biopsies were not performed, we do not know whether the buffer-related effects on ultrafiltration capacity were associated with changes in peritoneal morphology, such as differences in capillary density. Likewise, we did not measure effluent surrogate parameters, such as VEGF, and because sodium sieving was not determined in the PET studies, we cannot draw any conclusions regarding a mechanistic role of peritoneal aquaporin-1 function. The study was not controlled for fluid intake. Clinical investigation, bioimpedance analyses, BP measurements, and daily urine output, however, did not indicate any systematic difference in hydration status between groups over time. The peritonitis rate observed during treatment with conventional single-chamber fluid was similar to the incidence observed in a previous pediatric trial (30) and lower during treatment with the biocompatible PD solutions in keeping with observations in some (41,42) but not all adult patients (11,12). However, the number of peritonitis episodes and observation times in this study was too small to draw firm conclusions.
In summary, our prospective comparison of two pH-neutral, low-GDP PD fluids shows equally effective control of metabolic acidosis with lactate and bicarbonate buffer. Buffer-dependent changes in peritoneal solute and water transport over time suggest better long-term preservation of peritoneal membrane function with bicarbonate compared with lactate-based low-GDP PD fluid.
Disclosures
None.
Acknowledgments
We thank Bärbel Philippin and Markus Zorn for excellent technical assistance. The investigator-initiated trial was supported financially by Fresenius Medical Care. C.P.S. and F.S. have received research grants and lecture honoraria from Fresenius Medical Care.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Jörres A, Bender TO, Finn A, Witowski J, Fröhlich S, Gahl GM, Frei U, Keck H, Passlick-Deetjen J: Biocompatibility and buffers: Effect of bicarbonate-buffered peritoneal dialysis fluids on peritoneal cell function. Kidney Int 54: 2184–2193, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Witowski J, Korybalska K, Ksiazek K, Wisniewska-Elnur J, Jörres A, Lage C, Schaub TP, Passlick-Deetjen J, Breborowicz A, Grzegorzewska A, Ksiazek A, Liberek T, Lichodziejewska-Niemierko M, Majdan M, Rutkowski B, Stompór T, Sulowicz W: Peritoneal dialysis with solutions low in glucose degradation products is associated with improved biocompatibility profile towards peritoneal mesothelial cells. Nephrol Dial Transplant 19: 917–924, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Hekking LH, Zareie M, Driesprong BA, Faict D, Welten AG, de Greeuw I, Schadee-Eestermans IL, Havenith CE, van den Born J, ter Wee PM, Beelen RH: Better preservation of peritoneal morphologic features and defense in rats after long-term exposure to a bicarbonate/lactate-buffered solution. J Am Soc Nephrol 12: 2775–2786, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Mortier S, Faict D, Lameire NH, De Vriese AS: Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat model. Kidney Int 67: 1559–1565, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Wieczorowska-Tobis K, Brelinska R, Witowski J, Passlick-Deetjen J, Schaub TP, Schilling H, Breborowicz A: Evidence for less irritation to the peritoneal membrane in rats dialyzed with solutions low in glucose degradation products. Perit Dial Int 24: 48–57, 2004 [PubMed] [Google Scholar]
- 6.Kim SG, Kim S, Hwang YH, Kim K, Oh JE, Chung W, Oh KH, Kim HJ, Ahn C, Korean Balnet Study Group : Could solutions low in glucose degradation products preserve residual renal function in incident peritoneal dialysis patients? A 1-year multicenter prospective randomized controlled trial (Balnet Study). Perit Dial Int 28[Suppl 3]: S117–S122, 2008 [PubMed] [Google Scholar]
- 7.Haag-Weber M, Krämer R, Haake R, Islam MS, Prischl F, Haug U, Nabut JL, Deppisch R, behalf of the DIUREST Study Group : Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: A prospective randomized study. Nephrol Dial Transplant 25: 2288–2296, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Choi HY, Kim DK, Lee TH, Moon SJ, Han SH, Lee JE, Kim BS, Park HC, Choi KH, Ha SK, Han DS, Lee HY: The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: An open randomized prospective trial. Perit Dial Int 28: 174–182, 2008 [PubMed] [Google Scholar]
- 9.Zeier M, Schwenger V, Deppisch R, Haug U, Weigel K, Bahner U, Wanner C, Schneider H, Henle T, Ritz E: Glucose degradation products in PD fluids: Do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int 63: 298–305, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Schmitt CP, von Heyl D, Rieger S, Arbeiter K, Bonzel KE, Fischbach M, Misselwitz J, Pieper AK, Schaefer F, for the Mid European Pediatric Peritoneal Dialysis Study Group (MEPPS) : Reduced systemic advanced glycation end products in children receiving peritoneal dialysis with low glucose degradation product content. Nephrol Dial Transplant 22: 2038–2044, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Lee HY, Choi HY, Park HC, Seo BJ, Do JY, Yun SR, Song HY, Kim YH, Kim YL, Kim DJ, Kim YS, Kim MJ, Shin SK: Changing prescribing practice in CAPD patients in Korea: Increased utilization of low GDP solutions improves patient outcome. Nephrol Dial Transplant 21: 2893–2899, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Han SH, Ahn SV, Yun JY, Tranaeus A, Han DS: Mortality and technique failure in peritoneal dialysis patients using advanced peritoneal dialysis solutions. Am J Kidney Dis 54: 711–720, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Müller-Krebs S, Kihm LP, Zeier B, Gross ML, Deppisch R, Wieslander A, Henle T, Penndorf I, Oh J, Reiser J, Nawroth PP, Zeier M, Schwenger V: Renal toxicity mediated by glucose degradation products in a rat model of advanced renal failure. Eur J Clin Invest 38: 296–305, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Topley N, Alobaidi HM, Davies M, Coles GA, Williams JD, Lloyd D: The effect of dialysate on peritoneal phagocyte oxidative metabolism. Kidney Int 34: 404–411, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Topley N, Kaur D, Petersen MM, Jörres A, Williams JD, Faict D, Holmes CJ: In vitro effects of bicarbonate and bicarbonate-lactate buffered peritoneal dialysis solutions on mesothelial and neutrophil function. J Am Soc Nephrol 7: 218–224, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Plum J, Razeghi P, Lordnejad RM, Perniok A, Fleisch M, Fusshöller A, Schneider M, Grabensee B: Peritoneal dialysis fluids with a physiologic pH based on either lactate or bicarbonate buffer-effects on human mesothelial cells. Am J Kidney Dis 38: 867–875, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Ogata S, Naito T, Yorioka N, Kiribayashi K, Kuratsune M, Kohno N: Effect of lactate and bicarbonate on human peritoneal mesothelial cells, fibroblasts and vascular endothelial cells, and the role of basic fibroblast growth factor. Nephrol Dial Transplant 19: 2831–2837, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ogata S, Mori M, Tatsukawa Y, Kiribayashi K, Yorioka N: Expression of vascular endothelial growth factor, fibroblast growth factor, and lactate dehydrogenase by human peritoneal mesothelial cells in solutions with lactate or bicarbonate or both. Adv Perit Dial 22: 37–40, 2006 [PubMed] [Google Scholar]
- 19.Albrektsson A, Bazargani F, Wieslander A, Braide M: Peritoneal dialysis fluid-induced angiogenesis in rat mesentery is increased by lactate in the presence or absence of glucose. ASAIO J 52: 276–281, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Frischmann M, Spitzer J, Fünfrocken M, Mittelmaier S, Deckert M, Fichert T, Pischetsrieder M: Development and validation of an HPLC method to quantify 3,4-dideoxyglucosone-3-ene in peritoneal dialysis fluids. Biomed Chromatogr 23: 843–851, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Erixon M, Wieslander A, Lindén T, Carlsson O, Forsbäck G, Svensson E, Jönsson JA, Kjellstrand P: How to avoid glucose degradation products in peritoneal dialysis fluids. Perit Dial Int 26: 490–497, 2006 [PubMed] [Google Scholar]
- 22.Tranaeus A, The Bicarbonate/Lactate Study Group : A long-term study of a bicarbonate/lactate-based peritoneal dialysis solution—clinical benefits. Perit Dial Int 20: 516–523, 2000 [PubMed] [Google Scholar]
- 23.Schmitt CP, Haraldsson B, Doetschmann R, Zimmering M, Greiner C, Böswald M, Klaus G, Passlick-Deetjen J, Schaefer F: Effects of pH-neutral, bicarbonate-buffered dialysis fluid on peritoneal transport kinetics in children. Kidney Int 61: 1527–1536, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Haas S, Schmitt CP, Arbeiter K, Bonzel KE, Fischbach M, John U, Pieper AK, Schaub TP, Passlick-Deetjen J, Mehls O, Schaefer F: Improved acidosis correction and recovery of mesothelial cell mass with neutral-pH bicarbonate dialysis solution among children undergoing automated peritoneal dialysis. J Am Soc Nephrol 14: 2632–2638, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Montenegro J, Saracho RM, Martínez IM, Muñoz RI, Ocharan JJ, Valladares E: Long-term clinical experience with pure bicarbonate peritoneal dialysis solutions. Perit Dial Int 26: 89–94, 2006 [PubMed] [Google Scholar]
- 26.Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, Passlick-Deetjen J, Euro Balance Trial Group : The Euro-Balance Trial: The effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int 66: 408–418, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Simonsen O, Sterner G, Carlsson O, Wieslander A, Rippe B: Improvement of peritoneal ultrafiltration with peritoneal dialysis solution buffered with bicarbonate/lactate mixture. Perit Dial Int 26: 353–359, 2006 [PubMed] [Google Scholar]
- 28.Fischbach M, Stefanidis CJ, Watson AR, European Paediatric Peritoneal Dialysis Working Group : Guidelines by an ad hoc European committee on adequacy of the paediatric peritoneal dialysis prescription. Nephrol Dial Transplant 17: 380–385, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Schaefer F, Langenbeck D, Heckert K-H, Schärer K, Mehls O: Evaluation of peritoneal solute transfer by the peritoneal equilibration test in children. Adv Perit Dial 8: 410–415, 1992 [PubMed] [Google Scholar]
- 30.Schaefer F, Klaus G, Müller-Wiefel DE, Mehls O, The Mid-European Pediatric Peritoneal Dialysis Study Group (MEPPS) : Intermittent versus continuous intraperitoneal glycopeptide/ceftazidime treatment in children with peritoneal dialysis-associated peritonitis. J Am Soc Nephrol 10: 136–145, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Mortier S, De Vriese AS, Van de Voorde J, Schaub TP, Passlick-Deetjen J, Lameire NH: Hemodynamic effects of peritoneal dialysis solutions on the rat peritoneal membrane: Role of acidity, buffer choice, glucose concentration, and glucose degradation products. J Am Soc Nephrol 13: 480–489, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Kumar VB, Viji RI, Kiran MS, Sudhakaran PR: Endothelial cell response to lactate: Implication of PAR modification of VEGF. J Cell Physiol 211: 477–485, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Végran F, Boidot R, Michiels C, Sonveaux P, Feron O: Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res 71: 2550–2560, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Walenta S, Mueller-Klieser WF: Lactate: Mirror and motor of tumor malignancy. Semin Radiat Oncol 14: 267–274, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, Mueller-Klieser W: Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 51: 349–353, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W: High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 60: 916–921, 2000 [PubMed] [Google Scholar]
- 37.Zhai Y, Bloch J, Hömme M, Schaefer J, Hackert T, Philippin B, Schwenger V, Schaefer F, Schmitt CP: Buffer-dependent regulation of aquaporin-1 expression and function in human peritoneal mesothelial cells. Pediatr Nephrol 27: 1165–1177, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Raaijmakers R, Coester A, Smit W, Krediet RT, Schröder CH: Free water transport in children on peritoneal dialysis is higher with more biocompatible dialysis solutions, higher with older age and declines with time. Nephrol Dial Transplant 27: 1183–1190, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Litwin M, Wühl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Tröger J, Mehls O, Schaefer F: Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 16: 1494–1500, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Schneble F, Bonzel KE, Waldherr R, Bachmann S, Roth H, Schärer K: Peritoneal morphology in children treated by continuous ambulatory peritoneal dialysis. Pediatr Nephrol 6: 542–546, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Montenegro J, Saracho R, Gallardo I, Martínez I, Muñoz R, Quintanilla N: Use of pure bicarbonate-buffered peritoneal dialysis fluid reduces the incidence of CAPD peritonitis. Nephrol Dial Transplant 22: 1703–1708, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Furkert J, Zeier M, Schwenger V: Effects of peritoneal dialysis solutions low in GDPs on peritonitis and exit-site infection rates. Perit Dial Int 28: 637–640, 2008 [PubMed] [Google Scholar]