Summary
Background and objectives
CKD and multiple pregnancies bear important risks for pregnancy outcomes. The aim of the study was to define the risk for adverse pregnancy-related outcomes in multiple pregnancies in CKD patients in comparison with a control group of “low-risk” multiple pregnancies.
Design, setting, participants, & measurements
The study was performed in the Maternal Hospital of the University of Turin, Italy. Of 314 pregnancies referred in CKD (2000–2011), 20 were multiple (15 twin deliveries). Control groups consisted of 379 low-risk multiple pregnancies (314 twin deliveries) and 19 (15 twin deliveries) cases with hypertension-collagen diseases. Baseline data and outcomes were compared by univariate and logistic regression analyses.
Results
The prevalence of multiple pregnancies was relatively high in the CKD population (6.4%); all referred cases were in early CKD stages (I-II); both creatinine (0.68 to 0.79 mg/dl; P=0.010) and proteinuria (0.81 to 3.42 g/d; P=0.041) significantly increased from referral to delivery. No significant difference in demographic data at baseline was found between cases and low-risk controls. CKD was associated with higher risk of adverse pregnancy outcomes versus low-risk twin pregnancies. Statistical significance was reached for preterm delivery (<34 weeks: 60% vs 26.4%; P=0.005; <32 weeks: 53.3% vs 12.7%; P<0.001), small for gestational age babies (28.6% vs 8.1%; P<0.001), need for Neonatal Intensive Care Unit (60% vs 12.7%; P<0.001), weight discordance between twins (40% vs 17.8%; P=0.032), and neonatal and perinatal mortality (6.6% vs 0.8%; P=0.032).
Conclusion
This study suggests that maternal-fetal risks are increased in multiple pregnancies in the early CKD stages.
Introduction
Since the time of Cosmas and Damian (the twin brothers personifying medicine and surgery), legends have attributed both magic and danger to twins, in health and disease. Thus, multiple pregnancies are known for their high risks and relative unpredictability.
In the last few decades, so-called high-risk pregnancies have become more frequent and increasingly recognized, mainly as a reflection of the advances in maternal-fetal care. In spite of the growing interest, our knowledge is still limited and little is known in particular about the combination of several risk factors. This is the case with multiple pregnancies in CKD patients. Except for a few patients in the context of larger series, only sporadic, exceptional cases have been reported, many of which are in the context of kidney transplantation (1–13). Most of the available cases reported successful outcomes, presumably because of a baseline bias, probably a joint effect of the comprehensible unwillingness to report patients with a poor outcome, and of publication bias (1–13).
Multiple pregnancies are a growing challenge for at least two reasons. First, the increase in maternal age bears a higher incidence of twin pregnancy, especially above the ages of 35 and 40 years. Second, the use of assisted reproductive technologies, frequently using multiple embryo transfer, has also increased (14,15).
Consequently, twin pregnancies have increased by >50% and other multiple pregnancies (i.e., triplets, quadruplets, etc.) by 400% in the Western world, and the prevalence of twins, triplets, and quadruplets has now reached 3% of all pregnancies (16). The incidence of multiple pregnancies rises with parity, is influenced by genetic factors, and differs among ethnic groups. For example, twins account for 1 in 1000 newborns in Japan and 50 in 1000 newborns in Nigeria (17,18).
Multiple pregnancies bear a three- to five-fold higher risk of most maternal-fetal complications, including pregnancy-induced hypertension, preeclampsia, anemia, gestational diabetes, urinary tract infections, and hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome (19–22). The risk for intrauterine death is at least four- to five-fold higher than that recorded for singletons, along with the risk of low weight, prematurity, and malformations (23–26). Dichorionic fraternal pregnancies account for about 65%–70% of twin pregnancies, whereas monochorionic or identical pregnancies account for the remainder (27). The latter have specific and higher risks, particularly if monoamniotic, and are often analyzed separately (17,27).
Pregnancy in CKD patients is a recognized challenge for pregnancy-related outcomes and possibly also for the progression of CKD; because prevalence has been calculated as high as 3% in women in childbearing age, the occurrence of both CKD and multiple pregnancy can be considered rare but not exceptional (expected in roughly 1 in 1000 pregnancies) (28–32). Because both multiple pregnancies and CKD are acknowledged risk factors for adverse pregnancy-related outcomes, their combination can be expected to represent an explosive mix, deserving particular attention in the case of preconception counseling for assisted reproduction in CKD patients.
This study aimed to evaluate the outcomes recorded in multiple pregnancies in CKD patients observed and followed in synergy in two multidisciplinary units, one dedicated to CKD patients and the other to multiple pregnancies.
Materials and Methods
Study Setting and Inclusion Criteria
This study was conducted in the Maternal-Fetal Medicine Unit of Sant’Anna University Hospital (150 beds for obstetric patients) in Turin, Italy (29). The activity of the unit is divided into different smaller units, including one dedicated to CKD and one to multiple pregnancies. In both units, main baseline and outcome data have been gathered prospectively from the start of the activity. In the CKD unit, the multidisciplinary team is composed of nephrologists and obstetricians, and the activity started in 2000. This is the first series of multiple pregnancies analyzed from the archive, encompassing 314 pregnancies (20 multiple), with 233 singleton deliveries and 17 multiple deliveries (15 twins and 2 triplets) as of December 31, 2011.
Multiple pregnancies with maternal or fetal risks have been routinely followed by the Maternal-Fetal Unit since 1990. The outpatient unit dedicated to multiple pregnancies started regular activity in 2004. The multidisciplinary team consists of obstetricians, psychologists, and neonatologists. As of June 2011, 649 multiple pregnancies were on file.
A maternal disease or an obstetric risk factor was recorded in 270 patients, whereas the remaining 379 were low risk. Women were classified as “healthy women with low-risk pregnancies” if they were not known to have any of the medical or obstetric risk factors listed in the National Institute for Clinical Excellence intrapartum care guideline before the onset of labor (33,34). In the 270 patients with high-risk pregnancies, we recorded 6 therapeutic terminations, 29 spontaneous abortions, 29 triplets, and 17 ongoing pregnancies. Thus, 189 twin deliveries with risk factors were selected. Fifteen of the latter patients were affected by CKD and 15 patients were affected by conditions that may be considered at risk for CKD, including hypertension and collagen disease. These patients were selected as a second control group.
The remaining 159 twin deliveries were sorted into patients affected by obstetric risk factors (26.4%) and maternal risk factors (73.6%). In the latter subset, thyroid diseases accounted for 25.6%, thromboembolic risk for 10.25%, and infectious diseases and cardiovascular problems for 9.4% each, whereas psychiatric diseases, neurologic diseases, and obesity (without hypertension) accounted for 5.1%, with the rest being miscellaneous conditions.
In the low-risk group, there were 21 miscarriages, 35 triplet pregnancies, 3 quadruplet pregnancies, 6 pregnancy terminations, and 314 twin deliveries, which served as controls. To control for attrition biases, we tested a subset of multiple pregnancies referred before the 12th gestational week. However, because no significant difference was recorded between early and late referrals, the comparison took into account the overall noncomplicated twin population (35–38).
Definitions Utilized
Patients with CKD were stratified according to Kidney Disease Outcomes Quality Initiative guidelines (36,37). GFR calculation was based on preconception data and, in their absence, on data at the first routine visit. The Cockcroft and Gault formula was chosen because of the adjustment for weight, partially accounting for underweight or obesity, both of which were represented in our population. Modified Diet in Renal Disease and Chronic Kidney Disease Epidemiology Collaboration formulae were also applied, controlling for patients that would have been classified otherwise. After referral, creatinine clearance on 24-hour urine collection was considered as approximating GFR; proteinuria was assessed on 24-hour urine collection (29).
Hypertension was defined as systolic BP ≥140 and/or diastolic BP ≥90, or use of antihypertensive therapy (even if present before conception) (29).
Preeclampsia was strictly defined as hypertension accompanied by proteinuria ≥300 mg/24 h after 20 weeks of gestational age in a previously normotensive, nonproteinuric woman, in the absence of other signs or symptoms indicating a different nephrologic diagnosis (39,40). As the definition of superimposed preeclampsia is not unequivocal, we did not include it in this study (40).
A newborn was defined as small for gestational age (SGA) when the birth weight, adjusted for gestational age, was below the 10th percentile according to Italian birth weight references (32,41). Preterm delivery was defined as delivery before 37 completed weeks of gestational age, and early delivery was delivery before 34 completed weeks (29,31,32). Twins were categorized as having a weight discordance when their estimated weight difference exceeded 20% (42,43).
Prenatal and Intrapartum Care
CKD patients with multiple pregnancies are usually followed on alternate months in the CKD and multiple pregnancy units, which work in close contact. One important point in multiple pregnancies is the early definition of chorionicity (within the 12th week) as the basis for counseling on specific risks of the multiple pregnancies. Thereafter, the patients follow the basic controls and visits for multiple pregnancies, combined with those of CKD, as described elsewhere in detail (29–32,35).
Fetal ultrasounds and cardiotocography are performed according to chorionicity (35). Delivery is planned, according to the clinical status, between 37 and 38 gestational weeks in dichorionic pregnancies and between 35 and 36 weeks in monochorionic diamniotic pregnancies. Monochorionic monoamniotic and triplet pregnancies are an indication for caesarean delivery and follow specific protocols; because no difference was found in our previous series between spontaneous and assisted pregnancies, they were analyzed together (35).
Hospitalization is required in the presence of poorly controlled hypertension, worsening of renal function, proteinuria of new onset or with rapid worsening, and for any potentially severe problem of mother and/or fetus (35). Indications for early delivery are severe worsening of maternal conditions until 32 weeks, including severe preeclampsia or HELLP syndrome, poorly controlled hypertension, rapidly increasing nephrotic proteinuria, or rapid increase in serum creatinine, alone or in combination. Fetal worsening includes abnormal fetal heart rate tracings at any gestational age, absent end diastolic flow velocities at Doppler study of the umbilical arteries at or after 32 weeks of gestational age, or no fetal growth over 2 weeks at later gestational ages. The main indications for admission to the neonatal intensive care unit (NICU) are birth weight <1500 g, gestational age <34 weeks, Apgar score <7 at 5′, or need for intubation (29,35).
Statistical Analyses
Both units keep a dedicated database; in both, the start of observation is referral to the unit, whereas the end of observation is at the postpartum visit, 1 month after delivery. The databases were merged at the 2011 updating.
Common parameters were analyzed in CKD patients in comparison with the control group of low-risk multiple pregnancies; analyses were limited to twins and were performed separately for all patients and for dichorionic pregnancies. Stratification included referral to the unit before or after 12 weeks of gestational age, maternal age, parity, nationality (Italian versus other), CKD stage, proteinuria at referral, and spontaneous or induced pregnancy.
The main outcomes analyzed were caesarean section, prematurity (cutpoints: 37, 34, and 32 weeks), gestational week at delivery, weight, weight discordance >20%, prevalence of SGA, and need for hospitalization in the NICU.
Descriptive analyses were performed as appropriate (mean ± SD for parametric and median and range for nonparametric data). The chi-squared test, Fisher’s test, Kruskal–Wallis test, Mann–Whitney U test, ANOVA, and t test with Bonferroni correction were used for comparisons between patients and controls and among groups. Significance was set at <0.05. Multivariate logistic regression analysis was used to control for simultaneous effects of covariates. Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) were derived from the estimated regression coefficients. Statistical evaluation was performed using SPSS software for Windows (version 18.0; SPSS Chicago, IL).
Results
Overall Data
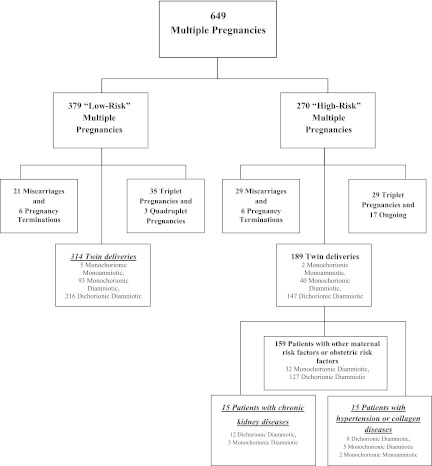
The flowchart of the study population is shown in Figure 1. In the study period (January 2000–December 2011), 314 pregnancies were observed in CKD patients; the overall prevalence of multiple pregnancies (6%) was high compared with the 3% prevalence reported in the overall European population.
Figure 1.
Flowchart of the study population.
Table 1 reports the main clinical characteristics of the 20 pregnancies referred to our unit. Of note, all patients were in the early CKD stages; however, counseling for interruption of multiple pregnancy in the context of more advanced CKD had not been performed in either unit. The diagnoses are different and encompass all the main causes of CKD in young patients. In three patients, diagnosis was made in pregnancy. In an additional seven patients, a previous diagnosis of CKD had been overlooked with respect to pregnancy. One patient was referred in the differential diagnosis between preeclampsia and glomerular disease (Table 1). No patient changed stage before delivery; a trend toward increasing proteinuria was observed particularly in patients already proteinuric at baseline (Table 1). Six patients underwent assisted fertilization for reasons such as endometriosis, reduced ovarian reserve, oligo-asthenospermia, and “idiopathic” causes.
Table 1.
Characteristics of the multiple pregnancies recorded in CKD patients (ordered according to referral)
| Case | Age | Kidney Disease | Week of Referral (Neph.) Week of Delivery | Chorionicity | GFR, (mL/min) Stage at Referral) | sCr at Referral and Delivery (mg/dL) | HT (Y-N) at Referral and Delivery | uPt (g/day) at Referral and Delivery | Sex and Weight | Problems SGA; Weight Discordance; NICU; Death |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | Membranous GNa | 22–27 | Dichorionic Diamniotic | 150.2 Stage 1 | 0.43 0.77 | N | 5.30; 21.90 | F 725 F 505 | Weight discordance, NICU for bothb |
| 2 | 34 | IgA GNc | 26–29 | Dichorionic Diamniotic | 103.6 Stage 1 | 0.70 0.85 | N–Y | 0.20; 12.00 | M 1330 M 700 | 1 SGA, weight discordance, NICU for both |
| 3 | 32 | HT urinary anomalies | 26–32 | Dichorionic Diamnioticd | 132.6 Stage 1 | 0.50 0.97 | Y | 0.15; 9.48 | F 2000 F 1085 | 1 SGA, weight discordance, NICU for both |
| 4 | 42 | PKD | 6–32 | Dichorionic Diamniotic | 88.5 Stage 2 | 1.11 1.10 | Y | 0.14; 0.47 | M 1380 F 1875 | 1 SGA, weight discordance, NICU for both |
| 5 | 30 | Urinary tract infections | 14–36 | Dichorionic Diamniotic | 103.1 Stage 1 | 0.68 0.63 | N | 0.00; 0.10 | M 2140 M 1950 | 1 SGA |
| 6 | 34 | Renal hypoplasia | 25–32 | Dichorionic Diamnioticd | 85.0 Stage 2 | 0.78 1.08 | N–Y | 1.48; 1.15 | M 1550 F 840 | 1 SGA, weight discordance, NICU for both |
| 7 | 35 | Urinary dilatation | 26–34 | Trichorionic Triamnioticd | 112.6 Stage 1 | 0.55 0.43 | N | 0.20; 0.35 | M 2420 M 1930 M 2050 | NICU for all |
| 8 | 34 | Single kidney | 32–32 | Monochorionic Diamniotic | 142.7 Stage 1 | 0.71 0.71 | N | 0.23; 0.23 | M 1950 M 1895 | NICU for both |
| 9 | 25 | Previous APN (scars) | 19–36 | Monochorionic Diamniotic | 140.4 Stage 1 | 0.58 0.56 | N | 0.00; 0.30 | M 2015 M 2540 | Weight discordance |
| 10 | 33 | Urinary dilatation | 25–34 | Dichorionic Diamnioticd | 148.7 Stage 1 | 0.45 0.66 | N–Y | 0.00; 0.10 | F 1910 M 1960 | None |
| 11 | 35 | Kidney stones | 18–37 | Dichorionic Diamniotic | 109.2 Stage 1 | 0.59 0.63 | N | 0.20; 0.10 | F 2470 F 2630 | None |
| 12 | 45 | Sponge kidney | 19–33 | Dichorionic Triamnioticd | 171.8 Stage 1 | 0.62 0.62 | Y | 0.38; 0.41 | M 1305 F 1540 | 1 miscarriage at 17 weeks, 1 SGA, NICU for both |
| 13 | 32 | Nephro-calcinosis | 33–36 | Dichorionic Diamniotic§ | 117.6 Stage 1 | 0.65 0.90 | Y | 0.10; 0.10 | M 2660 F2440 | None |
| 14 | 41 | Kidney stones | 22–36 | Dichorionic Diamniotic | 152.8 Stage 1 | 0.52 0.52 | N | 0.30; 0.30 | F 2200 F 2250 | None |
| 15 | 31 | PKD | 16–36 | Monochorionic Diamniotic | 134.3 Stage 1 | 0.45 0.52 | N | 0.00; 0.20 | M 2190 M 2410 | NICU for both |
| 16 | 32 | Diabetic nephropathy | 21–32 | Dichorionic Diamniotic | 122.6 Stage 1 | 0.52 0.65 | N–Y | 5.42; 10.00 | M 1270 F 1275 | 1 SGA, NICU for both, death of male twin |
| 17 | 36 | Kidney transplant | 5 | Dichorionic Diamniotic | 47.2 Stage 3 | 1.30 1.20 | N | 0.37; 0.40 | - | Miscarriage at 8 weeks |
| 18 | 32 | Familial nephropathy | 20–32 | Dichorionic Diamniotic | 69.8 Stage 2 | 1.06 1.39 | Y | 0.18; 4.00 | M 1200 F 1000 | 2 SGA, NICU for both, death of male twin |
| 19 | 28 | GN or PEe | 24–ongoing | Monochorionic Diamniotic | 119 Stage 1 | Ongoing | Y | 1.52; | -- | Ongoing |
| 20 | 38 | PKD | 19–ongoing | Dichorionic Diamniotic | 92.7 Stage 1 | Ongoing | N | 0.10; | -- | Ongoing |
GN, glomerulonephritis; PKD, polycystic kidney disease; PE, pre-eclampsia; GFR, glomerular filtration rate; HT, hypertension; uPt, 24 hours proteinuria: APN, acute pyelonephritis; Spont, spontaneous pregnancy; SGA, small for gestational age; NICU, Neonatal Intensive Care Unit; Weight discordance, at least 20% weight discrepancy; Neph, Nephrology Unit.
Biopsy after delivery.
SGA not assessed (delivery before the 28th week).
Previously classified as acute GN.
Assisted fertilization.
Referred in differential diagnosis with PE.
Table 2 reports a baseline comparison between multiple pregnancies in CKD, in low-risk pregnancies and in a subset of patients at risk for CKD due to hypertension or collagen disease. To note, there were no significant differences in baseline characteristics in CKD patients and controls, except for the week of referral, occurring later in the unit dedicated to kidney diseases in pregnancy. The referral week was similar for patients with and without CKD in the outpatient unit dedicated to twins; as a rule, the patients with CKD were referred earlier to the twins unit (median week of referral, 13 weeks; range, 8–24) than to the CKD unit (median week of referral, 22 weeks; range, 6–33). CKD was discovered in pregnancy in 10 of 20 patients; the two units work together and the patients are referred to the CKD unit after having performed blood and urine tests and kidney ultrasounds, hence the delay between first suspicion of CKD, diagnosis, and referral (Table 1). The prevalence of assisted fertilization was similar in all subsets (35) (Table 3). A baseline comparison between CKD and controls, limited to dichorionic diamniotic pregnancies, is also reported in Table 2. In spite of the lower number of patients, the comparisons led to similar results.
Table 2.
Main baseline characteristics of low-risk controls, patients with either chronic hypertension or collagen disease, and CKD patients
| Low Risk (n=314) | Chronic HTN or CD (n=15) | CKD (n=15) | |
|---|---|---|---|
| Age (yr) | 32.2±5.33 | 33.5±7.67 | 32.5±3.64 |
| BMI (kg/m2) | 22.7±3.90 | 28.3±8.48 | |
| Nulliparous | 188 (59.9) | 8 (53.3) | |
| Italian | 214 (68.2) | 13 (86.7) | |
| Gestational week at first control | 14 (6–34) | 12 (9–29) | Twins unit: 13 (8–24); |
| CKD unit: 22 (6–33) | |||
| Monochorionic diamniotic | 93 (29.6) | 5 (33.3) | |
| Spontaneous pregnancies | 240 (76.4) | 13 (86.7) |
Data are presented as mean ± SD, n (%), or median (range). Twin pregnancies only; triplets are excluded. All twin pregnancies: 10 patients with hypertension, 5 with collagen disease; dichorionic diamniotic: 6 patients with hypertension, 2 with collagen disease. HTN, hypertension; CD, collagen disease; BMI, body mass index.
P values were calculated by ANOVA and t test with Bonferroni correction, chi-squared test, Fisher’s test, Kruskal–Wallis test, Mann–Whitney U test. Statistical significance: P1, low-risk controls versus CKD patients; P2, low-risk controls versus patients with hypertension and collagen disease; P3, patients with hypertension and collagen disease versus CKD patients.
BMI: P2<0.05, P3<0.05; gestational week at first control: P1<0.01, P3<0.01.
BMI: P2<0.05, P3<0.05; gestational week at first control: P1<0.05.
Table 3.
Main outcomes recorded in twin deliveries in low-risk controls, patients with either chronic hypertension or collagen disease, and CKD patients
| All Twin Deliveries | Only Dichorionic Diamniotic | |||||||
|---|---|---|---|---|---|---|---|---|
| Low Risk (n=314) | Chronic HTN or CD (n=15) | CKD (n=15) | P Valuea | Low Risk (n=216) | Chronic HTN or CD (n=8) | CKD (n=12) | P Valuea | |
| Caesarean section | 236 (75.2) | 13 (86.7) | 15 (100) | 0.05 | 164 (75.9) | 8 (100) | 12 (100) | 0.05 |
| Mean gestational weeks | 35.1±2.29 | 33.7±1.75 | 33.3±2.91 | 0.001b | 35.2±2.40 | 33.5±1.92 | 32.9±3.02 | 0.001c |
| Preterm delivery ≤37 wk | 302 (96.2) | 15 (100) | 15 (100) | 0.55 | 205 (94.9) | 8 (100) | 12 (100) | 0.58 |
| Preterm delivery ≤34 wk | 83 (26.4) | 9 (60) | 9 (60) | <0.001b | 57 (26.4) | 5 (62.5) | 8 (66.7) | 0.001c |
| Preterm delivery ≤32 wk | 40 (12.7) | 4 (26.7) | 8 (53.3) | <0.001b | 29 (13.4) | 3 (37.5) | 7 (58.3) | <0.001c |
| Mean birth weight (g) | 2247.2±495.9 | 1994.5±375.7 | 1744.8±627.5 | <0.001b | 2271.1±500.59 | 2050.3±412.1 | 1639.4±650.86 | <0.001c |
| Weight discordance >20% | 56 (17.8) | None | 6 (40) | 0.01b | 40 (18.5) | None | 5 (41.7) | 0.05 |
| SGA | 51/626d (8.1) | None | 8/28 (28.6)b | <0.001b | 32/430 (7.4)e | None | 8/22 (36.4)b | <0.001c |
| Need for NICU | 80/628 (12.7) | 10/30 (33.3) | 18/30 (60) | <0.001b | 56/432 (13) | 4/16 (25) | 14/24 (58.3) | <0.001c |
| Neonatal and perinatal mortality | 5/628 (0.8) | None | 1/30 (3.3) | 0.30 | 4/432 (0.9) | None | 1/24 (4.2) | 0.29 |
Data are presented as mean ± SD, n (%), or median (range). Twin pregnancies only; triplets are excluded. HTN, hypertension; CD, collagen disease; SGA, small for gestational age; NICU, neonatal intensive care unit.
P values were calculated by ANOVA and t test with Bonferroni correction, chi-squared test, Fisher’s test, Kruskal–Wallis test, Mann–Whitney U test. Statistical significance: P1, low-risk controls versus CKD patients; P2, low-risk controls versus patients with hypertension and collagen disease; P3, patients with hypertension and collagen disease versus CKD patients.
Gestational week: P1<0.05; preterm delivery ≤34 weeks: P1<0.05, P2<0.05; preterm delivery ≤32 weeks: P1<0.001; birth weight: P1<0.05, P2<0.05; weight discordance >20%: P1<0.05, P3<0.05; SGA: P1<0.05, P3<0.01; need for NICU: P1<0.001, P2<0.005.
Gestational week: P1<0.05; preterm delivery ≤34 weeks: P1<0.01; preterm delivery ≤32 weeks: P1<0.001; birth weight: P1<0.05, P3<0.05; SGA: P1<0.001, P3<0.05; meed for NICU: P1<0.001.
Not evaluated for patient 1 (delivery at 27th week).
One missing datum in controls.
Outcome Analyses
Table 3 reports the main outcomes of twin pregnancies in CKD patients versus low-risk controls and versus patients with hypertension or collagen disease. The analysis was performed considering all twin deliveries and limiting the comparison to dichorionic diamniotic twins. In both cases, the outcomes were worse in CKD patients. The higher incidences of caesarean sections and of overall preterm deliveries (<37 weeks) did not reach statistical significance over a high baseline level; however, a significant difference was observed for all other major outcomes (birth weight, SGA, weight discordance, early preterm delivery, need for NICU, risk of death).
The data recorded in the small group of patients with maternal diseases at risk for CKD (hypertension and collagen disease) suggested an intermediate risk pattern, although statistical significance was not reached, perhaps due to the small number of patients analyzed (Table 3).
Two perinatal deaths were recorded in the CKD group: a male twin from a primiparous diabetic mother and a male twin from a tertiparous mother with a not-yet-defined familial nephropathy. The causes of death were cerebral hemorrhage after correction of great vessel transposition in patient 16 and infectious complication in patient 18; both children were preterm. Five deaths were recorded in the control group, and they were due to sepsis, respiratory failure, and multiple malformations.
By definition, no patient with essential preexisting hypertension was present in the low-risk group at baseline; during pregnancy, 19 of 314 patients developed hypertension (6.05%) and 17 of 314 developed preeclampsia (5.41%). HELLP syndrome occurred in 5 of 314 patients (1.59%). In the subset with hypertension or collagen disease, 1 of 5 normotensive women developed hypertension, whereas proteinuria developed in 7 of 10 patients with chronic hypertension.
All of the CKD patients who were hypertensive at referral (considering the first visit to either the twins unit or the CKD unit) remained hypertensive throughout pregnancy; hypertension developed in four of nine previously normotensive patients who delivered (44.44%).
Regarding kidney function, significant increases of both serum creatinine and proteinuria were observed in this small series of patients observed in the early CKD stages. Serum creatinine increased from 0.68 to 0.79 (P=0.01); although significant, the change was probably of scarce clinical significance. Conversely, 24-hour proteinuria showed a significant increase from 0.81 to 3.42 (P=0.04), in spite of very dispersed data (Table 1).
The distinction between preeclampsia and other causes of proteinuria or kidney disease may be impossible if additional data are not available. For example, patient 2 developed microhematuria, with red cell casts in the urine, typical of IgA nephropathy and not typical of preeclampsia.
Multivariate Analyses
A logistic regression analysis was carried out for the three outcomes that were significant in the univariate analysis and had the greatest clinical relevance: “very early” preterm delivery (<32 completed gestational weeks), SGA infant, and need for NICU. In spite of the relatively low number of patients in the CKD cohort, the differences were confirmed as statistically significant, with ORs ranging from 4.35 (95% CI, 1.79–10.53) for SGA to 9.88 for NICU (95% CI, 4.54–21.49) (Table 4).
Table 4.
Results of the logistic regression analysis
| OR (95% CI) | P Value | |
|---|---|---|
| Delivery before 32 completed gestational weeks | ||
| CKD | 7.50 (2.53, 22.27) | <0.001 |
| Age >30 yr | 0.78 (0.38, 1.59) | 0.49 |
| Parity >1 | 0.58 (0.28, 1.19) | 0.14 |
| Non-Italian | 1.05 (0.50, 2.20) | 0.89 |
| SGA | ||
| CKD | 4.35 (1.79, 10.53) | <0.001 |
| Age >30 yr | 1.02 (0.54, 1.95) | 0.92 |
| Parity >1 | 0.90 (0.50, 1.61) | 0.72 |
| Non-Italian | 0.93 (0.48, 1.77) | 0.82 |
| Need for NICU | ||
| CKD | 9.88 (4.54, 21.49) | <0.001 |
| Age >30 yr | 0.97 (0.58, 1.64) | 0.93 |
| Parity >1 | 0.93 (0.58, 1.50) | 0.78 |
| Non-Italian | 0.76 (0.44, 1.32) | 0.34 |
OR, odds ratio; CI, confidence interval; SGA, small for gestational age; NICU, neonatal intensive care unit.
Discussion
This study is the first to combine two high-risk situations, multiple pregnancy and CKD, with the aim of characterizing the risks as a guide for counseling and as help in the decision-making process in CKD patients who choose to undergo assisted fertilization techniques. Both situations are present in about 3% of pregnancies; with the continuous rise of multiple pregnancies and the higher awareness of the importance of early stages of CKD in young patients, this rare situation will presumably be encountered more frequently in the future (14–16,44). In our study, the prevalence of multiple pregnancies was higher (6%) compared with the European population; the particular attention to clinical problems in twin pregnancies may have played a role in enhancing referral.
Our study, conducted in the context of scarce information and a series of generally positive case reports, demonstrates a significant additional risk for adverse outcomes in multiple pregnancies in CKD patients (1–13). On a baseline of relatively high morbidity and mortality typical of multiple pregnancies, the availability of a large control group allowed comparison with low-risk twin pregnancies, performed by considering all twin pregnancies together or by limiting the analysis to the more common subset of dichorionic dizygotic twins (Table 2) (33,34). The cohort of at-risk multiple pregnancies was nonhomogeneous, combining both maternal and obstetric risks of different severity; from this large subset, a second, smaller control group was identified in 15 patients affected by diseases at risk for CKD (chronic hypertension or collagen disease) (Table 2).
The three groups were homogeneous for all baseline parameters, with the exception of week of referral (reflecting the usual referral from the multiple pregnancy unit to the nephrology unit) and of a high prevalence of patients in which CKD was either diagnosed or taken into consideration in pregnancy (10 of 20 patients) (Tables 1 and 2).
In this series of multiple pregnancies in CKD, all patients were in the early CKD stages (Table 1). None of the patients changed CKD stage and the small but significant increase in serum creatinine (0.68 at referral, 0.79 at delivery) was probably devoid of clinical significance, at least in the medium term. Nevertheless, the increase in proteinuria (from 0.80 to the nephrotic range) underlines the importance of closely monitoring this parameter over follow-up, also after pregnancy.
The presence of CKD, regardless of the normal renal function, significantly increased all of the major morbidity indexes: early and very early preterm delivery, birth weight, prevalence of SGA infants and need for NICU, an indirect marker of the severity of the tested morbidity parameters. Interestingly, the data recorded in the small group of patients with hypertension or collagen disease suggest that patients at risk for CKD may have an intermediate pattern (Table 3). Within the limits of small numbers (not allowing statistical significance), this observation suggests that the unfavorable outcomes observed in CKD patients are not merely due to hypertension or immune deregulation.
Our case series suggests an increase of perinatal mortality in multiple pregnancies over and above the relatively high background typical of twin pregnancies (Table 3) (14,18,21). Once more within the limits of relatively small numbers, the multivariate analysis comparing CKD patients and low-risk controls allowed quantification of the ORs, which were 4.35 for SGA, 7.50 for delivery before 32 completed gestational weeks, and 9.88 for the need for NICU (Table 4).
The risks for adverse pregnancy outcomes are remarkably different in singletons and twins with and without CKD (29,31,32). However, our previous data in stage 1 singletons indicated an OR of 8.99 for early preterm delivery, similar to the OR of 7.5 for delivery before 32 weeks presently found in twins, and an OR of 15.17 for need for NICU, corresponding to an OR of 9.88 in twins (Table 4) (29).
The early rise of the risk for adverse pregnancy-related events from the early stages of CKD is mostly unexplained, like the increased risk of cardiovascular events from the early stages of CKD. The relationship between kidney and placenta is being clarified in typical preeclampsia, in which placental ischemia leads to widespread activation/dysfunction of the maternal vascular endothelium via oxidative stress and an imbalance between proangiogenic and antiangiogenic factors (38–40,45–48). In our population, we can postulate that the presence of even mild renal damage may lower the threshold needed for evident endothelial kidney damage in pregnancy and, conversely, that factors of renal origin, thus far unknown, may affect the placental circulation, particularly in the later stages of pregnancy. The “larger” placentas of multiple pregnancies could enhance these pathologic responses.
Our data have the limitations shared by all studies dealing with a small sample size; however, this case series of 20 CKD patients with multiple pregnancies is the largest one available in the literature thus far. The importance of our study is enhanced by the availability of a control group uniformly followed in the same setting. Twin pregnancy outcomes are highly dependent on the policies and settings of care; thus, our study has the strength of a large control group allowing the expression of the data in terms of ORs, against a background of noncomplicated multiple pregnancies (Tables 3 and 4). Moreover, our data, obtained in a setting dedicated to the care of high-risk pregnancies (both for maternal diseases such as CKD and for obstetric reasons such as multiple pregnancies), are the result of a combination of prenatal multidisciplinary care and baseline conditions/diseases. An effect of referral of the most complicated patients to tertiary care centers may have played a role in increasing overall comorbidity and possibly in affecting outcomes. A regional database for high-risk or multiple pregnancies is needed to highlight this important point.
On these bases, we suggest that counseling for women with CKD and a multiple pregnancy should underline the higher risks of multiple pregnancies in CKD while admitting that there is still not enough experience to precisely quantify such risks. It should also be underlined that the choice of a tertiary care center, more capable of dealing with uncommon problems and complications, is probably the most pragmatic approach for maternal-fetal care. Assisted fertilization in CKD patients raises several issues on the borderline between clinical and ethical aspects, which are well beyond the scope of this analysis. They regard the often difficult balance between the odds for success with multiple implantation and the high risks linked to multiple pregnancies in CKD patients. Our data may indeed suggest that a policy of single embryo transfer in CKD patients is safer with regard to maternal-fetal outcomes. To note, in our small series, three of six patients who underwent assisted fertilization were diagnosed with CKD during pregnancy, suggesting that particular attention should be given to kidney diseases in the preliminary evaluation for assisted fertilization techniques, including renal ultrasounds and an evaluation of GFR, proteinuria, and microscopic urinalysis in the work-up.
Further systematic long-term studies and possibly multicenter studies are needed to better define the risks; earlier referral must be pursued in order to increase the diagnostic accuracy and improve therapeutic interventions. The availability of a long-term follow-up of the children will greatly help in overall counseling of CKD patients.
This study, the first aimed at assessing the risks of multiple pregnancies in CKD patients, underlines the importance of further investigation of the complex interactions between CKD and pregnancy and suggests that particular attention should be given to this potentially explosive combination.
Disclosures
None.
Acknowledgments
We thank Dr. Peter Christie for his careful language revision.
This study was approved by the Ethics Committee of the Maternal-Fetal Hospital of the University of Turin (n 335, protocol 11551/c28.2, 4/3/2011).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.MacLean AB, Sharp F, Briggs JD, MacPherson SG: Successful triplet pregnancy following renal transplantation. Scott Med J 25: 320–322, 1980 [DOI] [PubMed] [Google Scholar]
- 2.Boner G, Bott-Kanner G, Schweitzer A, Danon YL, Rosenfeld JB: Successful multiple pregnancy in renal transplant recipient. Int J Gynaecol Obstet 19: 251–254, 1981 [DOI] [PubMed] [Google Scholar]
- 3.Pavlović NB, Kostić S, Cvetković A, Stefanović V: Successful twin pregnancy in a renal transplant patient. Przegl Lek 42: 403–405, 1985 [PubMed] [Google Scholar]
- 4.Grunebaum AN, Minkoff H: Twin gestation and perinatal follow-up in a woman with severe chronic renal failure managed without dialysis. A case report. J Reprod Med 32: 463–465, 1987 [PubMed] [Google Scholar]
- 5.Burrows DA, O’Neil TJ, Sorrells TL: Successful twin pregnancy after renal transplant maintained on cyclosporine A immunosuppression. Obstet Gynecol 72: 459–461, 1988 [PubMed] [Google Scholar]
- 6.Prieto C, Errasti P, Olaizola JI, Morales JM, Andreś A, Medina C, Ortuño B, Purroy A, Rodicio JL: Successful twin pregnancies in renal transplant recipients taking cyclosporine. Transplantation 48: 1065–1067, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Biesenbach G, Stöger H, Zazgornik J: Successful pregnancy of twins in a renal transplant patient with Wegener’s granulomatosis. Nephrol Dial Transplant 6: 139–140, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Jimenez E, Gonzalez-Caraballo Z, Morales-Otero L, Santiago-Delpin EA: Triplets born to a kidney transplant recipient. Transplantation 59: 435–436, 1995 [PubMed] [Google Scholar]
- 9.Furman B, Wiznitzer A, Hackmon R, Gohar J, Mazor M: Multiple pregnancies in women after renal transplantation. Case report that rises a management dilemma. Eur J Obstet Gynecol Reprod Biol 84: 107–110, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Yoo J, Unnikrishnan D, Lwin LN, Villanueva HJ, Tannenberg AM: Successful triplet pregnancy in a patient on chronic haemodialysis. Nephrol Dial Transplant 19: 994–997, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Loeffler CL, Macri CJ, Bathgate SL, Freese L, Larsen JW: Autosomal dominant polycystic kidney disease in pregnancy complicated by twin gestation and severe preeclampsia: A case report. J Reprod Med 50: 370–372, 2005 [PubMed] [Google Scholar]
- 12.Skhiri H, Guedri Y, Achour A, Sabra A, Hadj YD, Bouraoui S, Frih A, Ben Dhia N, Sakkouhi M, Gahbiche M, Saad H, El May M: [Twin pregnancy after renal transplantation: First case reported in Tunisia]. Tunis Med 83: 240–242, 2005 [PubMed] [Google Scholar]
- 13.Nicovani V, Poblete H, Toro J, Carrera M, Pérez L: Successful multiple pregnancy (triplets) in a kidney transplant recipient: A case report. Transplant Proc 41: 2688–2690, 2009 [DOI] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists: Special Problem of Multiple Gestations. Educational Bulletin No. 253, Washington, DC, ACOG, 1998 [Google Scholar]
- 15.Norwitz ER, Edusa V, Park JS: Maternal physiology and complications of multiple pregnancy. Semin Perinatol 29: 338–348, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Martin JA, Park MM: Trends in twin and triplet births; 1980-97. Natl Vital Stat Rep 47: 1–16, 1999 [PubMed] [Google Scholar]
- 17.Hartley RS, Hitti J, Emanuel I: Size-discordant twin pairs have higher perinatal mortality rates than nondiscordant pairs. Am J Obstet Gynecol 187: 1173–1178, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Rao A, Sairam S, Shehata H: Obstetric complications of twin pregnancies. Best Pract Res Clin Obstet Gynaecol 18: 557–576, 2004 [DOI] [PubMed] [Google Scholar]
- 19.American College of Obstetricians and Gynecologists: Multiple Gestations: Complicated Twin, Triplet and High-Order Multifetal Pregnancy. Practice Bulletin, Vol. 104, No. 56, Washington, DC, ACOG, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Buhling KJ, Henrich W, Starr E, Lubke M, Bertram S, Siebert G, Dudenhausen JW: Risk for gestational diabetes and hypertension for women with twin pregnancy compared to singleton pregnancy. Arch Gynecol Obstet 269: 33–36, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Alexander GR, Kogan M, Martin J, Papiernik E: What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clin Obstet Gynecol 41: 114–125, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Conde-Agudelo A, Belizán JM, Lindmark G: Maternal morbidity and mortality associated with multiple gestations. Obstet Gynecol 95: 899–904, 2000 [PubMed] [Google Scholar]
- 23.Misra DP, Ananth CV: Infant mortality among singletons and twins in the United States during 2 decades: Effects of maternal age. Pediatrics 110: 1163–1168, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Sperling L, Kiil C, Larsen LU, Brocks V, Wojdemann KR, Qvist I, Schwartz M, Jørgensen C, Espersen G, Skajaa K, Bang J, Tabor A: Detection of chromosomal abnormalities, congenital abnormalities and transfusion syndrome in twins. Ultrasound Obstet Gynecol 29: 517–526, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Makhseed M, Al-Sharhan M, Egbase P, Al-Essa M, Grudzinskas JG: Maternal and perinatal outcomes of multiple pregnancy following IVF-ET. Int J Gynaecol Obstet 61: 155–163, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Smith-Levitin M, Skupski DW, Chervenak FA: Multifetal pregnancies. Curr Opin Obstet Gynecol 7: 465–471, 1995 [PubMed] [Google Scholar]
- 27.Sherer DM: Adverse perinatal outcome of twin pregnancies according to chorionicity: Review of the literature. Am J Perinatol 18: 23–37, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Hou S: Historical perspective of pregnancy in chronic kidney disease. Adv Chronic Kidney Dis 14: 116–118, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Piccoli GB, Attini R, Vasario E, Conijn A, Biolcati M, D’Amico F, Consiglio V, Bontempo S, Todros T: Pregnancy and chronic kidney disease: A challenge in all CKD stages. Clin J Am Soc Nephrol 5: 844–855, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imbasciati E, Gregorini G, Cabiddu G, Gammaro L, Ambroso G, Del Giudice A, Ravani P: Pregnancy in CKD stages 3 to 5: Fetal and maternal outcomes. Am J Kidney Dis 49: 753–762, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Nevis IF, Reitsma A, Dominic A, McDonald S, Thabane L, Akl EA, Hladunewich M, Akbari A, Joseph G, Sia W, Iansavichus AV, Garg AX: Pregnancy outcomes in women with chronic kidney disease: A systematic review. Clin J Am Soc Nephrol 6: 2587–2598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccoli GB, Conijn A, Attini R, Biolcati M, Bossotti C, Consiglio V, Deagostini MC, Todros T: Pregnancy in chronic kidney disease: Need for a common language. J Nephrol 24: 282–299, 2011 [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Clinical Excellence (NICE): Intrapartum care of healthy women and their babies during childbirth, 2007. Available at: http://www.nice.org.uk/nicemedia/pdf/IPCNICEguidance.pdf Accessed September 2012
- 34.Birthplace in England Collaborative Group: Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: The Birthplace in England national prospective cohort study. BMJ 343: d7400, 2011 [DOI] [PMC free article] [PubMed]
- 35.Vasario E, Borgarello V, Bossotti C, Libanori E, Biolcati M, Arduino S, Spinelli R, Delle Piane L, Revelli A, Todros T: IVF twins have similar obstetric and neonatal outcome as spontaneously conceived twins: A prospective follow-up study. Reprod Biomed Online 21: 422–428, 2010 [DOI] [PubMed] [Google Scholar]
- 36.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 37.Ikizler TA: CKD classification: Time to move beyond KDOQI. J Am Soc Nephrol 20: 929–930, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Steegers EAP, von Dadelszen P, Duvekot JJ, Pijnenborg R: Pre-eclampsia. Lancet 376: 631–644, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Young BC, Levine RJ, Karumanchi SA: Pathogenesis of preeclampsia. Annu Rev Pathol 5: 173–192, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM: Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Parazzini F, Cortinovis I, Bortolus R, Fedele L: [Standards of birth weight in Italy]. Ann Ostet Ginecol Med Perinat 112: 203–246, 1991 [PubMed] [Google Scholar]
- 42.Branum AM, Schoendorf KC: The effect of birth weight discordance on twin neonatal mortality. Obstet Gynecol 101: 570–574, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Blickstein I, Keith LG: Neonatal mortality rates among growth-discordant twins, classified according to the birth weight of the smaller twin. Am J Obstet Gynecol 190: 170–174, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Williams D, Davison J: Chronic kidney disease in pregnancy. BMJ 336: 211–215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM: Pre-eclampsia: Pathophysiology, diagnosis, and management. Vasc Health Risk Manag 7: 467–474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redman CW, Sargent IL: Latest advances in understanding preeclampsia. Science 308: 1592–1594, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Holden DP, Fickling SA, Whitley GS, Nussey SS: Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. Am J Obstet Gynecol 178: 551–556, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, Dudenhausen JW, Denk B, Stepan H: An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol 202: 161.e1–161.e11, 2010 [DOI] [PubMed] [Google Scholar]