Summary
Background and objectives
Congenital anomalies of the kidney and urinary tract (CAKUT) are the leading cause of ESRD in children, but the proportion of patients with individual CAKUT entities progressing to ESRD during adulthood and their long-term clinical outcomes are unknown. This study assessed the age at onset of renal replacement therapy (RRT) and patient and renal graft survival in patients with CAKUT across the entire age range.
Design, setting, participants, & measurements
Patients with CAKUT were compared with age-matched patients who were undergoing RRT for other renal disorders on the basis of data from the European Renal Association-European Dialysis and Transplant Association Registry. Competing risk and Cox regression analyses were conducted.
Results
Of 212,930 patients commencing RRT from 1990 to 2009, 4765 (2.2%) had renal diagnoses consistent with CAKUT. The proportion of incident RRT patients with CAKUT decreased from infancy to childhood and then increased until age 15–19 years, followed by a gradual decline throughout adulthood. Median age at RRT start was 31 years in the CAKUT cohort and 61 years in the non-CAKUT cohort (P<0.001). RRT was started earlier (median, 16 years) in patients with isolated renal dysplasia than in those with renal hypoplasia and associated urinary tract disorders (median, 29.5–39.5 years). Patients with CAKUT survived longer than age- and sex-matched non-CAKUT controls because of lower cardiovascular mortality (10-year survival rate, 76.4% versus 70.7%; P<0.001).
Conclusions
CAKUT leads to ESRD more often at adult than pediatric age. Treatment outcomes differ from those of acquired kidney diseases and vary within CAKUT subcategories.
Introduction
Congenital anomalies of the kidney and urinary tract (CAKUT) are the most common cause of ESRD in children. Bilateral renal hypoplasia and dysplasia with or without concomitant urinary tract malformations are present in more than 50% of children and adolescents commencing renal replacement therapy (RRT) (1–5). In contrast, CAKUT is a much less common cause of ESRD in adult patients undergoing RRT, accounting for less than 5% of a disease spectrum dominated by diabetic and nondiabetic glomerulopathies (6–9).
The natural course of CAKUT is very heterogeneous (10,11). Although the most severely affected newborns progress to ESRD within the first few months of life, kidney function improves in most children born with CAKUT, typically reaching a peak around age 3–4 years. Subsequently, kidney function remains stable in most children until puberty. During adolescence, accelerated progression of CKD to ESRD is frequently observed (10). Altogether, approximately 25% of children born with bilateral CAKUT and kidney dysfunction require RRT during the first two decades of life (2,10,11). However, little information is available about the long-term prognosis of patients with CAKUT as they advance into adult life.
The aim of this study was to assess the demographic characteristics of patients with CAKUT who were starting RRT and their outcomes on RRT across all age groups compared with patients without CAKUT. We used the database of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Registry, a large longitudinal database of patients receiving RRT throughout Europe that includes complete patient-level information from 12 countries with 150 million European residents.
Methods
Patient Population
Data on the incidence of RRT, time on dialysis, renal allograft, and patient survival in patients with CAKUT were extracted from the ERA-EDTA Registry.
The diagnosis CAKUT comprised the following ERA-EDTA primary renal disease codes (6): congenital renal hypoplasia (“hypoplasia”; code 60), oligomeganephronic hypoplasia (code 61), congenital renal dysplasia with or without urinary tract malformation (“dysplasia”; code 63), syndrome of agenesis of abdominal muscles (prune belly syndrome; code 66), pyelonephritis associated with neurogenic bladder (“neurogenic pyelonephritis”; code 21), pyelonephritis due to congenital obstructive uropathy with or without vesico-ureteric reflux (“obstructive pyelonephritis”; code 22), and pyelonephritis due to vesico-ureteric reflux without obstruction (“reflux pyelonephritis”; code 24). The few patients coded as “prune belly syndrome” (code 66) were combined with code 63, whereas patients with “oligomeganephronia” (code 61) were combined with code 60 for further analysis.
Patients with “pyelonephritis cause not specified” (code 20), “pyelonephritis due to acquired obstructive uropathy” (code 23), “pyelonephritis due to urolithiasis” (code 25), and “pyelonephritis due to other cause” (code 29) were not included in the analysis.
Incidence and prevalence data on patients with CAKUT from countries providing patient data from birth onward to the ERA-EDTA registry were analyzed (Austria, Denmark, Andalusia [Spain], Asturias [Spain], Basque country [Spain], Canary Island [Spain], Castille and Leon [Spain], Castile-La Mancha [Spain], Catalonia [Spain], Extremadura [Spain], Galicia [Spain], Valencian region [Spain], Finland, France, Greece, Iceland, the Netherlands, Norway, Romania, Sweden, and Scotland). The observation period was restricted to 1990–2009 but differed by country.
Incidence and prevalence data as well as age-dependent patterns of onset of RRT were analyzed with respect to patient survival, time on dialysis, and first renal allograft survival.
For comparison of patient and graft survival on RRT, two control groups (one for dialysis and one for graft survival) of patients with other underlying renal diagnoses matched for age, sex, country, year of start of RRT, and initial RRT modality were randomly selected from the registry. Furthermore, the CAKUT and non-CAKUT control patients did not differ with respect to the time on dialysis before transplantation and the proportion of pre-emptive transplantations.
The underlying primary renal diseases in the matched controls were glomerulonephritis (27.2%), diabetic nephropathy (11.9%), hypertension or renovascular disease (7.8%), and cystic kidney disease (9.7%). In 26.8%, other causes of ESRD were specified, and in 16.6% the renal disease was not classified.
Statistical Analyses
Incidence was defined as the number of patients with CAKUT starting RRT in the study period; point prevalence was defined as the total number of patients undergoing RRT on December 31, 2009. Both incidence and prevalence were expressed as absolute numbers and per million age-related population. EUROSTAT (the statistical office of the European Union), the national bureau of statistics, or the contributing national or regional registries provided the general population data. To investigate trends over time, the time periods 1990–1999 and 2000–2009 were compared. The risk for death from specific causes was calculated as incidence per person time on RRT, and 95% confidence intervals were used to assess statistically significant differences. All matched controls from both cohorts were used for comparison.
For the time-to-event data analysis for patient survival on dialysis or graft survival, a competing risk analysis was performed (12). P values to test for significance were calculated according to the method of Pepe and Mori (13). Cox regression models were used to analyze differences in patient and graft survival between subgroups. All analyses were adjusted for age, sex, cause of ESRD, RRT modality, period of RRT, and country of residence. All statistical analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC). P values <0.05 were considered to represent statistically significant findings.
Results
Patient Characteristics
From January 1, 1990, to December 31, 2009, a total of 212,930 patients started RRT in the area of the contributing registries. Of these, 4765 patients were classified as having a diagnosis of CAKUT. In the ERA-EDTA Registry population, CAKUT is a relatively rare diagnosis, accounting for 2.2% of the total patient cohort (Table 1). The incidence of RRT due to CAKUT disorders was stable over time (2.6–3.0 per million age-related population in any 5-year period between 1990 and 2009).
Table 1.
Characteristics of patients receiving renal replacement therapy in the European Renal Association-European Dialysis and Transplant Association Registry between January 1, 1990, and December 31, 2009
| Characteristic | All Patients | Patients with CAKUT |
|---|---|---|
| Prevalence 2009 (pmp) | 947.5 | 44.9 |
| Incidence (n) | 212,930 | 4,765 |
| Male patients (%) | 61.5 | 61.5 |
| Renal diagnoses (%) | ||
| Hypertensive or diabetic nephropathy | 46.9 | |
| Unknown/unspecified | 19.4 | |
| Glomerulonephritis | 14.4 | |
| Cystic kidney diseases | 7.1 | |
| Vasculitis | 2.0 | |
| Other | 8.0 | |
| CAKUT | ||
| Total | 2.2 | 100 |
| Neurogenic PN | 5.6 | |
| Obstructive PN | 22.9 | |
| Reflux PN | 41.9 | |
| Hypoplasia | 17.3 | |
| Dysplasia | 12.3 | |
| RRT modality (%) | ||
| Hemodialysis | 80.2 | 61.5 |
| Peritoneal dialysis | 17.2 | 26.1 |
| Pre-emptive transplantation | 2.6 | 12.4 |
| Mean age at start RRT (yr) | ||
| All | 61.4±16.6 | 35.1±21.0 |
| Male patients | 61.2±16.5 | 33.1±21.3 |
| Female patients | 61.7±16.8 | 38.3±20.1a |
| 1990–1999 | 58.4±16.7 | 32.9±19.7 |
| 2000–2009 | 62.9±16.3a | 36.6±21.5a |
| Neurogenic PN | 41.8±18.4b,c | |
| Obstructive PN | 34.1±21.4c,d | |
| Reflux PN | 38.5±18.7c,e | |
| Hypoplasia | 35.7±23.9c,e | |
| Dysplasia | 22.1±19.7c,f |
Values expressed with a plus/minus sign are the mean ± SD. CAKUT, congenital anomalies of the kidney and urinary tract; pmp, per million population; PN, pyelonephritis; RRT, renal replacement therapy.
Significant difference between sex or time periods (P<0.001).
Significantly different from patients with obstructive PN, reflux PN, hypoplasia, and dysplasia.
Significant difference (P<0.05).
Significantly different from patients with neurogenic PN, reflux PN, hypoplasia, and dysplasia.
Significantly different from patients with neurogenic PN, obstructive PN, and dysplasia.
Significantly different from patients with neurogenic, obstructive, and reflux PN and hypoplasia.
The median age at start of RRT was 31 years in patients with CAKUT and 61 years in the total ERA-EDTA Registry cohort. The incidence of RRT was lowest at age 5–9 years and increased to maximum around age 15–19 years, followed by a gradual decline throughout adulthood (Figure 1). In contrast, the number of patients with other primary renal diagnoses who started RRT increased steadily with age.
Figure 1.
Age distribution at onset of renal replacement therapy (RRT). Percentage of patients with congenital anomalies of the kidney and urinary tract (CAKUT) (closed bars) starting RRT at a given age compared with groups with non-CAKUT primary renal disease (hatched bars) in the European Renal Association-European Dialysis and Transplant Association registry.
Regarding the number of patients starting RRT in different age categories in 2000–2009 compared with 1990–1999, there was a clear tendency toward initiating RRT in more elderly patients. However, the number of young patients starting RRT did not change significantly over time.
Male patients with CAKUT were significantly younger at RRT start than female patients (mean difference, 5.2 years). However, this effect differed according to the type of CAKUT. Although male patients with obstructive (mean age, 31.5 years) or reflux (35.1 years) pyelonephritis were significantly younger than female patients (40.6 and 41.9 years, respectively; P<0.001), the age at start of RRT did not differ by sex in patients with renal dysplasia or hypoplasia. Women with neurogenic pyelonephritis initiated RRT at an even younger age than men (37.0 versus 44.7 years; P<0.001).
Irrespective of sex, patients with dysplasia required RRT at a significantly younger age than patients in the other CAKUT categories (Figure 2).
Figure 2.
Incidence and cumulative percentage of patients starting renal replacement therapy (RRT) by subcategory of congenital anomalies of the kidney and urinary tract (CAKUT). (A) Incidence per million age-related population. (B) Cumulative percentage.
Prevalence and Incidence of RRT in Patients with CAKUT
The point prevalence of RRT on December 31, 2009, was 947.5 cases per million population for the total ERA-EDTA Registry cohort and 44.9 cases per million population for patients with CAKUT. During the first decade of life the incidence of RRT for ESRD due to renal dysplasia was highest (1.2 per million age-related population) (Figure 2A), followed by obstructive pyelonephritis (0.8 per million age-related population) and hypoplasia (0.5 per million age-related population). Kidney failure due to reflux pyelonephritis requiring RRT increased steeply in incidence during the first two decades, peaking (1.7 per million age-related population) in the early twenties. Obstructive pyelonephritis resulted in a similar maximum RRT incidence in early adult life (1.5 per million age-related population), with a second peak in the elderly (age >70 years).
Figure 2B gives the cumulative percentage of patients with CAKUT receiving RRT by disease category. Median age at onset of RRT was 16 years in the dysplasia group, 32 years in those with hypoplasia, 29.5 years in the obstructive pyelonephritis group, 35 years in the reflux pyelonephritis group, and 39.5 years in patients with neurogenic pyelonephritis.
The kidney transplantation rate strongly depended on age. The fractions of patients with CAKUT who had undergone transplantation within 10 years were 90.2%, 84.4%, 73.6%, 44.2%, and 6.5% for patients starting dialysis at ages <18, 18–34, 35–49, 50–64, and >65 years, respectively.
The rate of preemptive kidney transplantation (12.4% overall) was highest in patients with renal dysplasia (23.4%) and lowest in those with neurogenic pyelonephritis (4.2%). This difference remained significant after adjustment for sex and age at start of RRT (P=0.001).
Patient Survival during RRT
The overall 10-year patient survival rates were 92%, 89.5%, 74.7%, 47%, and 10.4% in patients with CAKUT who started RRT at ages <18, 18–34, 35–49, 50–64, and ≥65 years, respectively. After adjustment for age, sex, RRT modality, time period of RRT, and country of origin, patient survival during RRT was associated with the type of underlying renal disease. The risk for death was highest in patients with neurogenic pyelonephritis, intermediate in those with renal dysplasia, and lowest in patients with renal hypoplasia and obstructive or reflux pyelonephritis (Table 2 and Figure 3). The causes of death differed between patients with CAKUT and matched patients without CAKUT: Fewer of the former died of cardiovascular causes (0.85/100 person-years for CAKUT versus 1.10 per 100 person-years for non-CAKUT) (Table 3). Within the CAKUT group, patients with neurogenic pyelonephritis were at increased risk for dying of cardiovascular disease, infection, and other or unknown causes compared with the other subcategories. Patients with renal hypoplasia had a two-fold increase in risk for death due to cardiovascular disease compared with patients in the dysplasia group (1.02 versus 0.47 cases per 100 person-years) (Table 3). Analysis of the results separately for death with dialysis versus death with transplantation showed that directions of differences in causes of death between the groups were very similar, although the risk for death was much lower for patients who underwent transplantation (Table 3).
Table 2.
Proportional hazard analysis for patient mortality on renal replacement therapy and renal graft loss in patients with congenital anomalies of the kidney and urinary tract in the European Renal Association-European Dialysis and Transplant Association Registry
| Variable | Patient Mortality with RRT | Graft Loss | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CAKUT category | ||||
| Neurogenic PN | 1.66 (1.24–2.21) | 0.006 | 1.32 (0.93–1.87) | 0.12 |
| Obstructive PN | 0.74 (0.56–0.99) | 0.04 | 0.95 (0.74–1.22) | 0.68 |
| Reflux PN | 0.75 (0.57–0.98) | 0.04 | 1.03 (0.79–1.32) | 0.85 |
| Hypoplasia | 0.73 (0.54–1.00) | 0.05 | 1.04 (0.77–1.40) | 0.81 |
| Dysplasia | 1.00 | 1.00 | ||
| RRT modality at start | ||||
| Hemodialysis | 1.56 (1.04–2.33) | 0.03 | 1.02 (0.81–1.28) | 0.89 |
| Peritoneal dialysis | 1.29 (0.85–1.96) | 0.23 | 0.89 (0.69–1.14) | 0.37 |
| Pre-emptive transplantation | 1.00 | 1.00 | ||
| Age | 1.06 (1.06–1.06) | <0.001 | 1.00 (1.00–1.01) | 0.39 |
| Female sex | 1.09 (0.95–1.25) | 0.24 | 1.08 (0.92–1.26) | 0.37 |
| Period after 2000 | 0.98 (0.86–1.13) | 0.81 | 0.78 (0.65–0.93) | 0.01 |
RRT, renal replacement therapy; HR, hazard ratio; CI, confidence interval; CAKUT, congenital anomalies of the kidney and urinary tract; PN, pyelonephritis.
Figure 3.
Patient survival during renal replacement therapy in patients with congenital anomalies of the kidney and urinary tract (CAKUT) from the European Renal Association-European Dialysis and Transplant Association Registry. Findings are shown by CAKUT category and for all patients without CAKUT.
Table 3.
Mortality rate due to specific causes in patients without congenital anomalies of the kidney and urinary tract by treatment modality
| Variable | Mortality Rate by Cause of Death (per 100 Person-Years) | ||||
|---|---|---|---|---|---|
| CVD | Cancer | Infection | Other | Unknown | |
| Dialysis | |||||
| Non-CAKUT | 3.72a | 0.68 | 1.49 | 2.09 | 1.87 |
| CAKUT | 2.05 | 0.51 | 1.28 | 1.62 | 1.51 |
| Neurogenic PN | 2.82b | 0.45 | 2.12b | 3.27b | 2.25b |
| Obstructive PN | 2.04 | 0.65 | 1.26 | 1.18 | 1.26 |
| Reflux PN | 1.86 | 0.45 | 1.07 | 1.55 | 1.55 |
| Hypoplasia | 2.79c | 0.47 | 0.85 | 1.40 | 1.47 |
| Dysplasia | 0.72 | 0.52 | 1.45 | 0.83 | 1.24 |
| Post-transplantation | |||||
| Non-CAKUT | 0.23 | 0.13 | 0.22 | 0.25 | 0.25 |
| CAKUT | 0.13 | 0.14 | 0.15 | 0.13 | 0.20 |
| Neurogenic PN | 0.41 | 0.10 | 0.51 | 0.41 | 0.61 |
| Obstructive PN | 0.12 | 0.10 | 0.02 | 0.06 | 0.14 |
| Reflux PN | 0.10 | 0.18 | 0.18 | 0.12 | 0.27 |
| Hypoplasia | 0.05 | 0.09 | 0.14 | 0.14 | 0.14 |
| Dysplasia | 0.16 | 0.16 | 0.16 | 0.16 | 0.04 |
CVD, cardiovascular disease; CAKUT, congenital anomalies of the kidneys and urinary tract; PN, pyelonephritis.
Significantly different from the CAKUT group
Within the CAKUT group: significantly different from the dysplasia subgroup (P<0.05).
Within the CAKUT group: significantly different from the other CAKUT subgroups (P<0.05).
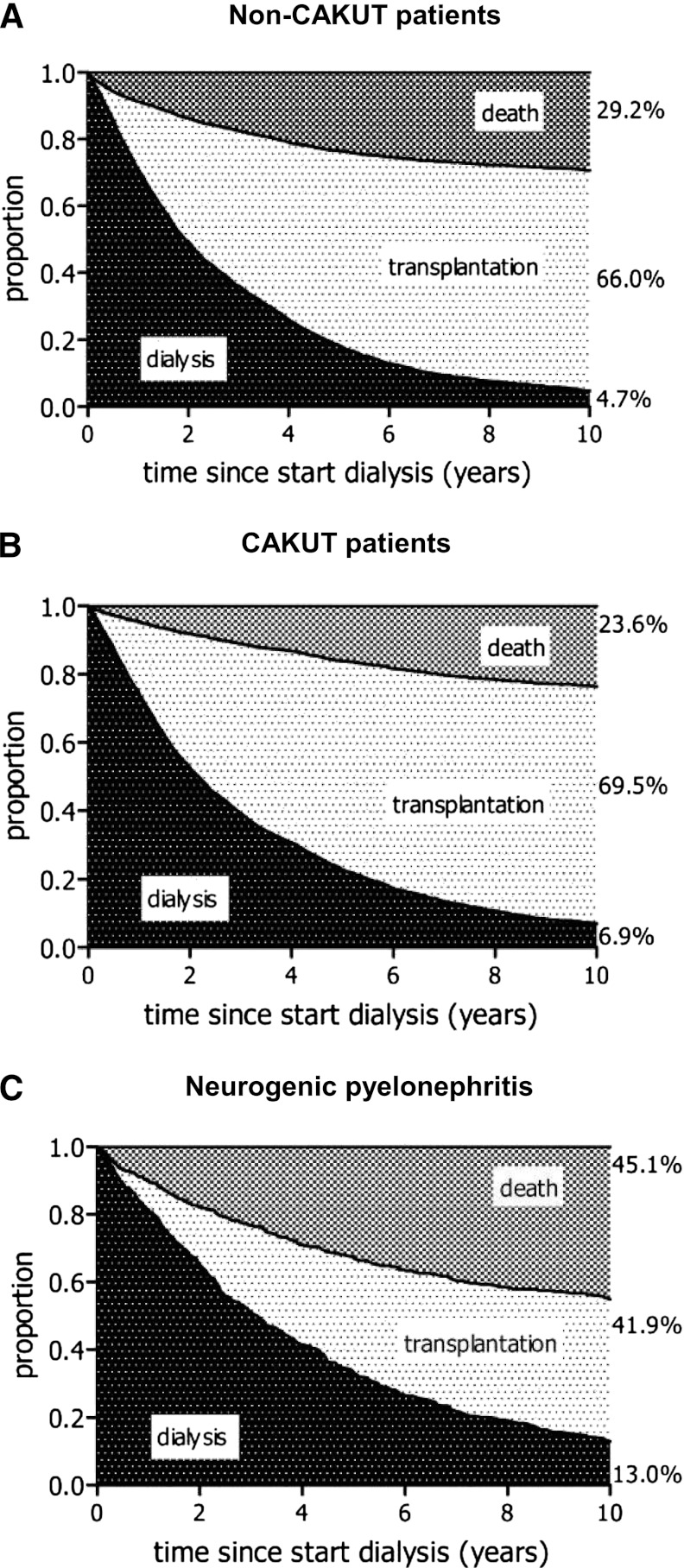
Competing risk analysis demonstrated that patients with CAKUT starting dialysis had a similar likelihood of receiving a kidney transplant within 10 years as patients without CAKUT (69.5% versus 66% after 10 years; P=0.14) but a significantly lower risk for death (23.6% versus 29.2% at 10 years; P<0.001). The CAKUT subgroup with neurogenic pyelonephritis were less likely to receive a transplant (41.9%; P=0.001 versus other CAKUT patients) and more likely to die (41.5%; P=0.001 versus other CAKUT patients) (Figure 4, A–C).
Figure 4.
Outcome of patients receiving renal replacement therapy. Competing risk analysis for patient survival and kidney transplantation during dialysis, comparing (A) patients with other primary renal diseases matched for age, sex, country, year of start of renal replacement therapy, and treatment modality at start with (B) all patients with congenital anomalies of the kidney and urinary tract (CAKUT) and (C) patients with neurogenic pyelonephritis from the European Renal Association-European Dialysis and Transplant Association Registry.
Graft Survival
Figure 5 gives the competing risk analysis for renal allograft (Figure 5A) and patient survival (Figure 5B) within the first 10 years after renal transplantation. In patients with CAKUT, the outcome with respect to graft (P=0.02) and patient (P=0.007) survival was superior compared with outcomes of patients without CAKUT, resulting in a higher probability that patients with CAKUT would be alive with a functioning graft after 10 years (67.1% versus 61.6%; P<0.001). In the Cox proportional hazard analysis, the only factor predicting graft survival in the CAKUT population was the treatment time period (improved outcome in the more recent period), whereas CAKUT subcategory, initial RRT modality, age at kidney transplantation, and sex did not have a significant effect on transplantation outcome (Table 2).
Figure 5.
Competing risk analysis for survival. (A) Graft survival. (B) Patient survival. Analysis compares patients who have congenital anomalies of the kidney and urinary tract (CAKUT) with those who do not have CAKUT (matched for age, sex, country, year of start of renal replacement therapy, and treatment modality at start) from the European Renal Association-European Dialysis and Transplant Association Registry.
Supplemental Table 1, part of the online Appendix, compares patient and graft survival of the total CAKUT cohort and CAKUT patients excluding those who have neurogenic pyelonephritis with survival in the total RRT population, age-matched RRT patients, and RRT patients excluding those with recurrent kidney diseases and diabetes.
Discussion
Limited information exists on the long-term natural course of CKD due to CAKUT. Epidemiologic information on the prevalence of congenital anomalies (including renal and urinary malformations) has been provided by the European Surveillance of Congenital Abnormalities Registry (14), covering almost 30% of the European birth population. However, no information on the postnatal course of these patients is available. Although a few single-center studies (10,11) and a single national registry (2) have assessed the course of CKD in children with CAKUT, we believe that our study is the first to provide information on the timing and outcomes of RRT in the individual phenotypic categories of CAKUT across the entire age range. Because comprehensive population-based data on all patients with CAKUT are not available, our analysis was limited to patients who progress to ESRD at any point during their lifetime. Hence, we cannot draw any conclusions regarding patients with milder forms of CAKUT who never require RRT. Still, our analysis of >4700 patients from 12 European countries who started RRT during the past two decades revealed several novel and remarkable insights regarding the natural course of this group of patients with rare kidney and urinary tract diseases.
Although the incidence of RRT was expectedly greater during childhood, it was surprising to note that more than two thirds of the patients progressed to ESRD at adult age, and 50% did not require RRT before the fourth decade of life. These figures are probably underestimations because an unknown number of adults with CAKUT entering ESRD programs without a pediatric history of renal disease may be misclassified as having CKD of “unspecified” or “other” origin. Indeed, adult CKD and ESRD registries usually report 20%–27% of patients without a specified renal diagnosis, compared with less than 5% in pediatric registries (1,15). In our data, 11.4% of the renal diagnoses among patients age <20 years were classified as “unknown” or “other,” compared with 19.6% among those >18 years. Furthermore, we did not include patients with “pyelonephritis, cause not specified” and “pyelonephritis due to other cause,” which are slightly more often coded for adults than for children and might include patients with congenital renal abnormalities as well.
Our findings suggest that in patients with a congenital reduction in nephron mass, CKD progression due to ongoing loss of remnant nephrons is probably a lifelong process. In this respect, CAKUT disorders may be a fascinating “experiment of nature” with which to study the mechanisms leading to progressive nephron loss in the absence of confounding immunologic and vascular pathomechanisms, which are major effectors of disease progression in most adult nephropathies.
Developed in the early 1970s, the ERA-EDTA disease codes (6) partly reflect outdated pathophysiologic concepts, such as the exclusive association of ESRD in the presence of reflux-associated, obstructive, or neurogenic urinary tract abnormalities with “pyelonephritis.” Ample experimental and clinical evidence suggests that renal function is commonly reduced at birth in patients with urinary tract malformation, secondary to a common underlying genetic abnormality or to early intrauterine urinary tract obstruction altering kidney development (16,17). Hence, bilateral renal hypoplasia or dysplasia is a far more likely cause of ESRD in most RRT patients classified as having “pyelonephritis” due to obstruction, reflux, or neurogenic bladder disorder, even more so as antibiotic prophylaxis and early corrective urologic surgery have greatly reduced the incidence of upper urinary tract infections in this population in the past three decades (18,19). The upcoming release of new ERA-EDTA disease codes might provide a more appropriate clinical and pathophysiologic classification of kidney diseases. However, for this retrospective analysis we were required to analyze the original EDTA disease codes in an approximative manner.
In the context of these changed ontologic concepts, the biphasic pattern of RRT incidence observed in the first two decades of life in patients with obstructive nephropathy and isolated renal dysplasia, with a dip around age 5–9 years, is consistent with the notion that early renal survival depends on the severity of renal dysplasia (10). Although children with severe dysplastic kidney disease require RRT from early infancy, those with less severe malformations characteristically undergo a transient period of stable or even increasing GFR resulting from compensatory hypertrophy of the remnant functioning nephrons. The functional “reserve” seems to be lowest in children with isolated renal dysplasia or hypoplasia, in whom RRT incidence peaks again at 5–14 years, whereas children with associated urinary tract abnormalities more frequently progress to ESRD around 15–25 years of age, with a spread into mid-adult life. A third peak of RRT incidence observed in elderly patients, mainly with obstructive nephropathies, may indicate admixture of acquired disorders in this age group.
The sharp increase in RRT incidence observed around puberty is in accordance with data from a population-based pediatric CKD registry comprising 1200 children in Italy (2 ) and was also noted in the prospective Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) trial (20). It is interesting to speculate whether this may be due to an increasing discrepancy between body size and functional nephron mass or whether the emerging production of sex steroid hormones at this age may influence renal survival. In this context, observations in patients with polycystic kidney disease (21), the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study (22), and a Canadian CKD study (23) have suggested that male sex might be a general risk factor for CKD progression. At first glance, our finding of earlier RRT onset in male patients appears to support this notion. However, the sex difference was largely confined to urinary tract abnormalities. It is possible that a major fraction of male patients in these categories had posterior urethral valves, a male-limited condition often associated with particularly severe renal dysplasia. In keeping with a major effect of sex-specific underlying kidney diseases on renal survival, a recent single-center CAKUT study from Italy showed that male sex predicted a shorter renal survival by univariate analysis but not after adjustment for diagnosis categories (11).
An issue of great concern for parents of children born with severe CAKUT is the uncertainty about the patients’ long-term well-being and survival. Mortality from childhood-onset ESRD is markedly increased even among children (24), although most survive into adult life. This study provides several important pieces of information about late outcomes of CAKUT. Besides the fact that most patients who need to start RRT will not do so before adulthood, the survival of patients with CAKUT receiving RRT is slightly better than that of age-matched patients with other underlying kidney diseases, such as type 1 diabetic nephropathy and other acquired and inherited glomerulopathies. This may be explained by the frequently retained urine production in patients with dysplastic kidneys, even on dialysis, and the consecutively lower incidence of fluid overload, hypertension, and metabolic abnormalities in patients with CAKUT compared with those who have other diseases leading to ESRD. Indeed, patients with CAKUT were less likely to die of cardiovascular causes than were those without CAKUT.
An exception to this rule was the subgroup of patients with ESRD related to neurogenic bladder abnormalities, who showed markedly poorer patient survival. These patients often need some form of urinary diversion (conduit or reservoir), which is associated with a higher risk for chronic and potentially invasive infections, an increased risk for malignancies, and a less favorable outcome after renal transplantation (25,26). In addition, these patients may have various neurologic comorbid conditions and complications that could affect their long-term survival.
Finally, after adjustment for age, sex, and disease subtype, patients with CAKUT had a better chance of survival while undergoing peritoneal dialysis compared with hemodialysis. This finding is consistent with previous findings in the ERA-EDTA Registry (27). In a large Canadian study (28) and in the U.S. Renal Data System (29), younger nondiabetic patients without comorbidity also had a survival benefit while receiving peritoneal dialysis, whereas no difference was seen in the total RRT cohort.
In conclusion, this study demonstrates that the traditional view of CAKUT as a rare pediatric kidney disorder of concern only to pediatric nephrologists and urologists should be revised. Many patients with moderate to severe renal hypoplasia or dysplasia and stable CKD during childhood progress to ESRD requiring RRT during adulthood. It will be important to develop effective transition strategies from pediatric to adult nephrology service, awareness of disease-specific risks and conditions, and continued good-quality medical care for this group of patients, who constitute the main cause of pediatric CKD but only a small fraction of the adult CKD and ESRD population. Furthermore, this study identifies important differences in the long-term phenotypic evolution of different subgroups of CAKUT. Efforts should be made to improve disease classification by comprehensive reference to the clinical, genetic, and molecular features of the disorders. More appropriate disease classification, ideally combined with life-long patient follow-up in dedicated registries, will contribute to improved prognostic counseling and personalized disease management among patients with congenital kidney malformations (30).
Disclosure
None.
Supplementary Material
Acknowledgments
We would like to thank the patients and staff of all the dialysis and transplant units who have contributed data via their national and regional renal registries. Furthermore, we gratefully acknowledge the following registries and persons for their participation in the data collection: R. Kramar (Austrian Registry); P. Castro de la Nuez and J. M. Munoz Terol (Andalusian Renal Registry); A. Magaz, J. Aranzabal, I. Lampreabe, and J. Arrieta (Basque Country Renal Registry); E. Arcos, J. Comas, R. Deulofeu, and J. Twose (Catalan Renal Registry and Catalan Transplant Organization); A. Hemke (Dutch End-Stage Renal Disease Registry); C. Gronhagen-Riska (Finnish Registry for Kidney Diseases); G.A. Ioannidis (Greek National Renal Registry); R. Palsson (Icelandic End-Stage Renal Disease Registry); T. Leivestad (Norwegian Registry); M. Lasalle and C. Couchoud (REIN Registry); G. Mirceascu, L. Garneata, and E. Podgoreanu (Romanian Registry); W. Metcalfe and K. Simpson (Scottish Renal Registry); S. Schon, A. Seeberger, L. Backman, and B. Rippe (Swedish Renal Registry); and O. Zurriaga (Registro de Enfermos Renales de la Comunidad Valenciana) for providing data.
The ERA-EDTA Registry is funded by the ERA-EDTA. This study was supported by a short-term fellowship awarded by the ERA-EDTA to E.W.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03310412/-/DCSupplemental.
References
- 1.Lewis M, Shaw J, Reid C, Evans J, Webb N, Verrier-Jones K: Demography and management of childhood established renal failure in the UK (chapter 13). Nephrol Dial Transplant 22[Suppl 7]: vii165–vii175, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Ardissino G, Daccò V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F, ItalKid Project : Epidemiology of chronic renal failure in children: Data from the ItalKid project. Pediatrics 111: e382–e387, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Orr NIT, McDonald SP, McTaggart S, Henning P, Craig JC: Frequency, etiology and treatment of childhood end-stage kidney disease in Australia and New Zealand. Pediatr Nephrol 24: 1719–1726, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Mong Hiep TT, Ismaili K, Collart F, Van Damme-Lombaerts R, Godefroid N, Ghuysen MS, Van Hoeck K, Raes A, Janssen F, Robert A: Clinical characteristics and outcomes of children with stage 3-5 chronic kidney disease. Pediatr Nephrol 25: 935–940, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ: Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27: 363–373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Renal Association-European Dialysis and Transplant Association: ERA-EDTA Registry Annual Report 2009. Academic Medical Center, Department of Medical Informatics, Amsterdam, The Netherlands, June 2011. Available at: http://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2009.pdf Accessed July 13, 2011
- 7.Neild GH: What do we know about chronic renal failure in young adults? II. Adult outcome of pediatric renal disease. Pediatr Nephrol 24: 1921–1928, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Nakai S, Suzuki K, Masakane I, Wada A, Itami N, Ogata S, Kimata N, Shigematsu T, Shinoda T, Syouji T, Taniguchi M, Tsuchida K, Nakamoto H, Nishi S, Nishi H, Hashimoto S, Hasegawa T, Hanafusa N, Hamano T, Fujii N, Marubayashi S, Morita O, Yamagata K, Wakai K, Watanabe Y, Iseki K, Tsubakihara Y: Overview of regular dialysis treatment in Japan (as of 31 December 2008). Ther Apher Dial 14: 505–540, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Byrne C, Ford D, Gilg J, Ansell D, Feehally J: UK Renal Registry 12th Annual Report (December 2009): chapter 3: UK ESRD incident rates in 2008: national and centre-specific analyses. Nephron Clin Pract 115[Suppl 1]: c9–c39, 2010 [DOI] [PubMed] [Google Scholar]
- 10.González Celedón C, Bitsori M, Tullus K: Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol 22: 1014–1020, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, Izzi C, Scolari F, Mattioli G, Allegri L, Ghiggeri GM: Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int 76: 528–533, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Verduijn M, Grootendorst DC, Dekker FW, Jager KJ, le Cessie S: The analysis of competing events like cause-specific mortality—beware of the Kaplan-Meier method. Nephrol Dial Transplant 26: 56–61, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Pepe MS, Mori M: Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med 12: 737–751, 1993 [DOI] [PubMed] [Google Scholar]
- 14.European Surveillance of Congenital Abnormalities: EUROCAT registry. Prevalence tables. 2012 Available at: www.eurocat-network.eu/accessprevalencedata/prevalencetables Accessed February 5, 2012
- 15.Neild GH: What do we know about chronic renal failure in young adults? I. Primary renal disease. Pediatr Nephrol 24: 1913–1919, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Woolf AS, Winyard PJD: Advances in the cell biology and genetics of human kidney malformations. J Am Soc Nephrol 9: 1114–1125, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Chevalier RL, Thornhill BA, Forbes MS, Kiley SC: Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol 25: 687–697, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Hewitt IK, Montini G: Pediatric febrile urinary tract infections: the current state of play. Ital J Pediatr 37: 57, 2011 [DOI] [PMC free article] [PubMed]
- 19.Craig JC, Simpson JM, Williams GJ, Lowe A, Reynolds GJ, McTaggart SJ, Hodson EM, Carapetis JR, Cranswick NE, Smith G, Irwig LM, Caldwell PHY, Hamilton S, Roy LP, Prevention of Recurrent Urinary Tract Infection in Children with Vesicoureteric Reflux and Normal Renal Tracts (PRIVENT) Investigators : Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med 361: 1748–1759, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen A-M, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F: Strict blood-pressure control and progression of renal failure in children. The ESCAPE Trial Group. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Johnson ES, Thorp ML, Platt RW, Smith DH: Predicting the risk of dialysis and transplant among patients with CKD: A retrospective cohort study. Am J Kidney Dis 52: 653–660, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F: Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106: 100–105, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Basiri A, Otoukesh H, Simforoosh N, Hosseini R, Farrokhi F: Kidney transplantation in children with augmentation cystoplasty. J Urol 178: 274–277, discussion 277, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Adams J, Mehls O, Wiesel M: Pediatric renal transplantation and the dysfunctional bladder. Transpl Int 17: 596–602, 2004 [DOI] [PubMed] [Google Scholar]
- 27.van de Luijtgaarden MW, Noordzij M, Stel VS, Ravani P, Jarraya F, Collart F, Schön S, Leivestad T, Puttinger H, Wanner C, Jager KJ: Effects of comorbid and demographic factors on dialysis modality choice and related patient survival in Europe. Nephrol Dial Transplant 26: 2940–2947, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S: Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant 27: 3568–3575, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Mehrotra R, Chiu Y-W, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Renkema KY, Winyard PJ, Skovorodkin IN, Levtchenko E, Hindryckx A, Jeanpierre C, Weber S, Salomon R, Antignac C, Vainio S, Schedl A, Schaefer F, Knoers NVAM, Bongers EMHF, EUCAKUT consortium : Novel perspectives for investigating congenital anomalies of the kidney and urinary tract (CAKUT). Nephrol Dial Transplant 26: 3843–3851, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.