Summary
Background and objectives
Pain, erectile dysfunction (ED), and depression are common yet frequently untreated in chronic hemodialysis patients. This study compared two management strategies for these symptoms in this patient population.
Design, setting, participants, & measurements
Pain, ED, and depression were assessed monthly during an observation usual care phase. Patients were then randomized to 12-month participation in either a feedback arm in which these symptoms were assessed monthly, renal providers were informed of patients' symptoms, and treatment was left treatment at their discretion; or a nurse management arm in which symptoms were assessed monthly and trained nurses were used to evaluate patients and generate and facilitate the implementation of treatment recommendations.
Results
Of 288 patients enrolled into observation between January 1, 2009 and March 30, 2010, 220 (76%) were randomized. Compared with the feedback approach, the results (shown as Δ symptom score [95% confidence interval]) indicated that nurse management was not associated with improved pain (0.49 [−0.56, 1.54]), ED (0.20 [−0.55, 0.95]), or depression (0.32 [−0.94, 1.58]). Relative to their symptoms during observation, feedback patients experienced small, statistically significant improvements in pain (−0.98 [−1.67, −0.28]), ED (−0.98 [−1.54, −0.41]), and depression (−1.36 [−2.19, −0.54]), whereas nurse management patients experienced small, statistically significant improvements in ED (−0.78 [−1.41, −0.15]) and depression (−1.04 [−2.04, −0.04]).
Conclusions
Compared with informing renal providers of their patients' pain, ED, and depression and leaving management at their discretion, a nurse-implemented management strategy does not improve these symptoms. Both approaches modestly reduced symptoms relative to usual care.
Introduction
Although it is life-sustaining, chronic hemodialysis is associated with a marked decrement in health-related quality of life (HRQOL) (1–6). One of the principal factors thought to contribute to impaired HRQOL in this patient population is a large burden of physical and emotional symptoms, of which pain, sexual dysfunction, and depression are particularly common, bothersome, and highly correlated with impaired HRQOL (7–18).
The high prevalence and importance of pain, sexual dysfunction, and depression in this patient population are particularly noteworthy because several recent studies demonstrate the safety and efficacy of pharmacologic agents for the treatment of these three symptoms (17–20). In patients on chronic hemodialysis, pharmacological analgesia and antidepressant medications reduce pain and depression, respectively, whereas phosphodiesterase-5 inhibitors are safe and efficacious for the treatment of erectile dysfunction (ED), the most common manifestation of sexual dysfunction in men (17,19,21). Nonetheless, research demonstrates that renal providers are commonly unaware of the presence of these symptoms in their dialysis patients and frequently do not implement these treatments (11,16,22,23). To date, there have been no studies investigating clinical strategies to improve the provision of symptom-alleviating therapy.
We sought to compare the effectiveness of two strategies for the management of pain, ED, and depression in chronic hemodialysis patients. We hypothesized that compared with sending renal providers written reports of their patients' pain, ED, and depression along with evidence-based algorithms to guide treatment, a strategy using trained nurses to evaluate patients and facilitate the implementation of treatment would lead to greater improvement in each of these symptoms. We focused on pain, ED, and depression rather than a single symptom because our goal was to test the effectiveness of potentially generalizable symptom management strategies for particularly common, bothersome, and treatable symptoms in those with ESRD rather than the approach to treatment of a single symptom in isolation (24,25).
Materials and Methods
Study Design
The Symptom Management Involving End-Stage Renal Disease study was a prospective, multicenter, unblinded, cluster randomized, effectiveness trial comparing two management strategies for pain, ED, and depression in adults receiving chronic, thrice-weekly outpatient hemodialysis (ClinicalTrials.gov #NCT00692419). Details of the study design and procedures were published previously (26). Study methods were approved by the University of Pittsburgh, VA Pittsburgh Healthcare System, and the Western Institutional Review Boards.
Study Participants
The trial was conducted at nine outpatient dialysis units in metropolitan Pittsburgh, Pennsylvania. Patients receiving chronic, thrice-weekly, outpatient hemodialysis who did not meet any exclusion criteria were recruited between January 2009 and March 2010. We assessed patients' cognitive function using the Mini-Cog and excluded patients with cognitive impairment based on Mini-Cog scores <3 (27). We also excluded non-English speakers, prisoners, patients participating in other clinical trials, and individuals considering transfer to peritoneal dialysis and/or undergoing evaluation for living-donor kidney transplantation to help ensure that study participants would remain on hemodialysis for the duration of the trial. We obtained written informed consent from eligible patients to participate.
Study Phases and Randomization
Patients were initially enrolled into an observation phase that lasted between 2 and 12 months depending on the date of enrollment. This was followed by a 12-month intervention phase. Data collection during the observation “usual care” phase enabled us to determine baseline levels and temporal trends in pain, ED, and depression, and to compare symptoms across the observation and intervention phases within individual patients. After the observation phase, we randomized patients within each dialysis unit to a “feedback” arm or “nurse management” arm specifically and solely based on their dialysis schedule (Monday/Wednesday/Friday versus Tuesday/Thursday/Saturday).
Data Collection
On a monthly basis throughout the study, all patients completed the Short-Form McGill Pain Questionnaire (SF-MPQ) and Patient Health Questionnaire-9 (PHQ-9) to assess pain and depression, respectively, and male participants completed the Sexual Health Inventory for Men (SHIM) to evaluate ED. The SF-MPQ includes 15 pain descriptors that are rated from 0 (no pain) to 3 (severe pain), with a summary score of 0–45 (17,28). We considered patients to have pain if they reported a score of ≥2 (moderate pain) on at least 1 of the 15 pain descriptors. The PHQ-9 is scored from 10 to 27, with higher scores denoting more severe depression (29). We considered patients with PHQ-9 scores ≥10 to have depression (29,30). We scored the SHIM from 5 to 25, with lower scores denoting more severe ED. We considered patients with SHIM scores <22 to have ED (31,32). To present results on all three questionnaires consistently, such that higher scores denote greater symptom severity, we recoded SHIM scores as 25 minus the raw score. We evaluated sexual dysfunction in women but did not analyze this as a primary outcome due to the lack of evidence-based treatment options. Study personnel conducted monthly dialysis chart reviews to abstract key laboratory values and all prescribed medications for pain, ED, and depression.
Interventions
For participants randomized to the feedback arm who had symptom scores indicating the presence of pain, ED, and/or depression, we mailed a standardized letter to the renal provider describing the presence and severity of their patient's symptom(s), along with evidence-based treatment algorithms for each relevant symptom of interest. The algorithms were derived from existing guidelines and modified by the investigative team to reflect recommended medication dose adjustments in ESRD (Supplemental Figures) (26). The decision on whether and which treatment(s) to implement was at the discretion of the renal provider.
For patients randomized to the nurse management arm, two nondialysis nurses who had completed training on the treatment of these symptoms reviewed patients' monthly symptom questionnaires, examined patients, formulated pharmacologic and/or nonpharmacologic treatment recommendations based on the same treatment algorithms and their clinical judgment, and discussed these recommendations with the patient. For treatments that the patient was interested in receiving and that involved starting a new medication, escalating the dose of an existing prescription medication, or referring the patient to another provider (e.g., pain specialist, behavioral health provider, or urologist), the nurse discussed the recommendation with the renal provider. If the provider agreed with the proposed treatment(s), the nurse facilitated the implementation of therapy. Because research nurses did not have prescribing authority, facilitating the implementation of treatment recommendations involved working with renal providers, dialysis unit personnel, and patients to institute therapy. For nurse recommendations that did not involve starting a new medication, increasing prescription medication doses, or referring the patient to a new provider, the nurse interventionist did not routinely discuss the recommendation with the provider, but rather provided education and/or instructions to the patient to modify existing treatment.
Statistical Analyses and Sample Size Estimates
We included patients who reported a symptom on ≥2 occasions during the observation phase in the primary analyses of the effects of the intervention on that symptom. We compared mean symptom scores at the first assessment during the observation period, mean number of monthly observations, and mean change in the symptom scores across study arms using the t test, Poisson regression, and mixed-effects linear regression models, respectively. In adjusted models, we used mixed-effects linear regression to compare changes in symptom scores between the two study arms over the duration of the intervention, and to compare symptom scores among individual patients across the observation and intervention phases. In addition to a patient-level random intercept and a random linear effect of time, these analyses included as fixed effects the intervention arm, symptom scores during the first observation month, site, dialysis schedule, and demographic and clinical covariates that we posited, a priori, to be potentially associated with one or more symptoms (e.g., age) and/or that were significantly imbalanced by intervention arm. We also included a linear effect of time in the model of ED only, due to its statistical significance. We report the effects of the intervention as the change (Δ) in symptom score, which reflect the mean change in scores on the symptom questionnaires. We report the number of treatment recommendations made and implemented during the intervention phase at the patient and monthly-assessment levels. We also present the change in symptom scores beginning with the first assessment point in the observation phase using box plots that depict median, 25th and 75th percentiles, and upper and lower adjacent values. All analyses were based on the intent-to-treat principle. Statistical analyses were conducted using SAS 9.2 software (SAS Institute, Cary, NC).
The power of the study was estimated using simulation that accounted for patient- and site-level random effects, including site-level variation in the treatment rates for each intervention, correlation of patient responses, patient nonresponse to therapy, and loss to follow-up. On the basis of the number of patients randomized and attrition observed during the observation period, this study had ≥80% power to detect an effect size of 0.5 for depression and greater power for pain and ED based on the higher prevalence of these symptoms.
Results
Study Population
We screened 591 patients, 209 of whom (35%) declined to participate, 42 (7%) were ineligible, and 25 (4%) were unable to be contacted. There were no differences in key demographic or clinical variables between patients who did and did not agree to be approached about study participation. Of the 315 patients who consented to participate, 27 exited the study before the first data collection point. We enrolled 288 patients who provided data during the observation phase, 68 of whom (24%) exited the study during this phase. The remaining 220 patients were randomized to the feedback arm (n=120) or nurse management arm (n=100) (Figure 1). There were no clinically significant differences in demographic characteristics or baseline clinical variables between the two study arms (Table 1).
Figure 1.
Recruitment and follow-up of the study population.
Table 1.
Comparison of baseline characteristics between intervention arms
| Characteristic | Overall Study Population (n=288)a | Feedback Arm (n=120) | Nurse Management Arm (n=100) | P Valueb |
|---|---|---|---|---|
| Demographic variables | ||||
| Age (yr) | 63.2±13.5 | 63.9±12.0 | 62.6±14.3 | 0.44 |
| Sex (male) | 163 (56.6) | 65 (54.2) | 56 (56.0) | 0.79 |
| Race (African-American) | 115 (39.9) | 48 (40.0) | 38 (38.0) | 0.76 |
| Education | 0.75 | |||
| Less than high school | 41 (14.3) | 17 (14.2) | 14 (14.1) | |
| High school equivalent | 92 (32.1) | 38 (31.7) | 36 (36.4) | |
| More than high school | 154 (53.7) | 65 (54.2) | 49 (49.5) | |
| Adequate health literacyc | 218 (84.2) | 88 (85.4) | 79 (84.9) | 0.92 |
| Married | 127 (44.3) | 55 (45.8) | 37 (37.0) | 0.19 |
| Employed | 33 (11.5) | 12 (10.0) | 10 (10.0) | 0.99 |
| Dialysis/clinical variables | ||||
| Dialysis vintage (yr) | 2.2 [3.4] | 2.1 [4.0] | 2.1 [3.2] | 0.45 |
| Dialysis adequacy (Kt/V) | 1.5±0.3 | 1.5±0.2 | 1.6±0.2 | 0.59 |
| Dialysis access | 0.62 | |||
| Catheter | 53 (18.9) | 21 (17.9) | 20 (20.6) | |
| AV fistula or graft | 228 (81.1) | 96 (82.1) | 77 (79.4) | |
| Monday/Wednesday/Fridayd | 185 (64.2) | 83 (69.2) | 58 (58.0) | 0.09 |
| Comorbidity | ||||
| Charlson comorbidity index score | 0.54 | |||
| 1–2 | 65 (22.9) | 25 (21.4) | 25 (25.0) | |
| 3–4 | 108 (38.0) | 45 (38.5) | 42 (42.0) | |
| >5 | 111 (39.1) | 47 (40.2) | 33 (33.0) | |
| Diabetes | 147 (51.8) | 61 (52.1) | 51 (51.0) | 0.87 |
| Peripheral vascular disease | 35 (12.3) | 16 (13.7) | 11 (11.0) | 0.55 |
| Laboratory values | ||||
| Hemoglobin (g/dl) | 11.6±1.1 | 11.8±1.0 | 11.4±1.1 | 0.01 |
| Albumin (g/dl) | 3.8±0.4 | 3.7±0.4 | 3.8±0.4 | 0.12 |
| Calcium (mg/dl) | 8.8±0.7 | 8.9±0.8 | 8.8±0.6 | 0.67 |
| Phosphorous (mg/dl) | 5.2±1.5 | 5.2±1.5 | 5.2±1.5 | 0.92 |
| Intact parathyroid hormone (pg/ml) | 263 [271] | 264 [274] | 250 [276] | 0.61 |
Data are presented as mean ± SD, median [interquartile range], or n (%). AV, arteriovenous.
Includes all patients enrolled into the observational phase.
Reflects statistical significance of comparisons of feedback arm with nurse management arm.
Denotes patients with Rapid Estimates of Adult Literacy in Medicine instrument scores >60.
Denotes dialysis treatment schedule.
Prevalence and Secular Trends in Symptoms
Overall, 179 patients (81%) reported pain, 66 patients (30%) reported depression, and 108 men (89%) reported ED on ≥2 monthly assessments during the observation phase and were included in analyses of intervention effectiveness. The maximum levels of pain, ED, and depression were reported as moderate or severe by 100%, 88%, and 70% of affected patients, respectively. Compared with the nurse management arm, a slightly greater proportion of patients in the feedback arm had pain on ≥2 assessments during the observation phase (86% versus 74%; P=0.03), with no differences in the proportion of patients with ED or depression across study arms or in the mean baseline levels of pain, ED, or depression (Table 2). During both observation and intervention phases, there were nominal improvements in pain and depression and worsening of ED over time compared with the first assessment during each of these phases (Table 2 and Figure 2). The differences in the magnitude of these secular trends in symptoms were not statistically significant. At the time of randomization, 72% of patients were prescribed an analgesic medication, 50% were prescribed an antidepressant, and 11% of men were prescribed medication for ED as part of usual care.
Table 2.
Comparison of baseline symptoms and change in symptoms during observation and intervention phases
| Symptom | Overall Study Population (n=288)a | Feedback Arm (n=120) | Nurse Management Arm (n=100) | P Valueb |
|---|---|---|---|---|
| Pain | ||||
| Patients reporting symptom ≥2 times during observation, n (%) | 224 (78) | 104 (87) | 75 (75) | 0.03 |
| First assessment SF-MPQ score during observation phase (mean ± SD)c | 13.1±10.2 | 12.1±9.5 | 12.8±10.8 | 0.63 |
| Average number of observations per patient during observation phase (mean ± SD) | 9.2±3.5 | 10.2±3.0 | 10.0±3.1 | 0.69 |
| Average change from first assessment during observation phase (mean ± SEM) | −2.75±0.46 | −2.40±0.64 | −3.17±0.76 | 0.44 |
| Average number of observations per patient during intervention phase (mean ± SD) | NA | 9.4±3.6 | 9.6±3.5 | 0.70 |
| Average change from first assessment during intervention phase (mean ± SEM) | NA | −3.70±0.51 | −3.12±0.54 | 0.18 |
| Erectile dysfunctiond | ||||
| Patients reporting symptom ≥2 times during observation, n (%) | 142 (87) | 61 (94) | 47 (85) | 0.13 |
| First assessment SHIM score during observation phase (mean ± SD)c | 14.7±6.3 | 14.6±6.5 | 15.1±6.0 | 0.69 |
| Average number of observations per patient during observation phase (mean ± SD) | 8.6±3.8 | 9.9±3.2 | 9.0±3.8 | 0.14 |
| Average change from first assessment during observation phase (mean ± SEM) | 0.87±0.29 | 0.82±0.39 | 0.33±0.45 | 0.41 |
| Average number of observations per patient during intervention phase (mean ± SD) | NA | 9.9±3.2 | 9.3±3.6 | 0.34 |
| Average change from first assessment during intervention phase (mean ± SEM) | NA | 0.71±0.32 | 0.62±0.34 | 0.77 |
| Depression | ||||
| Patients reporting symptom ≥2 times during observation, n (%) | 84 (29) | 38 (32) | 28 (28) | 0.56 |
| First assessment PHQ-9 score during observation phase (mean ± SD)c | 12.0±5.2 | 11.3±5.1 | 12.0±4.9 | 0.59 |
| Average observations per patient during observation phase (mean ± SD) | 8.7±3.4 | 10.1±2.8 | 9.1±3.1 | 0.21 |
| Average change from first assessment during observation phase (mean ± SEM) | −0.64±0.43 | −0.19±0.60 | −1.32±0.71 | 0.10 |
| Average observations per patient during intervention phase (mean ± SD) | NA | 8.8±3.7 | 8.7±4.0 | 0.92 |
| Average change from first assessment during intervention phase (mean ± SEM) | NA | −2.29±0.49 | −1.99±0.54 | 0.54 |
SF-MPQ, Short-Form McGill Pain Questionnaire; NA, data not applicable; SHIM, Sexual Health Inventory for Men; PHQ-9, Patient Health Questionnaire-9.
Includes all patients enrolled into observation period.
Denotes comparison of feedback arm with the management arm; Poisson regression used to compare number of observations.
First assessment denotes symptom score from first month of observation period.
Based on number of male patients: n=163 for overall study population, n=65 for feedback arm, n=55 for nurse management arm.
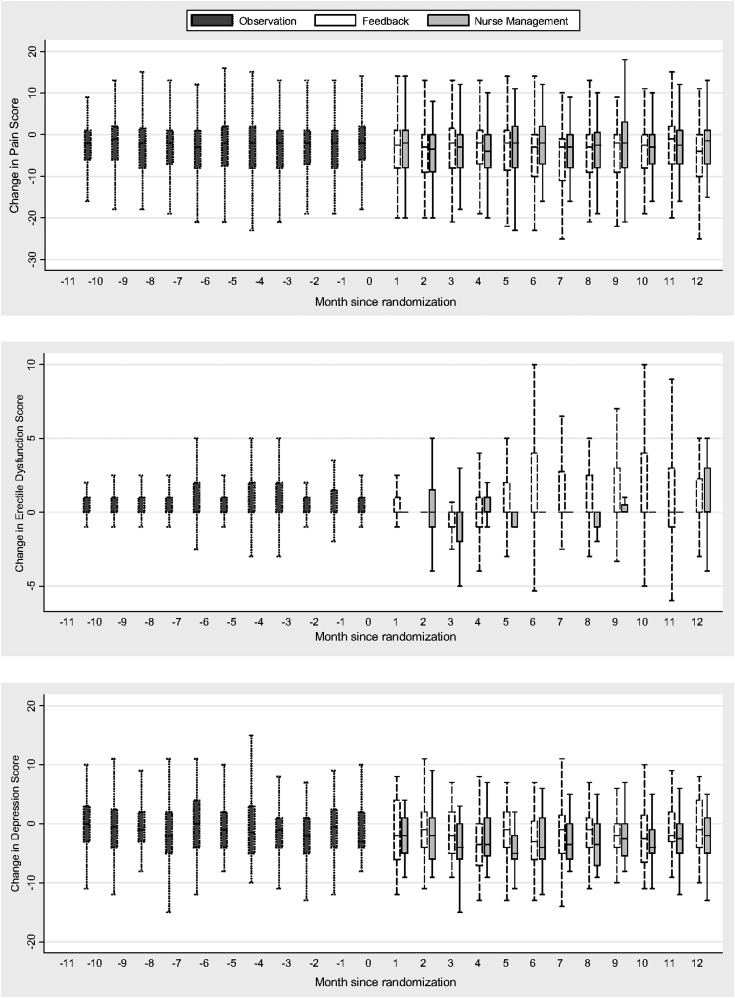
Figure 2.
Longitudinal change in symptom scores by study phase and intervention arm for patients with pain, erectile dysfunction, and depression.
Treatment Recommendations and Their Implementation
All 104 patients (100%) in the feedback arm who had pain during the observation phase reported pain during the intervention phase. Letters describing the presence and severity of pain and an accompanying treatment algorithm were sent to providers for 102 (98%) of these patients, and treatment was implemented by providers in 44 patients. All 61 of the men (100%) in the feedback arm who had ED during observation reported ED during the intervention and letters describing this symptom and its severity along with a treatment algorithm were mailed to providers for all 61 men (100%). Treatment was implemented by providers in three patients. Of 38 feedback patients who had depression during the observation phase, 37 (98%) reported this symptom during the intervention phase. Letters describing the presence and severity of depression and treatment algorithms were mailed to renal providers for all 37 patients (100%). Treatment was instituted by providers in 11 patients. Over the entire 12-month intervention phase, treatment was implemented on 72 occasions for pain, 4 occasions for ED, and 17 occasions for depression among patients in the feedback arm (Table 3). In 75% of instances in which a recommendation was implemented, there was documentation in the medical record of the specific date of implementation. The median number of days between the recommendation and the date of implementation of treatment was 28. Among feedback patients who were receiving pharmacologic treatment for pain, ED, or depression at the time of randomization, 49%, 14%, and 50%, respectively, were prescribed a new medication or had a change in the dose of an existing treatment during the intervention.
Table 3.
Treatment recommendations and treatments implemented during intervention phase
| Patient Level (n)a | Assessment Level (n)b | |||||
|---|---|---|---|---|---|---|
| Feedback arm | ||||||
| Pain (n=104) | ||||||
| Feedback arm | ||||||
| Pain (n=104) | ||||||
| Instances symptom reported | 104 | 920 | ||||
| Letters mailed to provider | 102 | 874 | ||||
| Treatments implementedc | 44 | 72 | ||||
| ED (n=61) | ||||||
| Instances symptom reported | 61 | 606 | ||||
| Letters mailed to provider | 61 | 573 | ||||
| Treatments implementedc | 3 | 4 | ||||
| Depression (n=38) | ||||||
| Instances symptom reported | 37 | 291 | ||||
| Letters mailed to provider | 37 | 270 | ||||
| Treatments implementedc | 11 | 17 | ||||
| Nurse management arm | ||||||
| Pain (n=75) | ||||||
| Instances symptom reported | 73 | 619 | ||||
| Conversations with patient about symptom | 73 | 613 | ||||
| Nurse recommendations made to and accepted by patient | 57 | 153 | ||||
| Nurse recommendations for new treatment made to provider | 27 | 46 | ||||
| Nurse recommendations for new treatment implementedd | 19 | 22 | ||||
| Erectile dysfunction (n=47) | ||||||
| Instances symptom reported | 46 | 429 | ||||
| Conversations with patient about symptom | 46 | 427 | ||||
| Nurse recommendations made to and accepted by patient | 27 | 75 | ||||
| Nurse recommendations for new treatment made to provider | 12 | 26 | ||||
| Nurse recommendations for new treatment implementedd | 5 | 8 | ||||
| Depression (n=28) | ||||||
| Instances symptom reported | 28 | 224 | ||||
| Conversations with patient about symptom | 28 | 215 | ||||
| Nurse recommendations made to and accepted by patient | 22 | 117 | ||||
| Nurse recommendations for new treatment made to provider | 11 | 16 | ||||
| Nurse recommendations for new treatment implementedd | 6 | 6 | ||||
Denotes number of patients with reports of symptoms and interventions recommended/implemented.
Denotes total number of reports of symptoms and interventions recommended/implemented over course of study.
Reflects the percentage of treatments implemented.
Reflects the percentage of nurse recommendations implemented.
In the nurse management arm, 73 of 75 patients (97%) who reported pain during the observation phase had this symptom during the intervention phase, 57 (73%) of whom were agreeable to a nurse-generated treatment recommendation (Table 3). In 27 of these 57 patients, novel treatment was recommended to the renal provider and was successfully implemented in 19 patients. Forty-six of 47 men (98%) who reported ED during observation had this symptom during the intervention, 27 of whom accepted a nurse-generated treatment recommendation. Novel therapy was recommended to the renal provider for 12 patients and was successfully implemented in 5 patients. All 28 patients in the nurse management arm (100%) who reported depression during the observation phase described this symptom during the intervention phase, 22 of whom accepted a nurse-generated treatment recommendation. New treatment was recommended to the provider for 11 patients, 6 of whom had the treatment successfully implemented. Over the 12-month intervention phase, nurse-generated treatment recommendations were successfully implemented on 22 occasions for pain, 8 occasions for ED, and 6 occasions for depression. Among nurse management patients who were receiving pharmacologic treatment for pain, ED, or depression at the time randomization, 58%, 28%, and 67%, respectively were prescribed a new medication or had a change in the dose of an existing treatment during the intervention.
Intervention Effects
In adjusted analyses, our results (presented as the Δ symptom score [95% confidence interval]) showed that patients in the nurse management arm did not experience improvements in pain, (0.49 [−0.56, 1.54]), ED (0.2; [−0.55, 0.95]), or depression (0.32 [−0.94, 1.58]) compared with patients in the feedback arm (Table 4). Relative to their symptom scores during the observational phase, patients in the feedback arm experienced small, albeit statistically significant reductions in pain (−0.98 [−1.67, −0.28]), ED (−0.98 [−1.54, −0.41]), and depression (−1.36 [−2.19, −0.54]) during the intervention phase. Similarly, patients in the nurse management arm experienced clinically small, statistically significant improvements in ED (−0.78 [−1.41, −0.15]) and depression (−1.04 [−2.04, −0.04]) from the observation to the intervention phase of the study.
Table 4.
Comparison of intervention effects on symptoms
| Intervention Effects | Pain | Erectile Dysfunction | Depression | |||
|---|---|---|---|---|---|---|
| Δ Symptom Scorea | 95% CI | Δ Symptom Score | 95% CI | Δ Symptom Score | 95% CI | |
| Nurse management versus feedback | 0.49 | −0.56, 1.54 | 0.20 | −0.55, 0.95 | 0.32 | −0.94, 1.58 |
| Feedback versus observation | −0.98b | −1.67, −0.28 | −0.98b | −1.54, −0.41 | −1.36b | −2.19, −0.54 |
| Nurse management versus observation | −0.46 | −1.30, 0.33 | −0.78c | −1.41, −0.15 | −1.04c | −2.04, −0.04 |
95% CI, 95% confidence interval.
Denotes mean change in symptom scores.
P<0.01.
P<0.05.
We also conducted sensitivity analyses in which we adjusted our primary models for interactions between the three symptoms. There were no clinically meaningful differences in the results accounting for such interactions; however, the nurse management arm was slightly less effective at treating ED in men with depression and slightly more effective at treating ED for men with pain. Finally, we conducted additional analyses using a composite outcome composed of all three symptoms. To generate this summary symptom outcome, we standardized all symptom scores and summed and re-standardized the scores. These analyses resulted in findings that were unchanged from our primary models.
Discussion
Compared with informing renal providers of their patients’ pain, ED, and depression and leaving treatment to their discretion, a symptom management strategy in which trained nurses evaluated patients, generated treatment recommendations, and facilitated the provision of therapy did not result in improvement in these symptoms. However, compared with usual care, both strategies led to clinically small, albeit statistically significant, reductions in symptoms. These findings should inform therapeutic approaches to the management of these symptoms in this patient population.
There are several likely reasons that our nurse management strategy was not more effective than feedback. First, not all symptomatic patients in the nurse management arm were willing to accept nurse-generated treatment recommendations. Polypharmacy is common in the dialysis population and some patients may have been uninterested in taking additional medications or considered their symptoms too mild to warrant more aggressive treatment (33). Patients may also have felt uncomfortable agreeing to therapy recommended by research nurses that were not members of their dialysis treatment team. Second, renal providers did not routinely agree to treatment recommendations generated by the nurse interventionists. This may relate to providers' unfamiliarity with or concern for adverse effects of pharmacologic treatments and/or reluctance to approve therapy recommended by individuals with whom they did not have established professional relationships. Future efforts to involve experienced dialysis nurses who are part of patients' treatment team in the management of symptoms may enhance the likelihood that therapeutic interventions would be acceptable to patients and renal providers.
We did observe small, albeit statistically significant improvements in symptoms in both study arms during the intervention phase relative to usual care. This suggests that screening, quantifying the severity of, and informing providers about their patients' pain, ED, and depression are potentially important. However, it is clearly not sufficient to result in substantial symptom alleviation. Even if patients are interested in being treated, there are several potential obstacles to the successful implementation of new treatment. First, many renal providers may be disinclined to prescribe pharmacologic or nonpharmacologic therapy. Recently published data from our group demonstrate that many renal providers believe it is the responsibility of primary care providers to institute treatment for pain, ED, and depression in patients on hemodialysis (34). However, past research demonstrates that 65%–80% of chronic dialysis patients do not have a primary care provider (35,36). It thus seems essential to make efforts to reconcile renal providers' views on the provision of symptom-directed therapy with the reality that many hemodialysis patients do not have primary providers and believe that their nephrologist serves this function. Second, there are likely financial/insurance-related barriers to the successful provision of treatment. Interestingly, we did not find differences in treatment between the Veterans Affairs site, which has a hospital-based formulary that provides medications at low cost, and non-Veterans Affairs sites. Nonetheless, attempts to elucidate which of these reasons explains the infrequent implementation of therapy will broaden our understanding of these symptoms and help inform future efforts to improve the provision of treatment to amenable patients.
Our findings underscore the important differences between “efficacy” and “effectiveness” studies. Several prior efficacy studies demonstrate that pharmacologic therapy for pain, ED, and depression is safe and efficacious in patients on chronic hemodialysis (17–20,37). However, these studies evaluated symptom-directed interventions in symptomatic patients who were interested in and eligible to receive such treatment. Conversely, our trial had much broader inclusion criteria and tested the effectiveness of symptom management strategies in the “real-world” clinical setting. Our findings demonstrate that establishing the safety and efficacy of treatment for these symptoms does not necessarily translate into their routine implementation and effectiveness in the clinical setting. This may explain why several past observational studies showed that patients on chronic hemodialysis with pain, ED, and depression commonly do not receive pharmacologic therapy for these symptoms (11,16,23). Implementation studies, which evaluate strategies to integrate efficacious treatments into clinical practice, may help improve the provision of symptom-alleviating care in candidate patients.
Our trial has limitations. First, although patients were randomized by dialysis schedule, individual providers were overseeing the care of patients from both study arms. This could have led to cross-over of treatments, biasing the results toward the null. Second, although our study was powered for the estimated effect sizes, the sample size was relatively small. Moreover, the limited number of patients who were prescribed therapy during the intervention may have reduced the capacity to observe a true effect of treatment. Third, patients were drawn from a single geographic area and were cared for by a relatively small number of renal providers (n=35), which may limit generalizability. Fourth, we used the presence of symptoms during the observation phase to define which patients were included in the analyses of the intervention effects. Fifth, although we included an observation phase that we believe reflected usual care, we did not have a control arm that was monitored contemporaneously with our interventions. Sixth, we did not formally assess whether patients were compliant with prescribed treatment. However, it is unlikely that potential noncompliance would differ across study arms. Seventh, we did not standardize the day of each month that symptoms were assessed. Finally, symptoms were identified using questionnaires administered during dialysis, which may not be the optimal time to assess symptoms. Future research on symptoms in patients on hemodialysis may benefit from approaches that rely on real-time data collection within the context in which patients live their lives.
In conclusion, a strategy for the management of pain, ED, and depression in which trained nurses generate treatment recommendations and work with patients and providers to facilitate their implementation does not result in improvements in these symptoms compared with informing renal providers of these symptoms and leaving the institution of therapy at their discretion. Both approaches help alleviate symptoms relative to usual care, but this effect is clinically insufficient. These findings should inform future efforts to determine the patient-, provider-, and system-level barriers to the provision of treatment for these and other symptoms and to identify those patients most amenable to, interested in, and likely to derive benefit from such therapy.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by a Department of Veterans Affairs Health Services Research and Development Merit Review award (IIR 07-190). The opinions expressed in this article are those of the authors and do not represent the views of the Department of Veterans Affairs or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editoral, “Improving Symptoms of Pain, Erectile Dysfunction, and Depression in Patients on Dialysis” on pages 5–7.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04450512/-/DCSupplemental.
References
- 1.Evans RW, Rader B, Manninen DL, Cooperative Multicenter EPO Clinical Trial Group : The quality of life of hemodialysis recipients treated with recombinant human erythropoietin. JAMA 263: 825–830, 1990 [PubMed] [Google Scholar]
- 2.Simmons RG, Abress L: Quality-of-life issues for end-stage renal disease patients. Am J Kidney Dis 15: 201–208, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Kimmel PL, Emont SL, Newmann JM, Danko H, Moss AH: ESRD patient quality of life: Symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am J Kidney Dis 42: 713–721, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Unruh ML, Weisbord SD, Kimmel PL: Health-related quality of life in nephrology research and clinical practice. Semin Dial 18: 82–90, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Kimmel PL, Patel SS: Quality of life in patients with chronic kidney disease: Focus on end-stage renal disease treated with hemodialysis. Semin Nephrol 26: 68–79, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Unruh M, Yan G, Radeva M, Hays RD, Benz R, Athienites NV, Kusek J, Levey AS, Meyer KB, HEMO Study Group : Bias in assessment of health-related quality of life in a hemodialysis population: A comparison of self-administered and interviewer-administered surveys in the HEMO study. J Am Soc Nephrol 14: 2132–2141, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Davison SN, Jhangri GS, Johnson JA: Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant 21: 3189–3195, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT: Physical symptoms and quality of life in patients on chronic dialysis: Results of The Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD). Nephrol Dial Transplant 14: 1163–1170, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Parfrey PS, Vavasour H, Bullock M, Henry S, Harnett JD, Gault MH: Symptoms in end-stage renal disease: Dialysis v transplantation. Transplant Proc 19: 3407–3409, 1987 [PubMed] [Google Scholar]
- 10.Weisbord SD, Carmody SS, Bruns FJ, Rotondi AJ, Cohen LM, Zeidel ML, Arnold RM: Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant 18: 1345–1352, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Davison SN: Pain in hemodialysis patients: Prevalence, cause, severity, and management. Am J Kidney Dis 42: 1239–1247, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Davison SN, Jhangri GS: The impact of chronic pain on depression, sleep, and the desire to withdraw from dialysis in hemodialysis patients. J Pain Symptom Manage 30: 465–473, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kimmel PL: Psychosocial factors in dialysis patients. Kidney Int 59: 1599–1613, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Kimmel PL: Depression in patients with chronic renal disease: What we know and what we need to know. J Psychosom Res 53: 951–956, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kimmel PL, Peterson RA, Weihs KL, Simmens SJ, Alleyne S, Cruz I, Veis JH: Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int 57: 2093–2098, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Rosas SE, Joffe M, Franklin E, Strom BL, Kotzker W, Brensinger C, Grossman E, Glasser D, Feldman HI: Prevalence and determinants of erectile dysfunction in hemodialysis patients. Kidney Int 59: 2259–2266, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Barakzoy AS, Moss AH: Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol 17: 3198–3203, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Seibel I, Poli De Figueiredo CE, Telöken C, Moraes JF: Efficacy of oral sildenafil in hemodialysis patients with erectile dysfunction. J Am Soc Nephrol 13: 2770–2775, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Levy NB, Blumenfield M, Beasley CM, Jr, Dubey AK, Solomon RJ, Todd R, Goodman A, Bergstrom RR: Fluoxetine in depressed patients with renal failure and in depressed patients with normal kidney function. Gen Hosp Psychiatry 18: 8–13, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Blumenfield M, Levy NB, Spinowitz B, Charytan C, Beasley CM, Jr, Dubey AK, Solomon RJ, Todd R, Goodman A, Bergstrom RF: Fluoxetine in depressed patients on dialysis. Int J Psychiatry Med 27: 71–80, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Rosas SE, Wasserstein A, Kobrin S, Feldman HI: Preliminary observations of sildenafil treatment for erectile dysfunction in dialysis patients. Am J Kidney Dis 37: 134–137, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Weisbord SD, Fried LF, Mor MK, Resnick AL, Unruh ML, Palevsky PM, Levenson DJ, Cooksey SH, Fine MJ, Kimmel PL, Arnold RM: Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol 2: 960–967, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Watnick S, Kirwin P, Mahnensmith R, Concato J: The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis 41: 105–110, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D: Collaborative care for patients with depression and chronic illnesses. N Engl J Med 363: 2611–2620, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Korff M, Katon WJ, Lin EH, Ciechanowski P, Peterson D, Ludman EJ, Young B, Rutter CM: Functional outcomes of multi-condition collaborative care and successful ageing: Results of randomised trial. BMJ 343: d6612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisbord SD, Shields AM, Mor MK, Sevick MA, Homer M, Peternel J, Porter P, Rollman BL, Palevsky PM, Arnold RM, Fine MJ: Methodology of a randomized clinical trial of symptom management strategies in patients receiving chronic hemodialysis: The SMILE study. Contemp Clin Trials 31: 491–497, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A: The mini-cog: A cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 15: 1021–1027, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Melzack R: The short-form McGill Pain Questionnaire. Pain 30: 191–197, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB: The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 16: 606–613, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watnick S, Wang PL, Demadura T, Ganzini L: Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis 46: 919–924, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM: Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 11: 319–326, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Cappelleri JC, Rosen RC: The Sexual Health Inventory for Men (SHIM): A 5-year review of research and clinical experience. Int J Impot Res 17: 307–319, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R: Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 4: 1089–1096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green JA, Mor MK, Shields AM, Sevik MA, Palevsky PM, Fine MJ, Arnold RM, Weisbord SD: Renal provider perceptions and practice patterns regarding the management of pain, sexual dysfunction, and depression in hemodialysis patients. J Palliat Med 15: 163–167, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Nespor SL, Holley JL: Patients on hemodialysis rely on nephrologists and dialysis units for maintenance health care. ASAIO J 38: M279–M281, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Shah N, Dahl NV, Kapoian T, Sherman RA, Walker JA: The nephrologist as a primary care provider for the hemodialysis patient. Int Urol Nephrol 37: 113–117, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Duarte PS, Miyazaki MC, Blay SL, Sesso R: Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int 76: 414–421, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.