Summary
Background and objectives
Patients with AKI after lung transplantation are at increased risk for CKD and death. Whether patients who completely recover from AKI have improved long-term outcome compared with patients who do not completely recover remains unknown.
Design, setting, participants, & measurements
This study retrospectively evaluated data on 657 patients who underwent lung transplantation from 1997 to 2009. Outcomes analyzed were the incidence of renal recovery after AKI and the association of this recovery with short- and long-term mortality. AKI was defined by an absolute increase in serum creatinine of ≥0.3 mg/dl or a percent increase in serum creatinine of ≥50% from baseline at any time during the first 2 weeks after transplantation.
Results
Four hundred twenty-four (65%) patients experienced AKI in the first 2 weeks after transplantation. Of these patients, complete renal recovery occurred in 142 (33%) patients. The incidence of in-hospital complications was similar between patients who recovered renal function and patients without recovery. At 1 year, the cumulative incidence of CKD was 14% and 22% (P=0.10) and patient survival rate was 81% and 76% (P=0.20) in patients with complete recovery from AKI and patients without recovery, respectively. Patients with completely recovered AKI had similar risk-adjusted long-term mortality compared with patients who did not recover (hazard ratio [95% confidence interval]=1.42 [1.15–2.05] versus 1.53 [1.01–2.00]).
Conclusions
Patients who recover completely from early AKI after lung transplantation have a similar risk for CKD and long-term mortality compared with patients who do not recover.
Introduction
AKI occurs in 50%–60% of patients after lung transplantation (1). Patients who develop AKI early in the postoperative period are at increased risk for morbidity and mortality compared with patients without AKI (2). Recent studies in different populations and hospital settings have shown that even the mildest form of AKI is associated with long-term adverse events (3). However, information is limited, especially in the transplant population, regarding the impact of recovery from an AKI event on relevant patient outcomes (4). Most studies that address renal recovery consider only critically ill patients requiring dialysis and declare renal recovery to be dialysis independency at hospital discharge (5). The effect of recovery from smaller—but still significant—postoperative AKI events in lung transplant patients remains largely unknown.
Therefore, the aim of our study is to evaluate whether lung transplant patients with complete recovery from AKI remain at high risk for adverse outcomes or whether their risk compares favorably with patients who did not experience AKI in the postoperative period.
Materials and Methods
Study Population
We performed a retrospective cohort study of 703 consecutive adult patients who underwent lung transplantation (other solid and/or combined organ transplants were not included) at Cleveland Clinic between August of 1997 and June of 2009. After Institutional Review Board approval, patient demographics, laboratory data, and outcomes were obtained from electronic medical records, paper charts, the Universal Transplant Database, and the Cardiothoracic Anesthesia Registry. Patients were excluded from analysis for the following reasons: missing data (35 patients), prior lung transplants (10 patients), and preoperative dialysis (1 patient); 657 patients remained for analysis.
Data Collection
Data collection included baseline demographic variables, patient characteristics, and comorbidities (Table 1). Perioperative data included hemodynamics parameters, requirement for vasopressors or inotropic agents, postoperative mechanical ventilation, fraction of inspired oxygen (FiO2), and partial pressure of oxygen in arterial blood (PaO2) at the time of intensive care unit (ICU) admission. Creatinine levels were recorded daily from the date of transplant until the date of discharge, at 1 and 6 months, and at 1, 2, 3, 4, and 5 years. The lung allocation score most proximate to transplant was collected. Cyclosporine and tacrolimus levels were collected for the first 2 weeks after transplantation. In-hospital postoperative complications were categorized as follows: cardiac events (atrial and ventricular arrhythmias, heart block, or permanent pacer placement); respiratory events (acute respiratory distress syndrome, aspiration, pleural effusion requiring thoracentesis, pneumothorax, pulmonary embolus, pulmonary hemorrhage, hemothorax/chylothorax, or respiratory failure); and infectious events (fungemia/bacteremia/line sepsis/septic shock, empyema, endocarditis, mediastinitis, wound infection, pneumonia, or urinary tract infection).
Table 1.
Baseline characteristics of lung transplant recipients stratified by recovery status after AKI
| No AKI (n=233; 35%) | AKI with Recovery (n=142; 22%) | AKI with No Recovery (n=282; 43%) | P Valuea | |
|---|---|---|---|---|
| Age (yr) | 57 (48–62) | 50 (40–59)b | 57 (50–62)c | <0.001 |
| Male | 129 (55%) | 86 (61%) | 173 (61%) | 0.32 |
| White | 219 (94%) | 130 (92%) | 258 (91%) | 0.51 |
| Primary diagnosis leading to transplantation | <0.001 | |||
| Chronic obstructive pulmonary disease | 102 (44%) | 36 (26%)b | 95 (33%)b | <0.001 |
| Idiopathic pulmonary fibrosis | 80 (34%) | 42 (30%) | 90 (32%) | 0.66 |
| Cystic fibrosis | 24 (10%) | 24 (17%) | 42 (15%) | 0.14 |
| α1-Antitrypsin deficiency | 9 (4%) | 6 (4%) | 14 (5%) | 0.83 |
| Pulmonary hypertension | 1 (1%) | 11 (8%)b | 8 (3%) | <0.001 |
| Other | 17 (7%) | 23 (16%)b | 33 (12%) | 0.02 |
| Type of lung transplantation | 0.01 | |||
| Double | 119 (51%) | 94 (66%) | 159 (56%) | |
| Single | 114 (49%) | 48 (34%) | 123 (44%) | |
| Pretransplantation comorbidites | ||||
| Diabetes | 23 (10%) | 18 (13%) | 58 (20%)b | <0.001 |
| Hypertension | 57 (24%) | 38 (27%) | 87 (31%) | 0.28 |
| Dyslipidemia | 48 (21%) | 21 (15%) | 65 (23%) | 0.14 |
| Medications | ||||
| RAAS blockade | 38 (16%) | 29 (21%) | 55 (20%) | 0.54 |
| Statin | 62 (27%) | 34 (25%) | 72 (26%) | 0.85 |
| Calcineurin inhibitors | 0.05 | |||
| TAC | 207 (89%) | 114 (80%) | 233 (83%) | |
| CsA | 26 (11%) | 28 (20%) | 49 (17%) | |
| Mean CsA level (ng/ml) | 429±68 | 421±60 | 426±86 | 0.91 |
| Mean TAC level (ng/ml) | 13±2.8 | 13±2.6 | 14±2 | 0.64 |
| Baseline creatinine (mg/dl) | 0.69±0.18 | 0.74±0.24b | 0.75±0.19b | <0.001 |
| Baseline eGFR (ml/min per 1.73 m2)d | 105±18 | 105±23 | 101±20 | 0.03 |
| Baseline eGFR<60 | 0 | 2 (1.4%) | 5 (1.7%) | 0.04 |
| LAS (n=366) | 36 (32–44) | 41 (35–56) | 39 (34–50) | 0.10 |
| Body mass index (kg/m2) | 25±8 | 25±5 | 25±5 | 0.33 |
Normally distributed continuous variables are expressed as mean ± SD. Non-normally distributed continuous variables are expressed as medians (25th to 75th percentiles). RAAS, renin-angiotension-aldosterone; TAC, tacrolimus; CsA, cyclosporine; eGFR, estimated GFR; LAS, lung allocation score.
P value for trend comparison across all three groups.
P<0.02 (Bonferonni correction) for comparison between AKI with recovery or no recovery and no AKI.
P<0.02 was considered significant (Bonferonni correction) for comparison between AKI with recovery and AKI with no recovery.
eGFR was estimated using the CKD Epidemiology Collaboration equation.
Definitions
AKI was defined by an abrupt (within 48 hours) absolute increase in the serum creatinine of ≥0.3 mg/dl from baseline or a percentage increase in the serum creatinine of ≥50% from baseline at anytime during the first 2 weeks after transplantation and classified according the AKI Network criteria into three stages: (1) stage 1 was defined as an increase in serum creatinine of ≥0.3 mg/dl or increase by 1.5- to 1.9-fold from baseline, (2) stage 2 was defined as a >2.0- to 2.9-fold increase in creatinine, and (3) stage 3 was defined as a >3.0-fold increase in creatinine or initiation of renal replacement therapy (6). Renal recovery after an AKI event was defined by return to baseline creatinine at any time during the initial hospital stay after transplantation (i.e., complete recovery). GFR was estimated using the CKD Epidemiology Collaboration equation. CKD was defined according to the clinical practice guidelines of the National Kidney Foundation (7) CKD stages 3 (estimated GFR [eGFR]=30–59 ml/min per 1.73 m2), 4 (eGFR =5–29 ml/min per 1.73 m2), and 5 (eGFR<15 ml/min per 1.73 m2). The onset of ESRD was defined by the need for renal replacement therapy for 8 weeks with no recovery on follow-up or need for kidney transplant.
Outcome Measures
The primary outcome was all-cause mortality on long-term follow-up. Secondary outcomes included (1) combined cumulative incidence of CKD, which was defined as the occurrence of stages 4 or 5 or ESRD, (2) hospital mortality, and (3) respiratory, cardiac, or infectious complications postlung transplantation. Death was determined using the medical records and Social Security Death Index.
Statistical Analyses
Descriptive statistics were calculated for the entire study population and expressed as mean ± SD for normally distributed continuous variables, median (25th to 75th percentiles) for non-normally distributed continuous variables, or n (%) for categorical variables. We compared variables between individuals with and without recovery after AKI by using the t, Wilcoxon rank sum, chi-squared, and Fischer exact tests as appropriate. Multivariable logistic regression analyses were performed to identify covariates associated with no recovery from AKI. Kaplan–Meier analysis assessed time to death and/or development of CKD stratified by recovery status after AKI. Multivariate Cox proportional hazard models were constructed to investigate independent associations between recovery status after AKI and CKD or patient death. Covariates used in the model were age, sex, race, pretransplant comorbidites (hypertension, diabetes, and dyslipidemia), body mass index, type of lung transplant, diagnosis of lung failure, baseline creatinine, lung allocation score at time of transplant, post-transplant comorbidites (cardiac, infectious, and pulmonary), FiO2/PaO2 at ICU admission, extracorporeal membrane oxygenation use, and post-transplant comorbidites (hypertension, diabetes, and dyslipidemia). P values<0.05 were considered significant. For multiple comparisons with three groups, the Bonferroni correction was applied, and P<0.02 was considered significant. All statistical analyses were performed using JMP 9.0 (SAS Institute, Cary, NC).
Results
Recovery from AKI after Lung Transplantation
Of 657 patients included in the final analysis, 424 (65%) patients developed AKI in the first 2 weeks after lung transplantation. Of patients with AKI, complete renal recovery occurred in 142 (33%) patients before hospital discharge. Table 1 represents the baseline clinical characteristics of patients who did not experience AKI, suffered AKI but recovered completely, and suffered AKI but did not recover completely. The mean age of the study cohort was 53±12 years, with 60% being male and 92% white. There were no differences in baseline characteristics between the patients who had AKI with complete recovery compared with the patients with no recovery, except that the latter group was older. Compared with patients with no AKI, patients who had AKI with complete recovery were younger and more likely to have pulmonary hypertension as the reason for transplant. However, patients with AKI with no recovery were more likely to be diabetic compared with patients with no AKI. Chronic obstructive pulmonary disease was more common in patients with no AKI. Baseline creatinine was higher in the groups who had AKI (regardless of if they recovered) compared with patients with no AKI. Confounded by the equation’s lack of accuracy to predict true GFR when renal function is in the normal range, Table 1 shows the baseline eGFR by CKD Epidemiology Collaboration equation for each group. Patients with no recovery had higher peak creatinine (1.9±1.2 versus 1.5±0.6 mg/dl, P<0.001) and creatinine at discharge (1.04±0.4 versus 0.68±0.2 mg/dl, P<0.001) compared with patients with renal recovery.
On univariate analysis, age, pulmonary hypertension, pretransplant diabetes and dyslipidemia, preoperative mechanical ventilation, reintubation after first extubation, and more severe AKI stage associated with no recovery from AKI. Except for hyperlipidemia, these variables remained independently associated with no recovery from AKI in the multivariable analysis (Table 2).
Table 2.
Predictors of nonrecovery from AKI after lung transplantation
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Age (yr) | 1.02 (1.01–1.04) | <0.001 | 1.03 (1.01–1.05) | <0.001 |
| Pulmonary hypertension | 0.34 (0.13–0.88) | 0.02 | 0.22 (0.06–0.69) | 0.001 |
| Pretransplantation diabetes | 1.70 (1.01–3.21) | 0.04 | 1.86 (1.00–3.59) | 0.04 |
| Pretransplantation dyslipidemia | 1.71 (1.01–2.94) | 0.04 | 1.30 (0.72–2.40) | 0.38 |
| Preoperative mechanical ventilation | 0.24 (0.06–0.77) | 0.02 | 0.22 (0.05–0.80) | 0.02 |
| Reintubation | 0.61 (0.40–0.94) | 0.02 | 0.48 (0.29–0.78) | 0.003 |
| AKIN | ||||
| AKIN-1 | Reference | Reference | ||
| AKIN-2 | 3.36 (1.45–9.18) | 0.003 | 3.92 (1.61–11.16) | 0.001 |
| AKIN-3 | 3.81 (2.05–7.68) | <0.001 | 6.78 (3.32–15.2) | <0.001 |
Multivariate model included only variables that were significantly associated with nonrecovery on univariate analysis. CI, confidence interval; AKIN, AKI network.
In-Hospital Morbidity and Mortality Associates with Recovery Status after AKI
Table 3 summarizes the perioperative hemodynamic and postoperative ICU events and in-hospital mortality. Patients with AKI versus no AKI had higher rates of in-hospital complications (cardiac, infectious, and pulmonary), perioperative requirement for pressor/inotropic agents, reintubation, and longer times on mechanical ventilation. Conversely, ICU complications were remarkably similar between AKI with recovery versus no recovery patients, except that patients with no recovery had more severe forms of AKI. The overall median (25th to 75th percentiles) hospital stay was 14 (10–25) days. The median length of stay was significantly longer in patients who had AKI, but it was not different between the recovery and no recovery groups [17 (12–32) versus 16 (12–28) days, P=0.12]. There were 35 (5%) deaths at hospital discharge, with a significantly higher in-hospital mortality rate based on AKI status: n=2 (1%) in the no AKI group, n=6 (4%) in the AKI with recovery group, and n=27 (10%) in the AKI with no recovery group (P<0.001 comparing AKI with no recovery versus no AKI; P=0.03 comparing AKI with recovery versus no AKI; P=0.05 comparing AKI with recovery versus AKI with no recovery).
Table 3.
Post-transplant hospital events stratified by recovery status after AKI
| No AKI (n=233; 35%) | AKI with Recovery (n=142; 22%) | AKI with No Recovery (n=282; 43%) | P Valuea | |
|---|---|---|---|---|
| Intraoperative use of inotrope/vasopressors | 141 (61%) | 104 (76%)b | 213 (76%)b | 0.001 |
| Intraoperative central venous pressure (cmH2O)c | 14±5 | 15±6 | 15±6 | 0.13 |
| Intraoperative mean arterial pressure (mm)c | 89±16 | 85±15b | 87±16b | 0.01 |
| Inotrope/vasopressors use in ICU | 141 (61%) | 104 (74%)b | 211 (75%)b | 0.001 |
| PaO2/FiO2 at ICU admission | 298±121 | 247±128b | 274±137b | <0.001 |
| Extracorporeal membrane oxygenation | 1 (0.5%) | 3 (2%) | 8 (3%)b | 0.08 |
| Reintubation | 34 (15%) | 57 (40%)b | 83 (30%)b | <0.001 |
| Days on mechanical ventilation | 2±2 | 4±7b | 4±6b | <0.001 |
| Primary graft dysfunction (n=146) | 2/49 (4%) | 4/21 (19%)b | 11/76 (14%)b | 0.08 |
| Cardiac complication in ICU | 30 (13%) | 41 (29%)b | 92 (32%)b | <0.001 |
| Respiratory complication in ICU | 37 (16%) | 45 (32%)b | 92 (33%)b | <0.001 |
| Infectious complication in ICU | 15 (6%) | 40 (28%)b | 67 (24%)b | <0.001 |
| Renal complication in ICU | ||||
| AKIN-1 | 124 (88%) | 184 (66%)d | <0.001 | |
| AKIN-2 | 6 (4%) | 30 (10%)d | <0.001 | |
| AKIN-3 | 12 (8%) | 68 (24%)d | <0.001 | |
| Renal replacement therapy | 7 (5%) | 38 (13%)d | <0.001 | |
| Median length of stay (25th to 75th percentile; d) | 11 (9–17) | 17 (12–32) | 16 (12–28)b | <0.001 |
| Death at hospital discharge | 2 (1%) | 6 (4%) | 27 (10%)b | <0.001 |
Plus–minus values are mean ± SD. Cardiac complication is asystole/atrial arrhythmia/ventricular tachycardia or fibrillation/heart block/permanent pacer. Respiratory complication is acute respiratory distress syndrome/aspiration/tapped pleural effusion/pneumothorax/pulmonary embolus/pulmonary hemorrhage/hemothorax/chylothorax/respiratory failure. Infection complication is fungemia/bacteremia/line sepsis/septic shock/empyema/endocarditis/mediastinitis/wound infection/pneumonia/urinary tract infection. ICU, intensive care unit; PaO2, partial pressure of oxygen in arterial blood; FiO2, fraction of inspired oxygen; AKIN, AKI network.
P value for trend comparison across all three groups.
P<0.02 was considered significant (Bonferonni correction) for comparison between AKI with recovery or no recovery and no AKI.
Hemodynamics at time of anesthesia induction.
P<0.02 was considered significant (Bonferonni correction) for comparison between AKI with recovery and AKI with no recovery.
CKD and All-Cause Mortality after Recovery from AKI
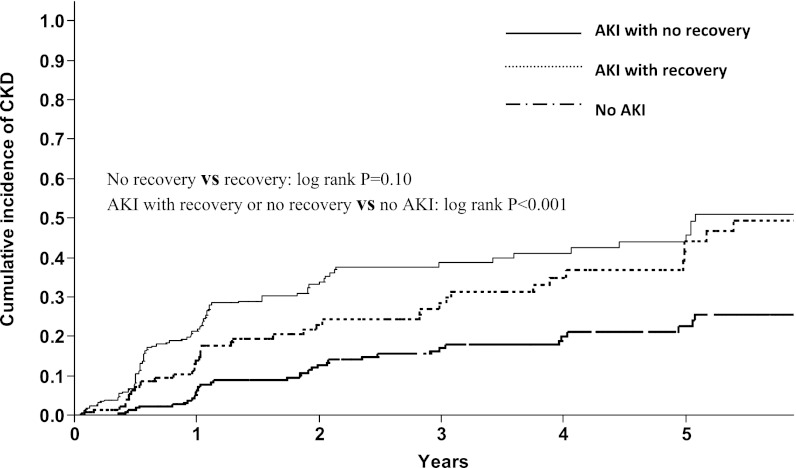
After a median follow-up of 1.6 (0.7–3.8) years, CKD developed in 164 (25%) patients. Figure 1 shows the cumulative incidence of CKD stratified by recovery status from AKI. At 1 year, the cumulative incidence of CKD was 6%, 14%, and 22% in the no AKI, AKI with recovery, and AKI with no recovery groups, respectively. At 5 years, the cumulative incidence was 22%, 44%, and 45% in the no AKI, AKI with recovery, and AKI with no recovery groups, respectively. The cumulative incidence of CKD was not different between the recovery and no recovery cohorts (P=0.10). Table 4 shows the findings of the multivariate Cox proportional hazard analysis investigating predictors of CKD development. After adjusting for appropriate covariates, the occurrence of an AKI event, whether recovered (hazard ratio [HR]=2.19, 95% confidence interval [CI]=1.34–3.55) or not recovered (HR=2.84, 95% CI=1.89–4.33), remained independently predictive of subsequent CKD in survivors.
Figure 1.
The cumulative incidence of CKD after lung transplantation was not different in patients with recovery or no recovery from AKI.
Table 4.
Risk of CKD and death after lung transplantation stratified by recovery status from AKI
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| CKD | ||||||||
| No AKI | Reference | Reference | Reference | Reference | ||||
| AKI with recovery | 2.13 | 1.36–3.34 | 2.73 | 1.71–4.37 | 2.39 | 1.47–3.86 | 2.19 | 1.34–3.55 |
| AKI with no recovery | 2.93 | 2.01–4.37 | 3.29 | 2.23–4.94 | 3.01 | 2.03–4.55 | 2.84 | 1.89–4.33 |
| Long-term mortality | ||||||||
| No AKI | Reference | Reference | Reference | Reference | ||||
| AKI with recovery | 1.53 | 1.43–2.40 | 1.68 | 1.22–2.30 | 1.74 | 1.41–2.44 | 1.42 | 1.15–2.05 |
| AKI with no recovery | 1.85 | 1.13–2.07 | 1.79 | 1.37–2.35 | 1.85 | 1.25–2.40 | 1.53 | 1.01–2.00 |
Model 1 was adjusted for age, sex, race, pretransplant comorbidites (hypertension, diabetes, and dyslipidemia), and body mass index. Model 2 was adjusted for model 1 plus type of lung transplant, diagnosis of lung failure, baseline creatinine, and lung allocation score at the time of transplant. Model 3 was adjusted for model 2 plus immediate post-transplant comorbidites (cardiac, infectious, and pulmonary), fraction of inspired oxygen/partial pressure of oxygen in arterial blood at intensive care unit admission, extracorporeal membrane oxygenation use, and post-transplant comorbidites (hypertension, diabetes, and dyslipidemia). HR, hazard ratio; CI, confidence interval.
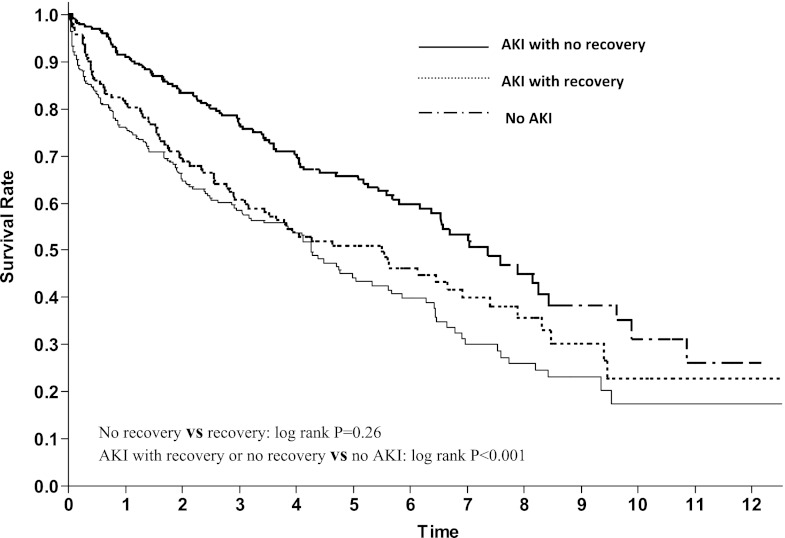
After a median follow-up of 2.9 (1.5–5.5) years, 325 (49%) patients died: 92 (39%) patients in the no AKI group versus 233 (55%) patients in the AKI group. The Kaplan–Meier analysis showed that 1-year patient survival was 91%, 81%, and 76% in the no AKI, AKI with recovery, and AKI with no recovery groups, respectively (Figure 2). There was no difference in the rate of mortality between the recovered and nonrecovered cohorts (P=0.26). After adjusting for demographics, pre- and post-transplant comorbid conditions, renal function, and in-hospital events, patients with completely recovered AKI (HR=1.42, 95% CI=1.15–2.05) and unrecovered AKI (HR=1.53, 95% CI=1.01–2.00) suffered the same degree of increased mortality post-transplant (Table 4).
Figure 2.
Long-term mortality after lung transplantation was similar in patients with recovery or no recovery from AKI.
Discussion
This study shows that AKI is a common complication after lung transplantation and that it is associated with higher rates of adverse hospital events and long-term mortality. Up to one-third of patients who had AKI recovered completely to baseline kidney function. Moreover, patients who recover from AKI remain at higher risk for morbidity and mortality compared with patients with no AKI to a similar degree as those patients with nonrecovered AKI. To our knowledge, little information is available to describe the association between renal recovery and long-term mortality in a large population of lung transplant recipients. This study highlights that even complete recovery from a prior AKI event does not protect the patient from suffering potential adverse outcomes that associate with AKI.
Previous studies have focused on the incidence, predictors, and outcomes of AKI events after lung transplant (1), and they often fail to use more contemporary definitions that highlight the occurrence and significance of less severe AKI events not requiring dialysis. Additionally, given that most definitions of renal recovery have focused on freedom from dialysis need at hospital discharge (8), the impact of recovery from a small but significant increment in creatinine remains largely unknown. We used very restrictive criteria of renal recovery (i.e., return to baseline creatinine) in an attempt to best describe the outcome of patients who were able to return to their baseline renal status after kidney injury. This strict definition of recovery was used to avoid misclassifying partially recovered AKI as complete recovery, which would otherwise bias the analysis. Applying such a definition, we found that 33% of patients who had AKI had complete recovery. Interestingly, a similar percentage has been reported in nontransplant patients who underwent coronary artery bypass graft surgery (9). To our surprise, there were no differences in the rate of in-hospital adverse events between the recovered and nonrecovered cohorts. Often, in the clinical setting, the expectation may be that patients who recover from an AKI event are healthier and less at risk. This study calls that expectation into question.
Other notable findings were seen in this study. We found that patients who required mechanical ventilation before surgery or reintubation after transplantation were more likely to develop AKI. Previous work suggested that renal vasoconstriction mirrors the degree of respiratory insufficiency (10) and renal function impairment that is more often observed in patients with severe respiratory failure and subsequent resolution after improvement in their respiratory condition (11). Additionally, of interest, despite the fact that patients with pulmonary hypertension were more likely to experience AKI, there was a trend for higher rate of recovery from such injury. In these patients generally, right-sided heart failure is present, and the resulting cardiorenal pathophysiology may impair renal perfusion and elicit a state of intense renal vasoconstriction (12). Thus, post-transplant, these patients may be simultaneously at higher risk of a hemodynamic-mediated temporary decline in GFR and more apt to recover function with eventual improvement in their circulatory status and renal perfusion, thus explaining return of their creatinine to, at least, their preoperative baseline.
We and others have shown that AKI, regardless of its severity, is associated with long-term mortality (13,14). Our current study adds to the literature that even patients who recover from such injury continue to have an increased risk of mortality on either short- or long-term follow-up. It is speculative why recovered AKI patients may develop complications post-transplant at the same rates as unrecovered patients. One hypothesis is that a less severe AKI event itself does not lead to damage but merely reflects a sicker patient population, which has less renal reserve that predictably may experience higher subsequent complication rates, regardless of whether they actually returned to their baseline creatinine after the initial event. Indeed, renal blood flow and clearance can remain impaired for a long period of time after an episode of AKI, despite apparent return of creatinine to baseline (15,16). Furthermore, AKI patients, regardless of recovery status, remain hospitalized for a longer duration and thus, may be at increased risk for multiple medical complications, such as infections, cardiac events, exposure to nephrotoxic medications, recurrent AKI events, and more importantly, graft dysfunction and failure, that could lead to increased risk of mortality irrespective of recovery state. Indeed, compared with the no AKI cohort, the rates of infectious, cardiac, and pulmonary complications were identical in the recovered and nonrecovered cohorts. However, it is also plausible that, biologically, an AKI event is more similar than different notwithstanding recovery status. Animal studies have shown that there is a decrease in density of peritubular capillaries and acute endothelial injury leading to vascular dropout and nephron loss followed by glomerular hypertrophy and fibrosis after sustaining AKI, irrespective of recovery state (17). These findings may explain the similar higher rates of CKD between both AKI cohorts.
There are notable limitations to our study. This study was a retrospective analysis and thus, is subject to the limitations inherent in this type of study. All retrospective investigations may be subject to selection bias, although our analysis included all patients undergoing specific lung transplant surgeries within a certain time period. Although our analysis adjusted for multiple baseline variables and perioperative characteristics, other unmeasured variables could significantly affect the association with outcomes and therefore, confound the results. We did not have data on proteinuria, which is an important risk factor for AKI as well as the definition of CKD. We did not have complete data on primary graft dysfunction (PGD), which is an important factor that predicts mortality in lung transplant patients. However, subanalysis for the 146 patients with complete data on graft dysfunction did not change the result when PGD (defined by PGD grade 3 at 72 hours) was used in the proportional hazard model. Additionally, in final model, we used factors that could be surrogate markers for PGD, such as PaO2/FiO2 and respiratory events. We did not have information on the occurrence of subsequent readmissions and/or AKI events after the initial transplant hospitalization. Additionally, the retrospective nature of this study precludes mechanistic insights into the association of renal recovery and long-term outcomes and whether a preventive strategy for AKI would have influenced patient outcomes. Finally, it is imperative to note that the results of our study apply to a very specific population with a high incidence of AKI and cannot necessarily be generalized to different populations.
In conclusion, our single-center experience shows that patients who recover completely from AKI early after lung transplantation continue to be at similar increased risk for CKD and mortality as patients who do not recover compared with patients with no AKI. This finding may imply that any AKI event, regardless of recovery, identifies a higher-risk cohort of patients that, predictably, may do worse over time and deserves closer attention.
Disclosures
None.
Acknowledgments
A.E.D. is supported by National Institutes of Health Grant 1K23HL093065-01A2.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rocha PN, Rocha AT, Palmer SM, Davis RD, Smith SR: Acute renal failure after lung transplantation: Incidence, predictors and impact on perioperative morbidity and mortality. Am J Transplant 5: 1469–1476, 2005 [DOI] [PubMed] [Google Scholar]
- 2.González Castro A, Llorca J, Suberviola Cañas B, Fernández-Miret B, Zurbano F, Miñambres E: Acute renal failure in lung transplantation: Incidence, correlation with subsequent kidney disease, and prognostic value. Arch Bronconeumol 44: 353–359, 2008 [PubMed] [Google Scholar]
- 3.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham PT, Slavov C, Pham PC: Acute kidney injury after liver, heart, and lung transplants: Dialysis modality, predictors of renal function recovery, and impact on survival. Adv Chronic Kidney Dis 16: 256–267, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Macedo E, Bouchard J, Mehta RL: Renal recovery following acute kidney injury. Curr Opin Crit Care 14: 660–665, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 8.Bagshaw SM, Mortis G, Godinez-Luna T, Doig CJ, Laupland KB: Renal recovery after severe acute renal failure. Int J Artif Organs 29: 1023–1030, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Mehta RH, Honeycutt E, Patel UD, Lopes RD, Shaw LK, Glower DD, Harrington RA, Califf RM, Sketch MH, Jr: Impact of recovery of renal function on long-term mortality after coronary artery bypass grafting. Am J Cardiol 106: 1728–1734, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Kilburn KH, Dowell AR: Renal function in respiratory failure. Effects of hypoxia, hyperoxia, and hypercapnia. Arch Intern Med 127: 754–762, 1971 [PubMed] [Google Scholar]
- 11.Kazory A, Ducloux D: Successful management of respiratory failure can improve renal function. Am J Crit Care 18: 10–11, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Navis G, Broekroelofs J, Mannes GP, van der Bij W, de Boer WJ, Tegzees AM, de Jong PE: Renal hemodynamics after lung transplantation. A prospective study. Transplantation 61: 1600–1605, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Wehbe E, Brock R, Budev M, Xu M, Demirjian S, Schreiber MJ, Jr, Stephany B: Short-term and long-term outcomes of acute kidney injury after lung transplantation. J Heart Lung Transplant 31: 244–251, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Arnaoutakis GJ, George TJ, Robinson CW, Gibbs KW, Orens JB, Merlo CA, Shah AS: Severe acute kidney injury according to the RIFLE (risk, injury, failure, loss, end stage) criteria affects mortality in lung transplantation. J Heart Lung Transplant 30: 1161–1168, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bull G, Joekes A, Lowe K: Renal function studies in acute tubular necrosis. Clin Sci 9: 379–404, 1950 [PubMed] [Google Scholar]
- 16.Hall JW, Johnson WJ, Maher FT, Hunt JC: Immediate and long-term prognosis in acute renal failure. Ann Intern Med 73: 515–521, 1970 [DOI] [PubMed] [Google Scholar]
- 17.Basile DP: Rarefaction of peritubular capillaries following ischemic acute renal failure: A potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004 [DOI] [PubMed] [Google Scholar]