Summary
Background and objectives
Oral nutritional supplementation (ONS) was provided to ESRD patients with hypoalbuminemia as part of Fresenius Medical Care Health Plan’s (FMCHP) disease management. This study evaluated the association between FMCHP’s ONS program and clinical outcomes.
Design, setting, participants, & measurements
Analyses included FMCHP patients with ONS indication (n=470) defined as 2-month mean albumin <3.8 g/dl until reaching a 3-month mean ≥3.8 g/dl from February 1, 2006 to December 31, 2008. Patients did not receive ONS if deemed inappropriate or refused. Patients on ONS were compared with patients who were not, despite meeting ONS indication. Patients with ONS indication regardless of use were compared with Medicare patients with similar serum albumin levels from the 2007 Centers for Medicare and Medicaid Services Clinical Performance Measures Project (CPM). Cox models calculated adjusted hospitalization and mortality risks at 1 year.
Results
Among patients with indication for ONS, 276 received supplements and 194 did not. ONS use was associated with 0.058 g/dl higher serum albumin overall (P=0.02); this difference decreased by 0.001 g/dl each month (P=0.05) such that the difference was 0.052 g/dl (P=0.04) in month 6 and the difference was no longer significant in month 12 . In analyses based on ONS use, ONS patients had lower hospitalization at 1 year (68.4%; P<0.01) versus patients without ONS (88.7%), but there was no significant reduction in mortality risk (P=0.29). In analyses based on ONS indication, patients with indication had lower mortality at 1 year (16.2%) compared with CPM patients (23.4%; P<0.01).
Conclusions
These findings suggest that ONS use was associated with significantly lower hospitalization rates but had no significant effect on mortality in a disease management setting.
Introduction
Multiple reports have stressed nutritional status as a predictor of morbidity and mortality in patients with ESRD (1–4). Hemodialysis patients are at increased risk of protein energy wasting of kidney disease for several reasons (3), including poor energy and protein intake, presence of ongoing inflammation related to multiple comorbidities and vascular access type, metabolic derangements, and hemodialysis-specific factors such as bioincompatibility and amino acid losses that may lead to a persistent catabolic state (1). As shown in the Dialysis Outcomes and Practice Patterns Study (DOPPS) and others, serum albumin levels <3.5 g/dl were associated with increased mortality (5). Despite risk of adverse outcomes associated with protein energy wasting of kidney disease, there have been relatively limited changes in the nutritional management of ESRD patients over the last decade. Indeed, as indicated in the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI) clinical guidelines, there is urgent need for studies that evaluate if nutritional intervention can increase nutrition markers and if this improves outcomes (3). We evaluated the effect of oral nutritional supplementation (ONS) in the ESRD Disease Management Demonstration of the Centers for Medicare and Medicaid Services (CMS), in which Medicare Advantage Plans were contracted to develop disease management programs that delivered coordinated care interventions to ESRD beneficiaries. One participating plan, the Fresenius Medical Care Health Plan (FMCHP), initiated ONS once serum albumin was <3.8 g/dl. We hypothesized that use of ONS for the hemodialysis population in the setting of disease management is associated with lower hospitalization and all-cause mortality.
Materials and Methods
FMCHP placed patients with a mean serum albumin <3.8 g/dl on ONS. A 3-month mean was used to determine ONS eligibility. Eligibility was broadened by August 2006 when FMCHP began placing patients with 2-month mean serum albumin <3.8 g/dl on ONS. In January 2007, FMCHP obtained serum albumin values for patients before enrollment in FMCHP disease management. When available, these pre-enrollment laboratory values determined ONS eligibility. Supplements were discontinued once three month average serum albumin was ≥3.8 g/dl. In 2006, patients were provided 24 cans per month of ONS Ensure Plus (Abbott Laboratories), and were advised to consume one can per day. The number of cans given to patients allowed for days off per week, the specifics of which were left to the patients’ discretion. In 2007, Glucerna (Abbott Laboratories) was added for patients with diabetes.
Patients enrolled in FMCHP from February 1, 2006 through December 31, 2008 were included in the analyses. FMCHP’s program was implemented in Texas, Massachusetts, and Pennsylvania in 2006, and expanded to Connecticut, Alabama, Tennessee, and California in 2007. For patients who enrolled multiple times (n=20), only the first enrollment was studied. Because the CMS Disease Management Demonstration evaluated only hemodialysis patients, peritoneal dialysis patients were excluded from patient outcomes analyses.
Data included the following: (1) FMCHP data collected for the CMS Disease Management evaluation, including ONS use, comorbidity conditions measured by the Fresenius Medical Care risk score (described below), and hospitalizations; (2) FMCHP data for the CMS ESRD Quality Incentive Payment Project Demonstration, which tested the association between incentive payments and achievement of intermediate markers, including information on patient enrollment and disenrollment in FMCHP, patient demographics, and serum albumin values; (3) CMS ESRD Clinical Performance Measures Project (CPM) data (6), which provided data for analyses of serum albumin levels for the comparison population; (4) data from fee-for-service claims, the Death Notification Form CMS-2746, and the Social Security Death Master File were used to identify patient outcomes for the comparison population; and (5) CMS-2728 form was used to obtain clinical and demographic data for the comparison population.
Several adjustments were calculated from available raw data. Patient age and time on dialysis were calculated at enrollment using date of birth and date of first dialysis treatment. Vascular access was defined as the first access recorded after enrollment in FMCHP. Baseline serum albumin was defined as mean serum albumin within the first 2 months of enrollment and was used to determine indication to receive ONS.
We used FMCHP’s risk stratification scale (FMCHP risk score) for comorbidity adjustment, which was developed for patients enrolled in a separate disease management program (Renaissance Health Care) (7). The scale is adapted from the Index of Coexisting Diseases instrument used in the HEMO study (8) and consists of two subsections: section 1, which is the presence and severity of 19 disease conditions (ischemic heart disease, congestive heart failure, hypertension, respiratory disease, cerebral vascular disease, etc.), and section 2, which examines demographic characteristics, psychosocial factors, patient adherence, and utilization of services. Each area is weighted based on importance as a predictor of future outcomes. Additive risk scores are generated and then separated into three categories. Risk scores 1–10, 11–29, and >29 were placed in the low, medium, and high categories, respectively, and used to assess comorbidity burden in our analyses.
Clinical and demographic variables were compared among patients eligible to receive ONS and who received the supplement with those who were eligible but did not receive ONS. Chi-squared analyses compared discrete variables, and the t test or Wilcoxon rank-sum test was used for continuous variables. Changes in distribution of serum albumin in those who received ONS were compared with those who did not receive ONS despite eligibility. Differences in serum albumin levels between the two groups were tested using a linear mixed model allowing multiple measures per patient, and included baseline ONS, month of enrollment, and interaction between ONS and month. We were not able to perform analyses separating the effect of crossover between ONS use and nonuse due to sample size reasons.
Our primary analysis was based on ONS use and compared FMCHP patients who received ONS with those who did not receive ONS despite eligibility (average serum albumin <3.8 g/dl in the first 2 months of enrollment). ONS use was determined based on whether the patient received a delivery of nutritional supplements during the first 3 months of enrollment.
In the analyses based on ONS use, the group that did not receive ONS included patients who refused the supplement and patients for whom supplementation was deemed inappropriate for unspecified reasons. To address potential residual confounding, we performed analyses based on ONS indication. This analysis compared all FMCHP patients with indication for ONS use, regardless of actually receiving ONS, with an outside comparison group with the same indication to receive ONS. For the external comparison population, we used the 2007 CMS CPM Project (6), which collected performance data from a national random sample of adult hemodialysis and peritoneal dialysis patients on a series of clinical measures as determined by experts in the community, and also based on KDOQI clinical practice guidelines.
The analyses based on ONS indication applied a similar approach to statistical methodologies used in randomized clinical trials, which analyzed patients according to treatment randomization regardless of whether treatment was received. In such an analysis, we conservatively assume that all patients with the appropriate indication received ONS, thereby including nonadherent patients in our treatment group. This approach also avoids informative censoring for reaching the albumin target and minimizes survival bias. Analyses based on ONS indication were restricted to FMCHP patients with a mean serum albumin <3.8 g/dl in the first 2 months of enrollment (n=470). Although treatment indication was originally based on a 3-month mean, analyses based on ONS use and analyses based on ONS indication both used a 2-month window because FMCHP’s 2-month mean to determine ONS eligibility was operational most of the demonstration period.
For the CPM comparison population, the 2006 baseline and 2007 follow-up periods were selected to coincide with FMCHP’s largest period of enrollment. In all analyses, serum albumin data from the CPM Project and FMCHP were limited to patients with measurements utilizing the Bromcresol Green laboratory method.
Adjusted hospitalization and mortality percentages were calculated for analyses based on ONS use and analyses based on ONS indication, and were derived from the predicted survival curve for the average FMCHP patient with indication to receive ONS. Hazard ratios (HRs) illustrating the relationship between ONS use and time to first hospitalization and death were calculated for analyses based on ONS use but not for analyses based on ONS indication (given data access limitations for the CPM comparison population). The HRs and mortality and hospitalization percentages were estimated using Cox models censoring patients at disenrollment, end of study period, or 4 days before transplant. In analyses based on ONS use, patients in the no-ONS group were also censored if ONS was received later in enrollment.
Statistical adjustments in each set of analyses varied based on data availability for both the treatment and control groups. In the analyses based on ONS use, statistical adjustments included age at enrollment, race, Hispanic ethnicity, years since ESRD onset at enrollment, catheter as vascular access, FMCHP risk score, and diabetes as a comorbidity. Missing indicators were used in several patients with missing vascular access (n=15) and FMCHP risk score data (n=41). Indication to receive ONS was defined during the first 2 months of enrollment and ONS use was defined as whether the patient received a delivery of ONS during the first 3 months of enrollment. As a result, time at risk began in the patient’s fourth month of enrollment.
In the analyses based on ONS indication, statistical adjustments included age at enrollment, race, Hispanic ethnicity, years since ESRD onset at enrollment, and diabetes as a comorbidity. Indication to receive ONS was defined during the first 2 months of enrollment and time at risk began in the patient’s third month of enrollment. Survival curves for the CPM comparison population were calculated using characteristics of the average FMCHP patient with indication to receive ONS.
All statistical tests were two-sided and an a priori level <0.05 was considered significant. Analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC).
Results
A total of 1377 dialysis patients enrolled in FMCHP between February 1, 2006 and December 31, 2008, and 51.7% received ONS (n=711) for a median cumulative duration of 5.0 months (interquartile range [IQR], 2–12) per patient. Patients were on continuous ONS use for a median of 2.0 months (IQR, 1.0–4.0), and were placed on ONS for a median of 2.0 episodes (IQR, 1.0–3.0) (424 patients received >1 course of ONS). After limiting to patients with the indication to receive ONS, there were 470 patients in our study in the first 2 months of enrollment, 59% (n=276) of whom received ONS within the first 3 months of enrollment.
Table 1 compares clinical and demographic characteristics of FMCHP patients who received ONS and those who did not despite eligibility. There were more patients in the “medium” risk score category on ONS (50.4% on ONS versus 41.2% not on ONS), and a higher percentage of patients without ONS had a missing risk score (5.4% for ONS patients and 13.4% for no-ONS patients). ONS patients had a higher median enrollment time of 13.5 months (IQR, 6.0–23.0) for ONS versus 9.0 months (IQR, 5.0–20.0) for no-ONS patients. Age at enrollment, race, Hispanic ethnicity, duration of ESRD, diabetes status, vascular access type, and baseline serum albumin levels, did not differ significantly between patients who received ONS versus those who did not.
Table 1.
Characteristics of FMCHP patients with and without ONS, restricted to patients with indication for ONS use
| Variable | ONS Received (n=276) | No ONS Received (n=194) | P Value |
|---|---|---|---|
| Sex | 0.49 | ||
| Female | 54.0 | 57.2 | |
| Male | 46.0 | 42.8 | |
| Race | 0.83 | ||
| Black | 44.2 | 45.4 | |
| White | 48.9 | 49.0 | |
| Other | 6.5 | 5.7 | |
| Ethnicity | 0.71 | ||
| Hispanic | 24.3 | 25.8 | |
| Non-Hispanic | 75.7 | 74.2 | |
| Diabetes as a comorbidity | 0.83 | ||
| Diabetes | 81.2 | 82.0 | |
| No diabetes | 18.8 | 18.0 | |
| Vascular access | 0.38 | ||
| Catheter | 18.5 | 18.6 | |
| Arteriovenous graft | 39.5 | 34.5 | |
| Arteriovenous fistula | 39.9 | 42.3 | |
| Missing | 2.2 | 4.6 | |
| FMCHP risk score categorya | 0.02 | ||
| Low (1–10) | 10.9 | 10.8 | |
| Medium (11–29) | 50.4 | 41.2 | |
| High (>29) | 33.3 | 34.5 | |
| Missing | 5.4 | 13.4 | |
| Age at enrollment (yr)b | 60.3±13.5 | 59.8±13.6 | 0.67 |
| Baseline serum albuminb,c | 3.5 (0.2) | 3.5 (0.3) | 0.40 |
| Years since ESRD onset at enrollmenta,d | 2.7 (1.0–5.3) | 2.8 (1.1–5.3) | 0.49 |
| Months enrolled in demonstrationd | 13.5 (6.0–23.0) | 9.0 (5.0–20.0) | <0.01 |
Data are shown as percentages, mean ± SD, or median (interquartile range). FMCHP, Fresenius Medical Care Health Plan; ONS, oral nutritional supplements.
The primary data source for date of ESRD onset is the date of first dialysis as recorded by FMCHP.
Data are shown as mean ± SD.
Baseline serum albumin is the average serum albumin in the first 2 months of enrollment.
Data are shown as median (interquartile).
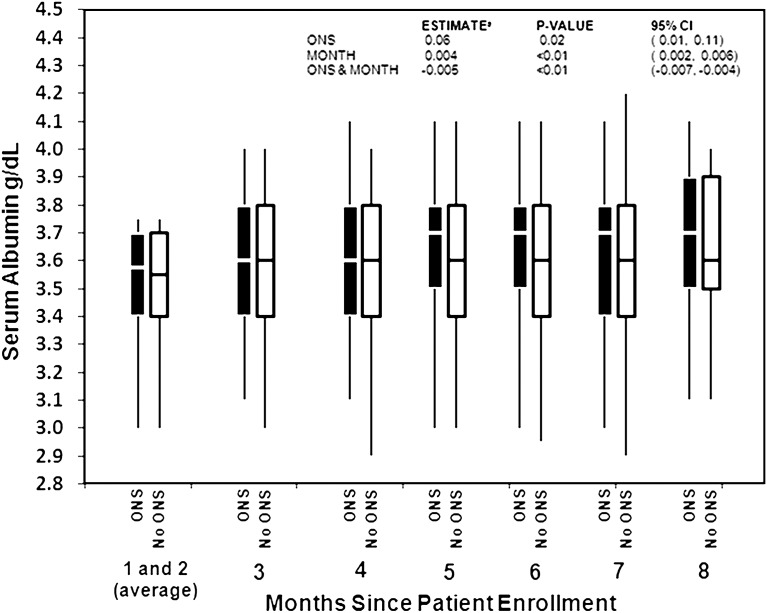
Because low serum albumin levels are associated with poor nutrition and increased mortality (5), we evaluated the change in serum albumin levels among patients who received ONS compared with those who did not. Figure 1 shows the change in the distribution of serum albumin values since patient enrollment. When ONS indication was determined (months 1 and 2) serum albumin distribution was similar between patients who received ONS and those who did not. In months 3 and 4, both groups experienced a slight increase in median serum albumin. By month 5, patients who received ONS had higher albumin levels. Patients who did not receive ONS had no further increase in serum albumin level. In months 7 and 8, there was no further increase in median serum albumin in the ONS group.
Figure 1.
Mean serum albumin (g/dl) by month of enrollment, among patients with indication for ONS. Boxes denote the 25th to the 75th percentiles, lines denote the median value, and bars denote the 5th and 95th percentiles. aEstimates of the effect of ONS and month on serum albumin were calculated using a linear mixed model that included ONS at baseline, month of enrollment, and the interaction between ONS and month of enrollment. ONS, oral nutritional supplements; 95% CI, 95% confidence interval.
Consistent results were found in a model testing the relationship between ONS and serum albumin. Patients who received supplements experienced higher serum albumin compared with patients without ONS. ONS use was associated with an overall mean serum albumin 0.058 g/dl higher (P=0.02); however, this difference decreased by 0.001 g/dl each month of enrollment (P=0.05). As a result, ONS use was associated with a serum albumin 0.055 g/dl higher at month 3 (P=0.03) and 0.052 g/dl at month 6 (P=0.04), but no significant difference was observed by month 12 (difference, 0.045; P=0.07; 95% confidence interval [95% CI], −0.004, 0.095 g/dl).
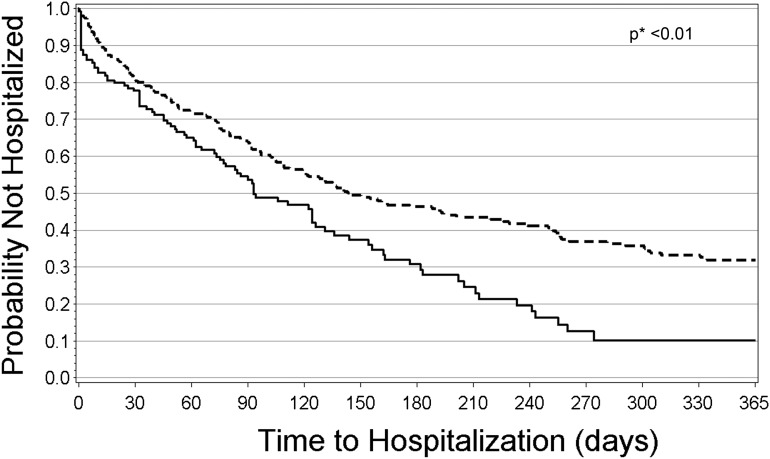
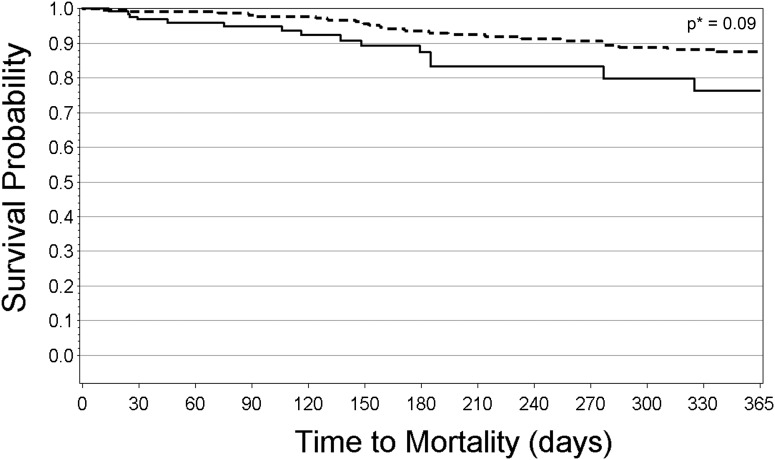
The relationships between ONS use and hospitalization or mortality were first evaluated using analyses based on ONS use. In the hospitalization analysis, median follow-up time (time from enrollment until an event or censoring) was 3.6 months (IQR, 1.2–9.9) in the ONS group and 2.0 months (IQR, 0.4–4.0) in the no-ONS group. In the mortality analysis, median follow-up time was 14.0 months (IQR, 5.6–21.0) in the ONS group and 3.1 months (IQR, 1.0–6.5) in the no-ONS group. Figures 2 and 3 show the Kaplan–Meier curves for hospitalization and mortality, respectively, for patients who received ONS compared with those who did not despite eligibility. In both figures, time at risk extends from 0 days to 365 days. Survival percentages after 365 days are not shown due to the small number of patients at risk past this point. Patients who received ONS had lower probability of being hospitalized, with an apparent difference at 35 days after enrollment into FMCHP (21% hospitalized in the ONS group versus 27% in the no-ONS group; P<0.01 for the difference in ONS and no-ONS hospitalization curves). This difference appeared to widen over time. Patient survival also differed in the ONS group compared with the no-ONS group with an apparent difference at 25 days (0.9% mortality for the ONS group versus 2.3% for the no-ONS group; P=0.02 for the difference in ONS and no-ONS survival curves).
Figure 2.
Unadjusted Kaplan–Meier hospitalization curve for FMCHP patients with ONS and FMCHP patients without ONS, among patients with indication to receive ONS (analyses based on ONS use). Dashed line indicates FMCHP patients with ONS in the first 3 months of enrollment, and straight line indicates FMCHP patients without ONS in the first 3 months of enrollment. *Log-rank test used to test for differences. FMCHP, Fresenius Medical Care Health Plan; ONS, oral nutritional supplements.
Figure 3.
Unadjusted Kaplan–Meier mortality curve for FMCHP patients with ONS and FMCHP patients without ONS, among patients with indication to receive ONS (analyses based on ONS use). Dashed line indicates FMCHP patients with ONS in the first 3 months of enrollment, and straight line indicates FMCHP patients without ONS in the first 3 months of enrollment. *Log-rank test used to test for differences. FMCHP, Fresenius Medical Care Health Plan; ONS, oral nutritional supplements.
We calculated 1-year adjusted hospitalization and mortality percentages for the analyses based on ONS use (Table 2). A 4-month minimum enrollment was used to evaluate indication for ONS in the first 2 months and ONS use in the third month, resulting in 395 patients in the final analyses. FMCHP patients who received ONS had lower adjusted hospitalization percentages at 1 year compared with those who did not receive ONS despite eligibility. Differences in hospitalization percentages achieved statistical significance, whereas there were no significant differences in mortality among patients on ONS compared with those who did not receive ONS. Hospitalization was 68.4% (95% CI, 60.6, 74.7) for the ONS group versus 88.7% (95% CI, 75.3, 94.8) for the no-ONS group (P<0.01). Mortality was 12.7% (95% CI, 0.0, 44.7) for the ONS group versus 20.5% (95% CI, 0.0, 63.6) for the no-ONS group (P=0.76).
Table 2.
Adjusteda hospitalization and mortality percentages of FMCHP patients with ONS and FMCHP patients without ONS at 1 year, among patients with indication to receive ONS (analyses based on ONS use)
| Hospitalizationb | Mortalityc | |
|---|---|---|
| FMCHP patients who received ONS with average serum albumin <3.8 g/dl within 2 mo of enrollment (n=235) | 68.4 (60.6, 74.7) | 12.7 (0.0, 44.7) |
| FMCHP patients who did not receive ONS with average serum albumin <3.8g/dl within 2 mo of enrollment (n=160) | 88.7 (75.3, 94.8) | 20.5 (0.0, 63.6) |
Data are expressed as percentages (95% confidence intervals). FMCHP, Fresenius Medical Care Health Plan; ONS, oral nutritional supplements.
Adjusted for age at enrollment, race, Hispanic ethnicity, years since ESRD onset at enrollment, diabetes as a comorbidity, FMCHP risk score category, and catheter as vascular access.
P<0.01. Significant differences between the survival estimates for patients with ONS and patients without ONS were tested using a z test.
P=0.76. Significant differences between the survival estimates for patients with ONS and patients without ONS were tested using a z test.
Adjusted HRs for hospitalization and mortality demonstrated a 34% reduction in hospitalization risk with ONS use (HR, 0.66; 95% CI, 0.50, 0.86; P<0.01) but no significant reduction in mortality risk (HR, 0.70; 95% CI, 0.36, 1.35; P=0.29).
In analyses based on ONS indication, we compared hospitalization and mortality percentages for FMCHP patients meeting eligibility for ONS within 2 months after enrollment, regardless of whether ONS was administered to CPM patients who also met the indication to receive ONS used by FMCHP (Table 3). Analyses adjusting for age, race, Hispanic ethnicity, duration of ESRD, and diabetes mellitus demonstrated that early ONS use was associated with significantly lower mortality percentages. In the FMCHP group, 16.2% (95% CI, 11.8, 20.3) of patients died within 1 year as opposed to 23.4% (95% CI, 21.2, 25.4) among CPM patients (P<0.01). Differences in hospitalization percentages were small and not statistically significant (FMCHP: 71.8%; 95% CI, 66.1, 76.6; and CPM: 72.2%; 95% CI, 70.0, 74.3; P=0.88).
Table 3.
Adjusteda hospitalization and mortality percentages of FMCHP patients and the 2007 ESRD CPM sampleb at 1 year, among patients with the indication for ONS use (analyses based on ONS indication)
| Hospitalizationc | Mortalityd | |
|---|---|---|
| FMCHP patients with average serum albumin <3.8 g/dl within 2 mo of enrollment (n=417)e | 71.8 (66.1, 76.6) | 16.2 (11.8, 20.3) |
| ESRD CPM patients with average serum albumin <3.8 g/dl in November/December 2006 (n=2425) | 72.2 (70.0, 74.3) | 23.4 (21.2, 25.4) |
Data are expressed as percentages (95% confidence intervals). FMCHP, Fresenius Medical Care Health Plan; ESRD CPM, CMS ESRD Clinical Performance Measures Project; ONS, oral nutritional supplements; CMS, Centers for Medicare and Medicaid Services.
Adjusted to the FMCHP study sample; adjusted for age at enrollment, race, Hispanic ethnicity, years since ESRD onset at enrollment, and diabetes as a comorbidity.
Data are from 2006 to 2008 for FMCHP and from 2007 for the ESRD CPM sample.
P=0.88. Significant differences between the survival estimates for FMCHP patients and CPM patients were tested using a z test.
P<0.01. Significant differences between the survival estimates for FMCHP patients and CPM patients were tested using a z test.
In this analysis, the FMCHP analysis population (n=417) is smaller than the analysis population used in Table 2 (n=470). In this analysis, the population was determined using data from the CMS Member Beneficiary Database, whereas the population in the other analysis was determined using FMCHP’s enrollment data.
Discussion
We evaluated the association of early administration of ONS on clinical outcomes. Key findings of our study suggest that a program of early ONS administration may be associated with reduced hospitalization rates. After adjusting for potential confounders, FMCHP patients who received ONS had significantly lower hospitalization percentages compared with those who did not receive ONS despite low serum albumin levels, although no significant reduction in mortality risk was noted. On the other hand, in the analyses based on ONS indication, FMCHP patients with the indication for ONS use had significantly lower mortality of 16.2% compared with 23.4% in the CPM population.
Our findings reveal that ONS use is associated with a small increase in mean serum albumin immediately after ONS initiation but that this increase does not persist. However, our analyses examining the relationship between ONS use and serum albumin levels were limited because of small sample sizes and inadequate statistical power. The study protocol that FMCHP used for starting/stopping ONS use was highly sensitive to month-to-month variation in albumin levels, resulting in a large amount of starting and stopping ONS use. Serum albumin level is a strong prognostic indicator for patients with ESRD. In the DOPPS, serum albumin concentrations <3.5 g/dl were associated with 38% greater risk of death (5). The increased mortality risk is particularly elevated at very low serum albumin (<2.5 g/dl), but prior evidence suggests that patients with near normal serum albumin values (3.5–3.9 g/dl) are also at increased risk (9).
Few studies have evaluated the effect of ONS on morbidity and mortality (4,10). A systematic review of 18 clinical trials evaluating ONS benefits concluded that studies demonstrating improved clinical outcomes are lacking (11). Furthermore, a recent review questions the value of ONS given the lack of evidence on improvement in patient outcomes (12).
More recently, a study by Cano et al. identified an increase in serum prealbumin (transthyretin) with ONS use (regardless of whether parenteral nutrition was added), corresponding with significantly reduced mortality (13). In addition, a study by Lacson et al. showed that ONS use was associated with higher survival when they evaluated patients in Fresenius Medical Care North America facilities with serum albumin ≤3.5 g/dl (14).
FMCHP’s disease management program demonstrates that ONS may be associated with improved hospitalization risk. A strength of our analysis is that improved outcomes from ONS use were observed in methodologies of both the analyses based on ONS use and analyses based on ONS indication. The approach to the analyses based on ONS indication minimizes potential for biases due to FMCHP only administering ONS to patients with a mean serum albumin <3.8 g/dl, and the possibility that patients who did not receive ONS may have died before initiation of ONS.
FMCHP’s program uniquely incorporates concrete interventions, rather than a sole focus on care coordination. FMCHP’s early initiation of ONS using a mean albumin threshold of 3.8 g/dl is an aggressive protocol. The KDOQI guidelines do not provide a serum albumin cut-off as to when ONS should be initiated (3). However, the European Society for Clinical Nutrition and Metabolism recommends initiation of ONS based on specific indicators, including body mass index <20 kg/m2 and serum albumin <3.5 g/dl (15).
Two different methodologies were used to explore the effect of ONS on patient outcomes. Nevertheless, our findings should be interpreted in the context of several limitations. In the analyses based on ONS use, there may be unadjusted differences between patients who did and did not receive ONS that influenced the difference in outcomes. We attempted to reduce this potential by performing analyses based on ONS indication, although it is likely that even with this analysis, unadjusted differences between the study population and the comparison CPM population exist. Other components of FMCHP’s program may have also contributed to the overall patient benefit compared with the CPM comparison group. For example, early dietitian intervention and close interaction with nurse care managers and so forth may have resulted in intangible patient benefits that improved patient outcomes. Using both methodologies allows us to explore the relationship between ONS use and patient outcomes from two different directions, and although significance levels varied, the consistent findings across both sets of analyses give promising evidence that ONS may be associated with improved patient outcomes.
In summary, protein energy wasting of kidney disease is a major issue in the long-term management of hemodialysis patients (3). More than half of our maintenance hemodialysis enrollees fulfilled the albumin criteria for ONS. For these reasons, the results of FMCHP’s ONS program are particularly noteworthy because early ONS use was associated with lower hospitalization rates but not with lower mortality.
Disclosures
F.K.P. was the principal investigator of the DOPPS, and S.R. is an investigator for the DOPPS.
P.F.S. and R.E.F. are employees of Fresenius Medical Care.
Acknowledgments
Editorial assistance was provided by Shauna A. Leighton, medical editor at Arbor Research Collaborative for Health.
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), Abbott (since 2009), and Baxter (since 2011), and Vifor Fresenius Renal Pharma (since 2011), without restrictions on publications.
The analyses upon which this publication is based were performed under the Centers for Medicare and Medicaid Services project entitled The Evaluation of the End-Stage Renal Disease (ESRD) Disease Management Demonstration (contract number 500-00-0028). This contract was entered with Arbor Research Collaborative for Health and has been sponsored by the Centers for Medicare and Medicaid Services of the Department of Health and Human Services. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The author assumes full responsibility for the accuracy and completeness of the ideas presented. Ideas and contributions to the author concerning experience in engaging with issues presented are welcomed.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editoral, “To Supplement or Not: That Is the Question” on pages 8–9.
References
- 1.Pupim LB, Ikizler TA: Uremic malnutrition: New insights into an old problem. Semin Dial 16: 224–232, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Toigo G, Aparicio M, Attman PO, Cano N, Cianciaruso B, Engel B, Fouque D, Heidland A, Teplan V, Wanner C: Expert working group report on nutrition in adult patients with renal insufficiency (part 2 of 2). Clin Nutr 19: 281–291, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Kopple JD: National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 37[Suppl 2]: S66–S70, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Cano NJ, Fouque D, Roth H, Aparicio M, Azar R, Canaud B, Chauveau P, Combe C, Laville M, Leverve XM, French Study Group for Nutrition in Dialysis : Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: A 2-year multicenter, prospective, randomized study. J Am Soc Nephrol 18: 2583–2591, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Combe C, McCullough KP, Asano Y, Ginsberg N, Maroni BJ, Pifer TB: Kidney Dialysis Outcomes Quality Initiative (KDOQI) and the Dialysis Outcomes and Practice Patterns Study (DOPPS): Nutrition guidelines, indicators and practices. Am J Kidney Dis 44[Suppl 2]: S39–S46, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare and Medicaid Services: Annual Report, End-Stage Renal Disease Clinical Performance Measures Project. Baltimore, MD, Department of Health and Human Services, Centers for Medicare and Medicaid Services, Office of Clinical Standards and Quality, 2008. Available at: http://www.esrdnetwork.org/assets/pdf/data/2008cpmannualreport.pdf. Accessed July 7, 2010
- 7.Sands JJ, Etheredge GD, Shankar A, Graff J, Loeper J, McKendry M, Farrell R: Predicting hospitalization and mortality in end-stage renal disease (ESRD) patients using an Index of Coexisting Disease (ICED)-based risk stratification model. Dis Manag 9: 224–235, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Athienites NV, Miskulin DC, Fernandez G, Bunnapradist S, Simon G, Landa M, Schmid CH, Greenfield S, Levey AS, Meyer KB: Comorbidity assessment in hemodialysis and peritoneal dialysis using the index of coexistent disease. Semin Dial 13: 320–326, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Lowrie EG, Lew NL: Death risk in hemodialysis patients: The predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 15: 458–482, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Sharma M, Rao M, Jacob S, Jacob CK: A controlled trial of intermittent enteral nutrient supplementation in maintenance hemodialysis patients. J Ren Nutr 12: 229–237, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Stratton RJ, Bircher G, Fouque D, Stenvinkel P, de Mutsert R, Engfer M, Elia M: Multinutrient oral supplements and tube feeding in maintenance dialysis: A systematic review and meta-analysis. Am J Kidney Dis 46: 387–405, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Friedman AN, Fadem SZ: Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol 21: 223–230, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Cano N, Fouque D, Roth H, Berrerd L, Maurizi K, Azar R, Aparicio M, Canaud B, Chauveau P, Combe C, Laville M, Leverve X: The French intradialytic nutrition evaluation study (FineS) [Abstract F-FC046]. J Am Soc Nephrol 16: 48A, 2005
- 14.Lacson E Jr, Wang W, Zebrowski B, Wingard R, Hakim RM: Outcomes associated with intradialytic oral nutritional supplements in patients undergoing maintenance hemodialysis: A quality improvement report. Am J Kidney Dis 60: 591–600, 2012 [DOI] [PubMed]
- 15.Cano N, Fiaccadori E, Tesinsky P, Toigo G, Druml W, Kuhlmann M, Mann H, Hörl WH, DGEM (German Society for Nutritional Medicine) ESPEN (European Society for Parenteral and Enteral Nutrition) : ESPEN Guidelines on Enteral Nutrition: Adult renal failure. Clin Nutr 25: 295–310, 2006 [DOI] [PubMed] [Google Scholar]