Abstract
Objective
The cause of death in murine models of sepsis remains unclear. The primary purpose of this study was to determine if significant lung injury develops in mice predicted to die following cecal ligation and puncture induced sepsis compared to those predicted to live.
Design
Prospective, laboratory controlled experiments.
Setting
University research laboratory.
Subjects
Adult, female, outbred ICR mice.
Interventions
Mice underwent cecal ligation and puncture (CLP) to induce sepsis. Two groups of mice were sacrificed at 24 and 48 hours post-CLP and samples were collected. These mice were further stratified into groups predicted to die (Die-P) and predicted to live (Live-P) based on plasma interleukin 6 (IL-6) levels obtained 24 hours post-CLP. Multiple measures of lung inflammation and lung injury were quantified in these two groups. Results from a group of mice receiving intratracheal normal saline without surgical intervention were also included as a negative control. As a positive control, bacterial pneumonia was induced with Pseudomonas aeruginosa to cause definitive lung injury. Separate mice were followed for survival until day 28 post-CLP. These mice were used to verify the IL-6 cut-offs for survival prediction.
Measurements and Main Results
Following sepsis, both the Die-P and Live-P mice had significantly suppressed measures of respiratory physiology but maintained normal levels of arterial oxygen saturation. Bronchoalveolar lavage (BAL) levels of pro and anti-inflammatory cytokines were not elevated in the Die-P mice compared to the Live-P. Additionally, there was no increase in the recruitment of neutrophils to the lung, pulmonary vascular permeability, or histological evidence of damage. In contrast, all of these pulmonary injury and inflammatory parameters were increased in mice with Pseudomonas pneumonia.
Conclusions
These data demonstrate that mice predicted to die during sepsis have no significant lung injury. In murine intra-abdominal sepsis, pulmonary injury cannot be considered the etiology of death in the acute phase.
Keywords: cytokine, interleukin 6, pulmonary, edema, neutrophil
Introduction
The burden that sepsis inflicts on society remains high. Sepsis affects 750,000 people every year in the United States and 210,000 die from the process (1), making it the second leading cause of death in non-coronary intensive care units (ICU) (2). Overall sepsis mortality remains high at approximately 30% with current treatment, and mortality increases with disease severity. Sepsis is defined as severe if there is evidence of organ dysfunction (3) and as the number of organs affected increases so does the mortality rate, reaching 70% when 3 or more organs are involved (4, 5). The organ that fails most frequently in septic patients is the lung (4).
Acute lung injury (ALI) and the more severe form, acute respiratory distress syndrome (ARDS) can be caused by a variety of pathological processes that affect the lung either directly or indirectly (6). Sepsis is the leading cause of both ALI and ARDS (7, 8). However, the majority of patients die of sepsis related multi-organ dysfunction syndrome (MODS), and respiratory failure alone does not increase the risk of death (9–11).
A variety of animal models have been developed to study ALI but none of them fully reproduce the human disease (12). Animal models of sepsis-induced ALI include the administration of endotoxin, live bacteria, or the creation of a nidus of infection such as CLP. The CLP model closely replicates the clinical picture of sepsis encountered in human patients and has become the most frequently used model of sepsis (13, 14). However, CLP has been used in fewer studies investigating lung injury relative to the other animal models (12). From previous studies that have utilized CLP, it is unclear if lung injury develops in this model (15–19).
The association between lung injury and mortality is still not well-understood in either human sepsis or animal models of the syndrome. Our study used a carefully characterized CLP model of sepsis to determine the extent of lung injury that develops following sepsis. Positive control mice were given intratracheal Pseudomonas aeruginosa (P. aeruginosa) to induce significant lung injury as a comparison. P. aeruginosa is a common cause of gram-negative nosocomial pneumonia (20) capable of causing severe or fatal infections (21). Previous experimental studies show that P. aeruginosa leads to the development of ALI, characterized by substantial alveolar protein-rich edema (22). The recruitment of airway neutrophils is a also major component of the initial host immune response to P. aeruginosa (23). Multiple cytokines that regulate host lung defense and inflammation are increased during this bacterial infection. A variety of histological abnormalities are seen in the lung including fibrinous exudate, polymorphonuclear leukocytes, hemorrhage, and alveolar septal necrosis (24). Impaired oxygenation may significantly affect pulmonary physiology and survival in P. aeruginosa infection. Mice receiving intratracheal normal saline are not expected to exhibit lung injury.
In our study, lung function and histological characteristics of CLP mice were obtained to evaluate for the presence of pulmonary injury. Since inflammation may occur without injury, several parameters of pulmonary inflammation were also analyzed. Septic mice were sampled and sacrificed at 24 and 48 hours in the acute phase of sepsis. Differences between predicted survivors and non-survivors, separated based on the IL-6 levels in the plasma collected at 24 hours post-CLP, were specifically investigated.
Materials and Methods
Animals
Female ICR mice (from Harlan Laboratories, Inc., Frederick, MD) were used. Mice were acclimated to the housing room in a temperature controlled room with a diurnal cycle of 12 hours light and 12 hours dark for at least 72 hours before experimentation. They were provided food and water ad libitum for the entire experiment. The experiments were approved by Boston University Animal Care and Use Committee.
Experimental Design
Forty eight mice were followed for survival until death or day 28 post-CLP and used to verify the IL-6 discrimination values for prediction of mortality. For all other experimental and control groups of mice, respiratory parameters were obtained 3 days prior to intervention to assess baseline respiratory physiology. Body weights were recorded at baseline and then measured daily until time of death or sacrifice. Groups of 10-20 mice underwent CLP followed by facial vein bleed 24 hours later to measure a complete blood count with differential and the IL-6 level in the plasma. Mice were stratified into Die-P and Live-P. On the day of sacrifice at 24 or 48 hours post-CLP, pulmonary physiology measures were repeated and pulse oximetry with a collar sensor (STARR Life Sciences Corp, Mouse Ox) was obtained. Mice were anesthetized with ketamine/xyalzine and underwent surgical dissection to expose the carotid artery for puncture and arterial blood gas analysis. The remainder of the murine blood volume was collected for cytokine analysis. After tracheal cannulation, a single lung BAL of the right side was performed. The right lower lobe was used for wet/dry weight ratios and the remaining three lobes stored at −20°C for MPO assay. Subsequently, the isolated left lung was insufflated with a standard 25 cm column height of formalin to ensure uniform expansion and collected for routine histological processing. Based on plasma IL-6 measurement, there were Live-P, n=5; Die-P, n= 8 at 24 hours and Live-P, n=7; Die-P, n= 7 at 48 hours. Mice with an indeterminate IL-6 level were excluded from further analysis.
Finally, a group of 8 mice received 50uL normal saline by intratracheal instillation (25) as a negative control. Another group of 8 mice were inoculated with 5×107 CFUs P. aeruginosa to induce lung injury as a positive control. The same parameters, as for the experimental mice, were measured. Eighteen hours after intratracheal administration of either normal saline or bacteria, all control mice underwent the above procedures and sacrifice.
Sepsis model
Cecal ligation and puncture was performed as first described (26) with minor modifications (27). Under continuous 3% isoflurane anesthesia, a 2 cm long midline abdominal incision was performed, the cecum ligated and then triple punctured with a 16 gauge needle to induce approximately 50% mortality. Antibiotic treatment with 25 mg/kg imipenem (Merck, West Point, PA) started 2 hours after surgery and continued every 12 hours for the first 5 days (for the mice followed for survival; mice sacrificed at 24 hours received the 2 doses on the first day and a third dose in the morning of the second day; mice sacrificed at 48 hours received the 2 doses on the first and second day and a fifth dose in the morning of the third day). Pain control for CLP mice was achieved with buprenorphine 0.05 mg/kg every 12 hours for the first two days post-operatively (a total of three doses for those sacrificed at 24 hours; four doses for the mice sacrificed at 48 hours or followed for survival).
Whole body plethysmography
Respiratory parameters were measured using a whole body plethysmography system (Buxco Research Systems, Wilmington, NC) prior to CLP and repeated at either 24 or 48 hours post-CLP, immediately prior to sacrifice. Measurements included respiratory rate, tidal volume, peak inspiratory and expiratory flow. Mice were individually placed into the chamber of the plethysmograph and left to acclimate for 10 minutes as previously described (28). Mice were conscious and unrestrained, and they showed normal exploratory behavior. Respiratory measurements were also collected at baseline and 18 hours after intervention for control mice, receiving normal saline or P. aeruginosa.
Blood and Lung Sampling
At 24 and 48 hours post CLP, 20 µl of blood was collected by facial vein puncture as described (29) for a complete blood count with differential with a Hemavet instrument (CDC Technologies, Oxford, CT). Following whole body plethysmography measurements at 24 and 48 hours, the mice were anesthetized with a solution of 87 µg/g Ketamine and 13 µg/g Xylazine in normal saline. Using a dissecting microscope, a midline ventral neck incision was made from the mandible to sternal notch. The fascia and strap muscles were bluntly dissected until the carotid artery was exposed, and it was clamped distally to engorge the vessel with blood. The carotid artery was punctured with a 30G heparin rinsed syringe and 100uL blood was collected for arterial blood gas (ABG). An iSTAT analyzer (Abbott Laboratories, Princeton, NJ) with CG4+ cartridges was used for ABG measurement. The remainder of the blood volume was collected in a separate syringe and mixed with 50uL of 169mM EDTA.
Single lung bronchoalveolar lavage (BAL) was performed followed by formalin fixation of the contralateral lung. The thorax was opened with a midline incision through the sternum to expose the heart and lungs. The left lung hilum was clamped closed. Right-sided BAL was performed by exposing the trachea and cannulating it with a polyethylene catheter and then the right lung was lavaged with a total of 2.5mL of warm Hanks’ Balanced Salt Solution (HBSS, Mediatech, Inc., Herndon, VA). The 0.5mL and two subsequent 1mL aliquots were centrifuged separately and the supernatant from the first wash was stored at −80°C for subsequent measurement of cytokines, total protein, albumin, and IgM. The supernatant from the second wash was discarded and the 3 pellets were resuspended in HBSS and combined. A total cell count was performed with a Beckman-Coulter particle counter model ZF (Coulter Electronics Inc., Hialeah, FL) and a differential count was obtained by counting 300 cells on cytospin slides stained with Diff-Quick (Baxter, Detroit, MI). Following lavage, the right hilum was clamped and tied off distally with 4-0 silk. The right lower lobe was used to determine the wet/dry ratio and the remainder of the right lung was stored for the myeloperoxidase assay. Next, a syringe filled with 10% buffered formalin at 25 cm water pressure was connected to the tracheal catheter and the left lung clamp was released to insufflate the lung for 2 minutes. The left lung was removed and placed into 10% formalin for fixation and histological analysis.
Histopathology
Formalin fixed lung tissue was embedded in paraffin and sectioned at 5 µm for routine histology. Slides were stained with hematoxylin and eosin and evaluated in a blinded manner by a board-certified pathologist (DGR). Edema, hyperemia, congestion, neutrophil margination and tissue infiltration, intra-alveolar hemorrhage and debris, cellular hyperplasia were evaluated to establish the presence or absence of lung injury as previously described (30).
Myeloperoxidase assay
The myeloperoxidase (MPO) assay was performed as previously described, with minor modifications (31). The absorbance was read at 450 nm and data was expressed as ΔOD (the difference between the average absorbance of the 3 substrate wells and the 3 background wells for the same sample).
Total protein, albumin, and IgM
BAL fluid total protein was measured with the Coomassie protein assay using Bradford Reagent (Sigma-Aldrich) for detection and bovine serum albumin for the standard and absorbance was read at 590nm. Albumin and IgM levels in the BAL fluid were measured by ELISA as previously described (32, 33).
Enzyme-linked immunosorbent assay (ELISA) and microarray immunoassay
An aliquot of the 1:10 diluted plasma collected at 24 hours from all the mice was used to determine the IL-6 concentration by ELISA as previously described (34). A separate plasma aliquot was stored and used to measure a variety of pro and anti-inflammatory cytokines using the sequential ELISA technique (35). This analysis was also performed on the supernatant of the BAL fluid.
Statistical analysis
Data were analyzed with Prism 5 (GraphPad Software). Results were reported as mean ± SEM unless otherwise noted. Non-parametric analyses were performed since the data was not normally distributed. For multiple group comparisons, a Kruskal-Wallis test with Dunn’s post-hoc test for pairwise comparisons. To demonstrate that bacteria pneumonia induced lung injury, these values were compared to normal saline values with the Mann Whitney test. Since the major goal of the study was to determine if mice predicted to die had lung injury compared to mice predicted to live, therefore these two groups were directly compared with the Mann Whitney test. Power analyses were performed with PS software version 3.0 using a Type I error probability of 0.05 with a power of 0.9 to determine the sample size necessary to reject the null hypothesis.
Results
Survival Prediction
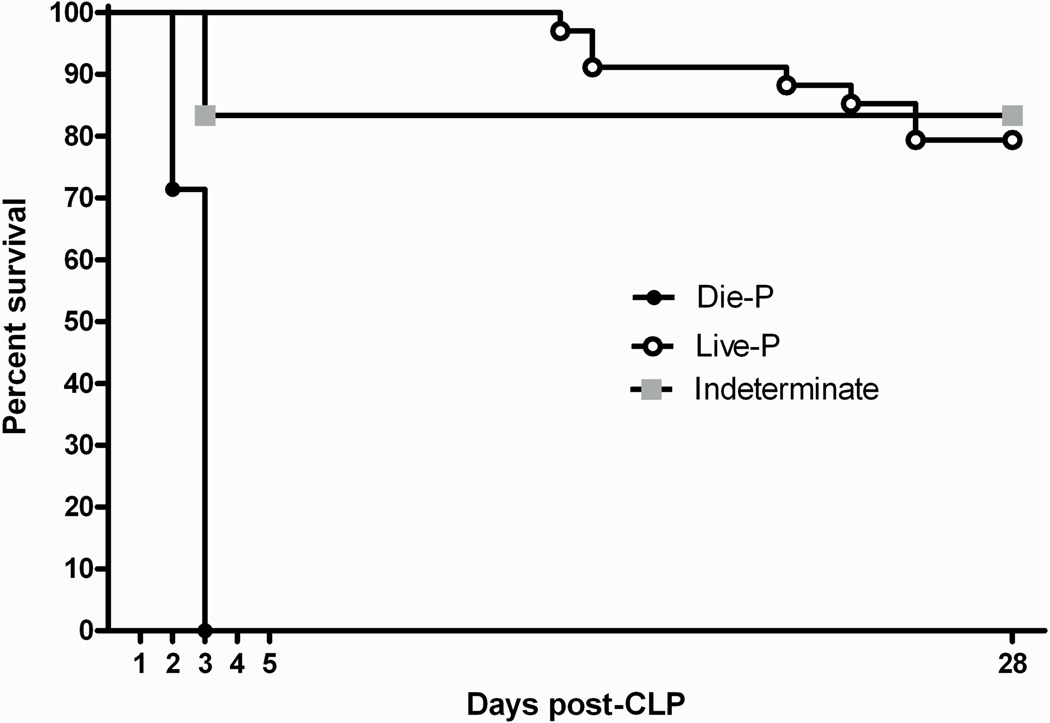
To perform a systematic assessment of potential lung inflammation or injury in the CLP induced sepsis model and correlate the results with outcome, mice needed to be sacrificed and samples collected prior to animals dying from sepsis. Plasma IL-6 accurately predicts mortality in CLP mice (36–38). A recently described dual cut-off system (29) that eliminates the compromise between sensitivity and specificity of a single cut-off was used to predict outcome in this study. Specifically, an IL-6 concentration over 12 ng/mL at 24 hours post-CLP predicted death by day 5 with 100% specificity (only true positives, Die-P) and a value under 1.5 ng/mL predicted survival by day 5 with 100% sensitivity (only true negatives, Live-P). IL-6 levels in between the 2 cut-offs were considered indeterminate. In the current study, this was verified in a group of 48 mice that had blood collected at 24 hours post-CLP and were followed for survival until day 28 (Figure 1). All mice in the Die-P group died before day 5 (100% specificity) while all of the Live-P survived in the same time-frame (100% sensitivity), confirming the accuracy of the IL-6 based prediction of outcome and actual survival. The predictive cut-offs were subsequently used to compare lung injury parameters in Live-P and Die-P mice.
Figure 1.
Confirmation of the accuracy of survival prediction based on IL-6 concentrations in plasma collected at 24 hours after cecal ligation and puncture. Blood was collected by facial bleeding and the plasma IL-6 levels were determined. Die-P mice (n=7) had plasma IL-6 levels > 12ng/ml, Live-P mice (n=34) had levels <1.5 ng/ml while the Indeterminate group (n=6) had IL-6 levels between those values. For predicting survival to day 5, the 24 hour IL-6 levels had 100% specificity and 100% sensitivity using the two distinct discrimination points. All of the Die-P mice succumbed within 72 hours of CLP.
Respiratory Physiology
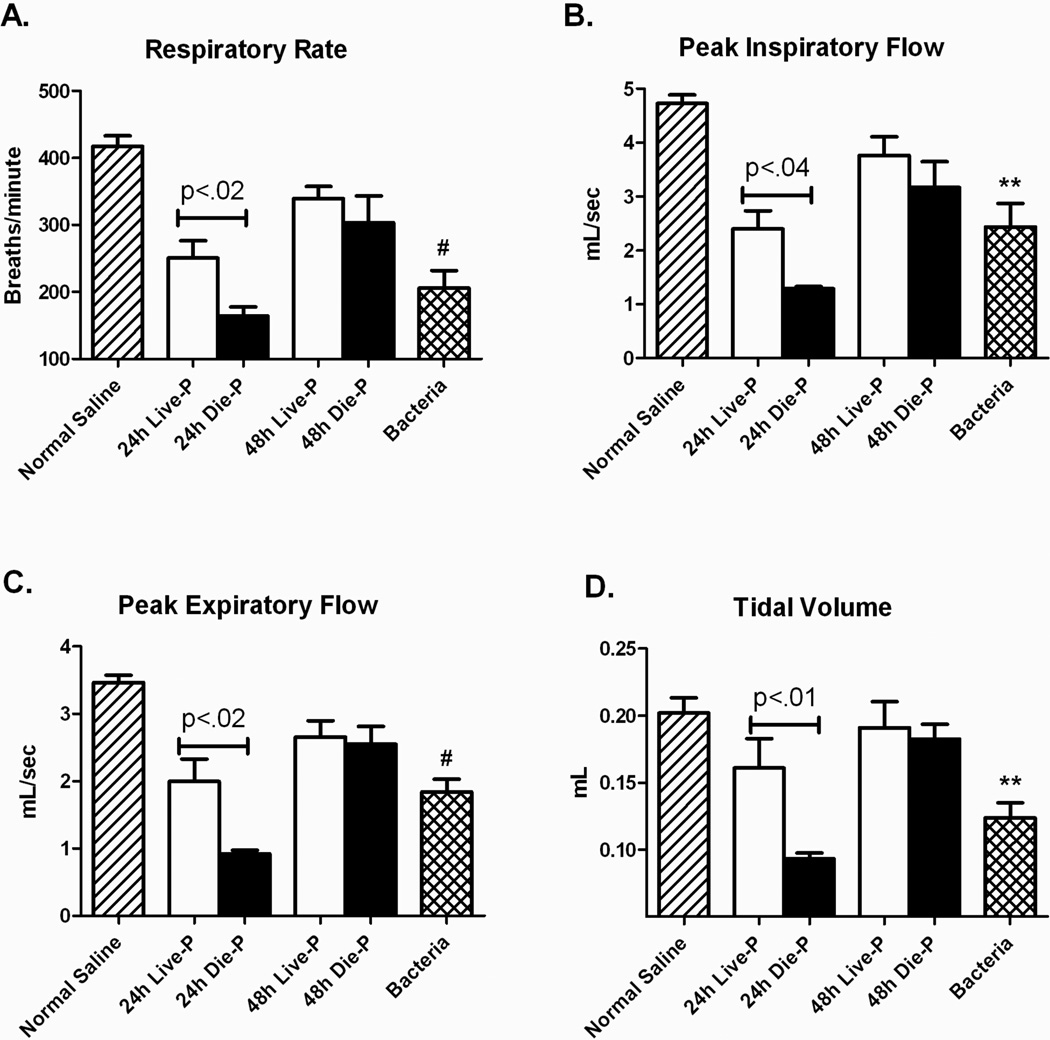
Physiological respiratory parameters were measured using whole body plethysmography and the values obtained prior to surgery for the mice undergoing CLP (data not shown) as well as values for the negative control mice without surgery were within published normal ranges for all the parameters measured (39). There were significant differences between mice given pulmonary exposure to normal saline compare to those with pneumonia in several respiratory parameters including respiratory rate, peak inspiratory flow (PIF) and peak expiratory flow (PEF) (Figure 2 A, B, and C). There were also significant decreases in these parameters between the mice predicted to live and those predicted to die at 24 hours which resolved by 48 hours. Despite these alterations, the tidal volume was equivalent for CLP mice compared to normal saline, except for 24h Die-P whose death was usually imminent (Figure 2 D). Even though the septic mice take fewer and slower breaths, both Live-P and Die-P animals were primarily capable of maintaining a normal tidal volume.
Figure 2.
Pulmonary respiratory parameters measured by whole body plethysmography 24 and 48 hours after CLP. Septic mice were separated in predicted to live until 5 days post cecal ligation and puncture, 24h Live-P (n=5) and 48h Live-P (n=7), or die by day 5, 24h Die-P (n=10) and 48h Die-P (n=4), based on the plasma IL-6 cut-offs. Two control groups of mice that were not subjected to cecal ligation and puncture, normal saline (n=8) and bacteria (n=8) were included in the analysis. The respiratory rate (A), peak inspiratory flow (B), and peak expiratory flow (C) were decreased in the septic mice predicted to die compared to the predicted to live group at 24 hours. By 48 hours, respiratory parameters for Live-P and Die-P mice were improving. Despite these changes, the tidal volume was equivalent for CLP mice compared to normal saline (D), except for 24h Die-P whose death was usually imminent. Values are mean ± SEM. There were significant differences between all the groups for all panels, p<.001. The differences between paired groups is indicated by the line. ** = p<0.01, #p<0.001 for bacteria versus normal saline.
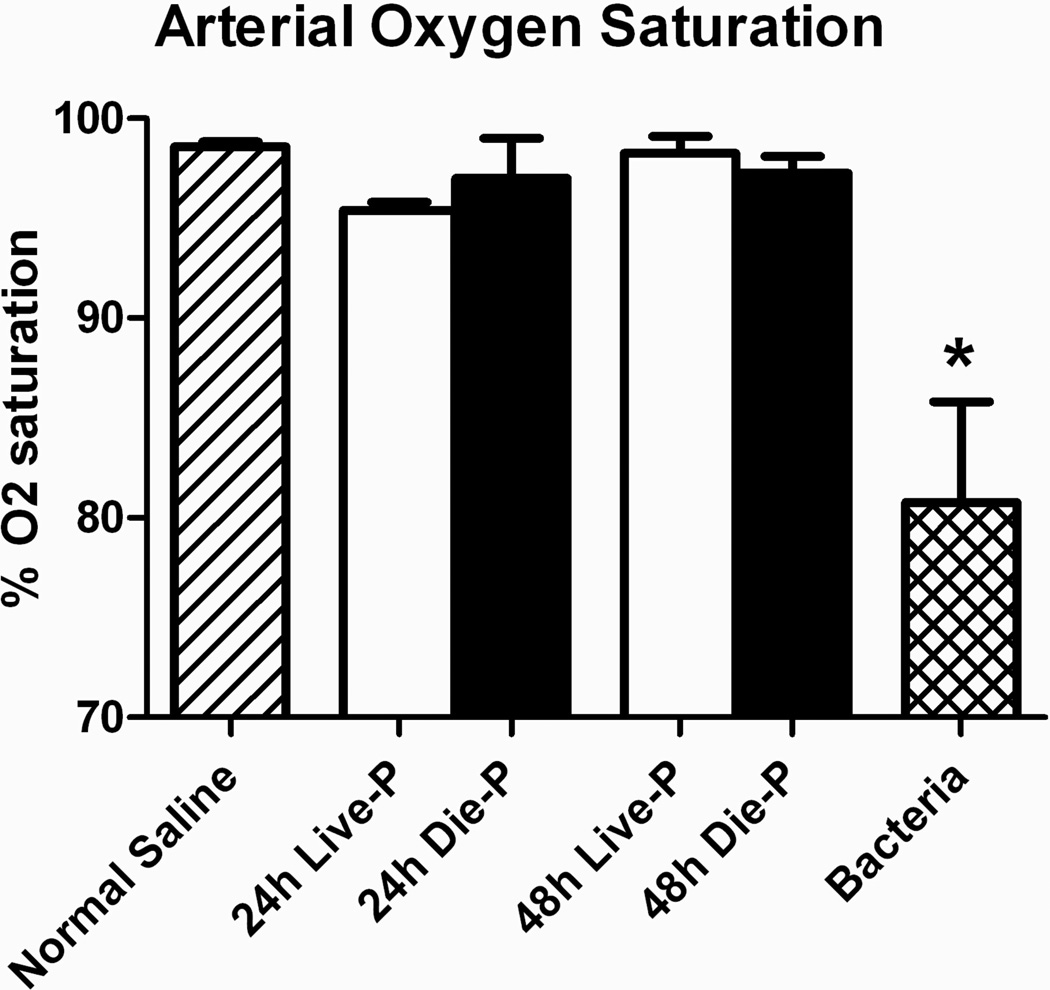
The most important pulmonary function is performing the gas exchange necessary for maintaining the supply of O2 to the tissues and eliminating CO2. Arterial oxygen saturation was measured from the carotid artery of mice immediately prior to sacrifice at 24 or 48 hours in mice subjected to CLP and at 18 hours for control mice receiving P. aeruginosa (Bacteria) or normal saline. As seen in Figure 3, the arterial oxygen saturation was maintained in CLP mice at both time points after sepsis for mice predicted to die, as well as live. Oxygen saturation was no different compared to normal saline controls, but gas exchange was markedly impaired in mice with bacterial pneumonia. Maintenance of a normal arterial oxygen saturation indicates that the septic mice, even those that are about to die, do not develop hypoxemia.
Figure 3.
Arterial oxygen saturation in early sepsis. Carotid artery puncture was performed to measure oxygen saturation of mice immediately prior to sacrifice 24 or 48 hours in post CLP mice and at 18 hours for control mice receiving P. aeruginosa (Bacteria) or normal saline. Group size was 24h Live-P (n=5), 24h Die-P (n=3); 48h Live-P (n=4); 48h Die=P (n=4), normal saline (n=5), bacteria (n=4). Values are mean ± SEM. There were significant differences between all the groups, p<0.01, but there was no difference between Live-P and Die-P mice at either 24 or 48 hours. * = p<0.05 bacteria versus normal saline.
Lung Injury
After evaluation of the function, a structural evaluation of the lungs was performed. The early, exudative phase of lung injury is characterized in patients by bilateral infiltrates on chest x-ray which corresponds to interstitial and alveolar edema formation (6).
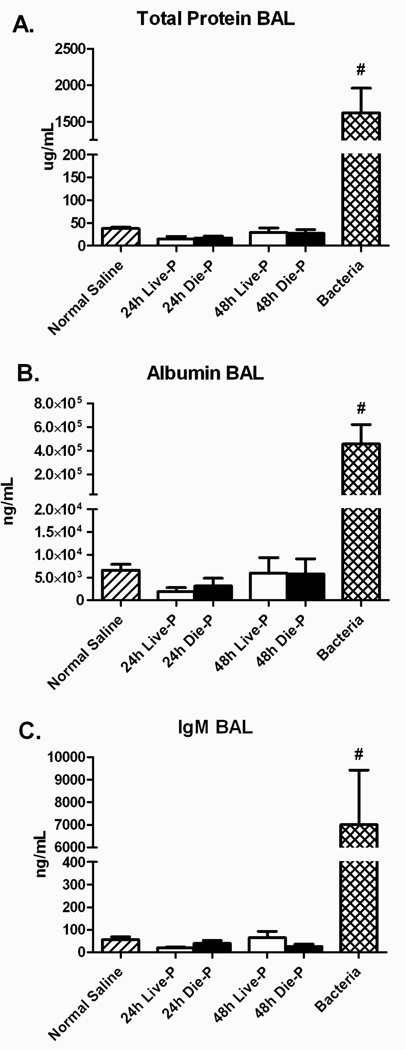
Pulmonary edema is composed of protein rich fluid resulting from an increase in vascular permeability (40–42). The concentrations of total protein, albumin, and IgM were measured in the BAL fluid. In this study, there were similar amounts of total protein, albumin, and IgM in CLP mice compared to normal saline, and no difference between Die-P and Live-P mice at either 24 or 48 hours (Figure 4 A, B and C). In contrast, bacterial pneumonia resulted in markedly increased levels compared to all other groups. This demonstrates that there was no significant protein extravasation into the airways of either Die-P or Live-P septic mice and suggests that the alveolo-capilary barrier remained intact. Our findings demonstrate that there is no significant lung injury in septic mice at 24 and 48 hours after the onset of sepsis. A sample size calculation was performed since there was a slight increase in total protein, albumin and IgM in the Die-P compared to the Live-P. To reach statistical significance would have required 672 mice in each group for the albumin measurements.
Figure 4.
Pulmonary alveolar protein content 24 and 48 hours after cecal ligation and puncture compared to normal saline and bacteria. Bronchoalveolar lavage was performed and the total protein (Panel A), albumin (Panel B) and IgM (Panel C) levels in the BA fluid were measured for 24h Live-P (n=5), 24h Die-P (n=6); 48h Live-P (n=7); 48h Die=P (n=7), normal saline (n=8), bacteria (n=8). Values are mean ± SEM. There were significant differences between all the groups, p<0.001, but there was no difference between Live-P and Die-P mice at either 24 or 48 hours. #p<0.001 for bacteria versus normal saline.
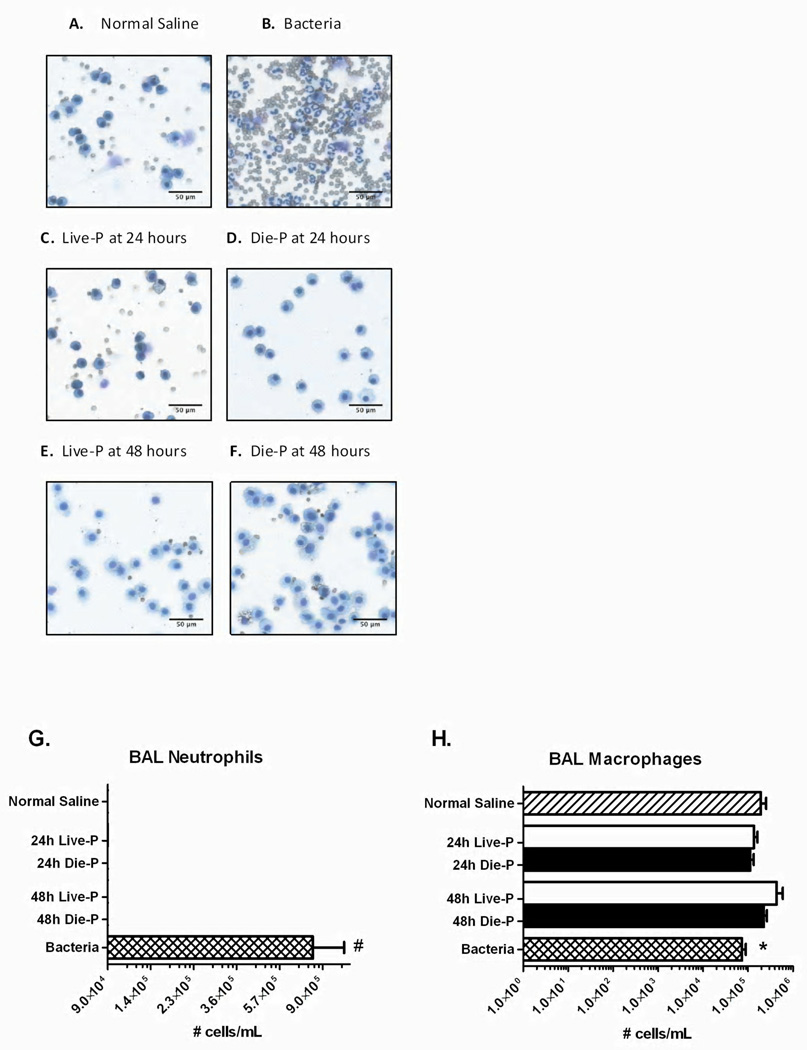
Accumulation of neutrophils in the lungs may cause severe degradation of the lung tissue through release of proteases and formation of reactive oxygen species (30, 43). To determine their presence in the alveolar space, lung parenchyma and circulation, neutrophil counts were measured in sacrificed mice. Photomicrographs of representative cytospin slides demonstrate only alveolar macrophages in BAL recovered from normal saline mice (Figure 5 A), Live-P mice (Figure 5 C&E) and Die-P (Figure 5 D&F). In contrast, bacterial pneumonia (Figure 5 B) showed a predominance of neutrophils with an increase in red blood cells. Quantitatively, BAL fluid at 24 and 48 hours after CLP showed no influx of neutrophils in the airways of septic mice (Figure 5 G) versus bacteria. Additionally, there were similar numbers of alveolar macrophages (Figure 5 H). There were so few neutrophils in the CLP mice that sample size calculation could not be performed.
Figure 5.
BAL cells 24 and 48 hours after cecal ligation and puncture. Panels A (normal saline), B (bacteria), C (24h Live-P), D (24h Die-P), E (48h Live-P), F (48h Die-P) show representative cytospin slides from each group of mice (Diff-Quick stain; 1000X magnification). Neutrophil extravasation in the airways did not occur in either mice predicted to die or live septic mice. Panel G shows the total neutrophil count, and Panel H the macrophages recovered from the airways by bronchoalveolar lavage 24h Live-P (n=5), 24h Die-P (n=5); 48h Live-P (n=7); 48h Die=P (n=9), normal saline (n=8), bacteria (n=8). Values are mean ± SEM. There were significant differences between all the groups, p<0.001, but there was no difference between Live-P and Die-P mice at either 24 or 48 hours. * = p<.05, #p<0.001 for bacteria versus normal saline.
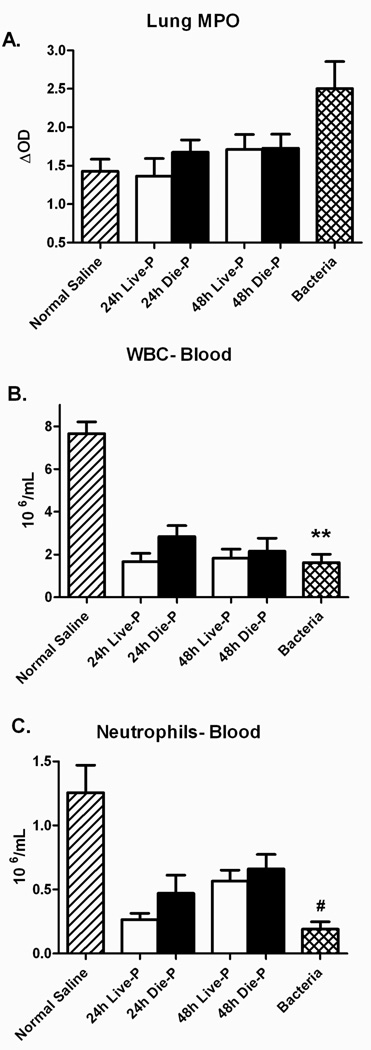
Myeloperoxidase (MPO) is frequently used to measure the presence of neutrophils in tissues (44, 45). MPO activity did not increase in any of the septic groups of mice compared to normal saline (Figure 6 A). However, mice receiving bacteria had a significant increase in lung MPO compared to the normal saline control. Leukopenia and neutropenia were present at the 24 and 48 hour time points in both groups of septic mice (Figure 6 B&C). Again, bacterial pneumonia led to a decrease in white blood cells and neutrophils compared to normal saline. The data presented in figures 5 and 6 indicates that there is no significant recruitment of neutrophils to the lungs of the septic mice that could lead to organ injury in these mice, regardless of their ultimate survival outcome.
Figure 6.
Pulmonary neutrophil sequestration and peripheral blood counts 24 and 48 hours after cecal ligation and puncture. Myeloperoxidase (MPO) levels in the lung homogenate were comparable to normal saline for Die-P and Live-P mice in Panel A (24h Live-P (n=5), 24h Die-P (n=5); 48h Live-P (n=6); 48h Die=P (n=7), normal saline (n=8), bacteria (n=8). The peripheral white blood cell and neutrophil counts were lower for the groups of septic mice compared to normal saline in Panel B (24h Live-P (n=5), 24h Die-P (n=5); 48h Live-P (n=6); 48h Die=P (n=7), normal saline (n=8), bacteria (n=8)). Septic mice had a decreased number of neutrophils in the blood 24 and 48 hours after cecal ligation and puncture but this did not translate in an increased recruitment to the lung. Values are mean ± SEM. There was no significant difference between the groups for MPO by Kruskal Wallis. For the other panels there was a significant difference between the groups, p<0.001, but there was no difference between Live-P and Die-P mice at either 24 or 48 hours. ** = p<0.01, #p<0.001 for bacteria versus normal saline.
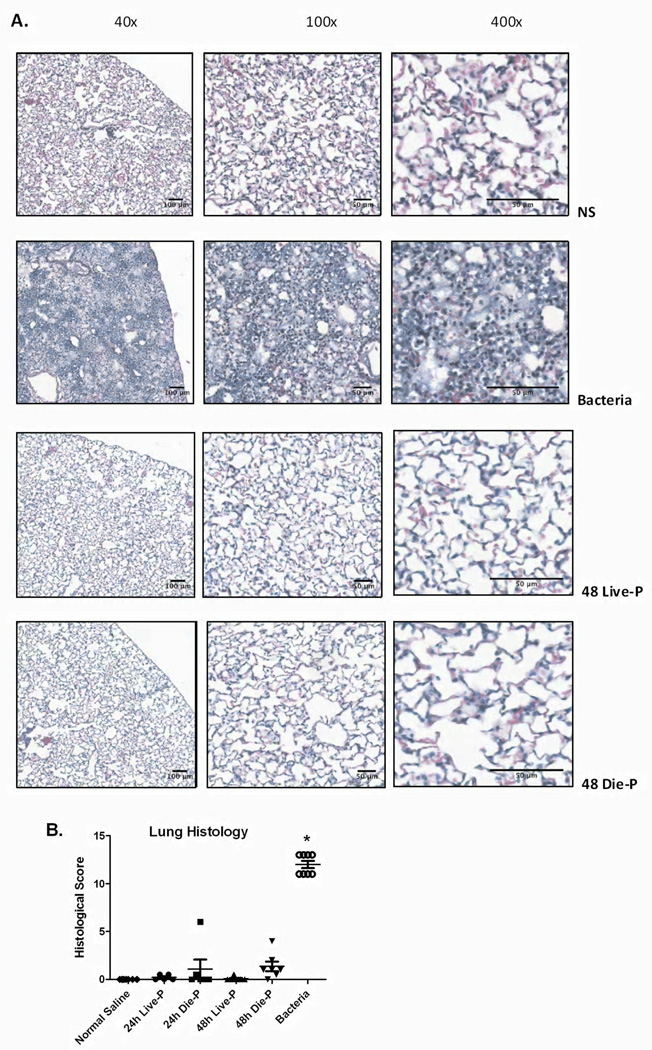
To confirm the lack of neutrophil recruitment and edema formation in the lungs of the septic mice, microscopic examination of H&E stained lung sections from all groups was performed. Figure 7 A shows representative images from 48 hour Die-P and Live-P mice, as well as a mouse given normal saline or bacteria. There were no visible neutrophils in the alveoli and interstitial spaces of either the Die-P or Live-P mice confirming the results from the BAL cytospin slides. No indication of protein leakage into the alveolar space was found with no detection of hyaline substance accumulation or edema fluid, confirming the protein measurements in figure 4. Conversely, mice given bacteria exhibited significant edema, hyperemia, congestion, neutrophil margination and infiltration, hemorrhage, and the presence of debris. Histological scoring of the lungs is shown in Figure 7 B, illustrating that the groups of septic mice and normal saline are comparable while bacteria is markedly elevated. All of these parameters demonstrate that there is no significant lung injury in CLP-induced sepsis at 24 and 48 hours in the acute phase.
Figure 7.
Histopathological evaluation of the lung 24 and 48 hours after cecal ligation and puncture. In Figure 7 A, images of hematoxylin and eosin stained slides at increasing magnifications are shown for 48 hour Die-P and Live-P mice, as well as a mouse given normal saline or bacteria. No appreciable signs of edema, protein leakage or alveolar neutrophil infiltration could be detected at any magnification in either Live-P or Die-P mice. Histological scoring is shown for each group in Figure 7 B (24h Live-P (n=5), 24h Die-P (n=5); 48h Live-P (n=6); 48h Die-P (n=7), normal saline (n=8), bacteria (n=8). The sections were evaluated in a blinded manner by a board certified pathologist. Values are mean ± SEM. There were significant differences between all the groups, p<0.001, but there was no difference between Live-P and Die-P mice at either 24 or 48 hours. * = p<.05 for bacteria versus normal saline.
Lung Inflammation
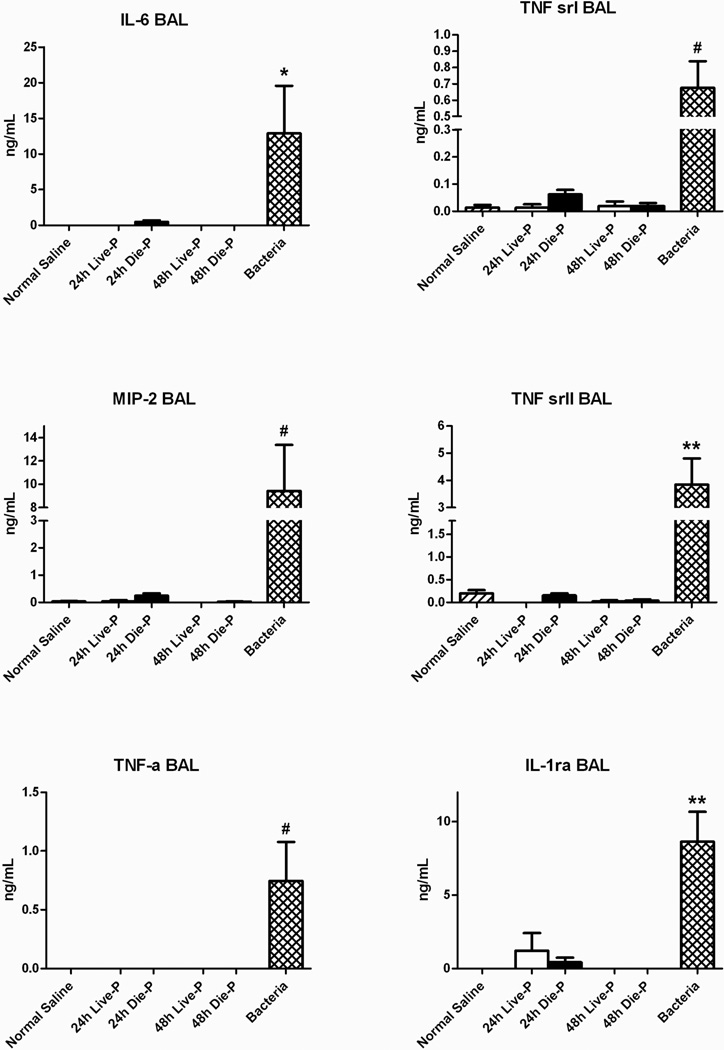
Finally, the presence of pulmonary inflammatory mediators was tested by measuring the local concentration of cytokines in the BAL fluid of the mice sacrificed at 24 and 48 hours. The systemic plasma levels were also measured to provide a general context for the local levels (data not shown). As expected, systemic levels of IL-6 were significantly increased in Die-P compared to Live-P. The BAL fluid concentration of 6 cytokines including interleukin-6 (IL-6), macrophage inflammatory protein-2 (MIP-2), tumor necrosis factor-alpha (TNF-α), TNF soluble receptor I and II (TNF sr I and TNF sr II), and interleukin-1 receptor antagonist (IL-1rα) was similar in the groups of septic mice as seen in Figure 8. Importantly, the bacterial pneumonia caused a significant increase in each of the BAL cytokines. Overall, only specific systemic cytokines were upregulated which did not translate into lung injury and there was no appreciable local inflammation.
Figure 8.
Local levels of cytokines at 24 and 48 hours after cecal ligation and puncture. There was no difference in BAL levels of IL-6, MIP-2, TNF-α, TNF sr I, TNF sr II, IL-1rα. BAL group size was 24h Live-P (n=5), 24h Die-P (n=8); 48h Live-P (n=3); 48h Die-P (n=6), normal saline (n=8), bacteria (n=8). Values are mean ± SEM. There were significant differences between all the groups, p<0.001, but there was no difference between Live-P and Die-P mice at either 24 or 48 hours. * = p<0.05, ** = p<.01, #p<0.001 for bacteria versus normal saline.
Discussion
Approximately 40% of sepsis patients develop ALI/ARDS, making sepsis the most common cause for ALI/ARDS (7, 8, 46). However, development of lung injury alone does not necessarily translate into increased mortality. Among the models used to study sepsis-induced ALI, CLP has received the least emphasis (12). The question of whether lung injury or inflammation develops in the septic mice predicted to die compared to those predicted to survive was investigated during the acute phase of sepsis when 100% of the mortality in the mice predicted to die will occur. The data provide evidence of increased systemic inflammation in the mice that were predicted to die, but no signs of lung injury for any of the septic mice, survivors and non-survivors, and consequently no correlation of lung injury with survival. The lung injury parameters used in this study were appropriate as demonstrated by the bacterial pneumonia model.
Stratification of septic mice into groups predicted to live or die based on plasma IL-6 has been successfully used before by our laboratory to show that high dose glucocorticoid therapy improves survival of the mice predicted to die (36). We also used it to demonstrate that increased early neutrophil recruitment at the focus of infection increases bacterial clearance and improves survival (29). Another group employed biomarker measurements to show that early antibiotic treatment also saves a portion of the mice that would be predicted to die (47). The biomarker prediction approach allows for powerful experiments in which mice that have been subjected to a similar septic insult but with different survival outcomes can be sacrificed and samples collected knowing their ultimate fate. In this study, an optimized dual cut-off system that allows a more precise separation of mice predicted to live or die (29) was used to investigate the pulmonary pathophysiology of sepsis, namely the development of lung injury or lung inflammation.
We evaluated lung injury 24 and 48 hours after CLP. Previous studies indicated that the pathological hallmarks of neutrophil recruitment and edema formation are present at 24 hours (15, 18, 48, 49). Other studies however indicate that lung injury may happen only later, with signs of it occurring at 48, 72 or even 96 hours post-CLP (16, 19, 50, 51). Yet, other studies suggest that lung injury does not develop when CLP is used as a single hit model but only occurs when it is preceded by a priming event like hemorrhagic shock (17, 52, 53), or succeeded by a direct pulmonary insult caused by immune complexes or lipopolysaccharide (54). Within this spectrum of the literature, our study concurs with those that do not find lung injury in the CLP model of sepsis. Since our study attempts to prove a negative, i.e. no lung injury, we rigorously quantified 12 different parameters of lung injury. A possible explanation for this overall range and the placement of our findings could be explained by the severity of the sepsis model used. CLP mortality rates are not always reported and recent papers emphasize the importance of this factor as the underlying pathology and/or success of treatment strategies varies with sepsis severity (55, 56). Most of the papers examining CLP-induced lung injury fail to report the mortality rate and those that do have 100% mortality within the first 5 days (16, 18, 48). A very severe model of sepsis that results in the death of all the animals could show signs of lung injury. Yet, findings in such a model would be hard to correlate with the pathophysiology in human sepsis as would findings from models producing overwhelming inflammation from endotoxin exposure (13, 57). The 50% mortality in our sepsis model closely reproduces a typical ICU mortality that ranges between 30 and 50% depending on severity and underlying condition (58). It allows us to compare septic mice that survive with septic mice that die in the same experiment, with all the mice sustaining a similar insult. Thus, pathological findings explaining mid-level severity in our model would have a higher relevance for human sepsis. However, the death of septic mice in the absence of lung injury, as observed in our study, can also be related to the situation in septic humans where lung injury alone does not increase the risk of death (9–11).
We also attempt to reproduce the clinical situation of human sepsis by providing broad spectrum antibiotic treatment for the mice. Our group and others have shown that this treatment greatly improves survival in sepsis, and we believe that further attempts to characterize this sepsis model or to discover therapeutic possibilities should use therapies already proven effective in human studies (47, 59). In septic humans, organ injury happens despite intensive treatment with appropriate antibiotics, fluid resuscitation and nutritional support (57, 60). The majority of the studies involving murine CLP do not report whether antibiotics are used, yet antibiotic treatment may delay the signs of lung injury by 24 hours in a model displaying 100% mortality (16). Therefore, we measured all parameters at both 24 and 48 hours. Of note, all of the mice died by 72 hours so the 48h Die-P data were collected within a time window no more than 24 hours prior to death. The fact that hypoxemia was not present in any of the septic mice throughout the acute phase, defined as the first 5 days post-CLP in survivors or until death in non-survivors (data not shown), indicates that lung injury was indeed absent.
Protein-rich lung edema caused by an increase in vascular permeability is the principal pathological finding early in the evolution of ALI/ARDS (61). The alveolar flooding and alveolar collapse (caused by interstitial edema) lead to a decrease in compliance. These changes, coupled with surfactant dysfunction (62) as well as ventilation and perfusion mismatching (63) are the primary causes for the perturbation of lung gas exchanges in ALI/ARDS. Protein content is not frequently measured but some studies report an increase (19, 51) and the studies that did not report injury indicate low protein levels (17, 53). We did not find evidence of lung edema as both dying and surviving septic mice had no extravasation of protein in their alveoli based on the levels measured in their BAL fluid. These results were confirmed by histological examination of the lungs. In the absence of edema, gas exchange in the lung should have been normal and this study confirmed by arterial blood gas that septic mice, even those that are succumbing to the disease, do not develop hypoxemia.
Conclusions
This study evaluated whether lung injury develops in the murine CLP model of sepsis. We found that the mice predicted to die demonstrate no signs of lung injury. Overall, the data strongly suggest that lung injury does not cause acute mortality in the murine CLP model of sepsis.
Acknowledgements
This work was supported by NIH grant GM 82962 and T32 GM 086308.
We thank Marc Lenburg, Ph.D., for helpful discussions regarding the statistical analysis and Dominic Beal for help with preparation of the cytospin slides. We also wish to thyank Dr. Colton from the Department of Epidemiology and Biostatistics for his review of the statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work was performed in the Department of Pathology and Laboratory Medicine, Boston University School of Medicine, 670 Albany Street, Room 441, Boston, MA 02118, USA.
The authors have not disclosed any potential conflicts of interest
References
- 1.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 Suppl):S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 2.Parrillo JE, Parker MM, Natanson C, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113(3):227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 3.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 6.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 7.Hudson LD, Milberg JA, Anardi D, et al. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 8.Newman JH. Sepsis and pulmonary edema. Clin Chest Med. 1985;6(3):371–391. [PubMed] [Google Scholar]
- 9.Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30(11):2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Ferring M, Vincent JL. Is outcome from ARDS related to the severity of respiratory failure? Eur Respir J. 1997;10(6):1297–1300. doi: 10.1183/09031936.97.10061297. [DOI] [PubMed] [Google Scholar]
- 11.Hudson LD. Survival data in patients with acute and chronic lung disease requiring mechanical ventilation. Am Rev Respir Dis. 1989;140(2 Pt 2):S19–S24. doi: 10.1164/ajrccm/140.2_Pt_2.S19. [DOI] [PubMed] [Google Scholar]
- 12.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4(10):854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 14.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81(1):137–143. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- 15.Asaduzzaman M, Rahman M, Jeppsson B, et al. P-selectin glycoprotein-ligand-1 regulates pulmonary recruitment of neutrophils in a platelet-independent manner in abdominal sepsis. Br J Pharmacol. 2009;156(2):307–315. doi: 10.1111/j.1476-5381.2008.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doerschug KC, Powers LS, Monick MM, et al. Antibiotics delay but do not prevent bacteremia and lung injury in murine sepsis. Crit Care Med. 2004;32(2):489–494. doi: 10.1097/01.CCM.0000109450.66450.23. [DOI] [PubMed] [Google Scholar]
- 17.Lomas-Neira J, Chung CS, Perl M, et al. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L51–L58. doi: 10.1152/ajplung.00028.2005. [DOI] [PubMed] [Google Scholar]
- 18.Tsujimoto H, Ono S, Mochizuki H, et al. Role of macrophage inflammatory protein 2 in acute lung injury in murine peritonitis. J Surg Res. 2002;103(1):61–67. doi: 10.1006/jsre.2001.6325. [DOI] [PubMed] [Google Scholar]
- 19.Yin K, Wilmanski J, Wang C, et al. Lung compartmentalization of inflammatory cells in sepsis. Inflammation. 2000;24(6):547–557. doi: 10.1023/a:1007077407302. [DOI] [PubMed] [Google Scholar]
- 20.Weber DJ, Rutala WA, Sickbert-Bennett EE, et al. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28(7):825–831. doi: 10.1086/518460. [DOI] [PubMed] [Google Scholar]
- 21.Fujitani S, Sun HY, Yu VL, et al. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest. 139(4):909–919. doi: 10.1378/chest.10-0166. [DOI] [PubMed] [Google Scholar]
- 22.Bir N, Lafargue M, Howard M, et al. Cytoprotective-selective activated protein C attenuates Pseudomonas aeruginosa-induced lung injury in mice. Am J Respir Cell Mol Biol. 45(3):632–641. doi: 10.1165/rcmb.2010-0397OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002;14(2):123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 24.Woods DE, Hwang WS, Shahrabadi MS, et al. Alteration of pulmonary structure by Pseudomonas aeruginosa exoenzyme S. J Med Microbiol. 1988;26(2):133–141. doi: 10.1099/00222615-26-2-133. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan S, Kim J, Remick DG. Acute pulmonary lipopolysaccharide tolerance decreases TNF-alpha without reducing neutrophil recruitment. J Immunol. 2008;181(12):8402–8408. doi: 10.4049/jimmunol.181.12.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 27.Ebong SJ, Call DR, Bolgos G, et al. Immunopathologic responses to non-lethal sepsis. Shock. 1999;12(2):118–126. doi: 10.1097/00024382-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Hemmila MR, Kim J, Sun JM, et al. Gene therapy with lipopolysaccharide binding protein for gram-negative pneumonia: respiratory physiology. J Trauma. 2006;61(3):598–605. doi: 10.1097/01.ta.0000233763.18853.5b. discussion 605-596. [DOI] [PubMed] [Google Scholar]
- 29.Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol. 2010;185(11):6930–6938. doi: 10.4049/jimmunol.1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982;3(1):35–56. [PubMed] [Google Scholar]
- 31.Schneider T, Issekutz AC. Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase. Application in a Brown Norway rat model of allergic pulmonary inflammation. J Immunol Methods. 1996;198(1):1–14. doi: 10.1016/0022-1759(96)00143-3. [DOI] [PubMed] [Google Scholar]
- 32.Moitra R, Beal DR, Belikoff BG, et al. Presence of preexisting antibodies mediates survival in sepsis. Shock. 37(1):56–62. doi: 10.1097/SHK.0b013e3182356f3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemzek JA, Call DR, Ebong SJ, et al. Immunopathology of a two-hit murine model of acid aspiration lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;278(3):L512–L520. doi: 10.1152/ajplung.2000.278.3.L512. [DOI] [PubMed] [Google Scholar]
- 34.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods. 2001;255(1–2):149–157. doi: 10.1016/s0022-1759(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 35.Osuchowski MF, Remick DG. The repetitive use of samples to measure multiple cytokines: the sequential ELISA. Methods. 2006;38(4):304–311. doi: 10.1016/j.ymeth.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Osuchowski MF, Connett J, Welch K, et al. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med. 2009;37(5):1567–1573. doi: 10.1097/CCM.0b013e31819df06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osuchowski MF, Welch K, Siddiqui J, et al. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177(3):1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 38.Remick DG, Bolgos GR, Siddiqui J, et al. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17(6):463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Vaickus LJ, Bouchard J, Kim J, et al. Assessing pulmonary pathology by detailed examination of respiratory function. Am J Pathol. 177(4):1861–1869. doi: 10.2353/ajpath.2010.100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brigham KL, Bowers R, Haynes J. Increased sheep lung vascular permeability caused by Escherichia coli endotoxin. Circ Res. 1979;45(2):292–297. doi: 10.1161/01.res.45.2.292. [DOI] [PubMed] [Google Scholar]
- 41.Brigham KL, Woolverton WC, Blake LH, et al. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest. 1974;54(4):792–804. doi: 10.1172/JCI107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fein A, Grossman RF, Jones JG, et al. The value of edema fluid protein measurement in patients with pulmonary edema. Am J Med. 1979;67(1):32–38. doi: 10.1016/0002-9343(79)90066-4. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg KP, Milberg JA, Martin TR, et al. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150(1):113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- 44.Allan G, Bhattacherjee P, Brook CD, et al. Myeloperoxidase activity as a quantitative marker of polymorphonuclear leukocyte accumulation into an experimental myocardial infarct-- the effect of ibuprofen on infarct size and polymorphonuclear leukocyte accumulation. J Cardiovasc Pharmacol. 1985;7(6):1154–1160. doi: 10.1097/00005344-198511000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Remick DG, Garg SJ, Newcomb DE, et al. Exogenous interleukin-10 fails to decrease the mortality or morbidity of sepsis. Crit Care Med. 1998;26(5):895–904. doi: 10.1097/00003246-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 46.Tsushima K, King LS, Aggarwal NR, et al. Acute lung injury review. Intern Med. 2009;48(9):621–630. doi: 10.2169/internalmedicine.48.1741. [DOI] [PubMed] [Google Scholar]
- 47.Vyas D, Javadi P, Dipasco PJ, et al. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R1048–R1053. doi: 10.1152/ajpregu.00312.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gold JA, Parsey M, Hoshino Y, et al. CD40 contributes to lethality in acute sepsis: in vivo role for CD40 in innate immunity. Infect Immun. 2003;71(6):3521–3528. doi: 10.1128/IAI.71.6.3521-3528.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu R, Dong W, Zhou M, et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176(8):805–813. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirsh M, Dyugovskaya L, Kaplan V, et al. Response of lung gammadelta T cells to experimental sepsis in mice. Immunology. 2004;112(1):153–160. doi: 10.1111/j.1365-2567.2004.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirsh M, Kaplan V, Dyugovskaya L, et al. Response of lung NK1.1-positive natural killer cells to experimental sepsis in mice. Shock. 2004;22(1):40–45. doi: 10.1097/01.shk.0000129758.81361.45. [DOI] [PubMed] [Google Scholar]
- 52.Ayala A, Chung CS, Lomas JL, et al. Shock-induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Am J Pathol. 2002;161(6):2283–2294. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lomas-Neira JL, Chung CS, Grutkoski PS, et al. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004;76(1):58–64. doi: 10.1189/jlb.1103541. [DOI] [PubMed] [Google Scholar]
- 54.Czermak BJ, Breckwoldt M, Ravage ZB, et al. Mechanisms of enhanced lung injury during sepsis. Am J Pathol. 1999;154(4):1057–1065. doi: 10.1016/S0002-9440(10)65358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rittirsch D, Flierl MA, Nadeau BA, et al. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14(5):551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rittirsch D, Huber-Lang MS, Flierl MA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barriere SL, Lowry SF. An overview of mortality risk prediction in sepsis. Crit Care Med. 1995;23(2):376–393. doi: 10.1097/00003246-199502000-00026. [DOI] [PubMed] [Google Scholar]
- 58.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Newcomb D, Bolgos G, Green L, et al. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock. 1998;10(2):110–117. doi: 10.1097/00024382-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10(2):79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 62.Gregory TJ, Longmore WJ, Moxley MA, et al. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest. 1991;88(6):1976–1981. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gunther A, Walmrath D, Grimminger F, et al. Pathophysiology of acute lung injury. Semin Respir Crit Care Med. 2001;22(3):247–258. doi: 10.1055/s-2001-15782. [DOI] [PubMed] [Google Scholar]