Abstract
AIM: To investigate retrospectively the long-term efficacy of various treatment strategies using adefovir dipivoxil (adefovir) in patients with lamivudine-resistant chronic hepatitis B.
METHODS: We included 154 consecutive patients in two treatment groups: the “add-on” group (n = 79), in which adefovir was added to ongoing lamivudine treatment due to lamivudine resistance, and the “switch/combination” group (n = 75), in which lamivudine was first switched to adefovir and then re-added later as needed. The “switch/combination” group was then divided into two subgroups depending on whether participants followed (group A, n = 30) or violated (group B, n = 45) a proposed treatment strategy that determined whether to add lamivudine based on the serum hepatitis B virus (HBV) DNA levels (< 60 IU/mL or not) after 6 mo of treatment (roadmap concept).
RESULTS: The cumulative probability of virologic response (HBV DNA < 60 IU/mL) was higher in group A than in the “add-on” group and in group B (P < 0.001). In contrast, the cumulative probability of virologic breakthrough was lower in the “add-on” group than in group B (P = 0.002). Furthermore, the risk of virologic breakthrough in the multivariate analysis was significantly lower in the “add-on” group than in group A (hazard ratio = 0.096; 95%CI, 0.015-0.629; P = 0.015).
CONCLUSION: The selective combination of adefovir with lamivudine based upon early treatment responses increased the odds of virologic breakthrough relative to the use of uniform combination therapy from the beginning of treatment.
Keywords: Chronic hepatitis B, Lamivudine-resistant, Adefovir, Combination therapy, Roadmap concept
INTRODUCTION
The aims of chronic hepatitis B (CHB) treatment are to achieve the sustained suppression of hepatitis B virus (HBV) replication and to prevent progression to cirrhosis, liver failure, and hepatocellular carcinoma[1]. Lamivudine is a safe and effective nucleoside analog that has been approved by the United States Food and Drug Administration as the first oral therapy for CHB. Lamivudine suppresses HBV replication by inhibiting the activity of HBV DNA polymerase, which leads to the normalization of serum alanine aminotransferase (ALT) levels, hepatitis B e antigen (HBeAg) seroconversion, and histological improvement[2-5]. However, some CHB patients who are treated with lamivudine over a long period of time develop resistance to the drug at a rate reaching approximately 70% after five years of administration[6]. This resistance leads to virologic breakthrough, re-elevation of the serum ALT levels, hepatic failure, and even death in some patients[3,4,7]. Despite the introduction of more potent antiviral agents for the treatment of CHB, lamivudine is still frequently used due to its lower cost.
Adefovir dipivoxil (adefovir) is a nucleotide analog that is effective against both wild-type and lamivudine-resistant forms of HBV[8-13]. However, adefovir resistance mutations develop more frequently in lamivudine-resistant patients than in treatment-naive patients[14-17]. Several recent studies have reported that the combination of adefovir with lamivudine results in a lower incidence of resistance to adefovir and has higher efficacy than adefovir monotherapy in patients with lamivudine resistance[18-21]. In reality, however, adefovir is frequently initiated as a monotherapy due to the high cost of combination therapy, and lamivudine is sometimes added later if needed. It has not yet been determined when and how to assess the appropriateness of the virologic response to adefovir monotherapy so that lamivudine can be added at the optimal time. In such situations, healthcare providers may consult the roadmap concept proposed by Keeffe et al[22]. The roadmap concept uses the treatment response at 6 mo to individualize ongoing antiviral management to minimize resistance and improve long-term efficacy. Although this concept is primarily based on the results of previous studies on treatment-naive CHB patients[23-25], a recent study suggested that the roadmap concept might be useful as a means of increasing the therapeutic efficacy during adefovir monotherapy in patients with lamivudine-resistant HBeAg-positive CHB[26].
To date, however, no studies have investigated whether treatment strategies based on the roadmap concept (first initiating adefovir monotherapy and then adding lamivudine based on early treatment response) are as effective as ab initio combination therapy in patients with lamivudine-resistant CHB.
In this study, we retrospectively compared the long-term efficacies of various treatment strategies using adefovir in patients with lamivudine-resistant CHB and analyzed factors associated with treatment efficacy. In particular, we focused on whether the long-term efficacies differed between patients treated in accordance with the roadmap concept and those treated with combination therapy from the start of treatment.
MATERIALS AND METHODS
Study sample
We screened the medical records of 255 consecutive patients who were prescribed adefovir 10 mg daily for the treatment of lamivudine-resistant CHB and followed up for more than 12 mo at Korea University Anam Hospital from May 2004 to February 2010. Of these 255 patients, 14 were excluded from the study because they had already been prescribed adefovir at another hospital. 20 patients were excluded because they received other drugs (entecavir, remofovir, or clevudine) prior to adefovir treatment. We also excluded another 67 patients with serum HBV DNA levels at month six that were below the detection limit (< 0.5 pg/mL) of the hybridization method and that were not measured with more sensitive quantitative techniques. The data were collected for the remaining 154 patients and analyzed retrospectively. Patients were divided into two groups depending on how adefovir was initiated: a “switch/combination” group and an “add-on” group. For the “switch/combination” group (n = 75), lamivudine was first switched to adefovir, and lamivudine was re-added later if necessary in cases of primary non-response and inadequate response (n = 31) or virologic breakthrough (n = 6). For the “add-on” group (n = 79), adefovir was added to ongoing lamivudine treatment due to lamivudine resistance.
Methods
Clinical information (including age, gender, duration of prior lamivudine treatment, body mass index, presence of liver cirrhosis, and Child-Pugh score) was obtained by reviewing the patient medical records. Data were also collected from laboratory tests that were performed prior to adefovir administration and every three months thereafter. These tests included routine complete blood counts; biochemical tests to measure the serum levels of ALT, aspartate aminotransferase, alkaline phosphatase, gamma glutamyl transpeptidase, albumin, total bilirubin, blood urea nitrogen, creatinine, and phosphorus; assays to determine the prothrombin time; and serologic studies to determine the HBeAg, anti-HBeAg antibody (anti-HBe), and HBV DNA levels.
The diagnosis of liver cirrhosis was based on liver biopsy features or on clinical, laboratory, radiologic and endoscopic data. Lamivudine resistance mutations (rtM204V/I or rtL180M) were detected using direct sequencing assays or restriction fragment mass polymorphisms at baseline in all patients included in our study. In the same manner, testing for adefovir resistance mutations was also performed in patients who exhibited virologic breakthrough during adefovir treatment. Prior to July 2007, the quantitative analyses of serum HBV DNA levels were conducted using a polymerase chain reaction (PCR) assay (COBAS Amplicor HBV Monitor, Roche Diagnostics, Indianapolis, IN, United States), which has a lower detection limit of 60 IU/mL; thereafter, a real-time PCR assay (COBAS TaqMan HBV test, Roche Diagnostics, Indianapolis, IN, United States) with a lower detection limit of 20 IU/mL was used.
Definition
Virologic response was defined as a decrease in HBV DNA to undetectable levels (HBV DNA < 60 IU/mL). Biochemical response was defined as a decrease in the serum ALT level to within the normal range. HBeAg seroclearance was defined as the loss of HBeAg from the serum with a decrease in serum HBV DNA to undetectable levels (HBV DNA < 60 IU/mL) in patients who had been seropositive for HBeAg before the initiation of adefovir. We defined virologic breakthrough as a confirmed increase in HBV DNA levels of more than 1 log10 IU/mL relative to the lowest HBV DNA levels observed during treatment. Biochemical breakthrough was defined as an increase in the serum ALT level above the upper limit of normal on at least two occasions after achieving normalization with treatment. Primary non-response was defined as a decrease of less than 2 log10 IU/mL in the HBV DNA level from baseline after six months of treatment. Inadequate response was defined as HBV DNA levels of 2000 IU/mL or greater after six months of treatment.
We based our treatment strategy for the “switch/combination” group on the roadmap concept, in which clinicians decide whether to add lamivudine based on the early treatment response after six months of adefovir monotherapy. However, if serum HBV DNA levels at six months were lower than 60 IU/mL, patients were regarded as having early treatment response and continued to receive adefovir monotherapy. However, if serum HBV DNA levels at six months were 60 IU/mL or higher, the response was considered inadequate, and lamivudine was added to the treatment regimen. The “switch/combination” group was then divided into subgroups A and B, in which the treatment strategy based on the roadmap concept was either satisfied or not satisfied, respectively.
Statistical analysis
The serum HBV DNA levels were logarithmically transformed for analysis. To evaluate differences in clinical aspects prior to adefovir treatment between the “switch/combination” group and the “add-on” group, continuous variables were compared using the two-sample t test, and categorical data were compared using the χ2 test. The cumulative probabilities of virologic and biochemical responses, HBeAg seroclearance, and virologic and biochemical breakthroughs were evaluated using Kaplan-Meier analysis. The log-rank test was used to evaluate differences between the two groups. We identified independent factors associated with treatment outcomes using the Cox proportional hazard model. Variables with P values < 0.1 in the univariate analysis were included in the multivariate analysis. P values < 0.05 were considered statistically significant. All data were analyzed using the Statistical Package for the Social Sciences (SPSS) for Windows (version 18, SPSS Inc., Chicago, IL, United States).
RESULTS
Baseline characteristics of the patients prior to adefovir treatment
Of the 154 patients included in the study, 109 (70.8%) were male, and 45 (29.2%) were female. The mean (± SD) age of the entire sample was 45.5 ± 11.8 years. 110 patients (71.4%) were seropositive for HBeAg, and 65 (42.2%) had liver cirrhosis. The mean levels of serum HBV DNA and ALT were 6.85 ± 1.01 log10 IU/mL and 200.1 ± 179.3 IU/L, respectively. The mean duration of prior lamivudine treatment before the initiation of adefovir was 28.5 ± 14.6 mo, and we conducted follow-up after the initiation of adefovir for an average of 33.8 ± 14.1 mo (median, 33; range 12-66). The “switch/combination” group included 75 patients, and the “add-on” group included 79 patients. The median durations of follow-up in the “switch/combination” group and the “add-on” group were 42 mo (range, 12-66) and 24 mo (range, 12-36), respectively. At baseline, there were no significant differences in the clinical and laboratory features between groups except for age and the presence of liver cirrhosis (Table 1).
Table 1.
Baseline characteristics of patients with lamivudine-resistant chronic hepatitis B
| "Switch/combination" group2 (n = 75) | "Add-on" group3 (n = 79) | P value1 | |
| Age (yr) | 43.3 ± 10.2 | 47.6 ± 12.9 | 0.022 |
| Male gender | 56 (74.7) | 53 (67.1) | 0.301 |
| BMI (kg/m2) | 24.1 ± 5.1 | 23.8 ± 3.2 | 0.583 |
| Positive for HBeAg | 57 (76.0) | 53 (67.1) | 0.221 |
| Positive for anti-HBe | 19 (25.3) | 25 (31.6) | 0.386 |
| HBV DNA (log10 IU/mL) | 6.75 ± 0.93 | 6.94 ± 1.08 | 0.233 |
| AST (IU/L) | 148.7 ± 134.5 | 141.1 ± 148.3 | 0.742 |
| ALT (IU/L) | 230.1 ± 213.4 | 199.5 ± 191.8 | 0.351 |
| Albumin (g/dL) | 4.4 ± 0.5 | 4.3 ± 0.6 | 0.166 |
| ALP (IU/L) | 83.4 ± 37.6 | 76.8 ± 27.6 | 0.211 |
| GGT (IU/L) | 60.5 ± 42.9 | 67.1 ± 71.1 | 0.494 |
| Total bilirubin (mg/dL) | 1.2 ± 0.9 | 1.4 ± 1.9 | 0.425 |
| Prothrombin time (INR) | 1.16 ± 0.29 | 1.15 ± 0.19 | 0.825 |
| Hemoglobin (g/dL) | 14.5 ± 1.8 | 14.2 ± 1.7 | 0.345 |
| WBC (/mm3) | 5279 ± 1771 | 4972 ± 1374 | 0.233 |
| Platelet (× 103/mm3) | 156 ± 68 | 145 ± 69 | 0.283 |
| BUN (mg/dL) | 12.5 ± 3.1 | 13.1 ± 4.4 | 0.325 |
| Creatinine (mg/dL) | 0.97 ± 0.15 | 0.92 ± 0.20 | 0.110 |
| Duration of prior lamivudine treatment (mo) | 28.7 ± 13.6 | 28.3 ± 15.5 | 0.886 |
| Cirrhosis | 25 (33.3) | 40 (50.6) | 0.030 |
All values are expressed as mean ± SD or number of patients (%).
P values were calculated using the two-sample t test for continuous variables and the χ2 test for categorical variables;
Lamivudine was first switched to adefovir dipivoxil (adefovir), and then lamivudine was re-added later as needed in cases of primary non-response, inadequate response or virologic breakthrough;
Adefovir was added to ongoing lamivudine treatment due to lamivudine resistance. BMI: Body mass index; HBeAg: Hepatitis B e Antigen; Anti-Hbe: Antibody to HBeAg; HBV DNA: Hepatitis B virus DNA; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; GGT: γ glutamyl transpeptidase; INR: International normalized ratio; WBC: White blood cell; BUN: Blood urea nitrogen.
Treatment efficacy in the "switch/combination" group and the "add-on" group
After the initiation of adefovir, virologic response was achieved in 51 of 75 (68.0%) patients in the “switch/combination” group during the follow-up period. In the “add-on” group, 51 of 79 (64.6%) patients achieved virologic response. There were no significant differences in the virologic response rate between the two groups (P = 0.249), with cumulative probabilities at the first and third years of 41.3% and 68.2% in the “switch/combination” group and 48.1% and 73.5% in the “add-on” group, respectively. In the multivariate analysis, however, virologic response was found to be significantly more common in the “add-on” group [hazard ratio (HR) = 1.646; 95%CI, 1.080-2.510; P = 0.021] and less frequent in patients with inadequate response (serum HBV DNA ≥ 2,000 IU/mL at 6 mo) (HR = 0.121; 95%CI, 0.069-0.212; P < 0.001) (Table 2).
Table 2.
Multivariate analysis for factors of virologic response (undetectable in polymerase chain reaction assay)
| Variables | HR | 95%CI | P value1 |
| Male gender | 0.958 | 0.621-1.477 | 0.846 |
| HBeAg (+) | 0.668 | 0.415-1.077 | 0.098 |
| HBV DNA (log10 IU/mL) | 0.810 | 0.644-1.018 | 0.071 |
| AST (IU/L) | 1.000 | 0.998-1.002 | 0.955 |
| ALT (IU/L) | 1.000 | 0.999-1.002 | 0.606 |
| Duration of prior lamivudine treatment (mo) | 1.011 | 0.998-1.024 | 0.112 |
| Inadequate response2 | 0.121 | 0.069-0.212 | < 0.001 |
| Treatment group3 "Add-on" vs "switch/combination" | 1.646 | 1.080-2.510 | 0.021 |
P-values were calculated with the Cox’s proportional hazard model; Variables with a P < 0.1 in the univariate analysis were included in the multivariate analysis;
Inadequate response was defined as serum HBV DNA levels of 2000 IU/mL or greater at 6 mo of treatment;
Patients were divided into two treatment groups: the "add-on" group, in which adefovir dipivoxil (adefovir) was added to ongoing lamivudine treatment due to lamivudine resistance, and the "switch/combination" group, in which lamivudine was first switched to adefovir and then re-added later as needed. HBeAg: Hepatitis B e Antigen; HBV DNA: Hepatitis B virus DNA; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; HR: Hazard ratio.
Biochemical response was achieved in 143 of 154 patients (the “switch/combination” group vs the “add-on” group: 92.0% vs 93.7%, respectively). There was no significant difference in the cumulative probability of biochemical response between the two groups (P = 0.190). In the multivariate analysis, inadequate response was the only independent factor associated with biochemical response (HR = 0.574; 95%CI, 0.402-0.820; P = 0.002).
Of our 110 patients who were HBeAg-positive at baseline, HBeAg seroclearance was achieved in 28 of 57 (49.1%) in the “switch/combination” group and in 23 of 53 (43.4%) in the “add-on” group. The incidence of HBeAg seroclearance did not differ significantly between the two groups (P = 0.326). The cumulative probabilities of seroclearance during the first and third years were 17.5% and 44.8% in the “switch/combination” group and 13.2% and 62.4% in the “add-on” group, respectively. Inadequate response was the only independent factor that was correlated with HBeAg seroclearance (HR = 0.214; 95%CI, 0.109-0.419; P < 0.001).
Virologic and biochemical breakthroughs during adefovir treatment were observed in 19 patients [17 of 75 (22.7%) in the “switch/combination” group and 2 of 79 (2.5%) in the “add-on” group] and 16 patients (20.0% vs 1.3%), respectively. Both breakthroughs were more common in the “switch/combination” group than in the “add-on” group, according to the Kaplan-Meier analysis (P < 0.05). The cumulative probabilities of virologic breakthrough at the first and third years were 2.7% and 23.2% in the “switch/combination” group and 0% and 3.8% in the “add-on” group (Figure 1). In the multivariate analysis, the risk of virologic breakthrough was significantly lower in the “add-on” group (HR = 0.130; 95%CI, 0.028-0.599; P = 0.009) and significantly higher in patients with inadequate response (HR = 5.251; 95%CI, 1.684-16.371; P = 0.004).
Figure 1.
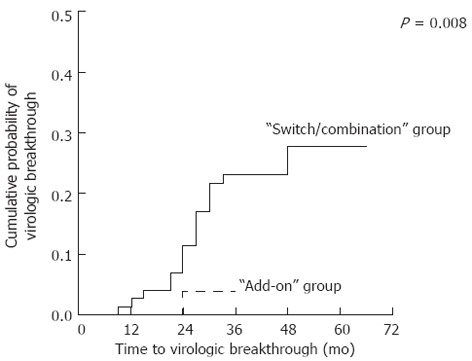
Cumulative probabilities of virologic breakthrough in the two treatment groups.
Treatment efficacy in the "switch/combination" group according to compliance with the treatment strategy based on the roadmap concept
As mentioned above, we used a roadmap concept-based treatment strategy, which recommends the addition of lamivudine based on an early treatment response after six months of adefovir monotherapy (< 60 IU/mL), for patients in the “switch/combination” group. group A included patients who were treated in accordance with the roadmap strategy. group B was made up of patients for whom the roadmap-dictated treatment strategy was not followed. Thirty patients were included in group A (27 maintained adefovir monotherapy, and 3 received concomitant lamivudine), and 45 patients were included in group B.
Patients in “switch/combination” group A were more likely to experience virologic response than those in group B (P < 0.001) or those in the “add-on” group (group C) (P < 0.001) (Figure 2A). In contrast, the cumulative probability of virologic breakthrough was significantly lower in the “add-on” group (0% at the first year, 3.8% at the third year) than in “switch/combination” group B (4.4% at the first year, 27.5% at the third year) (P = 0.002) but was not lower than in “switch/combination” group A (0% at the first year, 17.4% at the third year, 38.0% at the fifth year) (P = 0.114) (Figure 2B). In the multivariate analysis, however, the risk of virologic breakthrough was significantly lower in the “add-on” group than in “switch/combination” group A (HR = 0.096; 95%CI, 0.015-0.629; P = 0.015) (Table 3).
Figure 2.
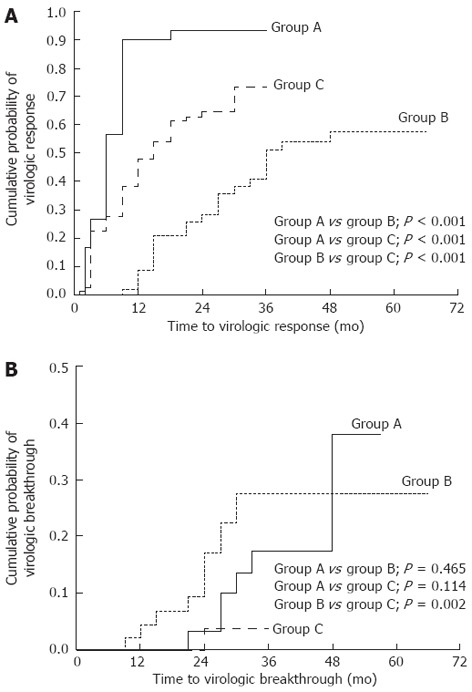
A comparison of the cumulative probabilities of virologic response and viral breakthrough according to different treatment strategies using adefovir dipivoxil in patients with lamivudine-resistant chronic hepatitis B. Groups A and B represent the patients who followed or violated the roadmap treatment strategy, respectively; Group C stands for those treated by adding adefovir dipivoxil in addition to ongoing lamivudine therapy. The roadmap treatment strategy was supposed to initiate adefovir monotherapy first and then add lamivudine when serum HBV DNA levels were ≥ 60 IU/mL at six months of treatment. A: There were significant differences in the cumulative probabilities of virologic responses between any two treatment groups (P < 0.001); B: The cumulative probability of virologic breakthrough was significantly lower in group C than in group B (P = 0.002) but not lower than group A (P = 0.114). However, there was a significant difference between groups C and A in the multivariate Cox’s regression model (Table 3).
Table 3.
Multivariate analysis for risk factors of virologic breakthrough
| HR | 95%CI | P value1 | |
| HBeAg (+) | 3.251 | 0.400-26.403 | 0.270 |
| Albumin (g/dL) | 0.552 | 0.194-1.573 | 0.266 |
| ALP (IU/L) | 1.004 | 0.994-1.014 | 0.427 |
| PT (INR) | 1.269 | 0.186-8.648 | 0.808 |
| Platelet (× 103/mm3) | 0.997 | 0.986-1.007 | 0.519 |
| Cirrhosis | 1.016 | 0.243-4.448 | 0.983 |
| Duration of prior lamivudine treatment (mo) | 0.99 | 0.945-1.038 | 0.691 |
| Inadequate response2 | 6.57 | –1.517-28.458 | 0.012 |
| Treatment groups3 | 0.029 | ||
| Group B vs group A | 0.668 | 0.149-2.984 | 0.597 |
| Group C vs group A | 0.096 | 0.015-0.629 | 0.015 |
P-values were calculated with Cox’s proportional hazard model; Variables with a P < 0.1 in the univariate analysis were included in the multivariate analysis;
Inadequate response was defined as serum HBV DNA levels of 2000 IU/mL or greater at 6 mo of treatment;
Treatment groups were divided into three groups according to the timing of the drug combination; groups A and B represent the patients who followed or violated the roadmap treatment strategy, respectively; group C included those participants who were treated by adding adefovir dipivoxil (adefovir) to ongoing lamivudine therapy. Roadmap treatment strategy was supposed to initiate adefovir monotherapy first and then add lamivudine later when serum HBV DNA levels were detectable (≥ 60 IU/mL) at six months of treatment. HBeAg: Hepatitis B e antigen; ALP: Alkaline phosphatase; PT: Prothrombin time; INR: International normalized ratio; HR: Hazard ratio.
In addition, patients with undetectable (< 60 IU/mL) serum HBV DNA levels after six months of adefovir monotherapy had cumulative probabilities of virologic breakthrough of 15.7% and 36.7% at three and five years of treatment, respectively, with continued adefovir monotherapy based on the roadmap concept strategy.
Mutations conferring adefovir resistance
Testing for mutations conferring adefovir resistance was performed in patients with primary non-response, inadequate response or virologic breakthrough during adefovir treatment. 28 (37.3%) and 6 (7.6%) patients were tested for genotypic resistance to adefovir in the “switch/combination” group and the “add-on” group, respectively. Among these patients, adefovir resistance mutations were detected in 10 and 2 patients in the “switch/combination” and “add-on” groups, respectively (Table 4). Adefovir resistance mutations were only identified in 6 (40%) of 15 patients with virologic breakthrough (5 of 13 in the “switch/combination” group and 1 of 2 in the “add-on” group).
Table 4.
Patterns of genotypic resistance to adefovir dipivoxil in patients with lamivudine-resistant chronic hepatitis B
| Mutation pattern | "Switch/combination" group1 (n = 75) | "Add-on" group2 (n = 79) |
| rtA181V | 2 | 0 |
| rtA181T | 3 | 1 |
| rtA181V + rtA181T | 0 | 1 |
| rtN236T | 3 | 0 |
| rtA181V + rtN236T | 1 | 0 |
| rtA181T + rtN236T | 1 | 0 |
| Total, n (%) | 10 (13.3) | 2 (2.5) |
rtA181V, alanine to valine substitution at rt181; rtA181T, alanine to threonine substitution at rt181; rtN236T, asparagine to threonine substitution at rt236.
Lamivudine was first switched to adefovir dipivoxil (adefovir), and then lamivudine was re-added later as needed in case of primary non-response, inadequate response, or virologic breakthrough;
Adefovir was added to ongoing lamivudine treatment due to lamivudine resistance.
Nephrotoxicity due to adefovir
After initiating adefovir therapy, increased serum creatinine levels (> 1.2 mg/dL and an increase of over 20% from baseline) were observed more than once in 9 of 154 patients (5.8%). Of 4 patients with elevated serum creatinine levels (> 1.2 mg/dL) at baseline, 2 showed a greater than 20% increase from baseline.
9 of 154 patients (5.8%) had hypophosphatemia at least once during adefovir treatment, and 5 experienced the condition at least twice. Of these 5 patients, 3 recovered from hypophosphatemia after taking oral KH2PO4. In 3 patients (1.9%), increased serum creatinine and hypophosphatemia were both observed, and one of the participants showed severe hypophosphatemia, with levels less than 1.5 mg/dL.
DISCUSSION
Despite the high incidence of resistance, lamivudine is still prescribed for the treatment of CHB because of its low cost relative to other available drugs. Therefore, the treatment of lamivudine-resistant CHB will remain a clinical challenge in the field of hepatology for the foreseeable future.
In South Korea, we are currently able to use adefovir or entecavir to treat patients with lamivudine resistance. Approximately 40% of patients with lamivudine resistance develop resistance to adefovir monotherapy after four years of treatment[21]; patients receiving entecavir monotherapy exhibited resistance at a rate of 35.9% after three years[27]. By contrast, when adefovir was added to ongoing lamivudine therapy, the rate of resistance to adefovir was just 0%-2% at two years and 4.4% at four years[18,19,21]. In addition, as time passed, the proportion of patients who maintained undetectable serum HBV DNA levels and serum ALT levels within the normal range was greater in the combination therapy group than in the adefovir monotherapy group[21]. Therefore, when lamivudine resistance was detected, we prescribed combination therapy as soon as possible. In patients already receiving adefovir monotherapy, we added lamivudine only when an incomplete response was observed.
In the present study, we found that the cumulative probability of virologic breakthrough at three years was just 3.8% in the “add-on” group compared with 23.2% in the “switch/combination” group. Upon multivariate analysis, we confirmed that the probability of virologic response (serum HBV DNA < 60 IU/mL) was significantly higher and that the risk of virologic breakthrough was significantly lower in the “add-on” group than in the “switch/combination” group. These results suggest that adding adefovir to lamivudine may be the most appropriate treatment currently available for patients with lamivudine resistance in Korea. However, many patients do not have access to combination therapy because of its cost. Therefore, from a cost-effectiveness point of view, a treatment strategy based on the roadmap concept is worth considering.
The roadmap concept was originally proposed for treatment-naive patients after researchers realized that early virologic suppression during antiviral treatment was closely related to long-term therapeutic efficacy and the development of viral resistance to a drug[22]. However, a recent study of the outcomes of adefovir rescue monotherapy suggested that the roadmap concept might also be applicable in patients with lamivudine resistance[26]. Our study also found that early treatment responses (at six months) in patients with lamivudine-resistant CHB were strongly correlated with long-term therapeutic outcomes, such as virologic response, biochemical response and HBeAg seroclearance.
In this study, there was a significant difference in the rate of treatment response between the “switch/combination” and “add-on” groups. However, this result could not be interpreted to indicate that the add-on combination treatment was superior to a stepwise combination treatment based on the roadmap concept because the “switch/combination” group included many patients whose treatment was not based on the roadmap concept. To address this question, we divided the “switch/combination” group into two sub-groups depending on whether the participants received treatment based on the roadmap concept, and then we compared the treatment outcomes in these subgroups with those in the “add-on” group. We found that the cumulative probability of virologic response was significantly better in the subgroup whose treatment conformed to the roadmap concept (group A) than in the “add-on” group. However, the multivariate analysis showed that the risk of virologic breakthrough was significantly higher in group A than in the “add-on” group, with three-year cumulative probabilities of 17.4% and 3.8%, respectively. Even if the serum HBV DNA became undetectable (< 60 IU/mL) following six months of adefovir monotherapy, the cumulative probability of virologic breakthrough reached 15.7% and 36.7% at three and five years of treatment, respectively, when adefovir monotherapy was continued based on the roadmap strategy. These results suggest that the roadmap concept-based treatment strategy, which initiates adefovir first and adds lamivudine back to the treatment regimen depending on the treatment response at six months, should not be recommended for patients with lamivudine-resistant CHB. Of course, because the longest follow-up duration in our “add-on” group was only 36 mo, the resistance rate in the “add-on” group might increase as time passes. However, that possibility appears to be very low, given that the resistance rate at 48 mo in the “add-on” group was just 4% in two previous studies[18,21].
Adefovir resistance mutations were identified in only 40% of patients with virologic breakthrough in this study. This finding might be explained by the following: First, it is possible that virologic breakthrough developed following low drug compliance among patients without mutations. Second, lamivudine-resistant HBV may be decreased by the use of adefovir; at the same time, however, the wild-type HBV, which does not respond well to adefovir, might exhibit increased growth. These two processes may lead to virologic breakthrough. Third, these findings might be due to methodological problems regarding the analysis of genetic variations.
In this study, HBV genotyping was not performed because almost all patients with CHB (> 95%) in Korea are infected with HBV genotype C[28-31]. In addition, previous studies have not shown any relationship between HBV genotype and response to nucleos(t)ide analogs[32]. Therefore, the routine genotyping of HBV appears to be uninformative in predicting therapeutic outcome, and genotyping might be meaningless for Korean patients with CHB.
Nephrotoxicity is a well-known side-effect of adefovir. In the present study, we investigated two of the primary nephrotoxic side-effects that can be caused by adefovir. Serum creatinine levels were increased in 5.8% of patients after initiating adefovir in this study. This figure is similar to that reported in other studies[13,17-19]. Some case reports have found that adefovir can induce Fanconi syndrome or hypophosphatemic osteomalacia[33-35]. In this study, 5.8% of patients showed hypophosphatemia, and some of them required the administration of oral KH2PO4. Therefore, clinicians must monitor these side effects more thoroughly in case patients require further treatment. Although the mechanism of nephrotoxicity due to adefovir has not been clearly determined, it is thought to occur mainly in the proximal tubule of the kidney. As a nucleotide analog, adefovir might serve as a substrate for DNA polymerase γ, which is responsible for the replication of mitochondrial DNA (mtDNA). Thus, adefovir is thought to inhibit mtDNA replication and impair cellular oxidative respiration[36,37]. In addition, the over-expression of renal tubular transporters, which increases the intracellular concentration of the drug, is implicated in adefovir nephrotoxicity[38-40].
In conclusion, our study reaffirmed that the ab initio combination of adefovir with lamivudine was more effective than switching to adefovir monotherapy in a stepwise fashion in patients with lamivudine-resistant CHB. Although early treatment response is closely related to long-term efficacy, stepwise combination therapy based on the roadmap concept carries a higher risk of virologic breakthrough than initiating combination therapy from the beginning of treatment.
COMMENTS
Background
Lamivudine is still being used in many patients with chronic hepatitis B (CHB) due to its lower cost, despite its higher risk of promoting viral resistance. Adefovir dipivoxil (adefovir) has been used to treat lamivudine-resistant CHB. It is known that the combination of adefovir with lamivudine results in a lower rate of resistance than adefovir monotherapy in patients with lamivudine-resistant CHB. Due to the high cost of combination therapy, however, adefovir is frequently initiated as a monotherapy, with lamivudine added back to the treatment regimen later as needed.
Research frontiers
A recent study suggested the potential usefulness of response-guided adefovir monotherapy based on the roadmap concept in patients with lamivudine-resistant CHB. However, no study has compared the effectiveness of response-guided stepwise combination therapy with that of ab initio combination therapy.
Innovations and breakthroughs
This study aimed to compare the long-term efficacies of various treatment strategies including adefovir in patients with lamivudine-resistant CHB, with a focus on whether long-term efficacy differs between patients treated in accordance with the roadmap concept and those treated with combination therapy from the start of treatment. The results suggest that, although an early virologic response helps to predict long-term efficacy, the treatment strategy conforming to the roadmap concept carries a higher risk of virologic breakthrough than does initiating combination therapy from the beginning of treatment.
Applications
For patients with lamivudine-resistant CHB, adefovir should be used ab initio in combination with ongoing treatment with lamivudine rather than switching to adefovir monotherapy with the later addition of lamivudine.
Terminology
The roadmap concept: The roadmap concept is a treatment strategy that is used to individualize ongoing antiviral management based on early treatment responses to minimize resistance and improve long-term efficacy.
Peer review
The authors present the results for 154 lamivudine-resistant CHB patients treated with adefovir and the long-term efficacies of various treatment strategies. The study is well designed, and the manuscript is well written.
Footnotes
Supported by A grant from the Korea Healthcare Technology R and D project, Ministry of Health and Welfare, South Korea, No. R06050496
Peer reviewer: Arturo Panduro, MD, PhD, Head of the Department of Molecular Biology in Medicine, Civil Hospital of Guadalajara Fray Antonio Alcalde/University of Guadalajara, Hospital No. 278 S.H., Guadalajara, 44280 Jalisco, Mexico
S- Editor Shi ZF L- Editor A E- Editor Zhang DN
References
- 1.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 2.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 3.Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 4.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 6.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567–572. doi: 10.1002/hep.510300221. [DOI] [PubMed] [Google Scholar]
- 8.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 9.Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 10.Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81–90. doi: 10.1053/j.gastro.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 11.Peters MG, Hann Hw H, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray Df D, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 12.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673–2681. doi: 10.1056/NEJMoa042957. [DOI] [PubMed] [Google Scholar]
- 13.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385–1391. doi: 10.1002/hep.21189. [DOI] [PubMed] [Google Scholar]
- 15.Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, Seo YS, Chung HJ, Moon MS, Kim SO, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006;55:1488–1495. doi: 10.1136/gut.2005.077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yim HJ, Hussain M, Liu Y, Wong SN, Fung SK, Lok AS. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology. 2006;44:703–712. doi: 10.1002/hep.21290. [DOI] [PubMed] [Google Scholar]
- 17.Fung SK, Chae HB, Fontana RJ, Conjeevaram H, Marrero J, Oberhelman K, Hussain M, Lok AS. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445–1451. doi: 10.1053/j.gastro.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 19.Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307–313. doi: 10.1002/hep.21534. [DOI] [PubMed] [Google Scholar]
- 20.Pellicelli AM, Barbaro G, Francavilla R, Romano M, Barbarini G, Mazzoni E, Mecenate F, Paffetti A, Barlattani A, Struglia C, et al. Adefovir and lamivudine in combination compared with adefovir monotherapy in HBeAg-negative adults with chronic hepatitis B virus infection and clinical or virologic resistance to lamivudine: a retrospective, multicenter, nonrandomized, open-label study. Clin Ther. 2008;30:317–323. doi: 10.1016/j.clinthera.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Vassiliadis TG, Giouleme O, Koumerkeridis G, Koumaras H, Tziomalos K, Patsiaoura K, Grammatikos N, Mpoumponaris A, Gkisakis D, Theodoropoulos K, et al. Adefovir plus lamivudine are more effective than adefovir alone in lamivudine-resistant HBeAg- chronic hepatitis B patients: a 4-year study. J Gastroenterol Hepatol. 2010;25:54–60. doi: 10.1111/j.1440-1746.2009.05952.x. [DOI] [PubMed] [Google Scholar]
- 22.Keeffe EB, Zeuzem S, Koff RS, Dieterich DT, Esteban-Mur R, Gane EJ, Jacobson IM, Lim SG, Naoumov N, Marcellin P, et al. Report of an international workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5:890–897. doi: 10.1016/j.cgh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001;34:785–791. doi: 10.1053/jhep.2001.27563. [DOI] [PubMed] [Google Scholar]
- 24.Lai CL, Leung N, Teo EK, Tong M, Wong F, Hann HW, Han S, Poynard T, Myers M, Chao G, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005;129:528–536. doi: 10.1016/j.gastro.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 25.Locarnini S, Qi X, Arterburn S, Snow A, Brosgart CL, Currie G, Wulfsohn MS, Miller MD, Xiong S. Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil (ADV) therapy for patients with chronic hepatitis B (CHB) (abstract) J Hepatol. 2005;42(Suppl 2):17. [Google Scholar]
- 26.Shin JW, Park NH, Jung SW, Park BR, Kim CJ, Jeong ID, Bang SJ, Kim do H. Clinical usefulness of sequential hepatitis B virus DNA measurement (the roadmap concept) during adefovir treatment in lamivudine-resistant patients. Antivir Ther. 2009;14:181–186. [PubMed] [Google Scholar]
- 27.Karino Y, Toyota J, Kumada H, Katano Y, Izumi N, Kobashi H, Sata M, Moriyama M, Imazeki F, Kage M, et al. Efficacy and resistance of entecavir following 3 years of treatment of Japanese patients with lamivudine-refractory chronic hepatitis B. Hepatol Int. 2010;4:414–422. doi: 10.1007/s12072-009-9162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae SH, Yoon SK, Jang JW, Kim CW, Nam SW, Choi JY, Kim BS, Park YM, Suzuki S, Sugauchi F, et al. Hepatitis B virus genotype C prevails among chronic carriers of the virus in Korea. J Korean Med Sci. 2005;20:816–820. doi: 10.3346/jkms.2005.20.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song BC, Cui XJ, Kim H. Hepatitis B virus genotypes in Korea: an endemic area of hepatitis B virus infection. Intervirology. 2005;48:133–137. doi: 10.1159/000081740. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Jee YM, Song BC, Shin JW, Yang SH, Mun HS, Kim HJ, Oh EJ, Yoon JH, Kim YJ, et al. Molecular epidemiology of hepatitis B virus (HBV) genotypes and serotypes in patients with chronic HBV infection in Korea. Intervirology. 2007;50:52–57. doi: 10.1159/000096313. [DOI] [PubMed] [Google Scholar]
- 31.Cho JH, Yoon KH, Lee KE, Park DS, Lee YJ, Moon HB, Lee KR, Choi CS, Cho EY, Kim HC. [Distribution of hepatitis B virus genotypes in Korea] Korean J Hepatol. 2009;15:140–147. doi: 10.3350/kjhep.2009.15.2.140. [DOI] [PubMed] [Google Scholar]
- 32.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, Choi JW, Kim TN, Eun JR. [A case of severe hypophosphatemia related to adefovir dipivoxil treatment in a patient with liver cirrhosis related to hepatitis B virus] Korean J Hepatol. 2008;14:381–386. doi: 10.3350/kjhep.2008.14.3.381. [DOI] [PubMed] [Google Scholar]
- 34.Izzedine H, Kheder-Elfekih R, Housset P, Sarkozy C, Brocheriou I, Deray G. Adefovir dipivoxil-induced acute tubular necrosis and Fanconi syndrome in a renal transplant patient. AIDS. 2009;23:544–545. doi: 10.1097/QAD.0b013e32832407f7. [DOI] [PubMed] [Google Scholar]
- 35.Wong T, Girgis CM, Ngu MC, Chen RC, Emmett L, Archer KA, Seibel MJ. Hypophosphatemic osteomalacia after low-dose adefovir dipivoxil therapy for hepatitis B. J Clin Endocrinol Metab. 2010;95:479–480. doi: 10.1210/jc.2009-2051. [DOI] [PubMed] [Google Scholar]
- 36.Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Tanji N, Tanji K, Kambham N, Markowitz GS, Bell A, D’agati VD. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Hum Pathol. 2001;32:734–740. doi: 10.1053/hupa.2001.25586. [DOI] [PubMed] [Google Scholar]
- 38.Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56:570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- 39.Ho ES, Lin DC, Mendel DB, Cihlar T. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000;11:383–393. doi: 10.1681/ASN.V113383. [DOI] [PubMed] [Google Scholar]
- 40.Mulato AS, Ho ES, Cihlar T. Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adefovir mediated by the human renal organic anion transporter 1. J Pharmacol Exp Ther. 2000;295:10–15. [PubMed] [Google Scholar]


