Abstract
AIM: To study the effect of salvianolate on tight junctions (TJs) and zonula occludens protein 1 (ZO-1) in small intestinal mucosa of cirrhotic rats.
METHODS: Cirrhosis was induced using carbon tetrachloride. Rats were randomly divided into the untreated group, low-dose salvianolate (12 mg/kg) treatment group, medium-dose salvianolate (24 mg/kg) treatment group, and high-dose salvianolate (48 mg/kg) treatment group, and were treated for 2 wk. Another 10 healthy rats served as the normal control group. Histological changes in liver tissue samples were observed under a light microscope. We evaluated morphologic indices of ileal mucosa including intestinal villi width and thickness of mucosa and intestinal wall using a pathological image analysis system. Ultrastructural changes in small intestinal mucosa were investigated in the five groups using transmission electron microscopy. The changes in ZO-1 expression, a tight junction protein, were analyzed by immunocytochemistry. The staining index was calculated as the product of the staining intensity score and the proportion of positive cells.
RESULTS: In the untreated group, hepatocytes showed a disordered arrangement, fatty degeneration was extensive, swelling was obvious, and disorganized lobules were divided by collagen fibers in hepatic tissue, which were partly improved in the salvianolate treated groups. In the untreated group, abundant lymphocytes infiltrated the fibrous tissue with proliferation of bile ducts, and collagen fibers gradually decreased and damaged hepatic lobules were partly repaired following salvianolate treatment. Compared with the untreated group, no differences in intestinal villi width between the five groups were observed. The villi height as well as mucosa and intestinal wall thickness gradually thickened with salvianolate treatment and were significantly shorter in the untreated group compared with those in the salvianolate treatment groups and normal group (P < 0.01). The number of microvilli decreased and showed irregular lengths and arrangements in the untreated group. The intercellular space between epithelial cells was wider. The TJs were discontinuous, which indicated disruption in TJ morphology in the untreated group. In the treated groups, the microvilli in the intestinal epithelium were regular and the TJs were gradually integrated and distinct. The expression of ZO-1 decreased in the small intestine of the untreated cirrhotic rats. The high expression rate of ZO-1 in ileal mucosa in the untreated group was significantly lower than that in the medium-dose salvianolate group (21.43% vs 64.29%, χ2 = 5.25, P < 0.05), high-dose salvianolate group (21.43% vs 76.92%, χ2 = 8.315, P < 0.01) and normal group (21.43% vs 90%, χ2 = 10.98, P < 0.01).
CONCLUSION: Salvianolate improves liver histopathological changes, repairs intestinal mucosa and TJ structure, and enhances ZO-1 expression in the small intestinal mucosa in cirrhotic rats.
Keywords: Salvianolate, Cirrhosis, Gut barrier, Tight junction, Zonula occludens protein 1
INTRODUCTION
In liver cirrhosis, disruption of the intestinal mucosal barrier function and increased mucosal intestinal permeability lead to bacterial translocation and endotoxemia[1], which increase susceptibility to infection, the most frequent and severe of which is spontaneous bacterial peritonitis[2,3]. Endotoxemia may affect liver function, contribute to systemic hemodynamic derangement in liver cirrhosis[4,5], and result in severe complications[6-8]. Therefore, restoring the integrity of the intestinal barrier is an important goal in preventing deterioration of liver function in patients with cirrhosis[3,9].
The intestinal mucosal barrier includes both secretory and physical preventive measures against the penetration of microbes. The secretory mechanism is realized through mucus secretion, local immunoglobulins and bile salts. The physical mechanism is represented by the intestinal epithelium[10,11], specifically its lack of permeability and active antimicrobial peptide production. The tight junctions (TJs) of the intestinal epithelium allow only the passage of very tiny molecules[12], preventing the transport of bacteria or macromolecules (lipopolysaccharides)[13]. Disruption of the intestinal TJ barrier results in a “leaky” TJ barrier, allowing paracellular permeation of toxic luminal substances. Altered intestinal TJ expression in patients is a molecular mechanism of intestinal hyperpermeability. It was demonstrated that patients with decompensated and compensated cirrhosis had significantly reduced expression of TJ proteins, higher Child-Pugh score, and decreased liver function[14]. As an important contributor to the development of liver cirrhosis, the physical barrier of the small intestine represents an ideal therapeutic target[15]. However, there are no reports on an effective means of preventing disruption of the intestinal physical barrier in liver cirrhosis.
Radix Salvia miltiorrhiza, a traditional Chinese medicinal herb known as “danshen” has been widely used for the treatment of various cardiovascular diseases[16,17]. Salvia miltiorrhiza (S. miltiorrhiza) extracts contain lipid-soluble diterpene quinones (tanshinones) and water-soluble phenolic acid derivatives such as salvianolic acid A and B and lithospermic acid B[18]. Recent pharmacological research has shown that S. miltiorrhiza eliminates oxygen free radicals, enhances antioxidant activity, decreases serum cytokine levels, and inhibits endotoxemia[19]. Moreover, S. miltiorrhiza can block the lethal toxicity of lipopolysaccharides in mice via suppression of tumor necrosis factor-α (TNF-α) release[20] and help to maintain the integrity of the endothelial junction structure[21]. Salvianolate is a new water-soluble phenolic compound and is one of the most bioactive compounds in S. miltiorrhiza Bge. However, in carbon tetrachloride (CCl4)-induced cirrhosis in rats, the effect of salvianolate on the physical barriers of the small intestine is less clear. A previous study demonstrated that salvianolate can reduce the endotoxin level, ameliorate injury to the intestinal mucosa, and inhibit the expression of TNF-α and interleukin-6 (IL-6) mRNA in the small intestine of cirrhotic rats[22]. Therefore, we used CCl4-induced cirrhotic rats to evaluate changes in the epithelial barrier of the ileal mucosa and the effect of different doses of salvianolate on TJs and zonula occludens protein 1 (ZO-1) in microvillus cells of the small intestine mucosa.
MATERIALS AND METHODS
A previous study demonstrated that salvianolate can reduce endotoxin levels in the portal vein, ameliorate injury to the intestinal mucosa, and inhibit cytokine gene expression in rats with CCl4-induced liver cirrhosis[22]. To further explore the mechanism of salvianolate in enhancement of the intestinal mechanical barrier, in the present study, we evaluated liver histopathological changes and morphologic indices of ileal mucosa using light microscopy, analyzed the ultrastructural changes using transmission electron microscopy and the expression of ZO-1, a TJ protein, using immunocytochemistry. The results of this study may provide a new strategy for the treatment of liver cirrhosis.
Animals
Ninety male Sprague-Dawley rats (weight: 180-220 g) were provided by the Department of Animal Care, Zhejiang Traditional University, Hangzhou, China. Experimental animals were housed in individual cages at 22 °C to 25 °C under a 12-h light/dark cycle and fed a standard laboratory diet and tap water ad libitum.
Experimental protocol
The rats were randomly divided into two groups: the normal control group (n = 10, group A) and the model group. All model group rats received a subcutaneous injection of 40% CCl4 in a 2:3 mixture with olive oil (0.3 mL/kg) once weekly for 12 wk. Liver cirrhosis was successfully induced in 55 rats at the end of 12 wk as shown by liver histological evaluation. The 55 model rats were further randomly divided into four subgroups: the untreated group (n = 14, group B), low-dose salvianolate-treated group (12 mg/kg) (n = 14, group C), medium-dose salvianolate-treated group (24 mg/kg) (n = 14, group D), and high-dose salvianolate-treated group (48 mg/kg) (n = 13, group E). Group A was injected intraperitoneally with 5% glucose solution once daily for 2 wk. Groups C to E were treated intraperitoneally with different doses of salvianolate dissolved in 5% glucose solution once daily for 2 wk. 40% CCl4 administration was continued for the 14-wk experimental period. At the end of the experimental period, all rats were anesthetized with 3% chloral hydrate and dissected. Blood samples from the portal vein and intestinal tissue were obtained for further analysis.
Assessment of histological changes in the liver and morphologic indices of ileal mucosa
At the end of the 14-wk experimental period, the abdominal cavity in each rat was opened by a horizontal incision along the mid-section and the liver and intestines were excised. Tissue samples were taken immediately and washed three times with cold physiological saline and fixed in 10% formalin solution. After fixation, tissue specimens were dehydrated and embedded in paraffin. Sections from each sample were cut at a thickness of 4 μm and stained with hematoxylin and eosin (Olympus BX50; Tokyo, Japan). We evaluated morphologic indices of the ileal mucosa including intestinal villi width, and thickness of the mucosa and intestinal wall using a pathological image analysis system (Leica Qwinv3, Jiangsu, China).
Ultrastructure with transmission electron microscopy
Ileum samples in the five groups were separated and fixed immediately with 2% glutaraldehyde, post-fixed with 1% osmium tetroxide, and embedded in resin (EMbed 812 Embedding Kit, Electron Microscopy Sciences Company, United States). Ultrathin sections were cut and stained with uranyl acetate and lead citrate. Samples were examined using a transmission electron microscope (Philips Tecnai 12, Holland) and analyzed by an electron microscope image analyzer (Erlangshen ES500W, Holland).
Immunohistochemistry
Immunohistochemical analysis was performed to study ZO-1 expression in the intestinal mucous membranes. In brief, slides were baked at 55 °C for 2 h, deparaffinized with xylene, and rehydrated. The sections were submerged in EDTA antigenic retrieval buffer and microwaved for antigenic retrieval, after which they were treated with 3% hydrogen peroxide in methanol to quench endogenous peroxidase activity. They were then incubated with 1% bovine serum albumin to block nonspecific binding. Sections were incubated with mouse anti-ZO-1 antibodies (Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) overnight at 4 °C. Normal goat serum was used as a negative control. After washing, tissue sections were treated with secondary antibody. Tissue sections were then counterstained with hematoxylin, dehydrated and mounted. The cytoplasm and stroma containing ZO-1 were stained as the buffy coat. The degree of immunostaining was reviewed and scored independently by two observers based on the proportion of positively stained epithelium and intensity of staining[23-25]. The proportion of ZO-1 expression-positive areas was scored as follows: 0 (≤ 5% positive area expression), 1 (6%-25% positive area expression), 2 (26%-50% positive area expression), and 3 (> 51% positive area expression). Staining intensity was graded according to the following criteria: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow-brown), and 3 (strong staining, brown). The staining index was calculated as the product of the staining intensity score and the proportion of positive cells. Using this method of assessment, we evaluated ZO-1 expression in the intestinal mucous membranes by determining the staining index with scores of 0, 1, 2, 3, 4, 6, or 9. The cutoff value for high and low expression levels was chosen based on a measure of heterogeneity using the log-rank test with respect to overall survival. An optimal cutoff value was identified as follows: a staining index score of ≥ 6 was used to define high ZO-1 expression and a staining index score of < 6 was used to indicate low ZO-1 expression.
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 (Chicago, IL, United States). Results of the mucosal morphologic indices were assessed by analysis of variance. Data were expressed as the means ± SD. The expression rate of ZO-1 protein was compared using the χ2 test. A P value of < 0.05 was considered statistically significant.
RESULTS
Liver histological evaluation
In the untreated group, hepatocytes showed a disordered arrangement, fatty degeneration was extensive, and swelling was obvious. Spotty necrosis and eosinophilic bodies appeared in the hepatic tissue. Disorganized lobules were divided by collagen fibers. Central veins disappeared and pseudolobules proliferated in the hepatic tissue. Abundant lymphocytes infiltrated the fibrous tissue with proliferation of bile ducts (Figure 1A). Collagen fibers gradually decreased and damaged hepatic lobules were partly repaired by salvianolate treatment. Fatty degeneration of hepatocytes was reduced and inflammatory cell infiltration decreased in the fibrous tissue, especially in the high-dose salvianolate group (Figure 1B-D).
Figure 1.
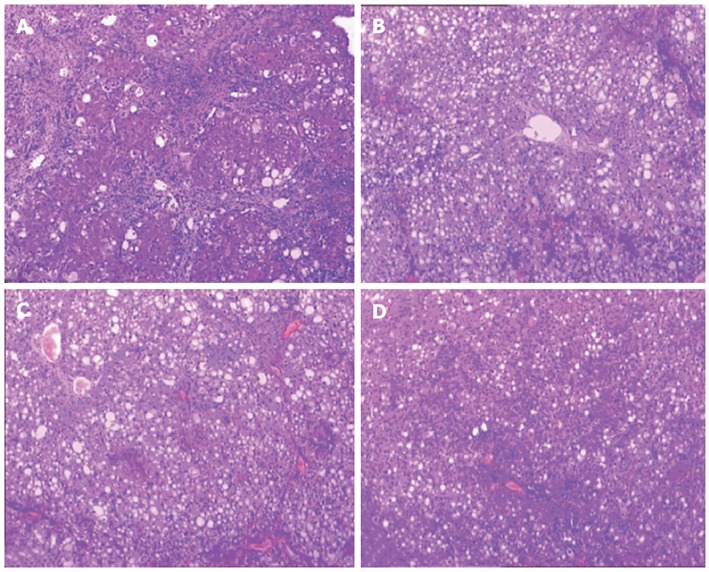
Liver histopathology in cirrhotic rats. A: Untreated group; B: Low-dose salvianolate-treated group; C: Medium-dose salvianolate-treated group; D: High-dose salvianolate-treated group (hematoxylin and eosin staining, × 200).
Morphologic indices of ileal mucosa
Histological changes observed in ileal tissue under light microscopy were previously published[22]. In that study, we found that salvianolate ameliorated injury to the intestinal mucosa. In the present study, we measured intestinal villi width and thickness of the mucosa and intestinal wall using a pathological image analysis system. There were no differences in intestinal villi width among the five groups (P > 0.05). Villi height was significantly shorter in group B than in groups C, D, E and the normal group (99.14 ± 14.57 μm vs 126.43 ± 22.12 μm, 128.71 ± 20.33 μm, 147.10 ± 19.48 μm and 221.82 ± 37.97 μm, respectively; P < 0.01). Mucosal thickness was significantly less in the untreated group than in groups C, D and E (131.43 ± 18.83 μm vs 179.02 ± 26.99 μm, 181.10 ± 30.71 μm and 230.28 ± 20.67 μm, respectively; P < 0.01). The intestinal walls were significantly thicker in groups C, D and E compared with group B (226.37 ± 40.66 μm, 229.95 ± 42.08 μm and 278.94 ± 39.94 μm vs 165.93 ± 23.02 μm, respectively; P < 0.01) (Table 1). These results show that the intestinal mucosa was gradually thickened and repaired by salvianolate treatment.
Table 1.
Microscopic evaluation scores of mucosa in the five groups (mean ± SD, μm)
| Group | n | Villi height | Villi width | Mucosa thickness | Intestinal wall thickness |
| A | 10 | 221.82 ± 37.97b | 42.08 ± 8.74 | 260.55 ± 16.57b | 337.49 ± 36.92b |
| B | 14 | 99.14 ± 14.57 | 38.84 ± 11.08 | 131.43 ± 18.83 | 165.93 ± 23.02 |
| C | 14 | 126.43 ± 22.12b | 40.28 ± 8.98 | 179.02 ± 26.99b | 226.37 ± 40.66b |
| D | 14 | 128.71 ± 20.33b | 40.59 ± 11.70 | 181.10 ± 30.71b | 229.95 ± 42.08b |
| E | 13 | 147.10 ± 19.48b | 41.32 ± 6.17 | 230.28 ± 20.67b | 278.94 ± 39.94b |
P < 0.01 vs group B.
Ultrastructural morphology of intestinal mucosa
In the normal group, regularly aligned microvilli and intact TJs were observed in the intestinal epithelium (Figure 2A). In the untreated group, the number of microvilli decreased and showed irregular lengths and arrangements. The intercellular space between epithelial cells widened. TJs were discontinuous, which indicated disruption in TJ morphology in the untreated group (Figure 2B). In the treated groups, the microvilli in the intestinal epithelium were regular and the TJs were gradually integrated and distinct (Figure 2C-E).
Figure 2.
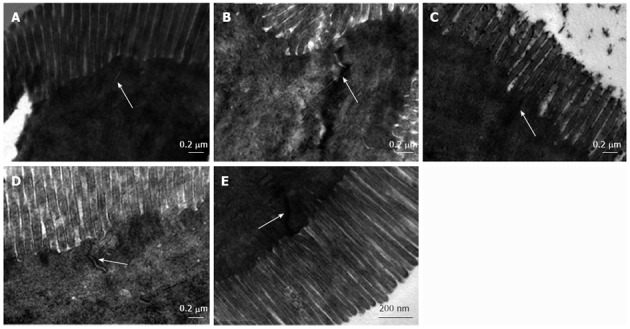
Tight junction structural morphology in ileal mucosa. A: Normal control group; B: Untreated group; C: Low-dose salvianolate-treated group; D: Medium-dose salvianolate-treated group; E: High-dose salvianolate-treated group (transmission electron microscopy, × 26 500).
Expression of ZO-1 in ileal epithelial mucosa shown by immunohistochemistry: Ileal epithelial mucosa from the five groups was assayed for ZO-1 expression using immunohistochemistry. Normal mice showed expression of ZO-1 at the apical pole of epithelial cells[26] and within the cytoplasm (Figure 3A). Nine (90%) rats in group A showed high expression of ZO-1. Staining of ZO-1 was less in group B; there was almost no expression at the apical pole of epithelial cells and slight expression within the cytoplasm (Figure 3B). The expression of ZO-1 gradually increased in epithelial cells following salvianolate treatment. Group B showed a high expression rate of 21.43%, which was significantly lower than that in group A (21.43% vs 90%, χ2 = 10.98, P < 0.01) (Figure 4). In the treated groups, brown grains again appeared at the apical pole of epithelial cells and within the cytoplasm of epithelial cells (Figure 3C-E). Groups C, D and E showed high ZO-1 expression rates of 57.14%, 64.29% and 76.92%, respectively. The expression rate in group B was significantly lower than that in groups D (21.43% vs 64.29%, χ2 = 5.25, P < 0.05) and E (21.43% vs 76.92%, χ2 = 8.315, P < 0.01) (Figure 4).
Figure 3.
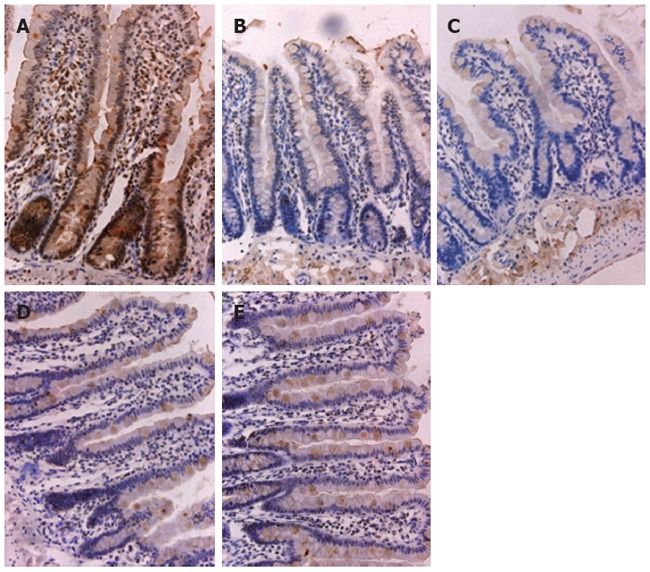
Immunohistochemical staining for zonula occludens protein 1 in ileal mucosa. A: Normal control group; B: Untreated group; C: Low-dose salvianolate-treated group; D: Medium-dose salvianolate-treated group; E: High-dose salvianolate-treated group (× 40).
Figure 4.
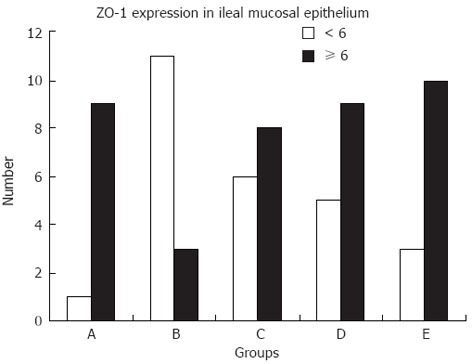
Expression of zonula occludens protein 1 in ileal mucosa. A: Normal control group; B: Untreated group; C: Low-dose salvianolate-treated group; D: Medium-dose salvianolate-treated group; E: High-dose salvianolate-treated group. The staining index was calculated as the product of the staining intensity score and the proportion of positive cells. We evaluated zonula occludens protein 1 (ZO-1) expression in the five groups by determining the staining index with scores of 0, 1, 2, 3, 4, 6, or 9. An optimal cutoff value was identified as follows: a staining index score of ≥ 6 was used to define high ZO-1 expression and a staining index score of < 3 was used to indicate low ZO-1 expression.
DISCUSSION
Protection of the intestinal barrier function and decreased bacterial translocation are important in patients with liver cirrhosis[27]. In our previous study[22], a significant increase in plasma endotoxins and high mortality were observed in untreated cirrhotic rats. Endotoxemia was significantly reduced and intestinal histopathological changes were improved by administration of salvianolate in cirrhotic rats. We showed that salvianolate has a direct anti-inflammatory effect on the intestine by inhibiting the expression of TNF-α and IL-6 mRNA. These favorable effects of salvianolate on the intestine can be partially explained by direct protection of the integrity of the mucosal barrier.
In this study, we observed improvement in liver histopathological changes (Figure 1) as well as thickening and gradual repair of the intestinal mucosa with salvianolate treatment (Table 1). To further elucidate the mechanisms of salvianolate in protecting the mucosal barrier in cirrhotic rats, we investigated the effect of salvianolate therapy on ultrastructural analysis using transmission electron microscopy and ZO-1 expression changes using immunocytochemistry.
The physical intestinal barrier comprises an intact layer of epithelial cells, which are tightly connected by a surrounding system of TJ strands that seal the lateral intercellular space[28]. The intercellular space between epithelial and endothelial cells is bridged by a set of specialized structures: TJs, or ZO; zonula adherens (ZA); desmosomes; and gap junctions. The TJ is an essential component of this barrier. The junctional complexes of the plasma membrane are not simply epithelial barriers in paracellular transport or barriers preventing diffusion across the plasma membrane, but also contain proteins involved in signal transduction and maintenance of the physiological epithelial cell state[29,30]. Increased permeability is caused by disruption of intercellular TJs in the intestine and plays an important role in the pathogenesis of chronic liver disease[31]. Previous studies have shown that impaired intestinal permeability may increase the risk of endotoxemia and spontaneous bacterial peritonitis in liver cirrhosis[14].
The intracellular complex of TJ-associated proteins includes ZO-1, ZO-2, ZO-3, cingulin, 7H6, symplekin and ZA-1[32]. This complex appears to link TJs to a perijunctional actomyosin ring, which supports and regulates TJ permeability in epithelial cells. ZO-1, one of the plaque proteins, was the first TJ protein to be characterized. It is a 225-kDa membrane-bound protein that localizes to the TJ. It binds the occluding and claudin transmembrane proteins, linking them to cytoskeletal actin[33]. ZO-1 may be the direct link between actin and the transmembrane proteins; it has its own specific function[34] and is a particularly important molecule in terms of the formation of TJs[35]. ZO-1 alterations may contribute to disturbances of the TJ barrier, which lead to enhanced intestinal permeability[36]. Disruption of this structure could lead to an imbalance in the normal interaction between aggressive factors and mucosal defense, resulting in increased intestinal permeability and bacterial translocation.
Further investigations revealed that mucosal damage was accompanied by ultrastructural changes in TJs. TJ injury is associated not only with increased transcellular permeability, but also with increased paracellular permeability because of the rearrangement of TJ proteins. In this study, we found that the expression of ZO-1 was decreased in the small intestine of untreated cirrhotic rats. Administration of salvianolate may recover the TJ structure and enhance the expression of ZO-1 in the small intestine of cirrhotic rats. This may explain the effect of salvianolate on the epithelial barriers of the small intestine in cirrhotic rats.
In summary, the epithelial barriers of the small intestine were destroyed in cirrhotic rats. These changes may promote bacterial translocation and intestinal endotoxemia. Salvianolate administration caused reduced mucosal damage and maintained the epithelial mucosal barrier function in the small intestine of cirrhotic rats. Furthermore, salvianolate may be a potent traditional Chinese medicinal herb for reducing the incidence of spontaneous bacterial peritonitis and influencing the natural history of liver cirrhosis. These results indicate that salvianolate may be a new therapy for liver cirrhosis.
COMMENTS
Background
In liver cirrhosis, disruption of the intestinal barrier function increases intestinal permeability and bacterial translocation, which contribute to endotoxemia and derangement in liver cirrhosis. Abnormalities of the epithelial mucosal barrier represented by tight junction structure damage play an important role in the pathogenesis of altered intestinal permeability. Improving the epithelial mucosal tight junctions (TJs) and epithelium tight junction proteins is a key goal in preventing intestinal endotoxemia in patients with cirrhosis.
Research frontiers
Recent studies have shown that the physical barrier of the intestinal mucosa plays an important role in intestinal defense mechanisms in hepatic cirrhosis. Previous experiments confirmed that salvianolate can reduce the endotoxin level, ameliorate injury to the intestinal mucosa and inhibit the expression of tumor necrosis factor-α and interleukin-6 mRNA in the small intestine of cirrhotic rats.
Innovations and breakthroughs
Disturbance of the TJs and zonula occludens protein 1 (ZO-1) alterations lead to increased intestinal permeability and bacterial translocation in hepatic cirrhosis. This study showed, for the first time, that salvianolate can restore ultrastructural changes in the intestinal mucosa and significantly increase TJ protein expression in cirrhotic rats. The authors demonstrated that the pharmacological activities of salvianolate represent an important cellular mechanism for preventing intestinal epithelial barrier function damage in liver cirrhosis.
Applications
By demonstrating the effects of salvianolate in maintaining the mucosal physical barrier function in the small intestine of cirrhotic rats, this study provides a new strategy for the treatment of liver cirrhosis. Salvianolate can be applied in clinical practice due to its potential pharmacological activities.
Terminology
Radix Salvia miltiorrhiza is a traditional Chinese medicinal herb known as “danshen”. Salvianolate is a water-soluble phenolic compound isolated from Radix Salvia miltiorrhiza.
Peer review
The authors have illustrated that the administration of salvianolate may recover the TJ structure and enhance the expression of ZO-1 in the small intestine of cirrhotic rats. This topic is of significant clinical importance and the results provide a new strategy for the treatment of spontaneous bacterial peritonitis.
Footnotes
Supported by Foundation of Chinese Medicine in Zhejiang Province Science and Technology, No. Z0102B002
Peer reviewer: Vasiliy I Reshetnyak, MD, PhD, Professor, Scientist Secretary of the Scientific Research, Institute of General Reanimatology, Petrovka 25-2, 107031 Moscow, Russia
S- Editor Gou SX L- Editor Webster JR E- Editor Zhang DN
References
- 1.Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR, Girona E, Gutiérrez A, Carnices F, Palazón JM, et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50:1482–1486. [PubMed] [Google Scholar]
- 2.Lata J, Stiburek O, Kopacova M. Spontaneous bacterial peritonitis: a severe complication of liver cirrhosis. World J Gastroenterol. 2009;15:5505–5510. doi: 10.3748/wjg.15.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi Y, Jeon WK, Hwang SJ, Kim BI, Sohn CI, Park DI, Cho YK, Kim HJ, Park JH. The role of the gut barrier function in the pathophysiology of viral liver cirrhosis. Hepatogastroenterology. 2011;58:1244–1247. doi: 10.5754/hge10338. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, Albillos A, Jiménez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 5.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 6.Zhang HY, Han DW, Wang XG, Zhao YC, Zhou X, Zhao HZ. Experimental study on the role of endotoxin in the development of hepatopulmonary syndrome. World J Gastroenterol. 2005;11:567–572. doi: 10.3748/wjg.v11.i4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961–965. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukui H. How leaky gut and endotoxemia induce bacterial infection in cirrhosis and gastrointestinal hemorrhage? J Gastroenterol Hepatol. 2011;26:423–425. doi: 10.1111/j.1440-1746.2011.06668.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HY, Han DW, Zhao ZF, Liu MS, Wu YJ, Chen XM, Ji C. Multiple pathogenic factor-induced complications of cirrhosis in rats: a new model of hepatopulmonary syndrome with intestinal endotoxemia. World J Gastroenterol. 2007;13:3500–3507. doi: 10.3748/wjg.v13.i25.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 11.Watson AJ, Chu S, Sieck L, Gerasimenko O, Bullen T, Campbell F, McKenna M, Rose T, Montrose MH. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 2005;129:902–912. doi: 10.1053/j.gastro.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR, Watson AJ. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140:1208–1218.e1-2. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 14.Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, Kaltezioti V, Charonis A, Nikolopoulou VN, et al. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42:439–446. doi: 10.1111/j.1365-2362.2011.02609.x. [DOI] [PubMed] [Google Scholar]
- 15.Căruntu FA, Benea L. Spontaneous bacterial peritonitis: pathogenesis, diagnosis, treatment. J Gastrointestin Liver Dis. 2006;15:51–56. [PubMed] [Google Scholar]
- 16.Yagi A, Fujimoto K, Tanonaka K, Hirai K, Takeo S. Possible active components of tan-shen (Salvia miltiorrhiza) for protection of the myocardium against ischemia-induced derangements. Planta Med. 1989;55:51–54. doi: 10.1055/s-2006-961824. [DOI] [PubMed] [Google Scholar]
- 17.Fung KP, Zeng LH, Wu J, Wong HN, Lee CM, Hon PM, Chang HM, Wu TW. Demonstration of the myocardial salvage effect of lithospermic acid B isolated from the aqueous extract of Salvia miltiorrhiza. Life Sci. 1993;52:PL239–PL244. doi: 10.1016/0024-3205(93)90471-e. [DOI] [PubMed] [Google Scholar]
- 18.Liu GT, Zhang TM, Wang BE, Wang YW. Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochem Pharmacol. 1992;43:147–152. doi: 10.1016/0006-2952(92)90271-j. [DOI] [PubMed] [Google Scholar]
- 19.Guo J, Sun K, Wang CS, Fang SP, Horie Y, Yang JY, Liu YY, Wang F, Liu LY, Fan JY, et al. Protective effects of dihydroxylphenyl lactic acid and salvianolic acid B on LPS-induced mesenteric microcirculatory disturbance in rats. Shock. 2008;29:205–211. doi: 10.1097/SHK.0b013e318070c61a. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Narula AS, Jobin C. Salvia miltiorrhiza water-soluble extract, but not its constituent salvianolic acid B, abrogates LPS-induced NF-kappaB signalling in intestinal epithelial cells. Clin Exp Immunol. 2005;141:288–297. doi: 10.1111/j.1365-2249.2005.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding M, Yuan YJ. Study on the mechanisms of an extract of Salvia miltiorrhiza on the regulation of permeability of endothelial cells exposed to tumour necrosis factor-alpha. J Pharm Pharmacol. 2007;59:1027–1033. doi: 10.1211/jpp.59.7.0016. [DOI] [PubMed] [Google Scholar]
- 22.Yang DH, Ye ZY, Jin B, He XJ, Zhang Q, Zhou WM, Xu WJ, Lu HX. Salvianolate inhibits cytokine gene expression in small intestine of cirrhotic rats. World J Gastroenterol. 2011;17:1903–1909. doi: 10.3748/wjg.v17.i14.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song LB, Liao WT, Mai HQ, Zhang HZ, Zhang L, Li MZ, Hou JH, Fu LW, Huang WL, Zeng YX, et al. The clinical significance of twist expression in nasopharyngeal carcinoma. Cancer Lett. 2006;242:258–265. doi: 10.1016/j.canlet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Geisler SA, Olshan AF, Weissler MC, Cai J, Funkhouser WK, Smith J, Vick K. p16 and p53 Protein expression as prognostic indicators of survival and disease recurrence from head and neck cancer. Clin Cancer Res. 2002;8:3445–3453. [PubMed] [Google Scholar]
- 25.Fukuoka J, Fujii T, Shih JH, Dracheva T, Meerzaman D, Player A, Hong K, Settnek S, Gupta A, Buetow K, et al. Chromatin remodeling factors and BRM/BRG1 expression as prognostic indicators in non-small cell lung cancer. Clin Cancer Res. 2004;10:4314–4324. doi: 10.1158/1078-0432.CCR-03-0489. [DOI] [PubMed] [Google Scholar]
- 26.Bullen TF, Forrest S, Campbell F, Dodson AR, Hershman MJ, Pritchard DM, Turner JR, Montrose MH, Watson AJ. Characterization of epithelial cell shedding from human small intestine. Lab Invest. 2006;86:1052–1063. doi: 10.1038/labinvest.3700464. [DOI] [PubMed] [Google Scholar]
- 27.Palma P, Mihaljevic N, Hasenberg T, Keese M, Koeppel TA. Intestinal barrier dysfunction in developing liver cirrhosis: An in vivo analysis of bacterial translocation. Hepatol Res. 2007;37:6–12. doi: 10.1111/j.1872-034X.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- 28.Hossain Z, Hirata T. Molecular mechanism of intestinal permeability: interaction at tight junctions. Mol Biosyst. 2008;4:1181–1185. doi: 10.1039/b800402a. [DOI] [PubMed] [Google Scholar]
- 29.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116:725–742. doi: 10.1242/jcs.00300. [DOI] [PubMed] [Google Scholar]
- 31.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 32.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 34.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 35.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci USA. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamada K, Shitara Y, Sekine S, Horie T. Zonula Occludens-1 alterations and enhanced intestinal permeability in methotrexate-treated rats. Cancer Chemother Pharmacol. 2010;66:1031–1038. doi: 10.1007/s00280-010-1253-9. [DOI] [PubMed] [Google Scholar]


