Abstract
AIM: To investigate the therapeutic effect of radical treatment and palliative treatment in stage IV pancreatic cancer patients.
METHODS: 81 patients were enrolled in the study. Radical treatment was performed on 51 patients, while 30 patients were put under palliative treatment. The procedural safety and interval survival for stage IV pancreatic cancer (IS-IV) was assessed by almost 2.5 years of follow-ups. The IS-IV of patients under the two kinds of treatment, and the effects of treatment timing and frequency on IS-IV, were compared.
RESULTS: The IS-IV of patients who received radical treatment was significantly longer than those who received palliative treatment (P < 0.001). The IS-IV of patients who received delayed radical or palliative treatment was longer than those who received accordingly timely treatment (P = 0.0034 and 0.0415, respectively). Multiple treatments can play an important role in improving the IS-IV of patients who received radical treatment (P = 0.0389), but not for those who received palliative treatment (P = 0.99).
CONCLUSION: The effect of radical treatment was significantly more obvious than that of palliative treatment, and multiple radical treatments may contribute more to patients than a single radical treatment.
Keywords: Cryosurgery, Stage IV pancreatic cancer, Iodine-125 seed
INTRODUCTION
Pancreatic cancer is the fourth-leading cause of cancer-related death in Western societies[1]. The incidence almost equals the mortality rate, since pancreatic cancer has one of the worst prognoses of all human malignancies. Less than 20% of pancreatic cancers are curable on diagnosis[2]. Without effective treatment, the overall survival for patients with advanced pancreatic cancer is usually less than 1 year, and for those with metastatic stage IV pancreatic cancer the median survival is only 3-4 mo[3,4]. Chemotherapy is currently the standard treatment for stage IV pancreatic cancer[5,6], but it cannot radically eliminate larger tumors. Radiofrequency ablation has been widely used in radical treatment of metastatic pancreatic cancer: in 2007, Spiliotis et al[7] reported the effect of radiofrequency ablation combined with palliative surgery on patients with stage IV pancreatic cancer; in 2010, Zou et al[8] reported the effect of intraoperative radiofrequency ablation combined with iodine-125 seed implantation on patients with stage IV pancreatic cancer. The median overall survival of patients in both reports was 10 mo, but laparotomy is necessary for this technique. Cryosurgery, which is widely accepted as an invasive technique for curing solid tumors, has emerged as a new therapy for pancreatic cancer. The combination of cryosurgery and the implantation of iodine-125 seeds (CandS), which eliminate residual tumors in the treatment of advanced pancreatic cancer, was first reported by us in 2008[9,10]. In our reports, the median survival was 16.2 mo, with 26 patients (53.1%) surviving for 12 mo or more. Overall 6-, 12-, 24-, and 36-mo survival rates were 94.9%, 63.1%, 22.8%, and 9.5%, respectively.
Here we aimed to compare the effects of radical treatment (CandS for intrapancreatic and extrapancreatic tumors) and palliative treatment (seed implantation for intrapancreatic tumors, CandS for extrapancreatic tumors) on stage IV pancreatic cancer, and assess the influence of treatment timing and frequency on the survival time of patients. To concentrate on the survival time of patients with stage IV cancer, the interval survival of stage IV pancreatic cancer (IS-IV) was used as the main evaluation index.
MATERIALS AND METHODS
Ethics
This study protocol received ethical approval from the Regional Ethics Committee of Guangzhou Fuda Cancer Hospital. Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Patient selection
This was a retrospective study of patients treated for metastatic pancreatic tumors in our tumor hospital from November 2008 through to August 2010. Before hospitalization, 145 patients received a comprehensive evaluation, with their tumors being considered unresectable. These evaluations were multidisciplinary decisions incorporating a radiologist, a gastrointestinal pancreatic surgeon, and an oncologist in our hospital. Their diagnoses were principally based on computed tomography (CT) imaging and CT-guided needle biopsy to obtain a definitive diagnosis as pancreatic adenocarcinoma histologically. An 18-gauge Tru-Cut (Baxter, Deerfield, IL, United States) biopsy needle was used percutaneously to obtain one to two cores of tissue from the solid pancreatic tumor. Patients received their final treatments in our hospital and almost 2.5 years of follow-ups were reviewed.
A patient was deemed unsuitable for surgery and chemotherapy due to any of the following reasons: (1) multifocal disease; (2) an unresectable primary tumor; (3) the patient refusing surgery and chemotherapy, or seeking further treatment after previous chemotherapy; (4) severe complications (e.g., hypertension, hydrothorax, and ascites); or (5) advanced age. Written informed consent was obtained from all patients. The inclusion criteria were as follows: (1) the Karnofsky performance status (KPS) score was ≥ 70; (2) the platelet count was ≥ 80 × 109/L, white blood cell count was ≥ 3 × 109/L, neutrophil granulocyte count was ≥ 2 × 109/L, or hemoglobin ≥ 90 g/L; (3) the prothrombin time international normalized ratio was ≥ 1.5; (4) the largest primary or metastatic tumor diameter was < 6 cm, as measured by preoperative CT; (5) the pancreatic tumor did not obviously invade the main pancreatic duct, postcava, duodenum or colon; (6) absence of level 3 hypertension, severe coronary disease, myelosuppression, respiratory system disease, acute and chronic infection; (7) the basic normal liver function and puncture release ascites < 1L; and (8) the patient was deemed incapable of cooperating during the procedure.
When the patient had primary or metastatic tumors with a diameter ≥ 6 cm, or obviously invading the main pancreatic duct, postcava, duodenum or colon, we preferentially performed the treatment in other ways[11,12], and so these patients were not enrolled in this study.
Percutaneous cryosurgery and iodine-125 seed implantation
According to the patients’ own selection, 51 patients were under radical treatment and 31 patients received palliative treatment. Their treatments had been reported by us[9,10]. In the radical treatment group, the CandS treatment of the pancreatic tumor was performed under double row helical CT (Somatom Emotion Duo; Siemens, Germany) and color ultrasound device (ALOKA SSD-5500SA; Aloka, Japan) guidance. Before the cryosurgery, the patients were administrated general or local anesthesia, and positioned for an upper abdominal incision. All cryosurgery were performed by Dr. Niu LZ and assistants (Zhou L and Zhang C). Based on the location of the pancreatic tumor, the cryoprobes were inserted percutaneously via the retroperitoneal, transhepatic, or transgastric approach. For tumors greater than 3 cm in length, more than two 1.7 mm cryoprobes (CRYO-42; Endocare, Irvine, CA, United State) were used, in an attempt to avoid puncturing the main pancreatic duct and duodenum. A 1-3 cycle freeze/thaw procedure was used with an argon gas-based cryosurgical unit (Endocare, United State)[9,10]. The iodine-125 seed (Syncor Pharmaceuticals, Shanghai, China) implantation was performed by PTC needle, either at the time of cryosurgery or after cryosurgery through the percutaneous approach under a 3D treatment planning system. The seeds (activity of a single seed 0.7 mCi, half-life period 1-6 mCi) were implanted at the tumor borderline. The number of seeds deployed depended on the tumor size (matching dose around 120Gy, usually ≤ 20 particles), with the seeds implanted at intervals of 0.5 cm. In the palliative treatment group, the iodine-125 seeds were implanted in all parts of the pancreatic tumor under ultrasound or CT guidance, and the planting density and quantity were both more than the radical treatment group. For extrapancreatic metastases in the two groups, the CandS treatment were performed percutaneously via the retroperitoneal or transabdominal approach at the same time, or after the treatment of the intrapancreatic tumor.
Postoperative treatment
Once cryoablation was completed, 1 mL of both fibrinogen and thrombin for each probe were injected into the sheath simultaneously. The patients were then observed in the intensive care unit for at least 6 h, and fasted for at least 24 h. Therapies of anti-infection and inhibition of pancreatic juice secretion were given for a few days. Patients under radical treatment received some special treatment: for patients under the transgastric approach of cryosurgery, antacid and stomach mucosa-protecting drugs were delivered for a few days; for patients under the transhepatic approach of cryosurgery, oppressing hemostasis, bellyband, and liver-protecting drugs were all administered for a few days.
Statistical analysis
Complications were recorded and classified in accordance with the Common Terminology Criteria of Adverse Events v4.0. Local tumor control and IS-IV were also evaluated. Radiographic local tumor control was assessed by image-guided tumor ablation criteria[13]. A post-operative plain abdominal CT was performed immediately after the removal of the cryoprobes for verification as to whether any major complications, such as pancreatic fistula, bile leakage, or intestinal fistula, had occurred. Abdominal ultrasound was performed at both 1 d and 1 wk after the cryoablation procedure. Follow-up dynamic CT abdominal scans of patients were carried out at 1 mo, and then at 3 to 4 mo intervals. The revised RECIST criteria (version 1.1) were used to assess the basic response of the intrapancreatic and extrapancreatic cancer[14]. Three diagnostic radiologists (Piao XH, Zhou Q, and Tang J) with 17, 20, and 13 years of clinical experience, respectively, determined whether progression or recurrence had occurred; reviewing CT scans in every case. Diagnoses were made independently, and the radiologists discussed with each other when they the results were different. The IS-IV was calculated from the date when a patient was first diagnosed as suffering stage IV pancreatic cancer, and compared using the Kaplan-Meier test with long-rank analysis. A significant difference was indicated by P < 0.05. All analyses were performed using GraphPad Software (San Diego, CA, United States).
RESULTS
Clinical data
Percutaneous cryoablation was performed on 81 patients (42-84 years of age, median age: 65 years; 43 male patients, 38 female patients). The patients of each treatment half were from both China (38 patients) and abroad (43 patients). Two-thirds of patients (54 patients) were treated in our hospital when diagnosed as having stage IV pancreatic cancer; one-third of patients were diagnosed and treated with chemotherapy (27 patients, 95 sessions) and/or radiation (9 patients, 24 sessions) in other hospitals first, and came to our hospital 2-14 mo later for further treatment. Liver metastases (75 lesions) were found in 47 patients, peritoneum and liver metastases (76 lesions) were found in 27 patients, and all other metastases (26 lesions) were found in seven patients. Diabetes (16 patients), hydrothorax/ascites (15 patients), and hypertension/coronary disease (8 patients) were common complications in these patients. Radical treatment was performed on 50 patients (79 sessions), and 31 patients (48 sessions) were put under palliative treatment. The therapeutic option of cryoablation was chosen according to the previously-stated criteria.
The mean intrapancreatic tumor diameter in the radical treatment group was 4.3 ± 0.9 cm (range: 3.2-5.7 cm). For the first treatment of the 50 lesions, 13 were treated by using 2 cryoprobes; 16 by 3 cryoprobes; and 21 by four cryoprobes. The mean intrapancreatic tumor diameter in the palliative treatment group was 4.5 ± 0.8 cm (range: 3.5-5.8 cm). For the first treatment of the 31 lesions, 6 were implanted by using about 20 iodine-125 seeds; 9 by about 30 seeds; and 16 by about 40 seeds.
The mean extrapancreatic tumor diameter in the radical treatment group was 3.5 ± 0.8 cm (range: 2.1-4.5 cm). For the first treatment of the 110 lesions, 46 were treated by using 2 cryoprobes; 43 by 3 cryoprobes; and 21 by four cryoprobes. The mean extrapancreatic tumor diameter in the palliative treatment group was 3.7 ± 0.9 cm (range, 2.3-5.0 cm). For the first treatment of the 67 lesions, 31 were treated by using 2 cryoprobes; 27 by 3 cryoprobes; and 9 by 4 cryoprobes.
Perioperative outcomes
All percutaneous cryoablations of primary and metastatic pancreatic tumors by ultrasound and CT monitoring were performed successfully. No severe complications, such as pancreatic fistula, bile leakage, and intestinal fistula were discovered post-cryoablation. In the radical treatment group, some common adverse effects were found and are shown below. Serum amylase, an important index of acute pancreatitis, increased in 25 sessions (31%) for 16 patients (32%) on the first day after the procedure, unaccompanied by ascites or leukocytosis, but returned to normal levels in the following 5 d after symptomatic treatment. Seven patients (14%) with diabetes after 12 sessions (15%) experienced a rise in fasting blood glucose levels up to 20-25 mmol/L on the first day post-cryoablation, which were well-controlled with insulin injections. A mild decrease in the platelet count occurred after 14 sessions (18%) for 10 patients (20%), which returned to normal within 8-13 d without any treatment. Abdominal distension and nausea occurred after 23 sessions (29%) for 14 patients (28%) on the first day post-cryoablation, and disappeared automatically the following day. Eight patients (16%) in 11 sessions (14%) complained of poor appetite after the cryosurgery and were found to have ascites by ultrasound, which improved the following 3-5 d without any treatment. Eleven patients (22%) in 17 sessions (22%) were found to have abdominal bleeding, 8 patients (16%) of whom developed fever and a mild increase in white blood cells, and neutrophil granulocytes; they improved in the following 7 d after symptomatic treatment. Similar adverse effects were also found in the palliative treatment group, and disappeared in 2 wk after symptomatic treatment. There were no treatment-related deaths or any conversions to chemotherapy.
Within 1 wk after the first CandS treatment, 64 patients (79%) experienced a ≥ 50% reduction in pain score, 57 patients (70%) experienced a 50% decrease in analgesic consumption, and 43 patients (53%) experienced a ≥ 20 increase in KPS score.
Influence of therapies, treatment timing, and treatment frequency on IS-IV of patients
The median follow-up period was 8 mo. Up to the date of the last follow-up of each patient, the median IS-IV of all patients was 7.12 mo (95%CI: 3.79-13.37). The median IS-IV of patients with radical treatment was 8 mo, and those with palliative treatment was 4 mo. The IS-IV of the two groups differed significantly by long-rank test (P < 0.001, Figure 1).
Figure 1.
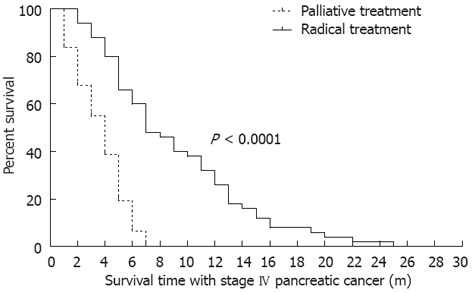
IS-IV of patients under radical or palliative treatment. All 81 patients had suffered from stage IV pancreatic cancer and died before October of 2011. There were 51 patients in the radical treatment group, and 30 patients in the palliative treatment group. The median follow-up period was 8 mo. The IS-IV of patients was accumulated from the early diagnosis of stage IV pancreatic cancer in our own or other hospitals.
Two-third of patients received CandS treatment 2 mo after the diagnosis of stage IV pancreatic cancer, and one-third of patients received CandS treatment 2-14 mo after diagnosis. The influences of treatment timing on IS-IV of patients were detected: for the radical treatment group, the median IS-IV of patients under timely treatment was 6 mo, and 13 mo for those under delayed treatment (P = 0.0034, Figure 2A); for the palliative treatment group, the median IS-IV of patients under timely treatment was 3 mo, and 5 mo for those under delayed treatment (P = 0.0415, Figure 2B). In the two groups, the patients who received CandS treatment ≥ 2 mo after diagnosis were associated with a longer IS-IV, which was more obvious in the radical treatment group.
Figure 2.
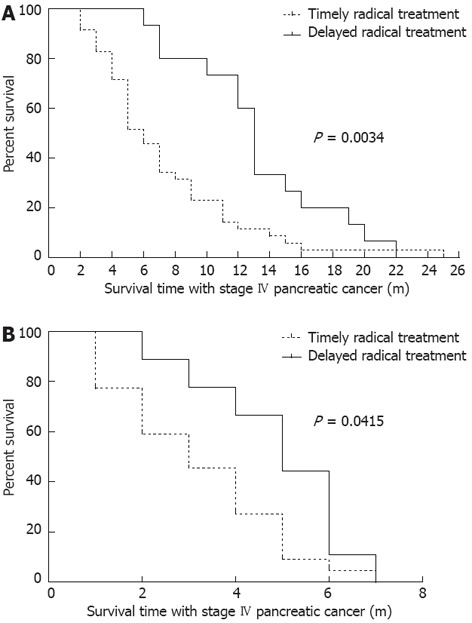
IS-IV of patients under timely or delayed treatment. A: Comparison between patients in the radical treatment group: 32 patients under timely treatment and 18 patients under delayed treatment; B: Comparison between patients in the palliative treatment group: 22 patients under timely treatment and 9 patients under delayed treatment.
According to disease progression, tumor recurrence and individual intents of patients, 31 patients (39%) received repeated CandS treatment when re-examined. For the radical treatment group, the median IS-IV of patients under single treatment was 7 mo, and 11 mo for those under multiple treatments (P = 0.0389, Figure 3A); for the palliative treatment group, the median IS-IV of patients under single treatment was 3 mo, and 4 mo for those under multiple treatments (P = 0.99, Figure 3B). Only in the radical treatment group did multiple treatments show a significant superiority in prolonging the IS-IV of patients.
Figure 3.
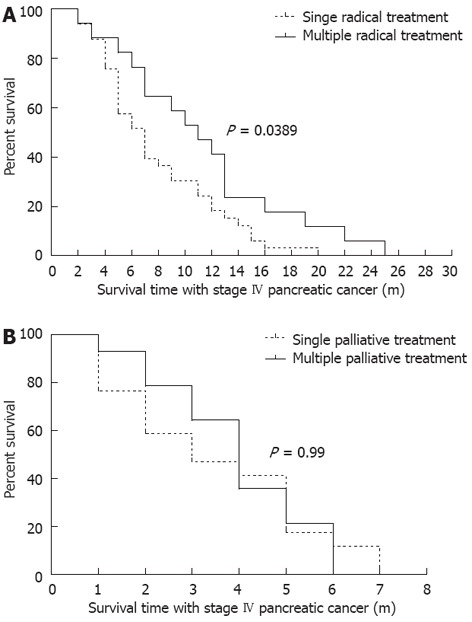
IS-IV of patients under different treatment frequency. A: Comparison among patients in radical treatment group, 17 patients under multiple radical treatment (2 times, by 10 patients; 3 times, by 4 patients; 4, 5 and 9 times, by 1 patient each) and 33 patients were under single radical treatment; B: Comparison among patients in palliative treatment group, 14 patients under multiple palliative treatment (2 times, by 11 patients; 3 times, by 3 patients) and 17 patients under single palliative treatment.
DISCUSSION
Cryoablation has been performed for hepatic, renal, breast, and prostate cancer, and it has shown acceptable results. During cryoablation, the formation of an oversized ice ball increases the complication rate and can endanger a patient’s life; an undersized ice ball can lead to the recurrence of the tumor at the edge of the frozen area[15]. As the volumes of primary or metastatic tumors are sometimes large, adhesions to other organs or tissues and invasive growth are often present. Percutaneous cryoablation cannot guarantee complete ablation, and combination with brachytherapy might be a better choice[16-20]. This combination of minimal invasive therapies (namely CandS treatment) can minimize the damage of radiotherapy and cryoablation, and increase the treatment response[21,22].
In ≥ 80% of patients with pancreatic cancer, the tumor is unresectable at time of diagnosis. Chemotherapy, palliative surgery, and radiofrequency ablation may be the best options for patients with metastases. Along with advances in the cryosurgical system and imageology, percutaneous cryosurgery has been increasingly successful in the treatment of pancreatic cancer[9,10]. This method avoids the risks of laparotomy, decreases the likelihood of complications[23-25], and improves the quality of life, but the data is still lacking as to whether it can extend the IS-IV of patients. Cryoablation and chemotherapy have different effects on patients with stage IV pancreatic cancer. In 2010, Stathis et al[26] reported that the median survival for patients treated with single-agent 5-FU or gemcitabine were 4.41 and 5.65 mo, respectively. Combined applications of other agents with gemcitabine had been frequently attempted in order to get a better IS-IV, but the statistical indication for such a benefit failed to materialize until now[4,27-30]. In our study, the IS-IV of patients under radical treatment was significantly longer than for those under palliative treatment (P < 0.0001), with a 4 mo extension of median IS-IV (8 mo vs 4 mo, respectively). The 1-year survival rate of patients in the radical treatment group was 32%, showing the greater superiority of radical treatment, as this was better than palliative treatment and chemotherapy. Interestingly, patients who delayed CandS treatment were associated with better IS-IV, regardless of radical or palliative treatment. For this reason we re-analyzed the data, and found that all the patients under delayed treatment had received chemotherapy before admission to our hospital, and so chemotherapy may be an important reason for the extension of IS-IV. It seems that if chemotherapy is delivered early in stage IV pancreatic cancer, it will change the systemic disease into local disease, and improve the benefit to survival time of patients. As for the frequency of CandS treatment, multiple treatments only showed a significant advantage in the radical treatment group (median survival: 11 mo vs 7 mo, P = 0.0389). Therefore, in order to get the best therapeutic effect, early chemotherapy, radical, and multiple treatments are all very important.
With regard to the complications associated with radical treatment, pancreatic fistula, bile leakage, and intestinal fistula were seldom observed in our study, maybe due to the evasion of patients with a high surgical risk when enrolling. Other minor complications associated with radical or palliative treatment were common after cryoablation of the pancreas and liver, including an increase of serum amylase and blood glucose, a decrease in the number of platelets, abdominal distension, ascites, fever, infection, and abdominal bleeding. All complications can be decreased to normal within 2 wk after symptomatic treatment. In effectively reducing tumors and removing obstructions, the physical strength and energy of patients in the two groups improved obviously and pains were reduced significantly. These achievements are inseparable with effective tumor reduction and close postoperative monitoring.
The present study represents an early experience, with a small number of patients. Hence, extrapolation of the results to clinical practice should be performed with caution. This was not a definitive study for assessing the effects of chemotherapy on IS-IV of patients, the effectiveness of CandS in the treatment of newly diagnosed stage IV pancreatic cancer, or for determining whether CandS treatment was as effective as surgery for pancreatic tumors.
In conclusion, percutaneous CandS treatment may have a useful role in the management of stage IV pancreatic cancer evading vital organs and those < 6 cm in diameter when surgery and chemotherapy are not options. To further increase the IS-IV of patients, close postoperative monitoring and multiple treatments will be needed for recurrent tumors.
COMMENTS
Background
Pancreatic cancer is the fourth leading cause of cancer-related death clinically. Less than 20% of pancreatic cancers are curable on diagnosis. Without effective treatment, the overall survival for patients with advanced pancreatic cancer is usually less than 1 year, and for those with metastatic stage IV pancreatic cancer, the median survival is only 3-4 mo. Treatment effects of radiation and chemotherapy are poor, and radiofrequency ablation can not be applied for patients percutaneously.
Research frontiers
This research concerns the field of ablation for pancreatic cancer. Currently, cryosurgery is the only useful method for in situ ablation of pancreatic cancer.
Innovations and breakthroughs
Three methods were used to treat different parts of the pancreatic cancer by percutaneous ablation, which increased the safety and efficiency of cryosurgery for patients. The relationship between treatment timing, treatment frequency, and survival time of patients with metastatic pancreatic cancer were analyzed.
Applications
Depending on the retrospective analysis of radical and palliative treatment on patients with stage IV pancreatic cancer, clinical evidence was provided concerning survival time, which will benefit the future application of this technology.
Terminology
Percutaneous cryosurgery: Under the guidance of B ultrasound or computed tomography (CT), cryoprobes are directly stuck into the tumor through skin, and cryoablation is performed in situ by the ultra-low temperature of the probe tip; Seed implantation: under the guidance of B ultrasound or CT, radioactive seeds are implanted into the tumor to act as brachytherapy; IS-IV: since the patients enrolled in this study were all suffered from metastatic tumors (stage IV pancreatic cancer), and many treatments had been carried out in other countries and hospitals before, the ideal evaluating method for survival time for our treatment is interval survival for stage IV pancreatic cancer.
Peer review
This study is the first retrospective analysis for the radical and palliative treatment of patients with stage IV pancreatic cancer, which clarified the benefits of percutaneous cryosurgery for this disease, along with the relationship between treatment timing, treatment frequency, and survival time of patients. These findings can provide important evidence for the clinical treatment of stage IV pancreatic cancer.
Footnotes
Supported by The Hai Zhu District Scientific and Technological Plan, No. 2010-Y-27; “Comprehensive Research of Pancreatic Cancer Cryotherapy”, Guangzhou, China
Peer reviewer: Dr. Devinder Kumar Dhawan, Professor, Department of Biophysics and Coordinator, Nuclear Medicine, Panjab University, Chandigarh 160014, India
S- Editor Lv S L- Editor Rutherford A E- Editor Zhang DN
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SP. Palliative chemotherapy for pancreatic malignancies. Surg Clin North Am. 2010;90:365–375. doi: 10.1016/j.suc.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Wolff RA. Chemotherapy for pancreatic cancer: from metastatic disease to adjuvant therapy. Cancer J. 2007;13:175–184. doi: 10.1097/PPO.0b013e318074e6c3. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S, Levine M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7:e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogelman D, Jafari M, Varadhachary GR, Xiong H, Bullock S, Ozer H, Lin E, Morris J, Cunningham P, Bennett B, et al. Bevacizumab plus gemcitabine and oxaliplatin as first-line therapy for metastatic or locally advanced pancreatic cancer: a phase II trial. Cancer Chemother Pharmacol. 2011;68:1431–1438. doi: 10.1007/s00280-011-1601-4. [DOI] [PubMed] [Google Scholar]
- 7.Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55–60. doi: 10.1007/s00423-006-0098-5. [DOI] [PubMed] [Google Scholar]
- 8.Zou YP, Li WM, Zheng F, Li FC, Huang H, Du JD, Liu HR. Intraoperative radiofrequency ablation combined with 125 iodine seed implantation for unresectable pancreatic cancer. World J Gastroenterol. 2010;16:5104–5110. doi: 10.3748/wjg.v16.i40.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu KC, Niu LZ, Hu YZ, He WB, He YS, Li YF, Zuo JS. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol. 2008;14:1603–1611. doi: 10.3748/wjg.14.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu KC, Niu LZ, Hu YZ, He WB, He YS, Zuo JS. Cryosurgery with combination of (125)iodine seed implantation for the treatment of locally advanced pancreatic cancer. J Dig Dis. 2008;9:32–40. doi: 10.1111/j.1443-9573.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- 11.Xu KC, Niu LZ, Zhou Q, Hu YZ, Guo DH, Liu ZP, Lan B, Mu F, Li YF, Zuo JS. Sequential use of transarterial chemoembolization and percutaneous cryosurgery for hepatocellular carcinoma. World J Gastroenterol. 2009;15:3664–3669. doi: 10.3748/wjg.15.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azizi A, Naguib NN, Mbalisike E, Farshid P, Emami AH, Vogl TJ. Liver metastases of pancreatic cancer: role of repetitive transarterial chemoembolization (TACE) on tumor response and survival. Pancreas. 2011;40:1271–1275. doi: 10.1097/MPA.0b013e318220e5b9. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16:765–778. doi: 10.1097/01.RVI.0000170858.46668.65. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59:229–243. doi: 10.1016/j.cryobiol.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen A, Galloway M, Landreneau R, d’Amato T, Colonias A, Karlovits S, Quinn A, Santucci T, Kalnicki S, Brown D. Intraoperative 125I brachytherapy for high-risk stage I non-small cell lung carcinoma. Int J Radiat Oncol Biol Phys. 1999;44:1057–1063. doi: 10.1016/s0360-3016(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 17.Chen QS, Blair HF. Thyroid uptake of 125iodine after prostate permanent brachytherapy. J Urol. 2004;172:1827–1829. doi: 10.1097/01.ju.0000140443.37787.3f. [DOI] [PubMed] [Google Scholar]
- 18.Grimm PD, Blasko JC, Sylvester JE, Meier RM, Cavanagh W. 10-year biochemical (prostate-specific antigen) control of prostate cancer with (125)I brachytherapy. Int J Radiat Oncol Biol Phys. 2001;51:31–40. doi: 10.1016/s0360-3016(01)01601-7. [DOI] [PubMed] [Google Scholar]
- 19.Holm HH, Juul N, Pedersen JF, Hansen H, Strøyer I. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. 1983. J Urol. 2002;167:985–988; discussion 985-988. doi: 10.1016/s0022-5347(02)80320-2. [DOI] [PubMed] [Google Scholar]
- 20.Stone NN, Stock RG, Unger P. Intermediate term biochemical-free progression and local control following 125iodine brachytherapy for prostate cancer. J Urol. 2005;173:803–807. doi: 10.1097/01.ju.0000152558.63996.29. [DOI] [PubMed] [Google Scholar]
- 21.Kaye KW, Olson DJ, Payne JT. Detailed preliminary analysis of 125iodine implantation for localized prostate cancer using percutaneous approach. J Urol. 1995;153:1020–1025. [PubMed] [Google Scholar]
- 22.Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J. 2006;28:200–218. doi: 10.1183/09031936.06.00014006. [DOI] [PubMed] [Google Scholar]
- 23.Kovach SJ, Hendrickson RJ, Cappadona CR, Schmidt CM, Groen K, Koniaris LG, Sitzmann JV. Cryoablation of unresectable pancreatic cancer. Surgery. 2002;131:463–464. doi: 10.1067/msy.2002.121231. [DOI] [PubMed] [Google Scholar]
- 24.Korpan NN. Cryosurgery: ultrastructural changes in pancreas tissue after low temperature exposure. Technol Cancer Res Treat. 2007;6:59–67. doi: 10.1177/153303460700600202. [DOI] [PubMed] [Google Scholar]
- 25.Korpan NN, Hochwarter G, Sellner F. Cryoscience and cryomedicine: new mechanisms of biological tissue injury following low temperature exposure. Experimental study. Klin Khir. 2009;(7-8):80–85. [PubMed] [Google Scholar]
- 26.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 27.Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, Cigolari S, Testa A, Maiello E, Lopez M. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer. 2002;94:902–910. [PubMed] [Google Scholar]
- 28.Scheithauer W, Schüll B, Ulrich-Pur H, Schmid K, Raderer M, Haider K, Kwasny W, Depisch D, Schneeweiss B, Lang F, et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol. 2003;14:97–104. doi: 10.1093/annonc/mdg029. [DOI] [PubMed] [Google Scholar]
- 29.Rocha Lima CM, Green MR, Rotche R, Miller WH, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 30.Oettle H, Richards D, Ramanathan RK, van Laethem JL, Peeters M, Fuchs M, Zimmermann A, John W, Von Hoff D, Arning M, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639–1645. doi: 10.1093/annonc/mdi309. [DOI] [PubMed] [Google Scholar]


