Abstract
AIM: To evaluate the protective role of AE-941, a matrix metalloproteinase (MMP) inhibitor, on ulcerative colitis (UC) in rats.
METHODS: Sprague Dawley (SD) rats were randomly divided into three groups: a control group, an AE-941 treatment group, and an UC model group. Rats were sacrificed on days 7, 21, or 56 following administration of treatment by enema and the disease activity index (DAI), colonic mucosa damage index (CMDI) and colonic expression of MMP-2 and MMP-9 were assessed.
RESULTS: DAI and CDMI scores in the UC model group increased significantly compared to the control group at all timepoints (P < 0.001), and also increased significantly at the 21- and 56-d timepoints compared to the AE-941-treated group (DAI: 21- and 56-d = 2.09 ± 0.25, 1.52 ± 0.30 vs 1.55 ± 0.28, 0.59 ± 0.19, respectively, P = 0.040 and 0.007, CMDI: 21- and 56-d = 3.03 ± 0.42, 1.60 ± 0.35 vs 2.08 ± 0.46, 0.86 ± 0.37, respectively, P = 0.040 and 0.005). Furthermore, the colonic expression of MMP-2 and MMP-9 in the UC model group increased significantly compared to the control group (P < 0.001), and also increased compared to the AE-941-treated group on the 21- and 56-d timepoints (MMP-2: 21- and 56-d = 0.6048 ± 0.0522, 0.4163 ± 0.0330 vs 0.3983 ± 0.0218, 0.1093 ± 0.0072, respectively, P = 0.010; MMP-9: 21- and 56-d = 0.6873 ± 0.0472, 0.4328 ± 0.0257 vs 0.5179 ± 0.0305, 0.2673 ± 0.0210, respectively, P = 0.010 and 0.040).
CONCLUSION: Expression of MMP-2 and MMP-9 increased significantly in rats with UC. AE-941 can reduce colonic mucosal damage by downregulating the expression of MMP-2 and MMP-9.
Keywords: AE-941, Extracellular matrix, Matrix metalloproteinase-2, Matrix metalloproteinase-9, Ulcerative colitis
INTRODUCTION
Ulcerative colitis (UC) is a type of nonspecific inflammatory bowel disease for which the etiology and disease mechanism are not completely clear. It has recently become apparent that the synthetic and degradative imbalance of the colonic extracellular matrix (ECM) may be associated with the progression of UC[1,2]. The aberrant expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) can cause a synthetic and degradative imbalance of the ECM, which then leads to a series of pathological reactions such as colonic mucosa inflammation, erosion, and ulceration[3,4]. TIMPs can inhibit the activity of MMPs, which are able to correct MMP-TIMP imbalances. Our previous research indicated that MMPs may be involved in the occurrence and development of UC.
Expression of MMP-2 and MMP-9 can be blocked by AE-941, a natural inhibitor of MMPs, which is a new and effective treatment of malignant tumors[5]. In this study, we used a rat model of UC and administered AE-941 by gastric lavage. We then observed the therapeutic effect of AE-941. We will discuss the mechanism of AE-941 alleviation of UC to provide a theoretical basis for a novel treatment for UC.
MATERIALS AND METHODS
Materials
Healthy male Sprague Dawley (SD) rats weighing 180-220 g and aged 4-8 wk were supplied by the SPF laboratory animal center of Dalian Medical University. 2,4,6-trinitrobenzenesulfonic acid solution (TNBS) was purchased from Sigma. AE-941, MMP-2 and MMP-9 polyclonal antibodies were supplied by Bioworld Technology. The MaxPoly Plus Anti-Mouse/Rabbit horseradish peroxidase IHC Kit was supplied by the Fujian Maixin Biological Technology Co. Primers, DNA markers (DL 2000), Takara RNA polymerase chain reaction (PCR) kit3.0 (AMV) kit were from Dalian, Takara Co. Ltd.
Animal treatment
A total of 54 SD rats were randomly divided into three groups: a control group, an AE-941 group, and an UC model group; each group had 18 rats. The SD rat model of UC was established by administering a mixture of TNBS (100 mg/kg) and 50% ethanol (0.25 mL) by enema. The control group was subject to enema and gastric lavage with normal saline. For the AE-941 group, TNBS was administered by enema and AE-941 was administered by gastric lavage (10 mg/kg once a day). The UC model group was subjected to TNBS enema and the gastric lavage was composed of a saline control. Rats from all groups were sacrificed on days 7, 21 and 56. Six rats per group were sacrificed each day, after the enema and colonic tissue 2.0-10.0 cm from the anus was collected for reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemical analysis.
Disease activity index and colonic mucosa damage index scoring
The rats were weighed and checked for behavior, stool consistency and the presence of gross blood in the stools every day. The scores were assigned as follows: percentage of body weight reduction (0, no change; 1, 1%-5%; 2, 6%-10%; 3, 11%-15%; 4, > 15%); stool consistency (0, normal; 2, loose; 4, diarrhea); and the presence of fecal blood (0, normal; 2, positive occult blood test; 4, visible bleeding)[6]. The disease activity index (DAI) was calculated as the sum of these scores.
Rats were sacrificed at the timepoints indicated and the entire colon was excised from the cecum to the anus and opened longitudinally. Macroscopic damage was evaluated using a validated [colonic mucosa damage index (CMDI)] scoring system with slight modifications[7]. The numerical rating score was as follows: 0, no inflammation; 1, local hyperemia without ulcers, and/or stool consistency; 2, ulceration without hyperemia; 3, ulceration and adhesions at one site; 4, two or more sites of inflammation and ulceration extending > 1 cm; 5, ulceration extending more than 2 cm.
MMP-2 and MMP-9 mRNA expression
mRNA was extracted from colonic tissue samples using Trizol according to the manufacturer’s protocols (Invitrogen) and RT-PCR was performed according to the instructions of the Takara RNA PCR kit 3.0 (AMV). An equal amount of cDNA from each sample was amplified using primers specific to each gene (Table 1). DNA amplification was done using a thermocycler under the following conditions: for MMP-2, 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 45 s, and extension at 72 °C for 90 s; for MMP-9, 35 cycles of denaturation at 94 °C for 30 s, annealing at 59 °C for 30s, and extension at 72 °C for 90 s; for β-actin, 35 cycles of denaturation at 94 °C for 30s, annealing at 62 °C for 30s, and extension at 72 °C for 90s. RT-PCR products were measured by photodensitometry using a gel image analysis system after agarose gel electrophoresis and ethidium bromide staining.
Table 1.
Oligonucleotide of primers of target genes
| mRNA species | mRNA | PCR product (bp) | |
| MMP-2 | Sense | 5’-ACCATCGCCCATCATCAAGT-3’ | 348 |
| Antisense | 5’-CGAGCAAAAGCATCATCCAC-3’ | ||
| MMP-9 | Sense | 5’-CCCTGCGTATTTCCATTCAT-3’ | 600 |
| Antisense | 5’-ACCCCACTTCTTGTCAGCGTC-3’ | ||
| β-actin | Sense | 5’-AAGCCTAAGGCCAACCGTGAAAAG-3’ | 241 |
| Antisense | 5’-TCAATGAGGTAGTCTGTCAGGT-3’ | ||
MMP: Metalloproteinase; PCR: Polymerase chain reaction.
Measurement of MMP-2 and MMP-9 protein expression
Immunohistochemistry was performed according to the Max Vision kit protocol. Image analysis software (Image-pro plus 6.0) was used to measure the light density of positive control cells in which the cytoplasm was tan-yellow or brown after 3,3’-diaminobenzidine staining. For each section, the positive integrated optical density (IOD) and total area of five representative visual fields without overlap were observed under high-power microscope (× 400). The ratio of IOD and total area represents the mean value of optical density, with a higher ratio indicating a higher level of protein expression.
Statistical analysis
All data were analyzed using the SPSS statistical package version 11.5. Data showed a normal distribution and are expressed as means ± SD. The responses of different experimental groups were analyzed using one-way analysis of variance. A P-value of < 0.05 indicates a statistically significant difference between different groups.
RESULTS
Results of disease activity index and colonic mucosa damage index
The DAI and CMDI of the UC model group was significantly higher than that of the control group (P < 0.05) at all timepoints. Compared with the UC model group, the DAI and CMDI values of the AE-941 treatment group were reduced on day 7, although not significantly (P > 0.05). On days 21 and 56 after treatment, the DAI and CMDI scores of the AE-941 treatment group were significantly lower than that of the UC model group (P < 0.05; Table 2).
Table 2.
Disease activity index and colonic mucosa damage index of three groups on different time (mean ± SD)
| Time |
Control group |
Model group |
AE-941 group |
|||
| DAI | CMDI | DAI | CMDI | DAI | CMDI | |
| 7th day | 0.06 ± 0.14 | 0.00 ± 0.00 | 2.89 ± 0.00a | 4.83 ± 0.55a | 2.76 ± 0.32 | 4.74 ± 0.39 |
| 21st day | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.09 ± 0.25a | 3.03 ± 0.42a | 1.55 ± 0.28c | 2.08 ± 0.46c |
| 56th day | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.52 ± 0.30a | 1.60 ± 0.35a | 0.59 ± 0.19c | 0.86 ± 0.37c |
P < 0.05 vs control group;
P < 0.05 vs model group. DAI: Disease activity index; CMDI: Colonic mucosa damage index.
Results of reverse transcription polymerase chain reaction
Expression of MMP-2 and MMP-9 mRNA in the colonic mucosa was significantly higher in the UC model group compared to the control group (P < 0.05). However, compared to the UC model group, MMP-2 and MMP-9 mRNA expression in the AE-941 treatment group was reduced by day 7, although not significantly (P > 0.05). On days 21 and 56, the two index values in the AE-941 treatment group were significantly lower than that of the model group (P < 0.05, Table 3, Figure 1).
Table 3.
Colonic mucosal mRNA expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 (mean ± SD)
| Time |
Control group |
Model group |
AE-941 group |
|||
| MMP-2 | MMP-9 | MMP-2 | MMP-9 | MMP-2 | MMP-9 | |
| 7th day | 0.0062 ± 0.0017 | 0.0068 ± 0.0096 | 0.8563 ± 0.1132a | 0.9936 ± 0.1187a | 0.7509 ± 0.0693 | 0.8375 ± 0.1054 |
| 21st day | 0.0056 ± 0.0012 | 0.0071 ± 0.0134 | 0.6048 ± 0.0522ae | 0.6873 ± 0.0472ae | 0.3983 ± 0.0218c | 0.5179 ± 0.0305c |
| 56th day | 0.0058 ± 0.0015 | 0.0069 ± 0.0011 | 0.4163 ± 0.0330ag | 0.4328 ± 0.0257ag | 0.1093 ± 0.0072c | 0.2673 ± 0.0210c |
P < 0.05 vs control group;
P < 0.05 vs model group;
P < 0.05 vs day 7 timepoint;
P < 0.05 vs day 21 timepoint. MMP: Matrix metalloproteinase.
Figure 1.
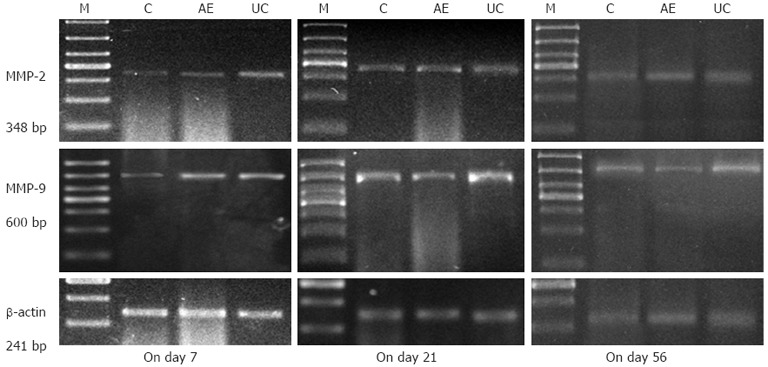
The colonic mucosal mRNA expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 in control, AE-941 and ulcerative colitis model groups at different timepoints. Expression of matrix metalloproteinases (MMP)-2 and MMP-9 in the colonic mucosa was significantly higher in the ulcerative colitis model group compared to the control group (P < 0.05). On days 21 and 56, the two index values in the AE-941 treatment group were significantly less than that of the model group (P < 0.05). M: Marker; C: Control group; AE: AE-941 group; UC: UC model group.
Results of immunohistochemistry
The expression of MMP-2 and MMP-9 protein in the colonic mucosa of the UC model group was significantly higher than in the control group (P < 0.05). Compared with the model group, expression of MMP-2 and MMP-9 protein in the AE-941 treatment group was reduced, although not significantly, by day 7 (P > 0.05). In contrast, by days 21 and 56 expression of MMP-2 and MMP-9 in the AE-941 treatment group was significantly lower than in the model group (P < 0.05) (Figure 2, Table 4).
Figure 2.
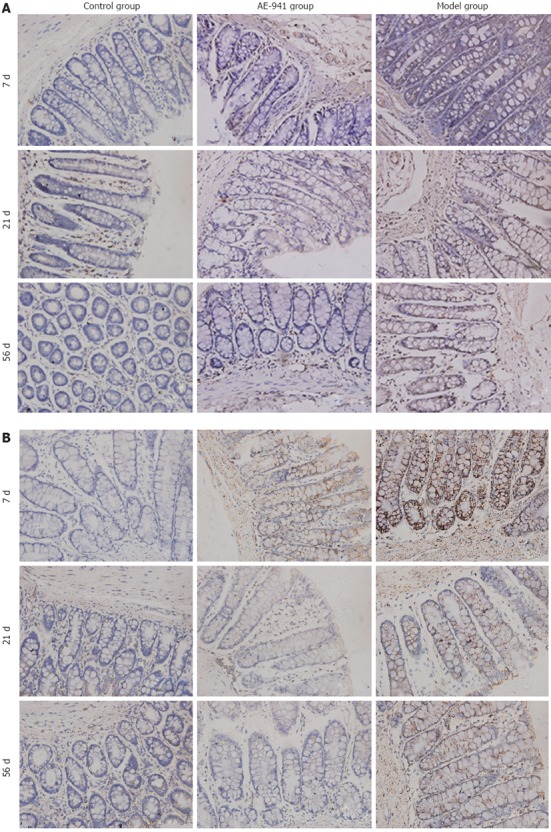
Colonic mucosal matrix metalloproteinase-2 and -9 protein expression in control, AE-941, and ulcerative colitis model groups at different timepoints. The expression of matrix metalloproteinases (MMP)-2 (A) and MMP-9 (B) protein in the colonic mucosa of the ulcerative colitis model group was significantly higher than in the control group (P < 0.05). By days 21 and 56 expression of MMP-2 and MMP-9 in the AE-941 treatment group was significantly less than in the model group (P < 0.05).
Table 4.
Colonic mucosal protein expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 (mean ± SD)
| Time |
Control group |
Model group |
AE-941 group |
|||
| MMP-2 | MMP-9 | MMP-2 | MMP-9 | MMP-2 | MMP-9 | |
| 7th day | 0.0053 ± 0.0037 | 0.0073 ± 0.0015 | 0.0986 ± 0.0084a | 0.0916 ± 0.0077a | 0.0923 ± 0.0071 | 0.0893 ± 0.0075 |
| 21st day | 0.0047 ± 0.0027 | 0.0067 ± 0.0011 | 0.0773 ± 0.0052a | 0.0748 ± 0.0067a | 0.0536 ± 0.0034c | 0.0554 ± 0.0061c |
| 56th day | 0.0057 ± 0.0026 | 0.0070 ± 0.0013 | 0.0453 ± 0.0036a | 0.0537 ± 0.0035a | 0.0324 ± 0.0025c | 0.0364 ± 0.0025c |
P < 0.05 vs control group;
P < 0.05 vs model group. MMP: Matrix metalloproteinase.
DISCUSSION
Our previous research indicated that MMPs may be involved in the occurrence and development of UC[8,9]. MMPs are a family of calcium- or zinc-dependent proteolytic enzymes that can remodel the ECM[10]. At least 28 different MMP enzymes have been identified to date[11]. MMP-2 and MMP-9 are the main matrix metalloproteinases that function to degrade collagen subtypes, including type IV and V, in the matrix[12]. The study of von Lampe demonstrated that MMP-2 was highly expressed in the mucosa of patients with UC[13]. Pirilä et al[14] identified MMP-2 overexpression in the colonic mucous membrane surrounding areas of inflammation in patients with UC. Medina also found that the expression and activity of MMP-9 was upregulated in colonic mucosa in the UC rat model[15]. These results suggest that overexpression of MMP-2 and MMP-9 is related to mucosal membrane injury, inflammation of the epithelium and tissue destruction. We therefore tested MMP-2 and MMP-9 expression levels in colonic mucosa using RT-PCR and immunohistochemistry. Our results showed that expression of two indices, DAI and CMDI were significantly higher in UC model animals than in the control group, and that decreased MMP-2 and MMP-9 expression is associated with an improvement in both DAI and CMDI. These data suggest that overexpression of MMP-2 and MMP-9 is related to mucosal injury and is particularly important for inflammation.
TIMPs are endogenous secretory proteins that can regulate the activity of MMPs[16]. A disequilibrium of MMPs and TIMPs can lead to a synthetic and degradative imbalance of the ECM, which induces a series of pathological reactions[17]. Our previous research showed that hyperdegradation combined with insufficient synthesis of colonic ECM resulted in mucosal injury, necrosis and ulceration that were attributed to unbalanced colonic mucosal expression of MMPs and TIMPs in patients with UC[18,19].
AE-941, also known as Neovastat, is a natural MMP inhibitor derived from shark cartilage, and is a potent inhibitor of MMP-2 and MMP-9 that can dissolve gelatin and elastic protein[20,21]. Béliveau and Falardeau et al[22,23] have shown that the activity of MMP-2, MMP-9 and MMP-12 is strongly inhibited by AE-941. Neovastat is a naturally occurring anti-angiogenic compound with multiple mechanisms of action that provide a broad therapeutic potential for a number of diseases[23]. This drug is currently in international Phase II trials for multiple myeloma, renal cell carcinoma, and non-small-cell lung cancer[24-26], and its inhibitory function may represent an innovative approach to the treatment of these diseases. The potent inhibition of MMP-2 and MMP-9 by AE-941 was used in our study of TNBS-induced UC in rats. Compared to the UC model group, the DAI and CMDI in the colon of AE-941-treated rats were significantly decreased at the 21- and 56-d timepoints. We found that expression of MMP-2 and MMP-9 mRNA and protein in the colonic mucosa of AE-941-treated rats was significantly lower than in the UC model group. Our results indicated that AE-941 is a potent MMP-2 and MMP-9 inhibitor that can relieve the intensity of chronic inflammation by decreasing the activity and expression of MMP-2 and MMP-9. We found no significant difference in the DAI, the CMDI, and colonic mucosal expression of MMP-2 and MMP-9 between the UC model animals and the AE-941 treatment group by day 7. A possible reason for this is that rats suffering the worst reaction to the TNBS enema at the day 7 evaluation were included in the UC model group. However, AE-941 did not achieve a response at the shorter (7-d) treatment time.
By comparing the colonic mucosa expression of MMP-2 and MMP-9 in UC model animals at different timepoints, we found that MMP-2 and MMP-9 expression decreased as the DAI and CMDI improved. Using Spearman analysis, we identified a positive correlation between the expression of both MMP-2 and MMP-9 and the severity of UC.
In conclusion, our study confirmed that colonic mucosa in UC expressed MMP-2 and MMP-9 excessively and this overexpression correlated with the severity of UC. Treatment with AE-941 decreased the DAI and CMDI of UC, as well as colonic mucosal expression of MMP-2 and MMP-9, which indicates that AE-941 can protect the colon by inhibiting the excessive expression of MMP-2 and MMP-9 in an UC model. Therefore, MMP inhibitors may become a new measure to treat UC.
ACKNOWLEDGMENTS
The authors thank Wan-Jing Zou for her excellent technical assistance for help with immunohistochemistry. We also thank the SPF laboratory animal center of Dalian Medical University.
COMMENTS
Background
Ulcerative colitis (UC) is a type of nonspecific inflammatory bowel diseases for which the pathogenesis is not completely clear and lacking of effective treatments. The aberrant expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) can cause a synthetic and degradative imbalance of the extracellular matrix, which then leads to colonic mucosa inflammation, erosion and ulceration. Therefore, in this study, AE-941, a natural MMP inhibitor (MMPI), was employed to verify its protective effect on the rat model of UC.
Research frontiers
In recent years, intense and extensive studies have shown that MMPs, TIMPs play an important role in the development of UC, while studies on the effects of exogenous MMPI on UC are not well documented. Therefore, studies are needed to verify the protective and therapeutic effects of MMPI on UC.
Innovations and breakthroughs
AE-941, a natural MMPI derived from shark cartilage, has been used in experimental and clinical treatment of tumors due to its potent inhibition of MMP-2 and MMP-9. However, its protective effects on UC remain largely unknown. Therefore, in this study, the authors verified the protective effects of AE-941 on UC in rats and provided a new therapeutic approach to UC.
Applications
Up till now, no satisfactory therapy for UC has been available. MMPI targeting MMPs may become a new and effective treatment modality for UC.
Peer review
This concise study reports interesting features on the role of metalloproteinase in ulcerative colitis. The study is well done, and the results are clearly presented.
Footnotes
Supported by Grants from Fund of the Education Department, Liaoning Province
Peer reviewer: Takayuki Yamamoto, MD, Inflammatory Bowel Disease Center, Yokkaichi Social Insurance Hospital, 10-8 Hazuyamacho, Yokkaichi 510-0016, Japan
S- Editor Shi ZF L- Editor O’Neill M E- Editor Zhang DN
References
- 1.Lakatos G, Sipos F, Miheller P, Hritz I, Varga MZ, Juhász M, Molnár B, Tulassay Z, Herszényi L. The behavior of matrix metalloproteinase-9 in lymphocytic colitis, collagenous colitis and ulcerative colitis. Pathol Oncol Res. 2012;18:85–91. doi: 10.1007/s12253-011-9420-9. [DOI] [PubMed] [Google Scholar]
- 2.Gao Q, Meijer MJ, Kubben FJ, Sier CF, Kruidenier L, van Duijn W, van den Berg M, van Hogezand RA, Lamers CB, Verspaget HW. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig Liver Dis. 2005;37:584–592. doi: 10.1016/j.dld.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Meijer MJ, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Hommes DW, Verspaget HW. Role of matrix metalloproteinase, tissue inhibitor of metalloproteinase and tumor necrosis factor-alpha single nucleotide gene polymorphisms in inflammatory bowel disease. World J Gastroenterol. 2007;13:2960–2966. doi: 10.3748/wjg.v13.i21.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mäkitalo L, Kolho KL, Karikoski R, Anthoni H, Saarialho-Kere U. Expression profiles of matrix metalloproteinases and their inhibitors in colonic inflammation related to pediatric inflammatory bowel disease. Scand J Gastroenterol. 2010;45:862–871. doi: 10.3109/00365520903583863. [DOI] [PubMed] [Google Scholar]
- 5.Gingras D, Boivin D, Deckers C, Gendron S, Barthomeuf C, Béliveau R. Neovastat--a novel antiangiogenic drug for cancer therapy. Anticancer Drugs. 2003;14:91–96. doi: 10.1097/00001813-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Porter SN, Howarth GS, Butler RN. An orally administered growth factor extract derived from bovine whey suppresses breath ethane in colitic rats. Scand J Gastroenterol. 1998;33:967–974. doi: 10.1080/003655298750027001. [DOI] [PubMed] [Google Scholar]
- 7.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YD, Yan PY. Expression of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in ulcerative colitis. World J Gastroenterol. 2006;12:6050–6053. doi: 10.3748/wjg.v12.i37.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YD, Mao JW. Expression of matrix metalloproteinase-1 and tumor necrosis factor-alpha in ulcerative colitis. World J Gastroenterol. 2007;13:5926–5932. doi: 10.3748/wjg.v13.i44.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Kim SK. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: the current situation and future prospects. Mar Drugs. 2009;7:71–84. doi: 10.3390/md7020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naito Y, Yoshikawa T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol Aspects Med. 2005;26:379–390. doi: 10.1016/j.mam.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3:409–421. doi: 10.2174/1389203023380549. [DOI] [PubMed] [Google Scholar]
- 13.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63–73. doi: 10.1136/gut.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirilä E, Ramamurthy NS, Sorsa T, Salo T, Hietanen J, Maisi P. Gelatinase A (MMP-2), collagenase-2 (MMP-8), and laminin-5 gamma2-chain expression in murine inflammatory bowel disease (ulcerative colitis) Dig Dis Sci. 2003;48:93–98. doi: 10.1023/a:1021790532723. [DOI] [PubMed] [Google Scholar]
- 15.Medina C, Videla S, Radomski A, Radomski MW, Antolín M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G116–G122. doi: 10.1152/ajpheart.00036.2002. [DOI] [PubMed] [Google Scholar]
- 16.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clutterbuck AL, Asplin KE, Harris P, Allaway D, Mobasheri A. Targeting matrix metalloproteinases in inflammatory conditions. Curr Drug Targets. 2009;10:1245–1254. doi: 10.2174/138945009789753264. [DOI] [PubMed] [Google Scholar]
- 18.Wang YD, Wang W. Protective effect of ilomastat on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2008;14:5683–5688. doi: 10.3748/wjg.14.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YD, Tan XY, Zhang K. Correlation of plasma MMP-1 and TIMP-1 levels and the colonic mucosa expressions in patients with ulcerative colitis. Mediators Inflamm. 2009;2009:275072. doi: 10.1155/2009/275072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dredge K. AE-941 (AEterna) Curr Opin Investig Drugs. 2004;5:668–677. [PubMed] [Google Scholar]
- 21.Gingras D, Nyalendo C, Di Tomasso G, Annabi B, Béliveau R. Activation of tissue plasminogen activator gene transcription by Neovastat, a multifunctional antiangiogenic agent. Biochem Biophys Res Commun. 2004;320:205–212. doi: 10.1016/j.bbrc.2004.05.151. [DOI] [PubMed] [Google Scholar]
- 22.Béliveau R, Gingras D, Kruger EA, Lamy S, Sirois P, Simard B, Sirois MG, Tranqui L, Baffert F, Beaulieu E, et al. The antiangiogenic agent neovastat (AE-941) inhibits vascular endothelial growth factor-mediated biological effects. Clin Cancer Res. 2002;8:1242–1250. [PubMed] [Google Scholar]
- 23.Falardeau P, Champagne P, Poyet P, Hariton C, Dupont E. Neovastat, a naturally occurring multifunctional antiangiogenic drug, in phase III clinical trials. Semin Oncol. 2001;28:620–625. doi: 10.1016/s0093-7754(01)90035-1. [DOI] [PubMed] [Google Scholar]
- 24.Gingras D, Renaud A, Mousseau N, Beaulieu E, Kachra Z, Béliveau R. Matrix proteinase inhibition by AE-941, a multifunctional antiangiogenic compound. Anticancer Res. 2001;21:145–155. [PubMed] [Google Scholar]
- 25.Latreille J, Batist G, Laberge F, Champagne P, Croteau D, Falardeau P, Levinton C, Hariton C, Evans WK, Dupont E. Phase I/II trial of the safety and efficacy of AE-941 (Neovastat) in the treatment of non-small-cell lung cancer. Clin Lung Cancer. 2003;4:231–236. doi: 10.3816/clc.2003.n.003. [DOI] [PubMed] [Google Scholar]
- 26.Escudier B, Choueiri TK, Oudard S, Szczylik C, Négrier S, Ravaud A, Chevreau C, Venner P, Champagne P, Croteau D, et al. Prognostic factors of metastatic renal cell carcinoma after failure of immunotherapy: new paradigm from a large phase III trial with shark cartilage extract AE 941. J Urol. 2007;178:1901–1905. doi: 10.1016/j.juro.2007.07.035. [DOI] [PubMed] [Google Scholar]


