Abstract
AIM: To investigate the adjunct anticancer effect of Astragalus polysaccharides in H22 tumor-bearing mice.
METHODS: To establish a solid tumor model, 5.0 × 106/mL H22 hepatoma cells were inoculated subcutaneously into the right armpit region of Kunming mice (6-12 wk old, 18-22 g). When the tumors reached a size of 100 mm3, the animals were treated as indicated, and the mice were randomly assigned to seven groups (n = 10 each). After ten days of treatment, blood samples were collected from mouse eyes, and serum was harvested by centrifugation. Mice were sacrificed, and the whole body, tumor, spleen and thymus were weighed immediately. The rate of tumor inhibition and organ indexes were calculated. The expression levels of serum cytokines, P-glycoprotein (P-GP) and multidrug resistance (MDR) 1 mRNA in tumor tissues were detected using enzyme-linked immunosorbent assay, Western blotting, and quantitative myeloid-derived suppressor cells reverse transcription-polymerase chain reaction, respectively.
RESULTS: The tumor inhibition rates in the treatment groups of Adriamycin (ADM) + Astragalus polysaccharides (APS) (50 mg/kg), ADM + APS (100 mg/kg), and ADM + APS (200 mg/kg) were significantly higher than in the ADM group (72.88% vs 60.36%, P = 0.013; 73.40% vs 60.36%, P = 0.010; 77.57% vs 60.36%, P = 0.001). The spleen indexes of the above groups were also significantly higher than in the ADM group (0.65 ± 0.22 vs 0.39 ± 0.17, P = 0.023; 0.62 ± 0.34 vs 0.39 ± 0.17, P = 0.022; 0.67 ± 0.20 vs 0.39 ± 0.17, P = 0.012), and the thymus indexes of the ADM + APS (100 mg/kg) and ADM + APS (200 mg/kg) groups were significantly higher than in the ADM group (0.20 ± 0.06 vs 0.13 ± 0.04, P = 0.029; 0.47 ± 0.12 vs 0.13 ± 0.04, P = 0.000). APS was found to exert a synergistic anti-tumor effect with ADM and to alleviate the decrease in the sizes of the spleen and thymus induced by AMD. The expression of interleukin-1α (IL-1α), IL-2, IL-6, and tumor necrosis factor-α (TNF-α) was significantly higher in the ADM + APS (50 mg/kg), ADM + APS (100 mg/kg) and ADM + APS (200 mg/kg) groups than in the ADM group; and IL-10 was significantly lower in the above groups than in the ADM group. APS could increase IL-1α, IL-2, IL-6, and TNF-α expression and decrease IL-10 levels. Compared with the ADM group, APS treatment at a dose of 50-200 mg/kg could down-regulate MDR1 mRNA expression in a dose-dependent manner (0.48 ± 0.13 vs 4.26 ± 1.51, P = 0.000; 0.36 ± 0.03 vs 4.26 ± 1.51, P = 0.000; 0.21 ± 0.04 vs 4.26 ± 1.51, P = 0.000). The expression level of P-GP was significantly lower in the ADM + APS (200 mg/kg) group than in the ADM group (137.35 ± 9.20 mg/kg vs 282.19 ± 20.54 mg/kg, P = 0.023).
CONCLUSION: APS exerts a synergistic anti-tumor effect with ADM in H22 tumor-bearing mice. This may be related to its ability to enhance the expression of IL-1α, IL-2, IL-6, and TNF-α, decrease IL-10, and down-regulate MDR1 mRNA and P-GP expression levels.
Keywords: Astragalus polysaccharides, Tumor inhibition rate, Cytokines, P-glycoprotein, Adjunct anticancer
INTRODUCTION
Cancer has become a major public health problem globally[1]. The World Health Organization predicts that by 2030 an estimated number of 21.4 million new cases of cancer and 13.2 million cancer deaths will occur annually around the world[2]. Surgery, radiotherapy, chemotherapy and endocrine therapy remains the classic cancer therapies[3]. For advanced tumors, chemotherapy is still the treatment of choice, and although these drugs are effective, they are associated with severe adverse events and drug resistance, especially multidrug resistance (MDR)[4].
Severe adverse events affect patients’ compliance. Drug resistance, especially MDR, is the leading cause of treatment failure in cancer therapy. Once the MDR occurs, chemotherapy is no longer effective even with doses of drugs high enough to overcome the resistance; toxic effects are observed and resistance mechanisms can be further stimulated[5]. One of the underlying mechanisms of MDR is cellular overproduction of P-glycoprotein (P-GP) which acts as an efflux pump for various anticancer drugs. P-GP is encoded by the MDR1 gene and its over-expression in cancer cells has become a therapeutic target for circumventing MDR. A potential therapeutic strategy is to co-administer efflux pump inhibitors, although such reversal agents might actually increase the side effects of chemotherapy by blocking physiological anticancer drug efflux from normal cells. Although great efforts have been made to overcome MDR with the first- and second-generation reversal agents available in current clinical use for other indications (e.g., verapamil, cyclosporine A and quinidine) or analogues of the first-generation drugs (e.g., dexverapamil, valspodar and cinchonine), few significant advances have been achieved. Clinical trials with the third-generation modulators (e.g., biricodar, zosuquidar and laniquidar) specifically for MDR reversal are being developed. The results however are not encouraging possibly because that the perfect reverser does not exist[6].
Traditional Chinese medicine (TCM) and herbal medicines in particular have been used in the treatment of cancer for thousands of years in China, Japan, South Korea and other Asian countries. These medicines are widely accepted as current forms of adjuvant therapy in cancer treatment in the United States and Europe[7,8]. TCM has been shown to play an adjunct anticancer role by inducing apoptosis and differentiation, enhancing the immune system, inhibiting angiogenesis and reversing MDR[9]. As adjunct anticancer agents, TCM has great advantages in terms of increasing the sensitivity of chemo-therapeutics, reducing the side effects and complications associated with chemotherapy, and improving patient quality of life and survival time[10]. In the search for new cancer therapeutics with lower toxicity and fewer side effects, TCM has shown promise[11].
The dried root of Astragalus membranaceus has a long history of medicinal use in TCM. Astragalus has demonstrated a wide range of potential therapeutic applications in immunodeficiency syndromes, as an adjunct cancer therapy, and for its adaptogenic effect on the heart and kidneys[12]. Astragalus extract inhibits destruction of gastric cancer cells by mesothelial cells through its anti-apoptosis effects[13]. The active pharmacological constituents of Astragalus membranaceus include various polysaccharides, saponins and flavonoids as well as L-arginine or L-canavanine[14,15]. Among these, Astragalus polysaccharides (APS) have been most widely studied. APS plays its adjunct anticancer role by improving immune function[16-19], counteracting the side effects of chemotherapeutic drugs[12,15,20,21]and increasing the sensitivity of chemo-therapeutics[13,17,18,22-25]. However, the mechanism underlying the adjunct anticancer property of APS, especially whether or not it involves the regulation of cytokines and reversal of MDR, is not completely clear.
Thus, the present study focuses on investigating the effect of APS on the expression of cytokines and P-GP in H22 tumor-bearing mice.
MATERIALS AND METHODS
Main reagents
APS (20 000-60 000 mol/L) was purchased from Shanxi Undersun Biomedtech Co. Ltd., China). Adriamycin (ADM), verapamil, and rifampicin (RFP) were purchased from the National Institutes for Food and Drug Control. Mouse interleukin-1α (IL-1α) enzyme-linked immunosorbent assay (ELISA) kit, mouse IL-2 ELISA kit, mouse IL-6 ELISA kit, mouse IL-10 ELISA kit and mouse tumor necrosis factor-α (TNF-α) ELISA kit, TaKaRaRNA PCRI Kit (AMV) Ver3.0, and TRIZOL reagent were purchased from Sigma Corporation, MO, United States. Goat anti-mouse IgG and fluorescein-affinity pure goat anti-rabbit IgG were purchased from Jackson ImmunoResearch Laboratories. Oligonucleotides and reagents for polymerase chain reaction (PCR) assay were purchased from Sigma Corporation.
Cell lines and culture
H22 hepatoma cells lines (purchased from Beijing Cowin Biotech Co. Ltd., Beijing, China) were cultured in cell culture vessels in vitro. The H22 cells were harvested and inoculated intraperitoneally for 9 d to the eight Kunming mice (KM). To establish the tumor-bearing mouse model, cells with ascites were harvested and inoculated subcutaneously into the right armpit region of of the mice.
Animals and trial groups
Male KM (age, 6-12 wk; weight, 18-22 g) were purchased from the Animal Center of the Third Xiangya Hospital. These animals were maintained at 25 ± 1 °C and 60% ± 5% humidity under a 12 h light-dark cycle. All experimental animals were housed under specific-pathogen-free conditions for 1 wk to get accustomed to the surroundings before initiation of the experiment. They were allowed free access to food and water throughout the study. All experimental protocols described in this study were approved by the Ethics Review Committee for Animal Experimentation of Central South University.
Mice were randomly assigned to one of the seven groups (n = 10 each): ADM group, ADM + RFP group, normal saline (NS) group, ADM + APS (50 mg/kg) group, ADM + APS (100 mg/kg) group, ADM + APS (200 mg/kg) group, ADM + VER group. All the agents were administered by intraperitoneal injection (ip): ADM 1.25 mg/kg, 0.2 mL, qod × 5 d; RFP 40 mg/kg, 0.2 mL, qd × 10 d; NS 0.4 mL, qd × 10 d; APS 50 mg/kg, 100 mg/kg, 200 mg/kg 0.2 mL, qd × 10 d; and VER 1 mg/kg, 0.2 mL, qd × 10 d. After ten days of treatment, the organ indexes and tumor inhibition rate were calculated, and compared with those of control group.
Modeling of the tumor-bearing mice
This model was created by subcutaneous injection of H22 cells as previously described[26-28]. Briefly, the H22 cells with ascites were harvested, diluted to a concentration of 5.0 × 106/mL with sterilized NS, and inoculated subcutaneously into the right armpit region of the mice. Each mouse was weighed immediately after inoculation. During this period, growth rate and tumor size were measured every two days by determining two perpendicular dimensions. Then the volume of each tumor was calculated using the following formula: volume = 1/2 × length × width2[28,29]. When the tumors reached a size of 100 mm3 (excluding maximum and minimum values) the animals were treated as indicated, and were randomly assigned to one of the groups mentioned above. Forty-eight hours after the final administration of tested drug on the 10th day of the experiment, blood samples were collected from the mice’s eyes and serum was harvested by centrifugation. Mice were killed by pulling and breaking of the cervical vertebra, and the whole body, tumor, spleen, and thymus were weighed immediately. Sera were stored at -70 °C and other specimens were stored in liquid nitrogen for further analysis[28,29].
Tumor inhibition rate and immune organ index
The inhibitory effect of experimental treatment on tumor growth was evaluated by tumor inhibition rate, and the influence of different drugs on the immune organs was evaluated by the immune organ index. The inhibitory rates of tumor growth were calculated as follows: inhibitory rate = (1-average tumor weight in the experimental group/average tumor weight of control group) × 100%[28,29]. The organ indexes of spleen and thymus were calculated as follows: organ index (%) = average weight of organ/(average body weight) × 100%.
Measurement of cytokines
The serum levels of cytokines were determined by ELISA according to the manufacturer’s instructions (eBioscience, United States). ELISA kits were employed for the measurement of the levels of IL-1α, IL-2, IL-6, IL-10, and TNF-α.
Western blotting analysis
RFP (P-GP inducer) and VER (P-GP antagonist) were used as positive controls. Western blotting analysis was performed as previously described[30-32]. Briefly, 200 mg of each tumor was frozen with liquid nitrogen and crushed in a mortar. The tumor samples were homogenized in a lysis buffer. Cells were washed twice with ice-cold PBS and total cell lysates were collected in sodium dodecyl sulfate (SDS) sample buffer (50 mmol Tris-HCl, pH 6.8, 100 mmol dithiothreitol (DTT), 2% SDS, 0.1% bromophenol blue, 10% glycerol). Cell lysates containing equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidine difluoride membranes. After blocking in 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20 (pH 7.6), membranes were incubated with the appropriate primary antibodies (goat anti-mouse IgG) at 4 °C, overnight, and exposed to the appropriate secondary antibody (goat anti-rabbit IgG) for 3 h at 37 °C. Immunoreactive proteins were visualized using the enhanced chemiluminescence system from Pierce (Rockford, IL, United States).
Quantitative real time reverse transcription-PCR
The MDR1 mRNA expression level in the H22 tumor-bearing mice was measured using quantitative reverse transcription (QRT)-PCR. Briefly, total RNA was extracted using the TRIzol reagent following the manufacturer’s instructions and reverse transcribed to cDNA using the Gene Amp RNA PCR kit in a DNA thermal cycler (Bio-Rad). QRT-PCR was performed with SYBR green PCR master mix in an ABI Prism 7700 real time PCR machine (Applied Biosystems, Foster City, CA, United States). The synthesized cDNA served as a template in a 25 μL reaction. A non-template control was included in all experiments. Primer sequences were as follows: P-GP GenBank, sense: 5’-TAATGCGACAGG AGATAGGCT-3’, and antisense: 5’-CCGCCATTGA CTGAAAGAACAT-3’; GAPDH GenBank: sense: 5’-GAGTCAACGGA TTTGGTCG-3’, and antisense: 5’-CGGAAGATGGTGATGGGATT-3’. QRT-PCR was performed at 94 °C for 4 min, followed by 40 cycles at 94 °C for 15 s, at 60 °C for 25 s, and at 72 °C for 25 s. Data were analyzed with Sequence Detector software (v1.9, Applied Biosystems, Foster City, CA, United States). The mean Ct value for duplicate measurements was used to detect the expression of target genes, which were normalized to a housekeeping gene, which was used as an internal control [glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] according to the 2-ΔΔCt formula.
Statistical analysis
For statistical analysis of cytotoxicity, results from P-GP and MDR1 mRNA expression assays were analyzed using SPSS 14.0 software (v14, SPSS Inc. Chicago, IL, United States). Differences among the experimental groups were analyzed by one-way analysis of variance. The real-time PCR data were analyzed using the SDS software on the ABI PRISM®7700 sequence detection system at a confidence limit of 95%. A P < 0.05 was considered statistically significant.
RESULTS
General health status of mice
There was no abnormality detected in the daily behavior, autonomic activities, ingestion, hydroposia, pelage, feces, or urine of any of the mice in each experimental group after drug administration. In addition, there was no abnormal secretion from the eyes, ears, noses, or mouths of the mice.
Tumor inhibition rates and the immune organ index
After mice were killed, the solid tumors were removed from tumor-bearing mice and are shown in Figure 1. The tumor inhibition rate and immune organ index are shown in Table 1. The mean weight of tumors in the treatment groups was significantly lower than in the NS group (P < 0.05). The mean weight of tumors in ADM + APS (50 mg/kg), ADM + APS (100 mg/kg), and ADM + APS (200 mg/kg) groups were significantly lower than in the ADM group (P < 0.05). The tumor inhibition rates in these groups were significantly higher than in the ADM group (P < 0.05). The spleen indexes in the ADM + RFP, ADM, and ADM + VER groups were significantly lower than that of the NS group (P < 0.05) and the thymus index of the ADM + APS (200 mg/kg) group was significantly higher than that of the NS group (P < 0.05). The spleen indexes of the ADM + APS (50 mg/kg), ADM + APS (100mg/kg), and ADM + APS (200mg/kg) groups were significantly higher than that of the ADM group (P < 0.05), and the thymus indexes of the ADM + APS (100 mg/kg), ADM + APS (200 mg/kg) group were significantly higher than the ADM group (P < 0.05). Collectively, these results showed that APS can act synergistically with AMD on tumor inhibition and can alleviate the decreased size of the spleen and thymus induced by AMD.
Figure 1.
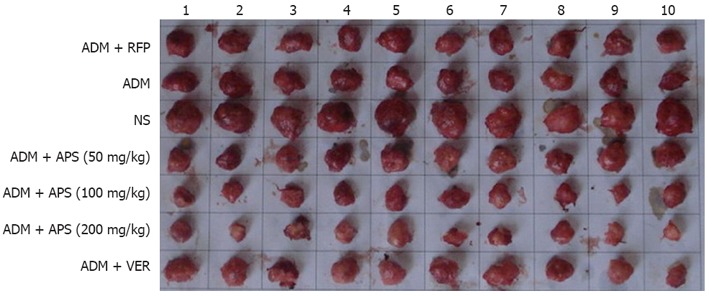
Solid tumors from tumor-bearing mice. ADM: Adriamycin; RFP: Rifampicin; NS: Normal saline; APS: Astragalus polysaccharides; VER: Verapamil.
Table 1.
Tumor inhibition rate and immune organ index (n = 10)
| Group | Tumor weight (g) | Inhibition rate (%) | Spleen index | Thymus index |
| ADM + RFP | 5.37 ± 1.31a | 60.00 | 0.51 ± 0.28a | 0.15 ± 0.04 |
| ADM | 5.32 ± 2.03a | 60.36 | 0.39 ± 0.17a | 0.13 ± 0.04 |
| NS | 13.42 ± 1.03 | - | 0.74 ± 0.21 | 0.15 ± 0.09 |
| ADM + APS (50 mg/kg) | 3.64 ± 1.54ac | 72.88c | 0.65 ± 0.22c | 0.15 ± 0.05 |
| ADM + APS (100 mg/kg) | 3.57 ± 1.66ac | 73.40c | 0.62 ± 0.34c | 0.20 ± 0.06c |
| ADM + APS (200 mg/kg) | 3.01 ± 1.95ac | 77.57c | 0.67 ± 0.20c | 0.47 ± 0.12ac |
| ADM + VER | 4.75 ± 1.86a | 69.45 | 0.50 ± 0.17a | 0.13 ± 0.03 |
P < 0.05 vs normal saline (NS) group;
P < 0.05 vs adriamycin (ADM) group. RFP: Rifampicin; APS: Astragalus polysaccharides; VER: Verapamil.
Expression levels of IL-α, IL-2, IL-6, TNF-α and IL-10
The expression levels of serum cytokines are shown in Table 2. The expression levels of IL-1α, IL-2, IL-6, and TNF-α were significantly higher in the ADM + APS (50 mg/kg), ADM + APS (100 mg/kg), and ADM + APS (200 mg/kg) groups than in the NS group (P < 0.05), particularly in the medium- and high-dose groups. Cytokine levels were lower in the ADM group than in the NS group. The expression levels of IL-1α, IL-2, IL-6, and TNF-α were also significantly higher (P < 0.05) in the ADM + APS (50 mg/kg), ADM + APS (100 mg/kg) and ADM + APS (200 mg/kg) groups than in the ADM group. The expression level of IL-10 was significantly lower (P < 0.05) in the ADM + APS (50 mg/kg), ADM + APS (100 mg/kg), and ADM + APS (200 mg/kg) groups than in either the NS or ADM groups. In summary, APS increased expression levels of IL-1α, IL-2, IL-6, and TNF-α and decreased IL-10 levels.
Table 2.
Expression of interleukin-1α, interleukin-2, interleukin-6, tumor necrosis factor-α, and interleukin-10 (n = 10)
| Group |
mean ± SD (pg/mL) |
||||
| IL-1α | IL-2 | IL-6 | TNF-α | IL-10 | |
| ADM + RFP | 5.89 ± 2.12 | 6.11 ± 2.9a | 10.99 ± 2.09a | 9.00 ± 1.21 | 55.98 ± 2.43 |
| ADM | 4.63 ± 3.2 | 5.23 ± 2.12a | 10.78 ± 3.13a | 8.01 ± 1.22 | 54.01 ± 2.33 |
| NS | 13.21 ± 2.01c | 11.35 ± 2.09c | 29.55 ± 8.97c | 23.1 ± 1.83c | 56.67 ± 4.32 |
| ADM + APS (50 mg/kg) | 14.34 ± 1.78c | 12.11 ± 3.08c | 41.57 ± 6.42ac | 29.97 ± 4.09c | 41.23 ± 3.12ac |
| ADM + APS (100 mg/kg) | 25.31 ± 2.98ac | 19.98 ± 3.21ac | 38.82 ± 5.88ac | 33.23 ± 3.99ac | 27.23 ± 7.68ac |
| ADM + APS (200 mg/kg) | 22.45 ± 4.01ac | 26.67 ± 7.21ac | 40.99 ± 5.54ac | 44.78 ± 3.98ac | 24.87 ± 5.78ac |
| ADM + VER | 7.34 ± 2.11 | 5.77 ± 2.11 | 9.34 ± 1.75a | 9.11 ± 2.01 | 50.66 ± 1.12 |
P < 0.05 vs normal saline (NS) group;
P < 0.05 vs adriamycin (ADM) group. IL: Interleukin; TNF-α: Tumor necrosis factor-α; RFP: Rifampicin; APS: Astragalus polysaccharides; VER: Verapamil.
Expression of P-GP in tumor tissue
The expression of P-GP in tumor tissue is shown in Figure 2. As assessed by computer-assisted gel analysis (Figure 3A), the ADM + RFP group exhibited a significantly higher P-GP expression than other groups. Different concentrations of APS and verapamil were found to down-regulate P-GP expression. APS down-regulated less P-GP expression than verapamil did. Also, APS (50-200 mg/kg) was found to down-regulate P-GP expression in a dose-dependent manner. The expression level of P-GP was significantly lower (P < 0.05) in the ADM + APS (200 mg/kg) and verapamil groups than in the ADM or ADM + RFP groups.
Figure 2.
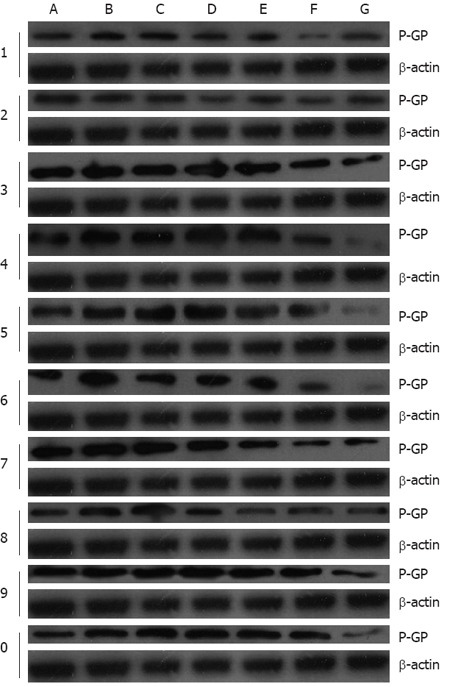
P-glycoprotein expression in tumor tissues after different chemotherapeutic treatment. A: Normal saline group (No.1-5); B: Adriamycin (ADM) group (No.1-5); C: ADM + rifampicin (RFP) group (No.1-5); D: ADM + astragalus polysaccharides (APS) (50 mg/kg) group (No.1-5); E: ADM + APS (100 mg/kg) group (No.1-5); F: ADM + APS (200 mg/kg) group (No.1-5); G: ADM + verapamil (VER) group (No.1-5); H: NS group (No. 6-10); I: ADM group (No. 6-10); J: ADM + RFP group (No. 6-10); K: ADM + APS (50 mg/kg) group (No. 6-10); L: ADM + APS (100 mg/kg) group (No. 6-10); M: ADM + APS (200 mg/kg) group (No. 6-10); N: ADM + VER group (No. 6-10). P-GP: P-glycoprotein.
Figure 3.
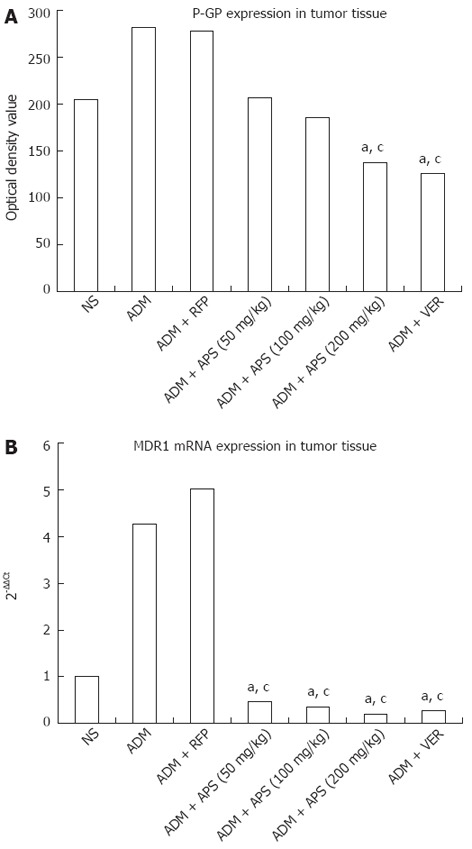
P-glycoprotein optical density values and MDR1 mRNA expression in tumor tissues after different chemotherapeutic treatment. A: P-glycoprotein (P-GP) optical density values; B: MDR1 mRNA expression. aP < 0.05 vs adriamycin (ADM) group; cP < 0.05 vs ADM + rifampicin (RFP) group. NS: Normal saline; APS: Astragalus polysaccharides; VER: Verapamil.
QRT-PCR detection of multidrug resistance 1 mRNA in tumor tissue
The expression of MDR1 mRNA in tumor tissue is shown in Table 3 and Figure 3B. The ADM and ADM + RFP group showed significantly higher MDR1 mRNA expression than the other groups (P < 0.05). Compared to the ADM group, the RFP group showed increased MDR1 mRNA expression, and the VER group and APS groups showed decreased MDR1 mRNA expression (P < 0.05). MDR1 mRNA expression decreased with increasing concentrations of APS (50-200 mg/kg). MDR1 mRNA expression was reduced in a dose-dependent manner.
Table 3.
Multidrug resistance 1 mRNA expression in tumor tissue (2-∆∆Ct) (n = 10)
| Group | 2-∆∆Ct ( mean ± SD ) |
| ADM + RFP | 5.02 ± 1.82 |
| ADM | 4.26 ± 1.51 |
| NS | 1.02 ± 0.05ac |
| ADM + APS (50 mg/kg) | 0.48 ± 0.13ac |
| ADM + APS (100 mg/kg) | 0.36 ± 0.03ac |
| ADM + APS (200 mg/kg) | 0.21 ± 0.04ac |
| ADM + VER | 0.28 ± 0.09ac |
P < 0.05 vs adriamycin (ADM) group;
P < 0.05 vs ADM + rifampicin (RFP) group. NS: Normal saline; APS: Astragalus polysaccharides; VER: Verapamil.
DISCUSSION
Guo et al[24] reported that treatment with APS along with vinorelbine and cisplatin significantly improves quality of life in patients with advanced non-small-cell lung cancer over vinorelbine and cisplatin alone. Cui et al[22] reported that hepatocarcinogenesis could be prevented in rats fed with the aqueous extract of Astragalus, which is mainly composed of Astragalus polysaccharides. There are also reports that APS act as an adjunct anticancer agent[13,18,23,25].
These results led the researchers to speculate that the adjunct anticancer role of APS might be related to immune function enhancement. However, the mechanism underlying these effects remains to be determined. In particular, it is not completely clear whether APS is involved in the regulation of cytokines and reversal of MDR.
Our conclusions regarding the sensitivity to chemotherapy drugs were partially supported by the results of these studies. Compared with the ADM group, the mean weight of tumors was significantly decreased (P < 0.05) and the inhibition rates of tumors were significantly increased (P < 0.05) within the APS treatment range of 50 to 200 mg/kg. The spleen and thymus index were also significantly increased. Collectively, these results show that APS exerts a synergistic anti-tumor effect with AMD, and alleviates the decreased sizes of the spleen and thymus induced by AMD (Table 1). The cytokines IL-1α, IL-2 and IL-6 are capable of inducing the proliferation of responsive T-cells. TNF-α has been proven to be an effective anticancer agent in in vitro and in vivo preclinical studies, by inducing apoptotic cell death and tumor necrosis. IL-10 inhibits the synthesis of IL-2 and TNF-α produced by activated macrophages and by helper T cells[33]. In the present study, APS was found to induce increase in IL-1α, IL-2, IL-6, and TNF-α expression and decrease in IL-10 expression (Table 2). APS effect on cytokine levels may be one of its adjunct anticancer mechanisms.
However, tumor immunology is a complex biological phenomenon, with the secretion, function and regulation of cytokines occurring through multiple mechanisms, and is mediated by a wide variety of cell populations. How APS induces expression of these cytokines merits further study.
It has been shown that drug resistance in tumor cells are related to MDR1 upregulation and P-GP over expression[34-36]. As a P-GP substrate, ADM can induce P-GP expression and consequently reduce its efficacy. In the present study, APS was found to enhance the chemo-sensitivity to ADM of H22 tumor-bearing mice. To determine whether APS is involved in P-GP expression, the P-GP inducer rifampin and P-GP inhibitor verapamil were used as positive controls. Western blotting analysis of P-GP expression and real-time PCR detection of MDR1 mRNA expression in tumor tissue revealed that APS (50 to 200 mg/kg) reduced P-GP protein and MDR1 mRNA expression in a dose-dependent manner (Figures 2, 3 and Table 3). The expression levels of P-GP and MDR1 were significantly decreased (P < 0.05) in the ADM + APS (200 mg/kg) treatment compared with the ADM or ADM + RFP treatment groups (P < 0.05). In the present study, APS was found to down-regulate MDR1 mRNA and P-GP expression levels, thereby increasing the intracellular concentration of chemotherapeutic drugs. This may be the mechanism behind its secondary anti-cancer effects.
It has been reported that APS exhibits several therapeutic advantages in terms of increasing sensitivity to chemo-therapeutics, reducing the side effects and complications associated with chemotherapy[12,15,20,21], and improving patient quality of life and survival time[24]. In addition, our present study reveals that APS can regulate cytokine and P-GP expression levels. Thus, APS is a promising candidate for therapeutics that exhibit a low toxicity and few side effects.
In summary, APS was found to exert a synergistic anti-tumor effect with Adriamycin in H22 tumor-bearing mice in vivo. This may be related to its ability to enhance the expression of IL-1α, IL-2, IL-6, and TNF-α, decrease IL-10, and down-regulate MDR1 mRNA and P-GP expression levels.
COMMENTS
Background
Cancer has been a major public health problem globally. Traditional Chinese medicine has great advantages in terms of increasing sensitivity to chemo-therapeutics, reducing side effects and complications associated with chemotherapy, and improving patient quality of life and survival. Astragalus membranaceus is widely accepted as a complementary and alternative medicine in cancer treatment. Astragalus polysaccharides (APS) are active constituents of Astragalus membranaceus.
Research frontiers
APS has been most widely studied mainly for its immunopotentiating properties, ability to counteract the side effects of chemotherapeutic drugs, and adjunct anticancer agent properties. However, the mechanism underlying the adjunct anticancer property of APS, specifically whether or not it involves the regulation of cytokines and reversal of multidrug resistance (MDR), is not completely clear. The present study focused on investigating the effect of APS on the expression of cytokines and P-glycoprotein (P-GP) in H22 tumor-bearing mice in vivo.
Innovations and breakthroughs
To date, there have been a limited number of studies regarding the adjunct anticancer property of APS. In the present study, APS was found to exert a synergistic anti-tumor effect with Adriamycin in H22 tumor-bearing mice. This may be related to its ability to enhance the expression of interleukin-1 α (IL-1α), IL-2, IL-6, and tumor necrosis factor-α, decrease IL-10, and down-regulate MDR1 mRNA and P-GP expression. This is the first study to report that the adjunct anticancer property of APS is partly through regulation of cytokines and P-GP expression in H22 tumor-bearing mice.
Applications
Traditional Chinese medicine has great advantages in terms of increasing sensitivity to chemo-therapeutics, reducing side effects and complications associated with chemotherapy, and improving patient quality of life and survival. APS could regulate cytokines and P-GP expression, and in the search for new cancer therapeutics with a lower toxicity and few side effects, APS is a promising candidate.
Terminology
APS, extracted and purified from Chinese medicinal herb Astragalus membranaceus roots, consist of several classes of neutral and acidic polysaccharides and glycoproteins. APS has also been observed to possess anti-tumor and anti-virus activities. Cytokine is a small protein released by cells and can exert a specific effect on the interactions between cells, on communications between cells or on the behavior of cells. P-GP is a plasma membrane protein which acts as a localized drug transport, actively exporting drugs out of the cell. Adjunct anticancer agents are a variety of medications that complement the chemotherapy regimen, help alleviate side effects of the treatment or symptoms of the cancer, or help potentiate the effect and activity of chemotherapy drugs.
Peer review
The manuscript reports on a possible adjunct anticancer effect of APS in tumor-bearing mice, using an in vivo animal model. Data on tumor inhibition were provided, and its possible effects on cytokines, MDR1 mRNA and P-GP expression were elucidated. APS may be a promising candidate for cancer therapeutics that exhibits a low toxicity and few side effects.
Footnotes
Peer reviewer: Mara Massimi, PhD, Department of Basic and Applied Biology, University of L’Aquila, Via Vetoio-Coppito, 67100 L’Aquila, Italy
S- Editor Gou SX L- Editor Ma JY E- Editor Zhang DN
References
- 1.Moving cancer up the global health agenda. Lancet. 2010;375:2051. doi: 10.1016/S0140-6736(10)60942-7. [DOI] [PubMed] [Google Scholar]
- 2.Kawahara N, Masui T, Roh JK, Hao XS, Hill D, Akaza H. What should we do to raise awareness on the issue of cancer in the global health agenda? Jpn J Clin Oncol. 2010;40 Suppl 1:i82–i85. doi: 10.1093/jjco/hyq132. [DOI] [PubMed] [Google Scholar]
- 3.Urruticoechea A, Alemany R, Balart J, Villanueva A, Viñals F, Capellá G. Recent advances in cancer therapy: an overview. Curr Pharm Des. 2010;16:3–10. doi: 10.2174/138161210789941847. [DOI] [PubMed] [Google Scholar]
- 4.Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199–334. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 5.Choi CH. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005;5:30. doi: 10.1186/1475-2867-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobili S, Landini I, Giglioni B, Mini E. Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets. 2006;7:861–879. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- 7.Wong R, Sagar CM, Sagar SM. Integration of Chinese medicine into supportive cancer care: a modern role for an ancient tradition. Cancer Treat Rev. 2001;27:235–246. doi: 10.1053/ctrv.2001.0227. [DOI] [PubMed] [Google Scholar]
- 8.Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo N, Li XK, Tang W. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends. 2010;4:297–307. [PubMed] [Google Scholar]
- 9.Ruan WJ, Lai MD, Zhou JG. Anticancer effects of Chinese herbal medicine, science or myth? J Zhejiang Univ Sci B. 2006;7:1006–1014. doi: 10.1631/jzus.2006.B1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konkimalla VB, Efferth T. Evidence-based Chinese medicine for cancer therapy. J Ethnopharmacol. 2008;116:207–210. doi: 10.1016/j.jep.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Ji DB, Ye J, Jiang YM, Qian BW. Anti-tumor effect of Liqi, a traditional Chinese medicine prescription, in tumor bearing mice. BMC Complement Altern Med. 2009;9:20. doi: 10.1186/1472-6882-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astragalus membranaceus. Monograph. Altern Med Rev. 2003;8:72–77. [PubMed] [Google Scholar]
- 13.Na D, Liu FN, Miao ZF, Du ZM, Xu HM. Astragalus extract inhibits destruction of gastric cancer cells to mesothelial cells by anti-apoptosis. World J Gastroenterol. 2009;15:570–577. doi: 10.3748/wjg.15.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittenhouse JR, Lui PD, Lau BH. Chinese medicinal herbs reverse macrophage suppression induced by urological tumors. J Urol. 1991;146:486–490. doi: 10.1016/s0022-5347(17)37830-8. [DOI] [PubMed] [Google Scholar]
- 15.Ma XQ, Shi Q, Duan JA, Dong TT, Tsim KW. Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. J Agric Food Chem. 2002;50:4861–4866. doi: 10.1021/jf0202279. [DOI] [PubMed] [Google Scholar]
- 16.Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Bao Y, Lam W, Li W, Lu F, Zhu X, Liu J, Wang H. Immunoregulatory and anti-tumor effects of polysaccharopeptide and Astragalus polysaccharides on tumor-bearing mice. Immunopharmacol Immunotoxicol. 2008;30:771–782. doi: 10.1080/08923970802279183. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Yang Y, Zhang X, Xu S, He S, Huang W, Roberts MS. Compound Astragalus and Salvia miltiorrhiza extract inhibits cell invasion by modulating transforming growth factor-beta/Smad in HepG2 cell. J Gastroenterol Hepatol. 2010;25:420–426. doi: 10.1111/j.1440-1746.2009.05981.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhao LH, Ma ZX, Zhu J, Yu XH, Weng DP. Characterization of polysaccharide from Astragalus radix as the macrophage stimulator. Cell Immunol. 2011;271:329–334. doi: 10.1016/j.cellimm.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Tin MM, Cho CH, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis. 2007;28:1347–1355. doi: 10.1093/carcin/bgl238. [DOI] [PubMed] [Google Scholar]
- 21.Zee-Cheng RK. Shi-quan-da-bu-tang (ten significant tonic decoction), SQT. A potent Chinese biological response modifier in cancer immunotherapy, potentiation and detoxification of anticancer drugs. Methods Find Exp Clin Pharmacol. 1992;14:725–736. [PubMed] [Google Scholar]
- 22.Cui R, He J, Wang B, Zhang F, Chen G, Yin S, Shen H. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother Pharmacol. 2003;51:75–80. doi: 10.1007/s00280-002-0532-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Ito H, Shimura K. Enhancing effect of antitumor polysaccharide from Astragalus or Radix hedysarum on C3 cleavage production of macrophages in mice. Jpn J Pharmacol. 1989;51:432–434. doi: 10.1254/jjp.51.432. [DOI] [PubMed] [Google Scholar]
- 24.Guo L, Bai SP, Zhao L, Wang XH. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: effects on quality of life and survival. Med Oncol. 2012;29:1656–1662. doi: 10.1007/s12032-011-0068-9. [DOI] [PubMed] [Google Scholar]
- 25.Liu XN, Zhang CY, Jin XD, Li YZ, Zheng XZ, Li L. Inhibitory effect of schisandrin B on gastric cancer cells in vitro. World J Gastroenterol. 2007;13:6506–6511. doi: 10.3748/wjg.v13.i48.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiaoguang C, Hongyan L, Xiaohong L, Zhaodi F, Yan L, Lihua T, Rui H. Cancer chemopreventive and therapeutic activities of red ginseng. J Ethnopharmacol. 1998;60:71–78. doi: 10.1016/s0378-8741(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 27.Gao L, Chen L, Fei XH, Qiu HY, Zhou H, Wang JM. STI571 combined with vincristine greatly suppressed the tumor formation of multidrug-resistant K562 cells in a human-nude mice xenograft model. Chin Med J (Engl) 2006;119:911–918. [PubMed] [Google Scholar]
- 28.Jin Y, Li J, Rong LF, Li YH, Guo L, Xu SY. Anti-hepatocarcinoma effects of 5-fluorouracil encapsulated by galactosylceramide liposomes in vivo and in vitro. World J Gastroenterol. 2005;11:2643–2646. doi: 10.3748/wjg.v11.i17.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Takahashi S, Imamura M, Okutani E, Zhang ZG, Chayama K, Chen BA. Earthworm fibrinolytic enzyme: anti-tumor activity on human hepatoma cells in vitro and in vivo. Chin Med J (Engl) 2007;120:898–904. [PubMed] [Google Scholar]
- 30.Mi Y, Lou L. ZD6474 reverses multidrug resistance by directly inhibiting the function of P-glycoprotein. Br J Cancer. 2007;97:934–940. doi: 10.1038/sj.bjc.6603985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Stevenson GD, Barrett HH, Furenlid LR, Wilson DW, Kastis GA, Bettan M, Woolfenden JM. Imaging recognition of inhibition of multidrug resistance in human breast cancer xenografts using 99mTc-labeled sestamibi and tetrofosmin. Nucl Med Biol. 2005;32:573–583. doi: 10.1016/j.nucmedbio.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding L, Chen XP, Zhang ZW, Jing K, Zhang WG. Human multi-drug resistant hepatocellular carcinoma induced in nude mice by B-ultrasonographically-directed orthotopic implantation: a new experimental model. Hepatobiliary Pancreat Dis Int. 2007;6:393–398. [PubMed] [Google Scholar]
- 33.Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. Scientific World J. 2011;11:2391–2402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dizdarevic S, Peters AM. Imaging of multidrug resistance in cancer. Cancer Imaging. 2011;11:1–8. doi: 10.1102/1470-7330.2011.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goda K, Bacsó Z, Szabó G. Multidrug resistance through the spectacle of P-glycoprotein. Curr Cancer Drug Targets. 2009;9:281–297. doi: 10.2174/156800909788166493. [DOI] [PubMed] [Google Scholar]
- 36.Mayur YC, Peters GJ, Prasad VV, Lemo C, Sathish NK. Design of new drug molecules to be used in reversing multidrug resistance in cancer cells. Curr Cancer Drug Targets. 2009;9:298–306. doi: 10.2174/156800909788166619. [DOI] [PubMed] [Google Scholar]


