Abstract
AIM: To investigate the associations between interleukin (IL)-1B and IL-1RN polymorphisms and gastric cancers among the Tibet, Hui and Han ethnicities.
METHODS: Genomic DNA was extracted from peripheral blood of 210, 205, and 202 healthy volunteers and from 155, 158, and 197 gastric cancer patients from the Tibet, Hui, and Han populations, respectively. Polymorphisms in IL-1B and IL-1RN were analyzed by denaturing high-performance liquid chromatography.
RESULTS: Carriers of the IL-1B-31 CC genotype had an increased risk of intestinal type gastric cancer [odds ratio (OR) = 2.17, P = 0.037] in the Tibet ethnicity. Carriers of the IL-1B 2/L genotype had an increased risk of both intestinal and diffuse types of gastric cancer (OR = 2.08, 2.31, P = 0.007, 0.016, respectively) in the Hui ethnicity. In the Han population, carriers of the IL-1B-31 CC, IL-1B-511CT, TT genotypes had increased risk of intestinal type gastric cancer (OR = 2.51, 2.74, 5.66, P = 0.005, 0.002, 0.000, respectively).
CONCLUSION: IL-1B and IL-RN genotypes may differentially contribute to gastric cancer among the Tibet, Hui, and Han ethnicities in the Qinghai area of China.
Keywords: Gastric cancer, Interleukin-1B, Interleukin-1RN, Polymorphism, Risk of gastric cancer
INTRODUCTION
Gastric cancer (GC) is the second leading cause of cancer-related death worldwide, and approximately 42% of these deaths occur in China[1,2]. Mortality of GC patients in China is the highest in the world, especially in the northwestern part of the country, which includes Qinghai province[3]. There are 56 different ethnicities living in China. The Han ethnicity represents the major ethnicity within Qinghai province, while the Tibet and Hui are minority nationalities. The incidence of GC in the Tibet and Hui populations is higher than that in the Han ethnicity. However, the study of minority ethnicities is not advanced due to fewer individuals and poorer economic conditions[4].
Interleukin (IL)-1B and IL-1RN belong to the IL-1 gene cluster. The IL-1 gene encodes both the glycoprotein IL-1β, which is a pro-inflammatory cytokine, and the IL-1 receptor antagonist (IL-1Ra), which is an anti-inflammatory cytokine. IL-1B is a potent inhibitor of gastric acid secretion and plays a major role in both initiating and amplifying the inflammatory response to Helicobacter pylori (H. pylori) infection[5-7]. IL-1RN encodes the IL-1Ra, an anti-inflammatory cytokine that competitively binds to IL-1 receptors and modulates the potentially damaging effects of IL-1[7,8]. Two biallelic polymorphisms in the IL-1B gene have been described, both C-T base transitions found at positions-511 (C>T) and -31 (T>C) bp from the translation initiation codon. IL-1RN has a variable number of identical tandem repeats polymorphism of 86 bp in intron 2. To date, over 50 studies have reported on the association between IL-1B and IL-1RN polymorphisms and GC risk[5]. While some studies have reported that IL-1B and IL-1RN polymorphisms are associated with increased GC risk in both Caucasians and Asians[5,6,9,10], other studies have shown inverse associations, especially in Asians[11,12]. Two studies chose a single ethnicity population in two different regions with different prevalence rates of GC as their subjects[10,13]. However, no study has examined several different ethnicities in a single geographical area at the same time. Thus, our study is the first to report such an examination.
Here, we investigated the associations between IL-1B-31, IL-1B-511 and IL-1RN polymorphisms and GC risk among the Tibet, Hui and Han ethnicities in the Qinghai area of China.
MATERIALS AND METHODS
Study subjects
Three ethnicities (Tibet, Hui and Han) from the Qinghai province of China were included in this study. Healthy controls included 210 Tibet, 205 Hui and 202 Han individuals who were enrolled from the Hainan Tibet Ethnicity Autonomous Prefecture, Minhe Hui Ethnicity Autonomous County, and Xining city in Qinghai province, respectively. Between December 2008 and October 2011, 155, 158 and 197 Tibet, Hui and Han individuals, respectively, with GC were enrolled from the Affiliated Hospital of Qinghai University. All recruited healthy controls were from families that had lived for a long time in that locality, did not marry other ethnicities for at least three generations, and were not related to each other. Both the age and sex of the healthy controls were matched to the patients and are shown in Table 1. None of these subjects had a history of systemic lupus erythematosus, diabetes mellitus, rheumatoid arthritis, or inflammatory bowel disease. Subjects with a family history of any cancer were excluded. All patients were histologically confirmed as having noncardiac GC. Patients and controls were interviewed with regard to smoking status. Individuals who smoked once a day for over 1 year were defined as smokers. The presence of H. pylori infection in the sera of patients and controls was measured using an enzyme-linked immunosorbent assay (Anti-H. pylori enzyme immunoassay, Huamei Biotech Inc., China). This study was approved by the Clinical Research Ethics Committee of the Qinghai University of Medical Sciences, and all patients provided signed informed consent.
Table 1.
Primer sequences, polymerase chain reaction and denaturing high-performance liquid chromatography conditions for detection of gene polymorphisms
| Gene | Primer sequence | PCR annealing temperature (°C) | PCR product size (bp) | DHPLC application type | Oven temperature (°C) |
| IL-1B-31 | F: AGAAGCTTCCACCAATACTC | 60 | 240 | Mutation | 59 |
| R: AGCACCTAGTTGTAAGGAAG | |||||
| IL-1B-511 | F: TGGCATTGATCTGGTTCATC | 58.5 | 306 | Mutation | 60.5 |
| R: GTTTAGGAATCTTCCCACTT | |||||
| IL-1 RN | F: CCCCTCGAGCAACATCC | 59 | 270-442 | VNTR | 50.0 |
| R: GGTCAGAAGGGCAGAGA |
DHPLC: Denaturing high-performance liquid chromatography; VNTR: Variable number of identical tandem repeats; PCR: Polymerase chain reaction; IL: Interleukin.
Analysis of the IL-1B-31, IL-1B-511 and IL1-RN polymorphisms
Genomic DNA was isolated from 5 mL of venous blood by the conventional proteinase K digestion and phenol/chloroform extraction method. Polymorphisms were analyzed by polymerase chain reaction (PCR)-based denaturing high-performance liquid chromatography (DHPLC). The corresponding primers have been by described by Lu et al[14] and are shown together with the PCR conditions, PCR annealing temperatures and DHPLC detection methods in Table 2. PCR was performed with a 25 mL reaction mixture containing 100 ng of genomic DNA, 1.0 mmol/L of primer, 0.2 mmol/L of dNTP, 2.0 mmol/L of MgCl2, and 1.0 U Taq DNA polymerase in 1× reaction buffer (Promega, Madison, WI, United States). DHPLC analysis was performed on a Transgenomic WAVE System. The detailed genotyping process has been previously described[14]. The PCR products were applied to the DHPLC column at an optimal oven temperature and eluted with a linear acetonitrile gradient at a flow rate of 0.9 mL/min (Figure 1A). The genotypes identified by DHPLC analysis were further confirmed by DNA sequencing using the ABI Prism 377 DNA Sequencer. The sizes of IL-1RN PCR products were analyzed by DHPLC based on the relationship between elution time and base pair number of the fragment (Figure 1B).
Table 2.
Selective characteristics and risk factors in patients with gastric cancer and controls from the Tibet, Hui and Han ethnicities n (%)
| Variable |
Tibet |
Hui |
Han |
||||||||||
| Cases (n = 155) | Controls (n = 210) | χ2 | P value | Cases (n = 158) | Controls (n = 205) | χ2 | P value | Cases (n = 197) | Controls (n = 202) | χ2 | P value | ||
| Age, yr | < 35 | 3 (1.94) | 5 (2.38) | 0.006 | 0.940 | 2 (1.27) | 2 (0.980) | 0.069 | 0.793 | 5 (2.54) | 5 (2.48) | 0.602 | 0.437 |
| 35-60 | 82 (52.90) | 110 (52.38) | 0.010 | 0.921 | 75 (47.46) | 102 (49.76) | 0.187 | 0.665 | 98 (49.75) | 106 (52.48) | 0.297 | 0.586 | |
| ≥ 60 | 70 (45.16) | 95 (45.24) | 0.000 | 0.988 | 81 (51.27) | 101 (49.24) | 0.142 | 0.706 | 94 (47.71) | 91 (45.04) | 0.285 | 0.593 | |
| Gender | Male | 116 (74.84) | 154 (73.33) | 0.105 | 0.746 | 116 (73.42) | 148 (71.20) | 0.067 | 0.795 | 146 (74.62) | 151 (74.75) | 0.003 | 0.952 |
| Female | 39 (25.16) | 56 (26.67) | 42 (26.58) | 57 (28.80) | 50 (25.38) | 51 (25.25) | |||||||
| Smoking | Yes | 99 (63.87) | 135 (64.29) | 0.007 | 0.935 | 28 (17.71) | 40 (19.51) | 0.188 | 0.665 | 131 (66.50) | 129 (63.86) | 0.200 | 0.655 |
| No | 56 (36.13) | 75 (35.71) | 130 (82.29) | 165 (80.49) | 66 (33.50) | 73 (36.14) | |||||||
| Hp | Positive | 101 (65.16) | 112 (53.33) | 5.134 | 0.023 | 100 (63.29) | 95 (46.34) | 10.311 | 0.001 | 124 (62.94) | 105 (51.98) | 4.903 | 0.027 |
| Negative | 54 (34.84) | 98 (46.67) | 58 (36.71) | 110 (53.66) | 73 (37.06) | 97 (48.02) | |||||||
Figure 1.
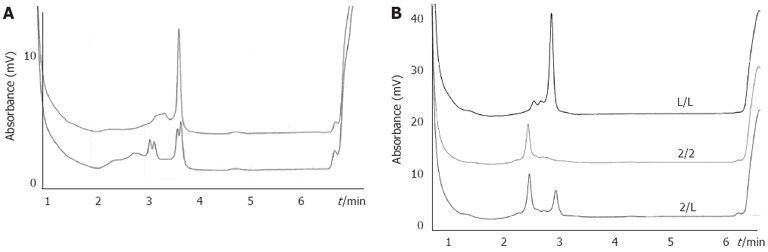
Typical denaturing high-performance liquid chromatography elution profiles for different genotypes. A: Representative denaturing high-performance liquid chromatography (DHPLC) profiles for different allelic polymerase chain reaction products containing the interleukin-1B (IL-1B)-311 C/T polymorphism site. In the first DHPLC, the CT genotype (lower panel) was discriminated from homozygous (upper panel). To determine the CC or TT genotype, the second DHPLC was run for the homozygous DNA mixed with a DNA sample known as the CC genotype. The profile of the CC genotype was unaltered, while that of the TT genotype changed into the same as the lower panel; B: DHPLC elution profiles of IL-1RN and IL-1RN variable number of identical tandem repeats were determined by elution time and base pair number of the fragment.
H. pylori antibody assays
Enzyme-linked immunosorbent assay for detection of H. pylori was performed according to the manufacturer’s instructions. After termination of the enzyme reaction, the absorbance at 630 nm was measured. Absorbance ratios (sample/negative control) equal to or greater than 2.1 were considered positive, and those below 2.1 were considered negative.
Statistical analysis
The data were analyzed using SPSS software (Version 13.0, SPSS, Chicago, IL, United States). The significance of the difference in the distribution of the polymorphisms among the different groups was calculated using the χ2 test. All allelic distributions were examined for deviations from their corresponding Hardy-Weinberg equilibrium. Multivariate logistic regression was used to obtain odds ratios (ORs) and 95%CI, adjusting for age, sex, smoking status and H. pylori infection. P < 0.05 (two-tailed) was considered statistically significant.
RESULTS
Clinical characteristics
The study population consisted of 210, 205 and 202 healthy controls and 155, 158 and 197 GC patients from the Tibet, Hui and Han ethnicities, respectively. Age, sex, smoking status, and H. pylori infection in the GC patients and control subjects are shown in Table 1. There were no statistically significant differences between the cases and controls with regard to age, sex, and smoking status in each ethnicity group. However, H. pylori infection was significantly higher in the cases compared to the controls (P = 0.023, 0.001 and 0.027, respectively) in each ethnicity group. The genotype frequencies of IL-1B-31, IL-1B-511 and IL-1RN in the controls in each ethnicity group were in agreement with the Hardy-Weinberg equilibrium (P > 0.05 for all).
IL-1B-31, IL-1B-511 and IL-1RN polymorphisms and GC risk
The frequencies of genotypes IL-1B-31, IL-1B-511 and IL-1RN among the Tibet, Hui and Han ethnicities are summarized in Table 3. In the Tibet ethnicity group, the IL-1B-31 CC genotype was significantly more frequent in GC patients (32.90%) compared with controls (24.29%) (χ2 = 4.98, P = 0.026). The risk of developing gastric cancer with this genotype was significantly increased (adjusted OR = 2.10, 95%CI: 1.09-4.04, P = 0.027).
Table 3.
Genotype distributions of interleukin-1B-31, interleukin-1B-511 and interleukin-1RN gene polymorphisms among gastric cancer cases and controls from the Tibet, Hui and Han ethnicities n (%)
| Genotype |
Tibet |
Hui |
Han |
|||||||||
| Cases | Controls | OR (95%CI)1 | P value | Cases | Controls | OR (95%CI)1 | P value | Cases | Controls | OR (95%CI)1 | P value | |
| IL-1B-31 | ||||||||||||
| TT | 23 (14.84) | 47 (22.38) | 1 | 46 (29.11) | 59 (28.78) | 1 | 40 (20.30) | 65 (32.18) | 1 | |||
| CT | 81 (52.26) | 112 (53.33) | 1.47 (0.81-2.66) | 0.208 | 79 (50.00) | 105 (51.22 | 0.97 (0.58-1.62) | 0.896 | 102 (51.77) | 98 (48.52) | 1.69 (1.03-2.76) | 0.036 |
| CC | 51 (32.90) | 51 (24.29) | 2.10 (1.09-4.04) | 0.027 | 33 (20.89) | 41 (20.00) | 1.03 (0.55-1.95) | 0.919 | 55 (27.92) | 39 (19.31) | 2.29 (1.28-4.10) | 0.005 |
| IL-1B-511 | ||||||||||||
| CC | 34 (21.94) | 55 (26.19) | 1 | 33 (20.89) | 43 (20.98) | 1 | 31 (15.74) | 65 (32.17) | 1 | |||
| CT | 80 (51.61) | 93 (44.29) | 1.37 (0.80-2.35) | 0.210 | 88 (55.70) | 110 (53.66) | 1.04 (0.59-1.83) | 0.888 | 101 (51.27) | 99 (49.09) | 2.16 (1.28-3.65) | 0.004 |
| TT | 41 (26.45) | 62 (29.52) | 1.02 (0.56-1.86) | 0.945 | 37 (23.41) | 52 (25.37) | 0.96 (0.50-1.84) | 0.891 | 65 (32.99) | 38 (18.81) | 3.53 (1.49-8.33) | 0.004 |
| IL-1RN | ||||||||||||
| L/L | 129 (83.22) | 185 (88.10) | 1 | 94 (59.49) | 156 (76.10) | 1 | 166 (84.26) | 171 (84.66) | 1 | |||
| 2/L | 26 (16.78) | 25 (11.90) | 1.50 (0.80-2.79) | 0.206 | 64 (40.50) | 49 (23.90) | 2.11 (1.31-3.40) | 0.002 | 31 (15.28) | 31 (14.44) | 1.03 (0.59-1.79) | 0.924 |
Adjusted for age, sex, smoking status and Helicobacter pylori infection. IL: Interleukin; OR: Odds ratio.
In the Hui nationality group, the IL-1RN L/2 genotype was significantly more frequent in GC patients (40.50%) compared with controls (23.90%) (χ2 = 11.47, P = 0.001). The risk of developing GC with this genotype was significantly increased (adjusted OR = 2.11, 95%CI: 1.31-3.40).
Unlike the results obtained for individuals belonging to either the Tibet or Hui populations, two genotype sites were associated with GC in the Han ethnicity. For the IL-1B-31 CT genotype, there was a significant difference between GC patients (51.77%) and controls (48.52%) (χ2 = 4.61, P = 0.032). There was also a significant difference in the CC genotype between GC patients (27.92%) and controls (19.31) (χ2 = 8.29, P = 0.004). The risk of developing GC in patients with IL-1B-31CT or CC genotypes was significantly increased (adjusted OR = 1.69, 2.29; 95%CI: 1.03-2.76, 1.28-4.10; P = 0.036, 0.005, respectively). In addition, for the IL-1B-511 genotypes, there was a statistically significant difference in the CT genotype distribution between GC patients (50.50%) and controls (49.09%) (χ2 = 8.70, P = 0.003). Additionally, there was also a statistically significant difference in TT genotype distribution between GC patients (32.67%) and controls (18.81%) (χ2 = 18.90, P = 0.000). The risk of developing GC in patients with the IL-1B-511 CT or TT genotypes was significantly increased (adjusted OR = 2.16, 3.53; 95%CI: 1.28-3.65, 1.49-8.33; P = 0.004, 0.004, respectively).
IL-1B-31, IL-1B-511 and IL-1RN polymorphisms and intestinal type GC risk
The identification of a genetic risk outline for GC could help the populations most at risk. Therefore, the prevalence of IL-1B-31, IL-1B-511 and IL-1RN polymorphisms in different GC subtypes were analyzed. In this study, there were 109, 106 and 139 cases (70.32%, 67.09% and 70.56%) of intestinal-type GC, and 46, 52 and 58 cases (29.68%, 32.91% and 29.44%) of diffuse or mixed-type GC in the Tibet, Hui and Han ethnicities, respectively. The frequencies of genotypes IL-1B-31, -511, and IL-1RN among intestinal-type GC and diffuse or mixed-type GC in all three populations are summarized in Table 4.
Table 4.
Genotype distributions of the interleukin-1B-31, interleukin-1B-511 and interleukin-1RN polymorphisms among different subtypes of gastric cancer in patients from the Tibet, Hui and Han ethnicities n (%)
|
Tibet |
Hui |
Han |
|||||||||||||||||||
| Genetype |
Intestinal cases |
Diffuse cases |
Intestinal cases |
Diffuse cases |
Intestinal cases |
Diffuse cases |
|||||||||||||||
| Controls | Cases | OR (95%CI)1 | P value | Cases | OR (95%CI)1 | P value | Controls | Cases | OR (95%CI)1 | P value | Cases | OR (95%CI)1 | P value | Controls | Cases | OR (95%CI)1 | P value | Cases | OR (95%CI)1 | P value | |
| IL-1B-31 | |||||||||||||||||||||
| TT | 47 (22.38) | 16 (14.68) | 1 | 7 (15.22) | 1 | 59 (28.78) | 33 (32.29) | 1 | 13 (25.00) | 1 | 65 (32.18) | 28 (20.14) | 1 | 12 (20.69) | 1 | ||||||
| CT | 112 (53.33) | 56 (51.38) | 1.46 (0.75-2.84) | 0.271 | 25 (54.35) | 1.48 (0.59-3.70) | 0.407 | 105 (51.22) | 51 (48.11) | 0.87 (0.49-1.54) | 0.618 | 28 (53.85) | 1.11 (0.51-2.39) | 0.792 | 98 (48.52) | 70 (50.36) | 1.68 (0.97-2.90) | 0.067 | 32 (55.17) | 2.00 (0.82-4.88) | 0.126 |
| CC | 51 (24.29) | 37 (33.94) | 2.17 (1.05-4.51) | 0.037 | 14 (30.43) | 1.86 (0.68-5.09) | 0.228 | 41 (20.00) | 22 (21.28) | 0.93 (0.46-1.90) | 0.845 | 11 (21.15) | 1.18 (0.46-3.01) | 0.734 | 39 (19.31) | 41 (29.50) | 2.51 (1.32-4.76) | 0.005 | 14 (24.14) | 1.79 (0.85-3.77) | 0.126 |
| IL-1B-511 | |||||||||||||||||||||
| CC | 55 (26.19) | 22 (20.18) | 1 | 12 (25.53) | 1 | 43 (20.98) | 23 (21.70) | 1 | 10 (19.23) | 1 | 65 (32.17) | 16 (11.51) | 1 | 15 (25.86) | 1 | ||||||
| CT | 93 (44.29) | 54 (49.54) | 1.48 (0.76-2.60) | 0.277 | 26 (55.32) | 1.28 (0.59-2.79) | 0.535 | 110 (53.66) | 58 (54.72) | 1.00 (0.53-1.89) | 0.998 | 30 (57.69) | 1.13 (0.48-2.62) | 0.784 | 99 (49.09) | 69 (49.64) | 2.74 (1.44-5.22) | 0.002 | 32 (55.17) | 1.45 (0.72-2.93) | 0.301 |
| TT | 62 (29.52) | 33 (30.28) | 1.25 (0.64-2.42) | 0.518 | 9 (19.15) | 0.62 (0.24-1.63) | 0.336 | 52 (25.37) | 25 (23.58) | 0.91 (0.43-1.92) | 0.811 | 12 (23.08) | 0.92 (0.34-2.50) | 0.872 | 38 (18.81) | 54 (38.85) | 5.66 (2.82-11.33) | 0 | 11 (18.97) | 1.23 (0.50-3.02) | 0.645 |
| IL-1RN | |||||||||||||||||||||
| L/L | 185 (88.10) | 93 (83.22) | 1 | 36 (84.78) | 1 | 156 (76.10) | 64 (60.38) | 1 | 30 (57.69) | 1 | 171 (84.66) | 116 (83.45) | 1 | 50 (86.21) | 1 | ||||||
| 2/L | 25 (11.90) | 16 (14.68) | 1.32 (0.66-2.66) | 0.439 | 10 (16.78) | 1.87 (0.79-4.43) | 0.158 | 49 (23.90) | 42 (39.62) | 2.08 (1.22-3.56) | 0.007 | 22 (42.31) | 2.31 (1.17-4.56) | 0.016 | 31 (14.44) | 23 (16.55) | 1.09 (0.59-1.97) | 0.807 | 8 (13.79) | 1.02 (0.43-2.39) | 0.971 |
Adjusted for age, sex, smoking status and Helicobacter pylori infection. IL: Interleukin; OR: Odds ratio.
For individuals belonging to the Tibet ethnicity group, the IL-1B-31 CC genotype was only associated with intestinal type GC (P = 0.037) with an adjusted OR of 2.17 (95%CI: 1.05-4.51).
In the Hui ethnicity group, the IL-1RN 2 genotype was associated with both intestinal and diffuse types of GC (P = 0.007, 0.016, respectively) with adjusted ORs of 2.08 and 2.31 (95%CI: 1.22-3.56, 1.17-4.56, respectively).
In the Han ethnicity group, the IL-1B-31 CC genotype was only associated with intestinal type GC (P = 0.005) with an adjusted OR of 2.51 (95%CI: 1.32-4.76). However, compared to the TT genotype, the GC risk in IL-1B-31 CT carriers did not achieve the threshold of statistical significance (P = 0.067) with an adjusted OR of 1.68 (95%CI: 0.97-2.90). Moreover, both the IL-1B-511 CT and TT genotypes were only associated with intestinal type GC (P = 0.002, 0.000) with adjusted ORs of 2.74 and 5.66 (95%CI: 1.44-5.22, 2.82-11.33, respectively).
No other significant associations were found when GC patients were sorted according to age, sex, and presence of H. pylori infection (data not shown).
DISCUSSION
Like many other malignancies, GC develops as a result of complex interactions between environmental risk factors (e.g., unhealthy lifestyle, smoking, uncontrolled over-drinking, unhealthy diet, and H. pylori infection) and genetic alterations[15]. This gene-environment interaction can alter gene expression and promote cell growth and carcinogenesis. The IL-1B and IL-1RN polymorphisms are implicated in cancer risk through their influences on IL-1B transcription.
Since El-Omar et al[5] reported that carriers of IL-1B-511 T or IL-1B-31 C were more susceptible to GC than other genotypes in 2000, other studies have reported on the associations between IL-1B and IL-1RN polymorphisms and GC risk in various populations but with mixed, or even conflicting results[6,7,10-14]. To date, at least four meta-analyses on the associations between IL-1B and IL-1RN polymorphisms and GC have been reported, however, their outcomes were different and even opposite[16-19]. In fact, the genetic/environmental interactions among different ethnic groups are quite complex. Thus, IL-1B and IL-1RN polymorphisms may play different roles in different populations or geographical areas.
Two studies have been performed to evaluate the differences in IL-1B and IL-1RN polymorphisms in patients of the same ethnicity - Chinese Han and Italian, respectively. Zeng et al[10] found that the IL-1B-511 T/T genotype frequency was significantly higher in patients with GC than in control subjects (25.0% vs 12.5%, χ2 = 6.7, P = 0.01) in the low GC prevalence region (Guangdong), but was similar (23.0% vs 23.0%) in the high prevalence region (Shanxi). Perri et al[13] did not find any association between GC occurrence and either IL-1B-511T or IL-1RN polymorphism when dividing the subjects between geographic areas displaying high prevalence rates (the North) or low prevalence rates (the South) in Italy.
To the best of our knowledge, this is the first study to examine the associations between IL-1 polymorphisms and GC risk among several ethnicities in one area at the same time. We examined these polymorphisms and their potential association with GC risk among the Tibet, Hui and Han ethnicities in the Qinghai area of China. We found associations between IL-1B-511, IL-1B-31 and IL-1 RN polymorphisms and GC risk among Tibet, Hui and Han ethnicities in the Qinghai area, which were not identical.
In the Tibet ethnicity group, the IL-1B-31 CC genotype was associated with GC. Additionally, our study showed that the IL-1B-31 CC genotype was only associated with intestinal type GC. Our results are consistent with those from several studies in Chinese and Caucasian populations, where IL-1B-31 CC polymorphisms were associated with an increased risk of GC[10,20,21]. However, these results are inconsistent with those from other studies in Asian and Caucasian populations[14,22,23].
In the Hui ethnicity group, the IL-1RN2 was associated with GC, and further study supported that the IL-1RN2 polymorphism was associated with both intestinal and diffuse types of GC. These findings were in agreement with those previously reported, including studies from Caucasian, Arab and Asian (including Chinese) populations[13,20,22,24]. However, our findings also differ from those reported in several other studies[14,25,26].
In the Han ethnicity group, the IL-1B-31 CT and CC genotypes and the IL-1B-511 CT and TT genotypes were associated with GC compared with the IL-1B-31 TT genotype and the IL-1B-511 CC genotype, respectively. Moreover, the IL-1B-31 CC genotype was also only associated with intestinal type GC. Importantly, although IL-1B-31 CT carriers displayed a trend in risk, they did not achieve the threshold of statistical significance. The IL-1B-511 CT and TT genotypes were only associated with intestinal type GC, compared with the CC genotype. While this is the same in Caucasian and Chinese populations[11,18,27,28], it differs from the findings observed in South Korean and Japanese populations in Asia[10,20]. This study also revealed that IL-1RN polymorphisms were not associated with increased risk of GC in the Han ethnicity group, which is similar to that seen in Japanese and South Korean populations[20].
It is interesting and important that the IL-1B-31, IL-1B-511 and IL-1RN polymorphisms and GC risk among the three ethnicities examined were not identical. Other studies have drawn similar conclusions. We believe that the differences among the ethnicities are related to different inherited gene backgrounds. This is supported by other studies which showed differential genetic/environmental interactions in different ethnic groups resulting in altered gene expression and altered effects on cell growth and tumorigenesis[29]. The gene distributions of IL-1B-31, IL-1B-511 and IL-1RN among the Tibet, Hui and Han ethnicities in Qinghai were different. Despite this, they were at least somewhat related to other Asian populations. The IL-1B-31 TT genotype was less frequent in the Tibet population (22.38%) than in the Han population (32.18%). The IL-1B-511 TT genotype was more frequent in the Tibet population (29.52%) than in the Han population (18.81%). The IL-1B-511 TT genotype was significantly lower in the Hui population (20.98%) than in the Han population (32.17%). In addition, the IL-1RN L was significantly lower in the Hui (76.10%) population compared to both the Tibet population (88.10%) and the Han population (84.66%). Ethnic origin is a crucial determinant of the frequency of genetic markers in all populations. Our data may reflect the influence of past selective pressures on the genotypes of Tibet, Hui and Han ethnicities over a long period of time. Han, Tibet and Hui ethnicities have different origins. The Han population is the major ethnicity, while the Tibet and Hui ethnicities are considered to be minorities in the Qinghai province of China. The selected healthy controls of the Tibet ethnicity were living in the Hainan Tibet Ethnicity Autonomous prefecture in Qinghai province, which belongs to the Tibet Anduo area. Shi et al[30] studied more than 5000 male samples from 73 East Asian populations and reconstructed the phylogenetic geography of the D-M174 lineage. The suggested frequency of D-M174 in Tibet (41.31%) was close to Japan (35.08%) but different from the Han ethnicity (< 5%). Thus, the Tibet gene feature is more similar to the Han ethnicity, but also has its own characteristics. The Hui ethnicity migrated from Central Asia, Persia and the Arab world. Yao et al[31] analyzed M*, N* and R* mtDNAs and found that the western Eurasian specific haplogroup frequency in the Hui population was 6.7%, but no western Eurasian type was found in Han Chinese samples from the same place. Since both the Tibet and Hui ethnicities practice endogamy, they tend to be ethnically homogeneous. Therefore, we believe that the Tibet, Hui, and Han ethnicities in the Qinghai area of China have different origins leading to different associations between IL-1B-31, IL-1B-511 and IL-1RN polymorphisms and GC risk.
Our study has some limitations. First, we did not consider education, consumption of alcohol, fresh fruits, and vegetables in the controls which could influence GC risk. Second, the altitude at which healthy controls lived was not considered. In this study, all of the Tibet controls and most of the Tibet patients lived in the plateau above 2800 meters of one another. For the Hui and Han populations, all controls and most patients lived within 2200 meters of each other. Finally, different eating habits among the groups were not investigated.
In conclusion, the present study shows different associations between the IL-1B-31, IL-1B-511 and IL-1RN polymorphisms and GC among the Tibet, Hui and Han ethnicities in the Qinghai area of China. No significant association was observed when GC patients were sorted by age, sex and the presence of H. pylori infection.
COMMENTS
Background
Studies suggest that polymorphisms in interleukin (IL)-1B and IL-1RN are associated with a differential risk of developing gastric cancer. However, there does not seem to be a consensus regarding these polymorphisms, since in some populations the polymorphisms are associated with increased disease occurrence, whereas in other populations, they are associated with a protective effect.
Research frontiers
There is no consensus regarding the associations between IL-1B, IL-RN polymorphisms and gastric cancers in different areas or ethnicities. The authors investigated the associations between IL-1B and IL-1RN polymorphisms and gastric cancers among the Tibet, Hui and Han ethnicities in China.
Innovations and breakthroughs
The outcomes of previous studies on the associations between IL-1B and IL-1RN polymorphisms and gastric cancer were different and even opposite. The genetic/environmental interactions among different ethnic groups are quite complex. Thus, IL-1B and IL-1RN polymorphisms may play different roles in different populations or geographical areas. This study suggests that carriers of the IL-1B-31 CC genotype had an increased risk of intestinal type gastric cancer in the Tibet ethnicity, carriers of the IL-1B 2/L genotype had an increased risk of both intestinal and diffuse types of gastric cancer in the Hui ethnicity, while carriers of the IL-1B-31 CC, IL-1B-511 CT, TT genotypes have an increased risk of intestinal type gastric cancer in the Han population.
Applications
The study results suggest that IL-1B and IL-RN genotypes may differentially contribute to gastric cancer among the Tibet, Hui and Han nationalities in the Qinghai area of China, and that IL-1B and IL-1RN polymorphisms may play different roles in different populations or geographical areas.
Terminology
Single nucleotide polymorphisms (SNPs) are short polymorphisms in the human DNA. SNPs occur once in every 300 nucleotides on average and can act as biological markers. When SNPs occur within a gene or in a regulatory region near a gene, they may play a more direct role in disease by affecting the gene’s function. SNPs can also be used to track the inheritance of disease genes within families.
Peer review
This is a good descriptive study in which authors investigated IL-1B and IL-1RN polymorphisms in Han, Tibetan and Hui ethnic populations in Qinghai Province of China and their associations with gastric cancer risk in these populations. The topic is interesting and of potential clinical implications.
Footnotes
Supported by Grants from the National Natural Science Foundation of China, No. 30860259; and the Youth Scientific Research Foundation of Qinghai University, No. 2008-QY-09
Peer reviewers: Harry HX Xia, MD, PhD, Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, NJ 07936-1080, United States; Dr. Xue-Mei Lu, Medical Research Center, 1st Teaching Hospital, Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China
S- Editor Huang XZ L- Editor Webster JR E- Editor Li JY
References
- 1.Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–315. doi: 10.1016/s0140-6736(03)13975-x. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Sun XD, Mu R, Zhou YS, Dai XD, Qiao YL, Zang SW, Huangpu XM, Sun J, Li LD, Lu FZ. Investigation and analysis on mortality of gastric cancer in China between 1990-1992. Zhonghua Zhongliu Zazhi. 2002;24:4–8. [PubMed] [Google Scholar]
- 4.Zhao JD, Sheng GS, Cao CZ, He JX, Ma XF, Wang LJ, Ji FX, Li JZ, Geng PL. Analysis on the morbidity feature among Tibet, Hui and Han nationalities with gastric cancer in Qinghai area. Zhonghua Neike Zazhi. 2009;48:955–956. [Google Scholar]
- 5.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 6.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 7.Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F, et al. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823–829. doi: 10.1053/gast.2001.28000. [DOI] [PubMed] [Google Scholar]
- 8.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 9.Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro Alves C, Campos ML, Van Doorn LJ, Caldas C, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364–371. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 10.Zeng ZR, Hu PJ, Hu S, Pang RP, Chen MH, Ng M, Sung JJ. Association of interleukin 1B gene polymorphism and gastric cancers in high and low prevalence regions in China. Gut. 2003;52:1684–1689. doi: 10.1136/gut.52.12.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato S, Onda M, Yamada S, Matsuda N, Tokunaga A, Matsukura N. Association of the interleukin-1 beta genetic polymorphism and gastric cancer risk in Japanese. J Gastroenterol. 2001;36:696–699. doi: 10.1007/s005350170033. [DOI] [PubMed] [Google Scholar]
- 12.Lee SG, Kim B, Choi W, Lee I, Choi J, Song K. Lack of association between pro-inflammatory genotypes of the interleukin-1 (IL-1B -31 C/+ and IL-1RN *2/*2) and gastric cancer/duodenal ulcer in Korean population. Cytokine. 2003;21:167–171. doi: 10.1016/s1043-4666(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 13.Perri F, Piepoli A, Bonvicini C, Gentile A, Quitadamo M, Di Candia M, Cotugno R, Cattaneo F, Zagari MR, Ricciardiello L, et al. Cytokine gene polymorphisms in gastric cancer patients from two Italian areas at high and low cancer prevalence. Cytokine. 2005;30:293–302. doi: 10.1016/j.cyto.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631–636. doi: 10.1093/carcin/bgh349. [DOI] [PubMed] [Google Scholar]
- 15.Lochhead P, El-Omar EM. Gastric cancer. Br Med Bull. 2008;85:87–100. doi: 10.1093/bmb/ldn007. [DOI] [PubMed] [Google Scholar]
- 16.Camargo MC, Mera R, Correa P, Peek RM, Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J, Schneider BG. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1674–1687. doi: 10.1158/1055-9965.EPI-06-0189. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Xia HH, Zhang JY, Dai LP, Xu XQ, Wang KJ. Association of interleukin-1 gene polymorphisms with gastric cancer: a meta-analysis. Int J Cancer. 2007;120:552–562. doi: 10.1002/ijc.22353. [DOI] [PubMed] [Google Scholar]
- 18.Kamangar F, Cheng C, Abnet CC, Rabkin CS. Interleukin-1B polymorphisms and gastric cancer risk--a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1920–1928. doi: 10.1158/1055-9965.EPI-06-0267. [DOI] [PubMed] [Google Scholar]
- 19.Xue H, Lin B, Ni P, Xu H, Huang G. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J Gastroenterol Hepatol. 2010;25:1604–1617. doi: 10.1111/j.1440-1746.2010.06428.x. [DOI] [PubMed] [Google Scholar]
- 20.He BS, Pan YQ, Xu YF, Zhu C, Qu LL, Wang SK. Polymorphisms in interleukin-1B (IL-1B) and interleukin 1 receptor antagonist (IL-1RN) genes associate with gastric cancer risk in the Chinese population. Dig Dis Sci. 2011;56:2017–2023. doi: 10.1007/s10620-010-1557-y. [DOI] [PubMed] [Google Scholar]
- 21.Sicinschi LA, Lopez-Carrillo L, Camargo MC, Correa P, Sierra RA, Henry RR, Chen J, Zabaleta J, Piazuelo MB, Schneider BG. Gastric cancer risk in a Mexican population: role of Helicobacter pylori CagA positive infection and polymorphisms in interleukin-1 and -10 genes. Int J Cancer. 2006;118:649–657. doi: 10.1002/ijc.21364. [DOI] [PubMed] [Google Scholar]
- 22.Al-Moundhri MS, Alkindy M, Al-Nabhani M, Al-Bahrani B, Burney IA, Al-Habsi H, Ganguly SS, Tanira M. Combined polymorphism analysis of glutathione S-transferase M1/G1 and interleukin-1B (IL-1B)/interleukin 1-receptor antagonist (IL-1RN) and gastric cancer risk in an Omani Arab Population. J Clin Gastroenterol. 2009;43:152–156. doi: 10.1097/MCG.0b013e31815853fa. [DOI] [PubMed] [Google Scholar]
- 23.Chang YW, Jang JY, Kim NH, Lee JW, Lee HJ, Jung WW, Dong SH, Kim HJ, Kim BH, Lee JI, et al. Interleukin-1B (IL-1B) polymorphisms and gastric mucosal levels of IL-1beta cytokine in Korean patients with gastric cancer. Int J Cancer. 2005;114:465–471. doi: 10.1002/ijc.20724. [DOI] [PubMed] [Google Scholar]
- 24.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. doi: 10.1038/35083631. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Xia HH, Xie W, Hu Z, Ye M, Li J, Cheng H, Zhang X, Xia B. Association between interleukin-1 gene polymorphisms and Helicobacter pylori infection in gastric carcinogenesis in a Chinese population. J Gastroenterol Hepatol. 2007;22:234–239. doi: 10.1111/j.1440-1746.2006.04379.x. [DOI] [PubMed] [Google Scholar]
- 26.Shin WG, Jang JS, Kim HS, Kim SJ, Kim KH, Jang MK, Lee JH, Kim HJ, Kim HY. Polymorphisms of interleukin-1 and interleukin-2 genes in patients with gastric cancer in Korea. J Gastroenterol Hepatol. 2008;23:1567–1573. doi: 10.1111/j.1440-1746.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Zeng Z, Wang S, Tian L, Wu J, Xue L, Lee CW, Zhang M, Goggins WB, Chen M, et al. IL-1B-511 polymorphism is associated with increased risk of certain subtypes of gastric cancer in Chinese: a case-control study. Am J Gastroenterol. 2010;105:557–564. doi: 10.1038/ajg.2009.644. [DOI] [PubMed] [Google Scholar]
- 28.Zhang WH, Wang XL, Zhou J, An LZ, Xie XD. Association of interleukin-1B (IL-1B) gene polymorphisms with risk of gastric cancer in Chinese population. Cytokine. 2005;30:378–381. doi: 10.1016/j.cyto.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Lee KA, Park JH, Sohn TS, Kim S, Rhee JC, Kim JW. Interaction of polymorphisms in the interleukin 1B-31 and general transcription factor 2A1 genes on the susceptibility to gastric cancer. Cytokine. 2007;38:96–100. doi: 10.1016/j.cyto.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Shi H, Zhong H, Peng Y, Dong YL, Qi XB, Zhang F, Liu LF, Tan SJ, Ma RZ, Xiao CJ, et al. Y chromosome evidence of earliest modern human settlement in East Asia and multiple origins of Tibetan and Japanese populations. BMC Biol. 2008;6:45. doi: 10.1186/1741-7007-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao YG, Kong QP, Wang CY, Zhu CL, Zhang YP. Different matrilineal contributions to genetic structure of ethnic groups in the silk road region in china. Mol Biol Evol. 2004;21:2265–2280. doi: 10.1093/molbev/msh238. [DOI] [PubMed] [Google Scholar]


