Abstract
Background
In some areas of the world mother-to-child transmission of HIV remains a significant problem in part due to widespread breastfeeding which is essential due to scarce supply of a safe replacement, protection conferred by breast milk against many enteric illnesses, and cultural norms. We propose that sustained, adequate levels of protective antibodies in breast milk will prevent transmission of HIV.
Methods
The HIV neutralizing human monoclonal antibody b12 (IgG1) has been expressed as an IgA2 in CHO cells and shown to retain full immunoreactivity and neutralizing activity as the parental IgG1. The expression plasmids containing the b12 heavy and light chains were also used to construct milk specific expression vectors using the GTC goat β-casein expression vector to direct expression of linked genes to the mammary gland with subsequent secretion into the milk. Female transgenic mice were generated and following parturition, their milk was tested for antibody immunoreactivity with gp120 and neutralization of HIV.
Results
When compared to CHO derived b12 IgA2 (or IgG1), immunoreactivity was retained. When tested for neutralization, milk derived b12 IgA2 was at least comparable to CHO derived antibody and in some cases superior to CHO derived antibody. Furthermore, milk that expressed b12 IgA2 was significantly more effective at mediating antibody dependent cell killing.
Conclusions
These results suggest it is possible to achieve functional HIV-specific mAb in the milk of transgenic mice and further investigations are warranted to explore ways for inducing this type of antibody response in the breast milk of HIV infected women.
Introduction
At the end of 2008, 2.1 million children under the age of 15 were HIV infected with the majority of these individuals contracting the virus from their infected mother. Transmission of HIV by breastfeeding accounts for approximately 40% of mother-to-child transmission (MTCT). In countries where safe, nutritional replacements are readily available, women are counseled to avoid breastfeeding and this guidance has significantly reduced the transmission of HIV by breastfeeding. There has been a reduction in MTCT to less than 1% in developed countries. However, in many parts of the world, safe replacement of breast milk is not readily available. Delivery of anti-retroviral therapy (ART) is not readily accomplished in many areas as it requires identification of HIV infected women, and is dependent upon testing programs and access to health care resources in addition to the drugs themselves. Therefore, it is essential that alternative means, including active or passive immunization, be developed to prevent MTCT through breastfeeding.
More direct infection by HIV can occur through breaks in the integrity of the epithelium, which may occur as a result of inflammation when infants are not exclusively breastfed or are exposed to pathogens. The mucosal barrier, as well as anti-viral properties of some components of breast milk, prevents the majority of infants from becoming infected despite repeated daily exposures to HIV. However, once the mucosal barrier has been crossed, HIV targets resting T cells and disseminates to the draining lymph nodes and the lymphoid. Therefore, a preventative vaccine must ensure that the immune response generated not only prevents entry into the mucosa and transgression through the mucosa, but also prevents infection of local cells that are exposed in areas of inflammation and trauma.
Maternal HIV specific antibodies in the form of secretory IgA, secretory IgM and IgG are found in breast milk. There are differences in the HIV specific antibody response between breast milk and that seen in the blood1, 2. This would suggest that in addition to homing of B cells primed to HIV at mucosal surfaces (such as genital tract, gastrointestinal tract) to the mammary gland, local stimulation and maturation of B cells in the mammary gland itself, is critical to the antibody component of breast milk. Given the multitude of components of breast milk which may vary with time, as well as differences in assay methodology, it remains unclear how effective the HIV specific antibody, induced during natural infection, is at preventing transmission3, 4. However, breast milk antibodies and the humoral immune response play a significant role in the control of a number of human viral diseases. Since maternal antibodies generally do not enter the circulation of infants through the gastrointestinal tract, they may function to prevent infection by neutralizing viral inoculum or preventing transmission across the epithelial cells either by immune exclusion or intracellular neutralization. Clearly, the more functional activity conferred by the antibody response, the more likely infection is inhibited. It can be hypothesized that, given the more active role of IgA antibodies in mucosal secretions, as compared to IgG antibodies, anti-HIV IgA antibodies at the mucosal surface may be highly effective in inhibiting HIV infection.
We believe that sustained, adequate levels of protective antibodies in breast milk will prevent transmission of HIV. Given the paucity of HIV specific IgA induced by natural infection, it will be necessary to develop strategies to deliver effective antibodies for secretion in the breast milk. Immunization may take the form of passive administration of milk derived antibodies or active induction of antibody producing cells. In this paper, we describe studies in which the neutralizing human monoclonal antibody b12 was expressed in both tissue culture and transgenic mouse milk, while demonstrating their neutralizing activity. These studies support exploration of our hypothesis, and proposed studies that sustained levels of protective antibody in milk will prevent the infection of infants during breastfeeding. While it has been shown that a secretory IgA2 variant of b12 protects macaques against vaginal simian/HIV challenge5, the use of an IgA2 antibody to prevent MTCT of HIV has not been previously explored.
Materials and Methods
Monoclonal antibodies, Virus and Cell Lines
The neutralizing antibody IgG1b12 (b12), directed to the CD4 binding site of gp120, was originally isolated in the laboratory of Dr. Dennis Burton of The Scripps Research Institute from an antibody phage display library prepared from an asymptomatic HIV-1-seropositive individual6. CHO-K1 cells were from American Type Culture Collection. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: SF162 (R5) from Dr. Jay Levy; 92HT593 (R5X4) from Dr. Neal Halsey; 89.6 (R5X4) from Dr. Ronald Collman; BaL (R5) from Dr. Suzanne Gartner, Dr. Mikulas Popovic and Dr. Robert Gallo; 93MW960 (clade C, R5) from Dr. Robert Bollinger and the UNAIDS Network for HIV; JR-FL (R5) from Dr. Irvin Chen; TZM-bl cells from Dr. John C. Kappes, Dr. Xiaoyun Wu and Transzyme, Inc. Isolate 67970 (CXCR4) was from Dr. David Montefiori.
Construction and production of anti-HIV b12 IgG1 and IgA variants
b12 VH and VL were PCR amplified respectively from b12 clones from Dr. Burton (The Scripps Research Institute, La Jolla, CA) using the specific primers to introduce restriction enzymes (5’ Nhe I and 3’ Hind III for VH; 5’ Nhe I and 3’ Not I for VL). The b12 VH fragment was cloned into the immunoglobulin expression vectors pHC-huCg1, and pHC-huCα1. The b12 VL was cloned into vector pLC-huCk. All of these immunoglobulin expression vectors were obtained from Dr. Gary McLean, containing the light chain, γ1 or α1 constant regions respectively. For constructing b12 IgA2, we amplified the α2 constant region from an IgA2 vector (6425pAH-ETEC6-IgA2m2, allotype m2) provided by Dr. Sherrie Morrison. b12 VH was then connected to the 5 end of α2 constant region by over-lap PCR. Unique restriction sites, 5’ Nhe and 3’ Xho I, were added to the fragment and replaced the b12A1 fragment in pHC-huCα1 vector using these two restriction enzymes.
To facilitate clonal expression, the complete immunoglobulin cassettes were cloned into IRES-based bicistronic expression vectors (Clontech). b12VL+ Ck fragment from pLC-huCk was cloned into pIRESneos3 vector using restriction sites 5’ Nhe I and 3’ Xba I. Heavy chain fragments of b12VH-Cγ1, b12VH-Cα1 and b12VH-Cα2 were respectively cloned into pIRESpuro3 vector using restriction sites 5’ Nhe I and 3’ Xho I. The plasmids carrying the b12 light chain and heavy chain were purified with the Maxiprep Kit (Qiagen). Purified plasmid encoding the b12 light chain was then transfected into CHO-K1 cells by lipofection (Invitrogen Life Technologies). After about two weeks selected by RPMI 1640 containing 800 µg/ml G418, the expressing clones were isolated using human κ chain capture ELISA. Cell lines expressing the b12 light chain were propagated and subsequently transfected with the IRESpuro3 plasmids encoding b12 heavy chains of the IgG1, IgA1 and IgA2 class individually. After around 2wk selected with medium containing 10 µg/ml puromycin and 800 µg/ml G418, the expressing cell clones that generated mature subclass b12 were isolated by subclass specific ELISA.
Construction and production of b12dIgA1
J chain was isolated from human heteromyeloma cells HMMA2.57 by Reverse Transcription PCR (RT-PCR) using primers derived from a human J chain sequence deposited in GenBank (accession number AAH38982). Forward primer was CTAGCTAGCATGAAGAACCATTTGC and reverse primer was TGCGATATCTTAGTCAGGATAGCAGG. The restriction sites 5’ Nhe I and 3’ EcoR V were added in primers. The J chain fragment obtained from PCR was cloned into pcDNA3.1 vector using Nhe I and EcoR V restriction sites. The purified J chain plasmid DNA was transfected into the cell line of b12A1/CHO by lipofection. The selected medium included 800 µg/ml G418, 10 µg/ml puromycin and 1 mg/ml zeocin. The positive clones were screened by J chain ELISA. Briefly, ELISA plate was coated using Goat anti-human κ chain and blocked using 0.1% BSA/PBS buffer. Bound antibody was detected using mouse anti-human J chain antibody followed by HRP conjugated goat anti-mouse IgG antibody.
Construction of b12 IgA2 milk specific vectors and expression in mice
The expression plasmids containing the b12 heavy and light chains were used as a source of the DNA to construct milk specific expression vectors. Restriction sites were introduced immediately upstream and downstream of the coding sequences, so that they could be cloned into the GTC goat β-casein expression vector. This vector carries the promoter and the downstream untranslated region of goat β-casein (pBC1, Invitrogen), and directs expression of linked genes to the mammary gland with subsequent secretion into the milk.
Two different versions of this vector were used. The gbc450 has the prokaryotic sequences flanked by Sal I sites, while gbc451 has them flanked by Not I sites. The prokaryotic sequences are removed prior to generation of transgenic animals. The upstream Nhe I and downstream Not I sites flanking the IgA2 heavy chain were converted to Sal I sites. The heavy chain could then be retrieved as a Sal I fragment, and ligated into the Xho I cloning site in the milk specific vector gbc451 to yield BC2470HC. The light chain was also subcloned by first changing the upstream Nhe I site to an Xho I site. The Xho I fragment containing the light chain was then ligated into the Xho I cloning site of the modified casein vector gbc450 to yield BC2526LC. These plasmids were used as sources of DNA to generate transgenic mice. Following removal of prokaryotic sequences, the DNA fragments were co-injected into pre-implantation mouse embryos and implanted into foster mothers. The resulting progeny were screened for the presence of the transgenes in their DNA. Female mice that carried the integrated beta casein linked to heavy and light chains of the antibody were grown to maturity and bred. Following parturition, the animal milk was collected and analyzed for the presence of the b12 antibody. All animal studies were approved by the GTC IACUC.
Neutralization assay
The neutralization activity of b12 variants was determined in vitro using a TZM-bl assay with a panel of five isolates, including an R5/clade C (93MW960) as well as SF162, JR-FL, 89.6 and 67970. Primary isolate virus was grown in PHA-stimulated peripheral blood mononuclear cells (PBMC) as previously described7, and detected titer on TZM-bl cells8 to determine TCID50. Serial two-fold dilutions of b12 variants were incubated with virus stock diluted to 100TCID50 for 1 hour, 37°C prior to the addition of TZM-bl cells (1×104 c/well). Plates were incubated for 48 hours, 37°C, 5% CO2 prior to measurement o f β-galactosidase activity by using β-galactosidase reagent from Promega as an indicator of HIV replication. Percent neutralization was determined based on control wells of virus, media, IC50 and IC90 values calculated by regression analysis.
Antibody dependent cell-mediated viral inhibition (ADCVI)
ADCVI activity was measured using HIV grown in PHA stimulated PBMC as previously described7. Neutrophils were obtained from peripheral blood of seronegative donors by Ficoll-Hypaque gradient centrifugation. Antibodies were titer in 96 well, round bottom plates in 50 µl media containing 20% heat-inactivated FBS. Target cells were PBMC productively infected with HIV-1 four days prior to use as previously described9 and 1×105 infected cells in 50µl were added per well. Within 10 minutes of combining of antibody and infected cells, neutrophils were added to the wells at 1×106 effector cells/well in 100 µl resulting in an effector to target, ratio of 10:1 (E:T). After 4 hours, in order to measure the surviving infectious virus, PHA stimulated PBMC were added as indicator cells (1×105/well). These indicator PBMC were incubated for seven days in the presence of IL-2, at which time ELISA was used to quantitate p247. IC50 values were determined by linear regression analysis and significance was ascertained by student’s t test. Control wells included no antibody, no effectors and no targets were used to determine background release of virus, maximum production of virus, or whether PMN alone were infected, respectively. Experiments were repeated three times.
Results
Construction and expression of b12 isotype variants in CHO cells
The monoclonal antibody b12 was isotype switched and expressed as an IgG1 IgA1, IgA2, and dimeric IgA1 (dIgA). For constructing isotype variants, immunoglobulin expression vectors were obtained from Dr. Gary McLean for light chain and γ1 or α1 constant regions. PCR products encompassing the variable regions of the light and heavy chains of the b12 antibody were cloned in frame into immunoglobulin expression vectors. A vector expressing α2 was generated by replacing the α1 constant region in the McLean vector with the IgA2 constant region which was isolated from an IgA2 vector (6425pAH-ETEC6-IgA2m2, allotype m2) provided by Dr. Sherrie Morrison. Since restriction site Hind III was not the unique site in A2 constant region, the b12 VH was connected to the A2 constant region using the overlap PCR technique. The whole b12A2 fragment was then cloned into the pHC-huCα1 vector to replace the b12A1 fragment with Nhe I and Xho I restriction sites. Following expression vector construction, all of plasmid DNA was verified by dideoxy sequencing.
To obtain the b12 isotype variant clonal expression, the complete immunoglobulin cassettes were cloned into IRES-based bicistronic expression vectors (Clontech). The rationale for using these vectors is based on the fact that both the gene of interest and the antibiotic resistance gene are encoded by the same mRNA, which not only facilitates clonal selection, but also maintains constant protein expression over time since the selective pressure is exerted on the entire expression cassette10. The b12 isotype variant clones were identified producing immunoglobulin at concentrations ranging from 1–30 µg/mL by κ chain capture ELISA. All antibodies were purified using protein L columns and quantitated using known concentrations of κ chain.
Clones expressing dIgA1 were derived by sequentially transfecting the J chain into a CHO cell line that was co-expressing b12 light chain and b12 α1 heavy chain. J chain is a 15kDa polypeptide covalently linked to the C-terminus of two IgA monomers. It is responsible for the intracellular assembly of IgA by modulating their structures and thereby effector functions. J chain is absolutely required for IgA polymerization11, 12.
Prior to functional assays, comparable immunoreactivity with gp120 was confirmed by ELISA. All clones displayed significant and comparable reactivity to HIVgp120, as assessed by ELISA (data not shown). Switch variants of b12 (IgG1, IgA1, IgA2 and dIgA1) were compared for neutralization of HIV using TZM-bl indicator cells. As shown in Table I, the IgA2 subclass and dimeric IgA1 required slightly more antibody for effective neutralization; however, this was not consistent across all isolates and all constructs retained neutralization activity.
Table I.
Neutralization of HIV-1 by b12 Isotype Variantsa
| IC90 | IC90 | IC90 | IC90 | IC50 | |
|---|---|---|---|---|---|
| SF162 | JR-FL | 89.6 | 93MW960 | 67970 | |
| IgG1 | 4.2 (1.4)b | 6.6 (1.0) | 15.1 (5.1) | 12.9 (3.8) | 5.8 (1.5) |
| IgA1 | 4.5 (1.5) | 7.5 (1.4) | 25.4 (10.1) | 33.4 (8.1) | 10.7 (3.4) |
| IgA2 | 6.7 (0.3) | 10.3 (1.7) | 37.7 (5.9) | 18.2 (1.5) | 13.7 (1.9) |
| dIgA1 | 4.4 (1.7) | 12.6 (1.5) | 33.4 (8.1) | 15.1 (2.6) | 11.3 (2.5) |
Neutralization of HIV was measured in TZM-bl cells and IC90 or IC50 determined by linear regression
Mean IC90 or IC50 with standard deviation in parenthesis
Production of b12 IgA2 in the milk of transgenic mice
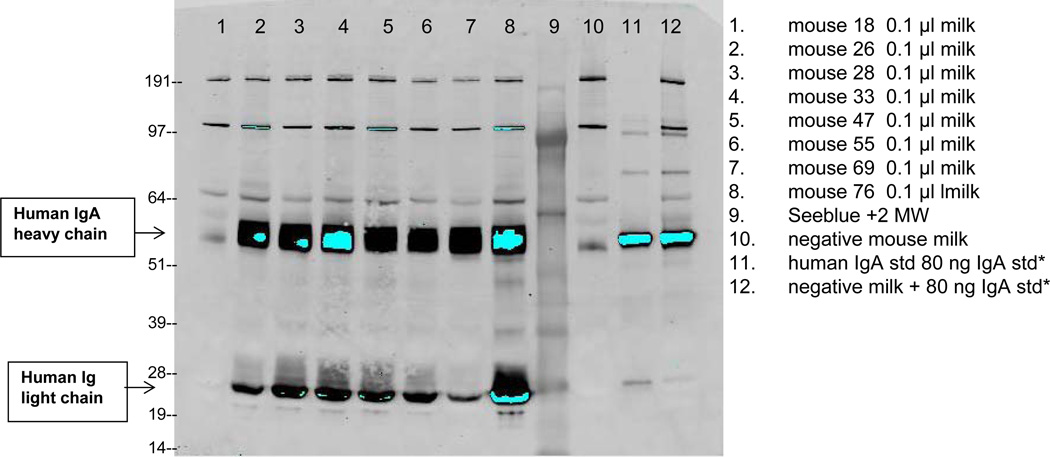
Milk specific expression vectors were constructed by directly cloning fragments encoding the b12 light chain and b12 A2 heavy chain into the GTC goat β-casein expression vector. This vector carries the promoter and downstream untranslated region of goat β-casein (pBC1, Invitrogen). It directs expression of linked genes to the mammary gland with subsequent secretion into the milk. The resulting plasmids, BC2470HC and BC2469LC with heavy or light chain linked to the β-casein promoter, were micro-injected into pre-implantation mouse embryos and implanted into foster mothers. The resulting progeny were screened for the presence of the transgenes in their DNA. The founder female mice that carried the integrated beta casein linked to the heavy and light chains of the antibody were grown to maturity and bred. Founder males were bred to wild-type mice and the F1 progeny tested for the presence of the transgene. Transgenic founder and F1 females were then bred and their milk tested for the presence of the b12 IgA antibody. Subsequent generations of these mice were expanded and their ability of the transgenic females to produce the b12 IgA was confirmed (data not shown). Lines were identified that produced more than 5 mg/ml as judged by Western blot analysis by comparison of with a known concentration of human IgA using Image J software (Figure 1). The milk was pooled from a number of lines and put through a clarification step to remove the colloidal milk proteins. The resulting clarified milk containing the b12 IgA2 antibody was then used for antigen binding and neutralization studies, as well as a source of purified b12 IgA2 antibody. It should be noted that pools of milk, testing, and purification were prepared on three separate occasions and there were no batch-to-batch differences in activity.
Figure 1. Identification of b12A2 antibody secreted in the milk of transgenic mice using western blot.
Lanes 1–8 are milk samples collected from individual mice; lane 9 is the molecular weight marker; lane 10 is milk from a negative control mouse; lane 11 is a known concentration of standard human IgA (Rockland 009-0106) at 80 ng/lane; lane 12 is negative control mouse milk spiked with standard human IgA. 80 ng of IgA standard is equivalent to 0.8 mg/ml in milk when loaded at 0.1 µl of milk per lane. Reactive bands were detected using biotinylated goat anti-human IgA alpha chain (Rockland 609-1606) followed by HRP conjugated streptavidin-IR800CW (Rockland 926-32230).
Neutralization of HIV by Milk Expressed b12 IgA2
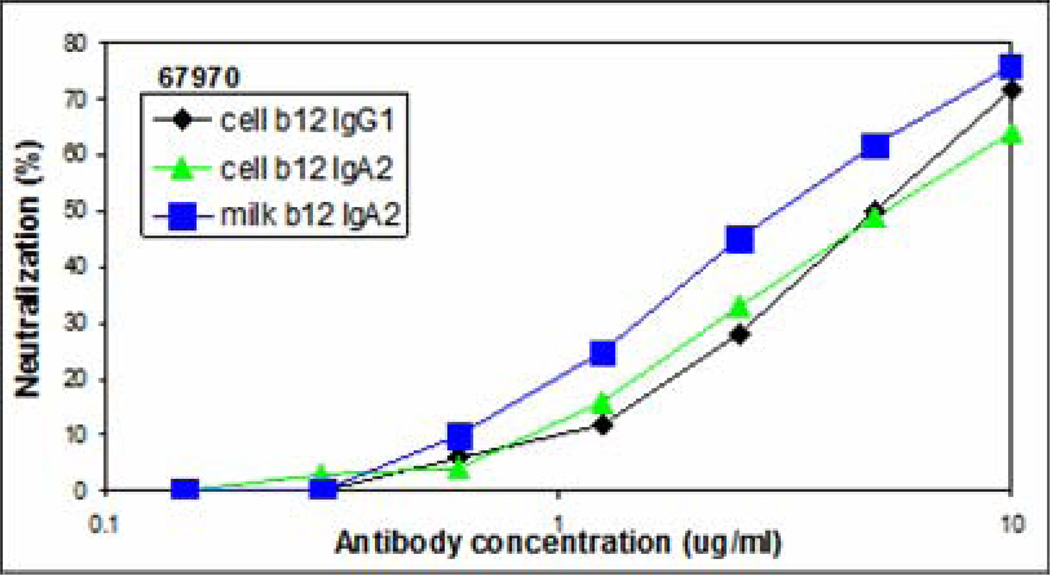
Both cell lines derived and milk derived b12 IgA2 were tested for immunoreactivity with gp120 by ELISA. Parental IgG1 b12 was used as the control and a goat anti-κ reagent was used for detection which equivalently recognizes the common light chain for all constructs. Immunoreactivity was similar for both cell line derived and milk derived b12 IgA2 (data not shown). Neutralization of virus was determined using TZM-bl cells with parental cell line derived IgG1 b12 used as a control. Furthermore, to determine if neutralization was a function of antibody alone or in concert with other milk proteins, b12IgA2 was purified from milk using protein L-chromatography. As shown in the representative experiments in Figure 2 and summarized in Table II for four individual isolates, b12IgA2 in milk was superior to cell derived IgA2 and b12 IgG1 control in neutralizing HIV (67970). Antibody b12IgA2 expressed in milk was significantly more effective at neutralizing HIV and this reached statistical significance for all isolates (p<0.05, Table II). This synergistic effect was lost when purified away from other milk components. It should be noted that for two isolates, a very high concentration of antibody is necessary to achieve IC90 values (i.e. 67970 and JR-FL). For these studies, virus stocks are prepared in PBMC resulting in a swarm of virus and it is our experience, and that of others, some of those particles are difficult to neutralize. While an IC90 value can be mathematically derived, we present the data for these isolates as IC50 to represent levels of antibody that may be more physiologically achieved.
Figure 2. Neutralization of HIV (67970) by b12 IgA2 Variants.
Cell-free HIV was incubated with serial dilutions of control b12 IgG1 antibody (♦), b12 IgA2 purified from CHO cells (▲) and b12 IgA2 expressed in milk (■) prior to the addition of TZM-bl cells. HIV was measured as b-galactosidase activity after 48 hours. Percent neutralization was determined by the formula ((control − test)/control)*100.
Table II.
Neutralization of HIV by Milk Derived b12 IgA2a
| CHO Purifiedb |
Milk Purified | Milk Expressed | |
|---|---|---|---|
| SF162 (IC90)c | 1.03 ± 0.52 | 0.79 ± 0.29 | 0.34 ± 0.13d |
| 67970 (IC50)c | 8.68 ± 3.05 | 12.94 ± 5.47 | 1.52 ± 0.73d |
| JR-FL (IC50)c | 7.27 ± 2.45 | 27.60 ± 16.17 | 2.72 ± 1.49d |
| 89.6 (IC90) c | 6.75 ± 2.63 | 8.37 ± 3.10 | 1.85 ± 1.53d |
Neutralization was measured using TZM-bl assay
b12 IgA2 antibody was purified from CHO cells, milk and tested as expressed in milk.
Virus isolate tested with indication of either IC90 or IC50 values were used as determined by linear regression analysis
Enhanced neutralization by milk-expressed b12 IgA2 reached statistical significance when compared to purified antibody (CHO or milk) with p<0.05
Antibody Dependent Cell-Mediated Viral Inhibition by Milk Expressed b12 IgA2
In addition to direct viral neutralization, antibody may also direct cell mediated inhibition of HIV as measured by ADCVI13. Neutrophils or PMN, which express IgA receptor were used in this assay as described previously7. In addition to measuring the ability of PMN effector cells to inhibit HIV, serial dilutions of antibody were also tested in the absence of PMN to identify the contribution of direct neutralization to the inhibitory effect. As shown in Table III, b12IgA2 was not as effective at directly neutralizing virus in the absence of PMN effector cells when compared to the neutralizing activity detected using the TZM-bl assay (Figure 2 and Table II). In fact, there was lack of neutralization for the majority of antibody/virus combinations when tested without PMN. However, in the presence of PMN, there was significant inhibitory activity with b12IgA2 in milk and consistently more effective than b12IgA2 purified from either CHO cells or milk. Thus, regardless of whether measuring neutralization of a single round of infection (TZM-bl) or cell-to-cell spread of HIV (ADCVI), b12IgA2 expressed in milk is significantly more effective at HIV inhibition.
Table III.
ADCVI activity of b12 IgA2
| Antibody Concentration for IC50 (µg/ml) a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibody | BaL Clade B, R5 |
SF162 Clade B, R5 |
93MW960 Clade C, R5 |
89.6 Clade B, R5X4 |
JR-FL Clade B, R5 |
|||||
| PMNb − |
PMNc + |
PMN − |
PMN + |
PMN − |
PMN + |
PMN − |
PMN + |
PMN − |
PMN + |
|
| b12 IgA2 produced in CHO | >20 | 2.1 | 9.5 | 0.3 | >20 | 10.0 | >20 | 14.5 | >20 | >20 |
| b12 IgA2 purified from milk | 13.1 | 2.2 | 2.1 | 0.3 | >20 | 17.5 | >20 | 17.1 | >20 | >20 |
| b12 IgA2 produced in milk | 19.3 | 2.3 | 6.6 | 0.4 | >20 | 3.3 | 4.8 | 3.9 | 9.6 | 6.3 |
The ADCVI activity was determined by IC50 that represents concentration (µg/ml) of antibody required for 50% inhibition of HIV.
ADCVI assays without neutrophils or PMN which is a measure of direct inhibitory or neutralizing activity
ADCVI with PMN to measure effector (PMN) mediated inhibition of HIV
Discussion
Many in vitro and in vivo studies have shown that specific human monoclonal antibodies to HIV result in significant neutralization and protection from infection either alone or in combination when tested in non-human primate models of infection14–19. Passive immunotherapy with human monoclonal antibodies has been shown to protect neonatal macaques from oral challenge with HIV17, 19–22. However, numerous studies have clearly demonstrated the general limitation of the humoral immune system to develop functionally effective neutralizing antibodies during natural infection or vaccination23–25. Therefore, novel means must be developed to provide neutralizing antibodies to prevent infection, especially for protecting the infants from the transmission of HIV by breastfeeding. In this study, we explore a novel immunotherapy to prevent breast milk transmission of HIV.
Immunoglobulins at mucosal surfaces represent an important line of defense against pathogens. While both IgG and IgA are found in mucosal secretions, structure-function relationships and the abundance of IgA suggests that IgA may be more effective on a molar basis at mucosal defense than IgG. First, due to the multimeric propensity of secretory IgA, the avidity of mucosal IgA, should enhance antibody binding with antigen, thus increasing antibody mediated conformational or structural changes in the antigen. The diverse and high level of glycosylation of IgA antibodies, in comparison to IgG, may further protect the mucosal surface from non-specific interference with microbial adherence26. Mucin, which is abundant in external secretions, binds sIgA which may further entrap sIgA coated virus in the mucus layer, preventing infection27,28, IgG antibodies also activate complement which induces inflammation and alters the integrity of the mucosal barrier. An in vitro study, in which the Fv region of an IgG anti-Clostridium difficile toxin A antibody was expressed as a monomeric IgA, dimeric IgA or IgG, clearly demonstrated that the superiority of dimeric IgA (pIgA) were over monomeric IgA or IgG, such that pIgA was six times more potent29. IgA antibodies provide protection against a number of viral infections including respiratory synctial virus30, human papilloma virus31, and rotavirus32. The data in this research have identified the potential of IgA anti-HIV antibodies in preventing HIV infection.
An important activity of antibodies is to participate with effector cells to destroy virus or virus infected cells. It is particularly important given the NK cell and neutrophil presence at mucosal surfaces acts as a first line of defense against pathogens. Traditionally, a four hour cytotoxicity assay has been used to measure antibody dependent cellular cytotoxicity; however, this assay has been adapted to measure viral inhibition as described by Forthal, et al13 as ADCVI or antibody-dependent cell-mediated viral inhibition. Although traditional ADCVI (or ADCC) assays are based on mononuclear cell populations, neutrophils are in fact an important component of the circulating ADCC-capable population. Neutrophils are the most prevalent leukocyte in breast milk and in circulation. They have both FcRγ and FcRα which mediate ADCVI for both isotypes. They are also non-infectable; therefore, the relative contribution of the IgA2 variants to ADCC can be compared to the parental IgG1 in neutrophils.
This study was a significant exploration to generate an immunotherapy to prevent breast milk transmission of HIV. Compared to CHO derived b12 isotype variants, immunoreactivity of b12A2 expressed in milk was retained. When tested for neutralization, milk derived b12 IgA2 was at least comparable to CHO derived antibody and in some cases superior to CHO derived antibody. Furthermore, milk expressed b12 IgA2 was significantly more effective at mediating antibody dependent cell killing. The value of the research is to not only prove that milk derived b12A2 could provide neutralizing activity, but also suggests that the means (active and/or passive) to induce such antibodies should be explored to prevent breast milk transmission of HIV. A passive immunization experiment in which an infusion of HIV hyperimmune globulin was given to pregnant women and their newborns was found safe and well-tolerated, though little efficacy to prevent MTCT was found33,34. Our study suggests that antibody isotypes may affect neutralizing activity which impacts passive immunotherapy. It may be possible to further augment the neutralization activity of broad neutralizing monoclonal antibody candidates such as PG16, P19, VRC01 and VRC02), to provide a more effective passive immunotherapeutic to prevent MTCT.
Acknowledgments
We are grateful for the generous contributions to this work of b12 genes provided by Dr. Dennis Burton (The Scripps Research Institute, Lo Jolla, CA), Ig expression vector from Dr. Gary McLean (University of Texas Health Sciences Center, Houston, TX) and IgA2 vector provided by Sherrie Morrison (University of California Los Angeles). We are also grateful to AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, as well as Dr. Jay Levy, Dr. Neal Halsey; Dr. Ronald Collman, Dr. Suzanne Gartner, Dr. Mikulas Popovic, Dr. Robert Gallo; Dr. Robert Bollinger, Dr. Irvin Chen for supporting HIV strain and Dr. John C. Kappes, Dr. Xiaoyun Wu, Dr. David Montefiori for providing TZM-bl cells. We also thank Miss Melissa Gawron for her efforts on editing and revising the manuscript.
This work was supported in part by Public Health Service grants AI63986, AI75932.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial conflict of interest.
References
- 1.Becquart P, et al. Compartmentalization of the IgG immune response to HIV-1 in breast milk. Aids. 1999;13:1323–1331. doi: 10.1097/00002030-199907300-00008. [DOI] [PubMed] [Google Scholar]
- 2.Duprat C, et al. Human immunodeficiency virus type 1 IgA antibody in breast milk and serum. Pediatr Infect Dis J. 1994;13:603–608. doi: 10.1097/00006454-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Becquart P, et al. Secretory anti-human immunodeficiency virus (HIV) antibodies in colostrum and breast milk are not a major determinant of the protection of early postnatal transmission of HIV. J Infect Dis. 2000;181:532–539. doi: 10.1086/315255. [DOI] [PubMed] [Google Scholar]
- 4.Van de Perre P, et al. Infective and anti-infective properties of breastmilk from HIV-1-infected women. Lancet. 1993;341:914–918. doi: 10.1016/0140-6736(93)91210-d. [DOI] [PubMed] [Google Scholar]
- 5.Mantis NJ, et al. Inhibition of HIV-1 infectivity and epithelial cell transfer by human monoclonal IgG and IgA antibodies carrying the b12 V region. J Immunol. 2007;179:3144–3152. doi: 10.4049/jimmunol.179.5.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roben P, et al. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duval M, Posner MR, Cavacini LA. A bispecific antibody composed of a nonneutralizing antibody to the gp41 immunodominant region and an anti-CD89 antibody directs broad human immunodeficiency virus destruction by neutrophils. J Virol. 2008;82:4671–4674. doi: 10.1128/JVI.02499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei X, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miranda L, et al. The neutralization properties of a HIV-specific antibody are markedly altered by glycosylation events outside the antigen-binding domain. J Immunol. 2007;178:7132–7138. doi: 10.4049/jimmunol.178.11.7132. [DOI] [PubMed] [Google Scholar]
- 10.Huang MT, Gorman CM. Intervening sequences increase efficiency of RNA 3' processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 1990;18:937–947. doi: 10.1093/nar/18.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krugmann S, Pleass RJ, Atkin JD, Woof JM. Structural requirements for assembly of dimeric IgA probed by site-directed mutagenesis of J chain and a cysteine residue of the alpha-chain CH2 domain. J Immunol. 1997;159:244–249. [PubMed] [Google Scholar]
- 12.Sorensen V, Rasmussen IB, Sundvold V, Michaelsen TE, Sandlie I. Structural requirements for incorporation of J chain into human IgM and IgA. Int Immunol. 2000;12:19–27. doi: 10.1093/intimm/12.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Forthal D, Gilbert P, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178:6596–6603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 14.Mascola JR, et al. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascola J, et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–40018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 17.Ferrantelli F, et al. Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS. 2003;17:201–209. doi: 10.1097/00002030-200302140-00003. [DOI] [PubMed] [Google Scholar]
- 18.Baba T, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 19.Ferrantelli F, et al. Complete protection of neonatal rhesus macaques against oral exposure to pathogenic simian-human immunodeficiency virus by human anti-HIV monoclonal antibodies. J Infect Dis. 2004;189:2167–2173. doi: 10.1086/420833. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann-Lehmann R, et al. Postnatal pre- and postexposure passive immunization strategies: protection of neonatal macaques against oral simian-human immunodeficiency virus challenge. J Med Primatol. 2002;31:109–119. doi: 10.1034/j.1600-0684.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann-Lehmann R, et al. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J Virol. 2001;75:7470–7480. doi: 10.1128/JVI.75.16.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann-Lehmann R, R R, Vlasak J, Smith BA, Baba T, Liska V, Montefiori DC, McClure HM, Anderson DC, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Katinger H, Stiegler G, Posner MR, Cavacini LA, Chou T, Ruprecht RM. Passive immunization against oral AIDS virus transmission: An approach to prevent mother-to-infant transmission? Journal of Medical Primatology. 2001;30:190–196. doi: 10.1034/j.1600-0684.2001.d01-52.x. [DOI] [PubMed] [Google Scholar]
- 23.Pilgrim A, et al. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 24.Zhou JY, Montefiori DC. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey J, et al. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active anti-retroviral therapy. J Virol. 2006;80:4758–4770. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4:590–597. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 27.Biesbrock AR, Reddy MS, Levine MJ. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991;59:3492–3497. doi: 10.1128/iai.59.10.3492-3497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnusson KE, Stjernstrom I. Mucosal barrier mechanisms. Interplay between secretory IgA (SIgA), IgG and mucins on the surface properties and association of salmonellae with intestine and granulocytes. Immunology. 1982;45:239–248. [PMC free article] [PubMed] [Google Scholar]
- 29.Stubbe H, Berdoz J, Kraehenbuhl J-P, Corthesy B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J Immunol. 2000;164:1952–1960. doi: 10.4049/jimmunol.164.4.1952. [DOI] [PubMed] [Google Scholar]
- 30.Walsh E, Falsey A. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 31.Rocha-Zavaleta L, Barrios t, Garcia-Carranca A, Valdespino V, Cruz-Talonia F. Cervical secretory immunoglobulin A to human papillomavirus type 16 (HPV-16) from HPV-16 infected women inhibit HPV16 virus-like particle-induced hemagglutination of mouse red blood cells. FEMS Immunol Med Microbiol. 2001;31:47–51. doi: 10.1111/j.1574-695X.2001.tb01585.x. [DOI] [PubMed] [Google Scholar]
- 32.Feng N, et al. Inhibition of rotavirus replication by a non-neutralizing, rotavirus VP6-specific IgA mAb. J Clin Invest. 2002;109:1203–1213. doi: 10.1172/JCI14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mofenson LM. Prevention of mother-to-child HIV-1 transmission--why we still need a preventive HIV immunization strategy. J Acquir Immune Defic Syndr. 2011;58:359–362. doi: 10.1097/QAI.0b013e318235517e. [DOI] [PubMed] [Google Scholar]
- 34.Onyango-Makumbi C, et al. Safety and efficacy of HIV hyperimmune globulin for prevention of mother-to-child HIV transmission in HIV-1-infected pregnant women and their infants in Kampala, Uganda (HIVIGLOB/NVP STUDY) J Acquir Immune Defic Syndr. 2011;58:399–407. doi: 10.1097/QAI.0b013e31822f8914. [DOI] [PMC free article] [PubMed] [Google Scholar]