Abstract
OBJECTIVE
We sought to establish the frequency of receiving >1 dose of epinephrine in children who present to the emergency department (ED) with food-related anaphylaxis.
PATIENTS AND METHODS
We performed a medical chart review at Boston hospitals of all children presenting to the ED for food-related acute allergic reactions between January 1, 2001, and December 31, 2006. We focused on causative foods, clinical presentations, and emergency treatments.
RESULTS
Through random sampling and appropriate weighting, the 605 reviewed cases represented a study cohort of 1255 patients. These patients had a median age of 5.8 years (95% confidence interval [CI]: 5.3– 6.3), and the cohort was 62% male. A variety of foods provoked the allergic reactions, including peanuts (23%), tree nuts (18%), and milk (15%). Approximately half (52% [95% CI: 48–57]) of the children met diagnostic criteria for food-related anaphylaxis. Among those with anaphylaxis, 31% received 1 dose and 3% received >1 dose of epinephrine before their arrival to the ED. In the ED, patients with anaphylaxis received antihistamines (59%), corticosteroids (57%), epinephrine (20%). Over the course of their reaction, 44% of patients with food-related anaphylaxis received epinephrine, and among this subset of patients, 12% (95% CI: 9–14) received >1 dose. Risk factors for repeat epinephrine use included older age and transfer from an outside hospital. Most patients (88%) were discharged from the hospital. On ED discharge, 43% were prescribed self-injectable epinephrine, and only 22% were referred to an allergist.
CONCLUSIONS
Among children with food-related anaphylaxis who received epinephrine, 12% received a second dose. Results of this study support the recommendation that children at risk for food-related anaphylaxis carry 2 doses of epinephrine.
Keywords: food allergy, anaphylaxis, emergency department, epinephrine
Food-related anaphylaxis is defined as an immunoglobulin E–mediated hypersensitivity reaction to an ingested food, which results in the rapid onset of multisystem and potentially life-threatening symptoms. Food allergies affect as many as 6% of children in developed countries, and by most estimates, this prevalence seems to be rising. 1 The Centers for Disease Control and Prevention recently reported that in 2007, ~3 million school-aged children in the United States had food allergies. This represents an 18% increase since 1997.2 The results of recent studies also support a dramatic rise in the incidence of anaphylaxis,3 and food allergy is the leading cause of anaphylaxis in children.4,5
Current practice guidelines recommend that all patients suspected of having an episode of food-related anaphylaxis be referred to an allergist, instructed to avoid the suspected food allergen, and prescribed self-injectable epinephrine.6,7 Consultation with an allergist will assist in identifying the offending food through careful history-taking and appropriate diagnostic testing. All patients with food allergies should be educated on the importance of vigilant avoidance of the responsible food allergen and readiness to treat allergic reactions in the event of unintentional exposure. The primary treatment for food-related anaphylaxis, similar to that for all other forms of anaphylaxis, is the prompt administration of epinephrine. Therefore, all patients with a history of food-related anaphylaxis should be prescribed and taught how to use self-injectable epinephrine, which delivers a 1-time premeasured dose of intramuscular epinephrine.6,7
The results of several small studies have suggested that it may be advisable for children with a history of food-related anaphylaxis to carry multiple doses of self-injectable epinephrine, 8–11 a recommendation that has important economic and logistic implications. Therefore, we sought to more accurately define the likelihood of receiving >1 dose of epinephrine for food-related anaphylaxis and to characterize the children for whom this was medically necessary.
PATIENTS AND METHODS
This multicenter medical chart review was performed as a part of the Multicenter Airway Research Collaboration, a division of the Emergency Medicine Network (www.emnet-usa.org). This study was an extension of an earlier pilot study.10 We have extended our review to encompass 2001–2006 at the pediatric emergency departments (EDs) at both Massachusetts General Hospital and Children’s Hospital Boston. The study was approved by the institutional review boards at both centers.
Patient Selection
We searched for all children who presented to the ED between January 1, 2001, and December 31, 2006, with a food-related acute allergic reaction using relevant International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes.5,12 These codes included 995.60 (anaphylactic shock because of unspecified food), 995.61–995.69 (anaphylactic shock due to specified food), 995.0 (other anaphylactic shock), 693.1 (dermatitis due to food), 995.7 (adverse food reaction, not otherwise classified), 558.3 (allergic gastroenteritis), and 692.5 (contact dermatitis due to food). In addition, random samplings of the codes 995.3 (allergy, unspecified), 995.1 (angioedema), and 708.X (urticaria) were reviewed to identify cases of food-related acute allergic reactions within nonspecific allergy codes. Patients younger than 18 years were included in this study.
Data Collection
A structured chart review was performed to collect the following data: patient demographics, medical history, clinical presentation, pre-ED and ED therapy, and disposition. The charts were reviewed by 2 physicians, including a pediatric allergist. The study team met monthly to discuss progress and resolve questions about data abstraction.
Definitions
A food-related acute allergic reaction was defined as an acute episode of immunoglobulin E–mediated symptoms in which the onset was temporally related to a known or suspected food allergen. Anaphylaxis was defined on the basis of the diagnostic criteria established by the Second Symposium of the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network.6 Specifically, food-related anaphylaxis was defined as an acute allergic reaction involving 2 or more organ systems or hypotension alone after exposure to a likely food allergen. Hypotension was defined as a systolic blood pressure of <(70mm Hg + [age × 2]) for children <10 years old and <90 mm Hg for children 10 to 18 years old.6
Statistical Analysis
We used a stratified sampling method to reflect the population of patients within each International Classification of Diseases, Ninth Revision, Clinical Modification code. By using the survey module in Stata 10.0 (Stata Corp, College Station, TX), sample weights were assigned to account for unequal probabilities of selection, oversampling, and nonresponse. Data are expressed as mean ± SE and proportion (95% confidence interval [CI]). Comparisons between nonanaphylaxis and anaphylaxis groups were by evaluated using χ2 tests. Because of the relatively small number of children with the main outcome (ie, receipt of >1 dose of epinephrine), multivariable analysis was performed by using unweighted visit numbers to explore risk factors for repeat epinephrine use. A 2-sided P value of <.05 was considered statistically significant.
RESULTS
Overview
Within the 6-year period, we reviewed the medical charts of 605 children who presented to the ED with a food-related acute allergic reaction. With appropriate weighting, this represented a study cohort of 1255 patients. Approximately half (52% [95% CI: 48 –57]) of these cases met criteria for food-related anaphylaxis. There were no fatal cases identified.
Demographic Factors
The patients were predominantly male (62%). The mean age was 5.8 years, and the majority of the children were white. In comparison to patients whose presentation did not meet criteria for anaphylaxis, patients with anaphylaxis were older and less frequently from minority race/ethnic groups. The gender distribution between these 2 groups was not significantly different (Table 1).
TABLE 1.
Demographic Characteristics and Medical History of Children Who Presented to the ED With a Food-Related Acute Allergic Reaction
| Overall (N = 1255), % (95% CI) | No Anaphylaxis (N = 597), % (95% CI) | Anaphylaxis (N = 658), % (95% CI)a | P | |
|---|---|---|---|---|
| Demographic characteristic, n (%) | ||||
| Age, median, y (95% CI) | 5.8 (5.3–6.3) | 4.8 (4.1–5.45) | 6.7 (5.9–7.4) | .003 |
| Male | 62 (57–66) | 62 (55–69) | 62 (56–68) | .97 |
| Race/ethnicity | .02 | |||
| White | 40 (35–44) | 31 (25–38) | 47 (41–53) | |
| Black | 27 (23–32) | 31 (24–38) | 24 (19–29) | |
| Hispanic | 12 (9–16) | 16 (10–22) | 9 (6–12) | |
| Asian | 9 (6–12) | 10 (5–14) | 8 (5–12) | |
| Other race | 12 (8–15) | 12 (7–17) | 12 (7–16) | |
| Medical history, n (%) | ||||
| Known allergy to offending allergen | 41 (36–46) | 37 (29–44) | 44 (38–51) | .11 |
| Known allergic problems | 67 (63–72) | 56 (49–64) | 77 (73–82) | <.001 |
| Previous allergic reactions to other sources | 62 (57–68) | 59 (49–69) | 64 (57–71) | .39 |
| Asthma | 41 (35–47) | 31 (22–40) | 48 (40–55) | .008 |
| Hay fever | 20 (15–25) | 16 (8–23) | 23 (16–29) | .16 |
| Eczema | 33 (27–39) | 37 (27–47) | 31 (24–37) | .33 |
| Hives | 1 (0–2) | 2 (0–5) | 0 | .12 |
| Angioedema | 0 | 0 | 0 | — |
| Patient owns EpiPen | 40 (34–45) | 30 (23–37) | 48 (41–55) | .001 |
| Other documented medical problems | 11 (8–14) | 9 (5–14) | 13 (9–17) | .25 |
| Patient on any chronic medications | 37 (33–42) | 26 (19–32) | 48 (42–55) | <.001 |
Anaphylaxis was defined as an allergic reaction involving ≥2 organ systems or hypotension. Hypotension was defined as systolic blood pressure of less than (70 + [age × 2]) for children younger than 10 years and systolic blood pressure of <90 mm Hg for children aged 10 to 18 years.
Atopic Disease
A history of atopic disease at presentation was common. Forty-one percent of all patients reported a known allergy to the offending food, and a similar percentage (40%) owned a self-injectable epinephrine device before their presentation to the ED. Sixty-seven percent of patients reported another known allergic problem, including allergic reactions to other sources (ie, other foods, medications, venom, or latex) (62%), asthma (41%), or eczema (33%). Patients with anaphylaxis more commonly reported a known allergic problem, especially asthma. A greater percentage of patients with anaphylaxis owned a self-injectable epinephrine device before their presentation to the ED (Table 1). Compared with patients who received 1 dose of epinephrine, patients who received >1 dose of epinephrine more commonly had a known allergy to the offending food (47% vs 69%; P = .02).
Setting and Allergens
Home was the most common setting for exposure to the inciting food allergen (70%). Patients were also exposed at school or day care (12%), restaurants (9%), and other locations (9%). Peanuts (23%), tree nuts (18%), and milk (15%) were the most common triggers for all food-related acute allergic reactions. The specific food allergens were not significantly different in the anaphylaxis versus nonanaphylaxis groups, with 2 exceptions. Specifically, a greater percentage of anaphylactic reactions were caused by milk, whereas a smaller percentage were caused by eggs (Table 2).
TABLE 2.
Presentation and Clinical Course of Children Who Presented to the ED With a Food-Related Acute Allergic Reaction
| Overall (N = 1255), % (95% CI) | No Anaphylaxis (N = 597), % (95% CI) | Anaphylaxis (N = 658), % (95% CI)a | P | |
|---|---|---|---|---|
| Arrive to ED by ambulance | 34 (30–39) | 25 (19–32) | 43 (37–49) | <.001 |
| Time since exposure | <.001 | |||
| <1 h | 20 (15–24) | 22 (15–29) | 17 (12–23) | |
| 1–3 h | 59 (53–64) | 51 (42–60) | 66 (59–72) | |
| 4–6 h | 9 (6–11) | 5 (2–9) | 12 (8–16) | |
| 7–12 h | 2 (0.4–4) | 3 (0–6) | 1 (0.4–1) | |
| >12 h | 11 (7–15) | 18 (11–25) | 4 (1–7) | |
| Location of exposure | .18 | |||
| Home | 70 (65–75) | 72 (64–80) | 69 (62–75) | |
| School/day care | 12 (8–16) | 15 (7–21) | 10 (6–14) | |
| Restaurant | 9 (6–13) | 7 (3–11) | 11 (6–16) | |
| Other | 9 (6–12) | 7 (3–11) | 10 (6–14) | |
| Location immediately before ED arrival | .001 | |||
| Home | 72 (67–77) | 77 (69–85) | 68 (62–73) | |
| School/day care | 11 (7–14) | 13 (7–20) | 8 (5–12) | |
| Restaurant | 3 (1–5) | 2 (0–4) | 4 (1–7) | |
| Doctor’s office/clinic | 5 (3–8) | 4 (0–9) | 6 (3–9) | |
| Outside hospital | 5 (3–7) | 2 (0–4) | 8 (4–11) | |
| Other | 4 (2–6) | 2 (0–4) | 6 (3–9) | |
| Specific food trigger that caused current reaction | ||||
| Peanuts | 23 (19–27) | 23 (17–29) | 23 (18–28) | .96 |
| Tree nuts | 18 (14–22) | 17 (11–22) | 19 (15–24) | .49 |
| Seeds | 1 (0–3) | 0 | 2 (0–5) | — |
| Fruits and vegetables | 11 (8–15) | 15 (9–20) | 9 (5–13) | .07 |
| Shellfish | 7 (5–10) | 8 (4–12) | 7 (4–10) | .86 |
| Fish | 4 (2–7) | 5 (1–8) | 4 (1–7) | .79 |
| Food additives | 1 (0–2) | 1 (0–3) | 1 (0–3) | .91 |
| Milk products | 15 (12–19) | 11 (6–16) | 19 (14–24) | .02 |
| Eggs | 8 (5–11) | 12 (6–17) | 5 (2–7) | .02 |
| Wheat | 1 (0.1–2) | 1 (0–3) | NC | — |
| Other foodb | 19 (15–23) | 17 (12–23) | 21 (15–26) | .45 |
| Signs and symptoms | ||||
| Skin rash | 76 (72–80) | 79 (73–85) | 73 (68–79) | .18 |
| Itching | 33 (28–37) | 32 (25–39) | 33 (27–39) | .80 |
| Swelling | 52 (47–57) | 56 (49–64) | 48 (42–54) | .11 |
| Angioedema | 3 (2–4) | 3 (1–6) | 3 (2–4) | .76 |
| Trouble swallowing | 14 (11–18) | NC | 27 (21–33) | — |
| Trouble breathing/shortness of breath | 23 (19–26) | 2 (0.2–4) | 41 (35–47) | <.001 |
| Wheezing | 21 (18–25) | 3 (1–5) | 38 (32–43) | <.001 |
| Hoarse voice | 6 (4–8) | NC | 11 (7–16) | — |
| Stridor | 3 (2–4) | NC | 6 (3–8) | — |
| Nausea/vomiting | 19 (16–23) | 2 (0–5) | 35 (29–41) | <.001 |
| Abdominal pain/cramps | 3 (2–5) | 1 (0–2) | 5 (3–8) | .01 |
| Diarrhea | NC | 0 | NC | — |
| Dizziness/fainting | 2 (1–3) | 0 | 3 (1–5) | .004 |
| Altered mental status | 1 (0.2–2) | 0 | 2 (0.4–4) | .02 |
| Organ system involvement | ||||
| Respiratory | 38 (34–43) | 4 (1–6) | 70 (64–76) | <.001 |
| Cutaneous | 93 (91–95) | 93 (89–97) | 93 (90–96) | .84 |
| Gastrointestinal | 30 (26–35) | 2 (0–5) | 56 (49–62) | <.001 |
| Cardiac | 3 (1–4) | 0 | 5 (3–8) | <.001 |
NC indicates noncalculable.
Anaphylaxis was defined as an allergic reaction involving ≥2 organ systems or hypotension. Hypotension was defined as systolic blood pressure of less than (70 + [age × 2]) for children younger than 10 years and systolic blood pressure of <90 mm Hg for children aged 10 to 18 years.
Other foods included less frequently reported foods (eg, soy or barley) and foods with multiple potential allergens (eg, cookies or pizza).
Clinical Features
The majority of patients (80%) presented within 3 hours of exposure to the food allergen. Patients with anaphylaxis more commonly arrived by ambulance and presented to the ED earlier than patients without anaphylaxis. The clinical presentation of almost all patients (93%) included cutaneous signs and/or symptoms. Among the patients who met criteria for anaphylaxis, cardiac involvement was rare (5%). However, respiratory (70%) and gastrointestinal (56%) signs/symptoms were common (Table 2). More patients who received >1 dose of epinephrine reported difficulty breathing than patients who received 1 dose of epinephrine (71% vs 51%; P = .02).
Therapy
A slight majority of patients (61%) received treatment before their arrival to the ED. Among the patients who received pre-ED treatment, 84% received antihistamines, 40% received epinephrine, and 13% received inhaled β-agonists (Table 3). Patients who received pre-ED epinephrine most frequently were administered their own self-injectable epinephrine device (72%). Twenty-three percent of all patients received 1 dose of epinephrine, and 2% received >1 dose before their arrival to the ED. Among the subset of patients with food-related anaphylaxis, these percentages were slightly greater (31% and 3%, respectively) (Table 3).
TABLE 3.
Treatments Received by Children Who Presented to the ED With a Food-Related Acute Allergic Reaction
| Overall (N = 1255), % (95% CI) | No Anaphylaxis (N = 597), % % (95% CI) | Anaphylaxis (N = 658), % (95% CI)a | P | |
|---|---|---|---|---|
| Pre-ED treatments | ||||
| Pre-ED treatments (<3 h before triage) | 61 (56–66) | 52 (45–60) | 69 (63–75) | .001 |
| Epinephrine | 40 (34–46) | 27 (18–36) | 49 (41–56) | .001 |
| Benadryl | 77 (71–83) | 74 (64–84) | 79 (72–85) | .44 |
| Other antihistamines | 7 (4–10) | 7 (2–12) | 7 (3–12) | .88 |
| Steroids | 8 (6–10) | 2 (1–2) | 12 (9–16) | <.001 |
| Intravenous fluids | 2 (1–2) | 1 (0–1) | 2 (2–3) | .02 |
| Inhaled 3-agonists | 13 (10–17) | 4 (1–7) | 20 (15–25) | <.001 |
| Oxygen | 1 (1–2) | 0 | 2 (1–3) | <.001 |
| Other | 6 (3–10) | 9 (1–16) | 5 (1–8) | .27 |
| No. of pre-ED epinephrine doses | <.001 | |||
| 0 | 76 (72–79) | 86 (81–91) | 66 (61–72) | |
| 1 | 23 (19–27) | 14 (9–19) | 31 (25–36) | |
| >1 | 2 (1–2) | 0 | 3 (2–3) | |
| Administrator of pre-ED epinephrine | ||||
| Patient’s own device | 72 (66–77) | 86 (79–92) | 66 (58–74) | .002 |
| Emergency medical services | 14 (10–18) | NC | 16 (11–22) | — |
| Outside hospital | 10 (7–12) | NC | 11 (8–15) | — |
| Doctor’s office/clinic | 8 (4–12) | NC | NC | — |
| ED treatments | ||||
| Oxygen | 2 (1–3) | NC | 3 (2–5) | — |
| Intravenous line established | 19 (17–21) | 7 (5–8) | 30 (26–34) | <.001 |
| Intravenous fluids given | 22 (16–28) | 13 (6–19) | 23 (17–30) | .03 |
| No. of epinephrine doses given in ED | <.001 | |||
| 0 | 86 (84–89) | 93 (89–97) | 80 (76–84) | |
| 1 | 13 (11–16) | 7 (3–11) | 19 (15–23) | |
| >1 | 0.3 (0.2–0.4) | 0 | 1 (0.4–1) | |
| ED antihistamines given | 60 (55–65) | 61 (53–68) | 59 (53–65) | .73 |
| Benadryl | 92 (89–94) | 99 (98–99) | 85 (80–90) | <.001 |
| Other H1 blockers | 1 (0–2) | NC | 2 (0–4) | .02 |
| H2 blockers | 20 (16–24) | 7 (4–10) | 33 (26–40) | <.001 |
| ED steroids given | <.001 | |||
| Prednisone | 28 (24–33) | 19 (14–25) | 37 (31–42) | |
| Methylprednisolone (Solu-Medrol) | 10 (8–11) | 4 (2–6) | 15 (12–18) | |
| Other | 3 (2–4) | 1 (0.6–1) | 5 (2–7) | |
| None | 59 (54–63) | 75 (69–82) | 43 (37–50) | |
| Inhaled β-agonists given | 8 (6–9) | 2 (0.4–3) | 14 (11–16) | <.001 |
| Inhaled anticholinergics given | 1 (1–1) | NC | 1 (1–2) | — |
| Additional medications given | 2 (1–3) | NC | 4 (2–6) | — |
| Overall treatments | ||||
| Total No. of epinephrine doses given | <.001 | |||
| 0 | 64 (59–68) | 79 (73–85) | 50 (44–56) | |
| 1 | 33 (29–38) | 21 (15–27) | 44 (38–50) | |
| >1 | 3 (2–4) | 0 | 6 (4–7) | |
| Total No. of epinephrine doses given among patients receiving any epinephrine | <.001 | |||
| 1 | 92 (90–94) | 100 | 88 (86–91) | |
| >1 | 8 (6–10) | 0 | 12 (9–14) |
NC indicates non-calculable; H1, histamine 1; H2, histamine 2.
Anaphylaxis was defined as an allergic reaction involving ≥2 organ systems or hypotension. Hypotension was defined as systolic blood pressure of less than (70 + [age × 2]) for children younger than 10 years and systolic blood pressure of <90 mm Hg for children aged 10 to 18 years.
In the ED, 20% of the patients with food-related anaphylaxis received epinephrine, and 1% received >1 dose. Epinephrine was most frequently given subcutaneously (74%). Patients with anaphylaxis frequently received other types of medications, including anti-histamines (59%), corticosteroids (56%), intravenous fluids (23%), and inhaled β-agonists (13%) (Table 3).
Over the course of their reaction, 44% of the patients with food-related anaphylaxis received 1 dose, and 6% received >1 dose of epinephrine. Accordingly, among patients with food-related anaphylaxis who received epinephrine, 12% (95% CI: 9 –14) received >1 dose (Fig 1). Most patients (59%) received the second dose within 1 hour of the first dose. Multivariable analysis (Table 4) revealed that children >10 years old and those treated at an outside hospital were more likely to receive >1 dose of epinephrine. Patients who owned a self-injectable epinephrine device were not statistically more likely to receive >1 dose of epinephrine.
FIGURE 1.
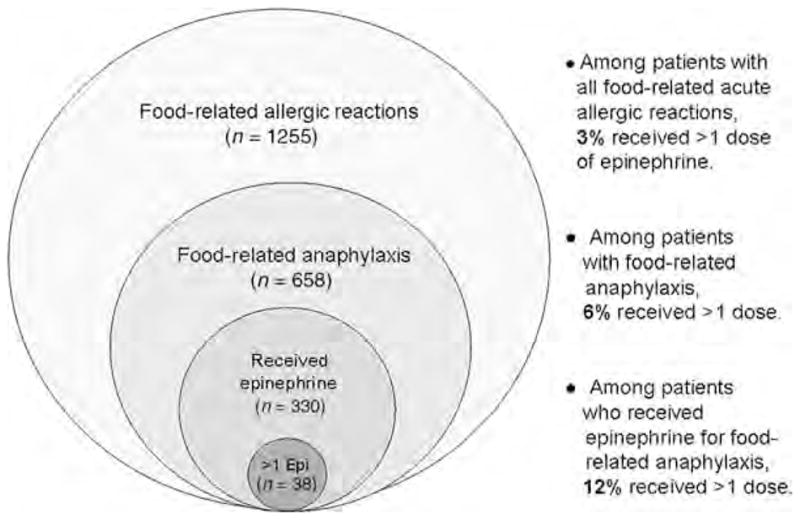
Percentages of patients who received >1 dose of epinephrine within different population groups.
TABLE 4.
Multivariable Model of Factors Associated With Number of Treatments With Epinephrine Among Children Who Presented to the ED With Food-Related Anaphylaxis
| Odds Ratio | 95% CI | P | |
|---|---|---|---|
| Age group | |||
| <5 y | 1.0 (reference) | ||
| 5–10 y | 1.7 | 0.5–5.7 | .39 |
| >10 y | 3.9 | 1.4–11.0 | .01 |
| Patient owns EpiPen | 1.6 | 0.6–4.0 | .36 |
| Outside hospital location immediately before ED arrival | 5.3 | 1.9–15.2 | .002 |
Anaphylaxis was defined as an allergic reaction involving ≥2 organ systems or hypotension. Hypotension was defined as systolic blood pressure of less than (70 + [age × 2]) for children younger than 10 years and systolic blood pressure of <90 mm Hg for children aged 10 to 18 years.
Disposition
Most patients (88%) were discharged from the hospital. Among all discharged patients, 36% (95% CI: 30–42) received instructions to avoid the offending allergen, 43% (95% CI: 37– 49) were prescribed a self-injectable epinephrine device, and 22% (95% CI: 17– 27) were referred to an allergist. When comparing patients with and without anaphylaxis, the frequencies of food-avoidance instructions (35% vs 38%; P = .64) and allergy referrals (21% vs 24%; P = .60) were not significantly different. However, patients with anaphylaxis were more frequently prescribed self-injectable epinephrine (38% vs 51%; P = .04). Among those patients who met diagnostic criteria for anaphylaxis, only 14% ([95% CI: 10– 17]) of patients were assigned an ED discharge diagnosis that included the term “anaphylaxis.”
The remaining patients (22%) were admitted to the floor, the ICU, or an observation unit. Patients with anaphylaxis were more frequently admitted (35% vs 7%; P ≤ .001). In contrast to the patients discharged from the ED, 68% (95% CI: 62–74) of all the admitted patients received food-avoidance instructions, 94% (95% CI: 89 –98) were prescribed self-injectable epinephrine, and 69% (95% CI: 63–75) were referred to an allergist. The majority of patients with anaphylaxis who were admitted to the hospital were assigned a discharge diagnosis that included the term “anaphylaxis” (79%).
DISCUSSION
The results of this study provide a comprehensive review of food-related anaphylaxis in 2 pediatric EDs over a 6-year period. To date, it represents (to our knowledge) the largest study of the causative agents, clinical features, and emergency treatments of food-related anaphylaxis reported in children. Despite the emergent nature of anaphylaxis and its potential for morbidity and mortality, there have been sparse data on the epidemiology, management, and outcome of this disease. Historically, the study of anaphylaxis has been complicated for several reasons. First, the definition of anaphylaxis has been highly variable, and an accepted clinical definition was lacking until recently.13 Second, the presentations of anaphylaxis are varied and can mimic those of other disorders. Third, it was shown previously that when symptoms do not seem life-threatening, physicians are less likely to categorize even multisystem complaints as anaphylaxis.14 Cognizant of these potential pitfalls, we have reviewed a broad range of allergy-related diagnosis codes to capture cases that meet diagnostic criteria for food-related anaphylaxis irrespective of the diagnosis code assigned.
The primary treatment of food-related anaphylaxis is epinephrine, and its prompt administration is recommended by all current practice guidelines. 6,7 Estimates suggest that there are ~150 to 200 deaths each year that result from food-related anaphylaxis,15 and previous studies have shown that delayed or lack of administration of epinephrine is associated with an increased risk of morbidity and mortality. 15,16 The results of our study indicate that food-related anaphylaxis continues to be underrecognized and inadequately treated in the ED setting. Only 13% of cases that met the criteria for anaphylaxis were assigned an ED discharge diagnosis that included the term “anaphylaxis.” Half of the patients with food-related anaphylaxis did not receive epinephrine either before their arrival or while in the ED. In the ED, epinephrine was most frequently administered subcutaneously, although current guidelines indicate that the optimal route of administration is intramuscular.6,17 In addition, patients received both antihistamines and corticosteroids more frequently than epinephrine despite the lack of evidence for their utility as first-line treatments of anaphylaxis.18
Results of several studies have suggested that it may be advisable for children with a history of food-related anaphylaxis to carry multiple doses of self-injectable epinephrine. Most recently, Järvinen et al8 reported, on the basis of questionnaire data within a referral population, that 19% of food-related anaphylactic reactions in children were treated with >1 dose of epinephrine. Similarly, survey data of 113 patients with food-related acute allergic reactions in the United Kingdom revealed that among children who received epinephrine, 10% reported receiving a second dose.9 Also, Oren et al10 reviewed 39 cases of children and adults with food-related allergic reactions who presented to the Massachusetts General Hospital ED and found that 16% were treated with >1 dose of epinephrine.
We describe a similar percentage of patients who received multiple doses of epinephrine as in these previous smaller reports. However, we note that this percentage is highly dependent on the population being evaluated. Among all the patients who presented to the ED with a food-related acute allergic reaction, only 3% received >1 dose of epinephrine over the course of their reaction. When the population is narrowed to those with food-related anaphylaxis, 6% of patients received >1 dose. Among patients with anaphylaxis who received epinephrine, 12% received >1 dose (Fig 1). We found that older children and those transferred from outside hospitals were at greater risk for receiving >1 epinephrine treatment; however, other risk factors remain unclear. Until these risk factors are better understood, it may be advisable to prescribe multiple doses of self-injectable epinephrine to all patients at risk of food-related anaphylaxis, especially those in settings where access to emergency care is less readily available.19
Previous reviews have cited risk factors of food-related anaphylaxis in childhood to include older age, asthma, peanut/tree nut allergy, and previous reactions involving the respiratory tract.20 Our population of patients with food-related anaphylaxis was significantly older than those with food-related allergic reactions that did not meet criteria for anaphylaxis. Multivariate analysis also supported that children older than 10 years were more likely to receive >1 dose of epinephrine. This may represent increased difficulties in symptom recognition in younger children or a true divergence in anaphylaxis risk.
Our data indicate that children with food-related anaphylaxis more commonly had a history of atopic disease, especially asthma. This is consistent with previous studies that have found that asthmatic children may have more severe food-related allergic reactions. 15 In addition, approximately half (44%) of the children with anaphylaxis had a known allergy to the offending food, and this percentage was even higher among patients who received >1 dose of epinephrine (69%). This highlights the high incidence of unintentional exposures and importance of providing patients with appropriate food-avoidance education. This recommendation is supported by the results of other studies, which have revealed that the majority of fatal reactions occurred in individuals who were aware of their food allergies but believed they were eating something safe.21
Our results confirm previous findings that the most common food allergens in children include peanuts, tree nuts, milk, egg, fish, and shellfish.22 However, reactions to peanuts or tree nuts were not more frequent in our patients with anaphylaxis, which could suggest that the potential for anaphylaxis is not allergen-specific. It was surprisingly that 11% of reactions in our review were reported to be triggered by a fruit or vegetable, foods typically believed to have low allergenicity. This may reflect the difficulty in deciphering the specific trigger of a food-related allergic reaction at the time of the event and underscore the need for referral to an experienced allergist.
Although home was the most common setting for exposure, approximately one-third of cases occurred in other locations, which emphasizes the need for food-allergic patients to have emergency medications available at all times. The location of exposure was not associated with multiple epinephrine treatment (data not shown), but patients who were transferred from an outside hospital were more likely to receive >1 dose of epinephrine. This association most likely reflects the severity of their presentation rather than being a risk factor for multiple epinephrine doses per se.
Our findings support previous observations regarding trends in symptomatology in childhood anaphylaxis. Specifically, almost all patients in our population with food-related anaphylaxis presented with cutaneous signs or symptoms. In addition, respiratory and gastrointestinal involvement occurred more frequently than cardiac manifestations, which distinguishes the presentation of childhood anaphylaxis from the typical presentation in adults.3 When compared with patients who received 1 dose of epinephrine, patients who received a second dose more commonly presented with difficulty breathing. This finding may provide guidance to physicians determining whether a patient may require multiple doses of epinephrine.
Multiple studies have illustrated deficiencies in the disposition of patients with anaphylaxis. In 2004, Clark et al23 reported that 16% of patients discharged from 21 North American EDs with a food-related acute allergic reaction were prescribed self-injectable epinephrine, and 12% were referred to an allergist. A similar community-based study from 1990 to 2000 reported slightly higher percentages (36% and 31%, respectively).24 In our more current review, less than half of the patients (43%) were prescribed self-injectable epinephrine, and smaller percentages were referred to an allergist or instructed on food avoidance. These data highlight a missed opportunity for emergency medical staff to provide patients with the means to appropriately manage possible future reactions. The percentages were more reassuring among patients admitted to the hospital, but this most likely reflects a population of patients with more severe and readily recognizable presentations of anaphylaxis.
A potential limitation of our study is reliance on the medical chart and the possibility that the documentation was inaccurate or incomplete. In addition, it is possible that limiting our review to the ED may have overestimated the percentage of patients receiving epinephrine, because less severe allergic reactions may be managed in other settings. However, studies have shown that the majority of patients with anaphylaxis are treated in the ED25; therefore, it would follow logically that ED visits would reflect rates of repeat epinephrine use. Also, our data may not be nationally representative, because the 2 hospitals evaluated were located in an urban, academic setting.
CONCLUSIONS
Food-related anaphylaxis is a growing health care concern with numerous clinical challenges and unresolved questions. In the current study, among children who presented to the ED with food-related anaphylaxis and received epinephrine, 12% received a second dose. This finding supports the recommendation that children at risk for food-related anaphylaxis carry 2 doses of self-injectable epinephrine. Given that children often require medications in multiple locations, consideration should be given to cost-saving approaches such as having unassigned second doses available at schools and day cares. Additional study is warranted to evaluate the long-term outcomes of children who experience an episode of food-related anaphylaxis and methods to improve and standardize their care.
WHAT’S KNOWN ON THIS SUBJECT
The primary treatment of food-related anaphylaxis is epinephrine, and its prompt administration is recommended by current practice guidelines. On the basis of limited data, it has been recommended that children with a history of food-related anaphylaxis carry multiple doses of self-injectable epinephrine.
WHAT THIS STUDY ADDS
Among children presenting to the ED with food-related anaphylaxis who received epinephrine, 12% received a second dose. This finding supports the recommendation that children at risk for food-related anaphylaxis should carry 2 doses of self-injectable epinephrine.
Acknowledgments
Dr Rudders is supported by National Institutes of Health training grant NRSA T32-AI-007512.
ABBREVIATIONS
- ED
emergency department
- CI
confidence interval
Footnotes
FINANCIAL DISCLOSURE: Dr Camargo has consulted for Dey (Napa, CA) and is principal investigator of an investigator-initiated research grant from Dey that partly supported the present study; the other authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
References
- 1.Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009;60:261–277. doi: 10.1146/annurev.med.60.042407.205711. [DOI] [PubMed] [Google Scholar]
- 2.Branum AM, Lukacs SL. Food allergy among US children: trends in prevalence and hospitalizations. [Accessed November 16, 2009];NCHS data brief No 10. Available at: www.cdc.gov/nchs/data/databriefs/db10.pdf. [PubMed]
- 3.Decker WW, Campbell RL, Manivannan V, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122(6):1161–1165. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Silva IL, Mehr SS, Tey D, Tang MLK. Paediatric anaphylaxis: a 5-year retrospective review. Allergy. 2008;63(8):1071–1076. doi: 10.1111/j.1398-9995.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 5.Braganza SC, Acworth JP, McKinnon DR, Peake JE, Brown AF. Paediatric emergency department anaphylaxis: different patterns from adults. Arch Dis Child. 2006;91(2):159–163. doi: 10.1136/adc.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 7.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol. 2005;115(3 suppl 2):S483–S523. doi: 10.1016/j.jaci.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Järvinen KM, Sicherer SH, Sampson HA, Nowak-Wegrzyn A. Use of multiple doses of epinephrine in food-induced anaphylaxis in children. J Allergy Clin Immunol. 2008;122(1):133–138. doi: 10.1016/j.jaci.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Uguz A, Lack G, Pumphrey P, et al. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy. 2005;35(6):746–750. doi: 10.1111/j.1365-2222.2005.02257.x. [DOI] [PubMed] [Google Scholar]
- 10.Oren E, Banerji A, Clark S, Camargo CA. Food induced anaphylaxis and repeated epinephrine treatments. Ann Allergy Asthma Immunol. 2007;99(5):429–432. doi: 10.1016/S1081-1206(10)60568-6. [DOI] [PubMed] [Google Scholar]
- 11.Kelso JM. A second dose of epinephrine for anaphylaxis: how often needed and how to carry. J Allergy Clin Immunol. 2006;117(2):464–465. doi: 10.1016/j.jaci.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Clark S, Gaeta TJ, Kamarthi GS, Camargo CA. ICD-9-CM coding of emergency department visits for food and insect sting allergy. Ann Epidemiol. 2006;16(9):696–700. doi: 10.1016/j.annepidem.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Simons FER, Sampson HA. Anaphylaxis epidemic: fact or fiction? J Allergy Clin Immunol. 2008;122(6):1166–1168. doi: 10.1016/j.jaci.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Klein JS, Yocum MW. Underreporting of anaphylaxis in a community emergency room. J Allergy Clin Immunol. 1995;95(2):637–638. doi: 10.1016/s0091-6749(95)70329-2. [DOI] [PubMed] [Google Scholar]
- 15.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 16.Gold MS, Sainsbury R. First aid anaphylaxis management in children who were prescribed an epinephrine auto-injector device (EpiPen) J Allergy Clin Immunol. 2000;106(1 pt 1):171–176. doi: 10.1067/mai.2000.106041. [DOI] [PubMed] [Google Scholar]
- 17.Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol. 1998;101(1 pt 1):33–37. doi: 10.1016/S0091-6749(98)70190-3. [DOI] [PubMed] [Google Scholar]
- 18.Sheikh A, Ten Broek V, Brown SG, Simons FE. H1 Anti-histamines for the treatment of anaphylaxis: Cochrane systematic review. Allergy. 2007;62(8):830–837. doi: 10.1111/j.1398-9995.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 19.Carr BG, Branas CC, Metlay JP, Sullivan AF, Camargo CA. Access to emergency care in the United States. Ann Emerg Med. 2009;54(2):261–269. doi: 10.1016/j.annemergmed.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemp AS. EpiPen epidemic: suggestions for rational prescribing in childhood food allergy. J Paediatr Child Health. 2003;39(5):372–375. doi: 10.1046/j.1440-1754.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 21.Pumphrey R. Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol. 2004;4(4):285–290. doi: 10.1097/01.all.0000136762.89313.0b. [DOI] [PubMed] [Google Scholar]
- 22.Arias K, Wasserman S, Jordana M. Management of food-induced anaphylaxis: unsolved challenges. Curr Clin Pharmacol. 2009;4(2):113–125. doi: 10.2174/157488409788184981. [DOI] [PubMed] [Google Scholar]
- 23.Clark S, Bock SA, Gaeta TJ, Brenner BE, Cydulka RK, Camargo CA. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol. 2004;113(2):347–352. doi: 10.1016/j.jaci.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 24.Campbell RL, Luke A, Weaver AL, et al. Prescriptions for self-injectable epinephrine and follow-up referral in emergency department patients presenting with anaphylaxis. Ann Allergy Asthma Immunol. 2008;101(6):631–636. doi: 10.1016/S1081-1206(10)60227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohlke K, Davis RL, DeStefano F, Marcy SM, Braun MM, Thompson RS. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization. J Allergy Clin Immunol. 2004;113(3):536–542. doi: 10.1016/j.jaci.2003.11.033. [DOI] [PubMed] [Google Scholar]


