Background: ENaC activity is regulated by multiple factors that affect channel density, open probability, or both.
Results: Mutations within the wrist domain altered the response of ENaC to external Na+ or to shear stress.
Conclusion: The wrist domain participates in transmitting peripheral structural changes to the channel gate.
Significance: Our results enhance our understanding of the regulation of ENaC by external factors.
Keywords: Acid-sensing Ion Channel (ASIC), Allosteric Regulation, ENaC, Shear Stress, Sodium Channels
Abstract
The epithelial Na+ channel (ENaC) is regulated by a variety of external factors that alter channel activity by inducing conformational changes within its large extracellular region that are transmitted to the gate. The wrist domain consists of small linkers connecting the extracellular region to the transmembrane domains, where the channel pore and gate reside. We employed site-directed mutagenesis combined with two-electrode voltage clamp to investigate the role of the wrist domain in channel gating in response to extracellular factors. Channel inhibition by external Na+ was reduced by selected mutations within the wrist domain of the α subunit, likely reflecting an increase in channel open probability. The most robust changes were observed when Cys was introduced at αPro-138 and αSer-568, sites immediately adjacent to the palm domain. In addition, one of these Cys mutants exhibited an enhanced response to shear stress. In the context of channels that have a low open probability due to retention of an inhibitory tract, the response to external Na+ was reduced by Cys substitutions at both αPro-138 and αSer-568. We observed a significant correlation between changes in channel inhibition by external Na+ and the relative response to shear stress for the α subunit mutants that were examined. Mutants that exhibited reduced inhibition by external Na+ also showed an enhanced response to shear stress. Together, our data suggest that the wrist domain has a role in modulating the channel's response to external stimuli.
Introduction
The epithelial Na+ channel (ENaC)3 mediates Na+ uptake across the apical membrane of high resistance, Na+-transporting epithelia in the kidney, lung, and colon (1, 2). ENaC is a member of the ENaC/degenerin family of ion channels that are composed of pore-forming subunits that share similar overall secondary structures, featuring a large extracellular loop connected to two transmembrane domains (TM1 and TM2) and short intracellular N and C termini (2). The resolved structure of an acid-sensing ion channel (ASIC1) provided important clues to the structural organization of other members in the ENaC/degenerin family. The extracellular region has clearly defined domains composed primarily of α helices (termed thumb, finger, and knuckle) or β strands (termed palm and β ball) (3, 4). The channel pore and gate reside within the two TMs (3–5). The transmembrane α helices are coupled to the palm domain β strands by short linkers referred to as the wrist domain (see Fig. 1) (3, 4).
FIGURE 1.
Structure and sequence alignments of the wrist domains. A, a model of an ASIC monomer with an enhanced view of the wrist domain (boxed) is shown. The side chains of selected residues adjacent to and within the wrist region (Leu-71, Tyr-72, Pro-73, and His-74 following TM1 and Lys-423, Ala-424, Tyr-425, and Glu-426 preceding TM2) are shown. B, sequence alignments of the transmembrane and wrist domains of ASIC and the three mouse (m) ENaC subunits. TM1 and TM2 are indicated. Conserved sites are highlighted in yellow. ENaC and ASIC1 wrist domain residues are boxed. Sites tested in this study are indicated by asterisks.
ENaC/degenerin channels are regulated by a variety of extracellular factors that are thought to interact at specific sites within the extracellular region. For example, H+ binding at specific sites within ASIC1 has been proposed to induce movements within the extracellular region that are transmitted to the TMs and the channel gate (3, 5). A loop linking the ninth β strand in the palm domain with the fourth α helix in the thumb domain (β9-α4 loop) is in close proximity to residues in the wrist domain and the TMs (see Fig. 1) (3). Several investigators have suggested that this loop has a role in transmitting structural transitions in the channel periphery to the gate (2, 3, 6, 7).
We have examined the role of the wrist domain in transmitting conformational changes from extracellular domains to the channel pore. We observed that the responses of ENaC to changes in the external [Na+] and to laminar shear stress (LSS) were altered by selected mutations within the wrist domains of the three subunits. Our results support the hypothesis that the wrist domain has a role in transmitting conformational changes in the extracellular regions of ENaC subunits to the channel gate.
EXPERIMENTAL PROCEDURES
Chemicals
Methanethiosulfonate (MTS) reagents were obtained from Toronto Research Chemicals (Toronto, Canada). 5 mm solutions of 2-sulfonatoethyl methanethiosulfonate sodium salt (MTSES) and 1 mm solutions of 2-(trimethylammonium)ethyl methanethiosulfonate bromide (MTSET) were prepared freshly in a high Na+ bath solution (110 mm NaCl, 2 mm KCl, 2 mm CaCl2, and 10 mm HEPES, pH 7.4) immediately before use. A 10 mm stock solution of 1,2-ethanediyl bismethanethiosulfonate (MTS-2-MTS), a bifunctional Cys-reactive reagent, was prepared in dimethyl sulfoxide and diluted 1:1000 in the high Na+ bath solution immediately prior to use. Copper(II) phenanthroline (Cu(Phen)3) was prepared from a 200 mm copper sulfate stock solution in water and a 200 mm 1,10-phenanthroline stock solution in dry ethanol, diluted to the final concentration of 16 μm 1,10-phenanthroline and 4 μm copper sulfate in the high Na+ bath solution.
Site-directed Mutagenesis and in Vitro Transcription
The mutants used in the study were generated with a QuikChange II XL kit (Stratagene, La Jolla, CA) using wild-type or mutant mouse ENaC α, β, or γ subunit cDNAs as templates. Nucleotide sequencing was performed to confirm the targeted mutations. cRNAs were synthesized with T3 mMESSAGE mMACHINE (Ambion, Inc.) and examined by agarose gel electrophoresis to confirm the appropriate size of the cRNA.
ENaC Expression and Two-electrode Voltage Clamp
Oocytes were harvested from Xenopus laevis using a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Stage V–VI Xenopus oocytes were injected with 1 ng of cRNA/subunit of mouse ENaC. The injected oocytes were incubated in modified Barth's saline (88 mm NaCl, 1 mm KCl, 2.4 mm NaHCO3, 15 mm HEPES, 0.3 mm Ca(NO3)2, 0.41 mm CaCl2, 0.82 mm MgSO4, 10 μg/ml sodium penicillin, 10 μg/ml streptomycin sulfate, and 100 μg/ml gentamycin sulfate, pH 7.4) at 18 °C. All experiments were performed at room temperature 20–28 h following injection.
Channel Inhibition by Extracellular Na+
The Na+ self-inhibition response (8) was measured in oocytes expressing wild-type or mutant ENaCs. Oocytes were clamped at −60 mV while whole cell Na+ currents were recorded continuously. In the first 60 s, oocytes were perfused with the high Na+ bath solution and then with a low Na+ bath solution (1 mm NaCl, 109 mm N-methyl-d-glucamine, 2 mm KCl, 2 mm CaCl2, and 10 mm HEPES, pH 7.4) for another 60 s. The pH of the low Na+ bath solution was adjusted with HCl, and this solution has a high [Cl−], which limits the maximal channel activation that could be achieved by removal of both Na+ and Cl− (9). To initiate Na+ self-inhibition, the low Na+ bath solution was rapidly replaced with the high Na+ bath solution. At the end of each experiment, 10 μm amiloride was added to the bath to determine the amiloride-insensitive component of the whole cell Na+ current. The rate constant for the Na+ self-inhibition response (τ) and the extent of the Na+ self-inhibition response (ratio of the steady-state current to the peak current) were calculated as described previously (7), with the peak current (Ipeak) measured as the maximal inward current immediately after the switch from the low Na+ bath solution to the high Na+ bath solution and with the steady-state current (Iss) measured 40 s after Ipeak.
Channel Activation by LSS
ENaC activation by LSS was examined as described previously (7, 10). Whole cell Na+ currents were continuously recorded while oocytes were clamped at a holding potential of −60 mV. Oocytes were perfused with the high Na+ bath solution for the first 30 s to measure basal currents (Ibasal) and subsequently perfused through a vertical pipette placed near the surface of the oocyte to determine LSS-activated currents (ILSS). The flow rate via the vertical pipette was adjusted to 1.6 ml/min, corresponding to 0.14 dynes/cm2 of shear stress (10). At the end of the experiment, perfusion was changed back to bath perfusion with 5 μm benzamil added to determine the benzamil-insensitive component of the whole cell current. Given the variability in the response of channels to LSS between different batches of oocytes, ILSS/Ibasal values for each mutant were normalized to those of wild-type channels from the same batch of oocytes to determine the relative magnitude of the LSS response, as described previously (7, 10). Time constants (τ) of ENaC activation by LSS were determined by fitting the first 40 s of current following the initiation of vertical perfusion with an exponential equation using Clampfit 10.2 (Molecular Devices) as described previously (7, 10).
Modification of Cys Mutants with Cu(Phen)3 or MTS Reagents
To examine if chemical modifications of engineered Cys residues within the wrist domain affect channel activity, the Na+ self-inhibition response was measured prior to and following the treatment of oocytes with the cross-linking reagent Cu(Phen)3 (16 μm 1,10-phenanthroline and 4 μm copper sulfate (2-min exposure)) or the bifunctional MTS reagents (10 μm (2-min exposure)). The effects of sulfhydryl-reactive MTS reagents bearing different charges (1 mm for MTSET or 5 mm for MTSES) were also examined. At the end of each experiment, 10 μm amiloride was delivered to the chamber to assess the amiloride-insensitive component of the whole cell current.
Statistical Analysis
Data are expressed as the means ± S.E. Experiments were repeated with a minimum of two batches of oocytes obtained from different frogs. Electrophysiological data were analyzed with Clampfit 10.2 and Origin 8.1 (OriginLab). Statistical comparisons were made using Student's unpaired t test or one-way analysis of variance (ANOVA) followed by the Bonferroni test. A p value of <0.05 was considered statistically significant.
RESULTS
Response to Shear Stress or to External Na+ Is Altered by Mutations in the Wrist Domain of the α Subunit
ENaC gating is regulated by a variety of external factors, including proteolytic processing, extracellular ions, and mechanical forces (1, 2). Channel inhibition by extracellular Na+, a process referred to as Na+ self-inhibition, and channel activation by LSS both reflect changes in channel open probability (Po) (11–13). To investigate the role of the wrist domain in ENaC gating, individual residues within the α subunit wrist domain were substituted with Cys, including the four sites immediately preceding β1 (Phe-135, Ser-136, Tyr-137, and Pro-138) and four sites directly following β12 (Ser-568, Val-569, Thr-570, and Met-571) (Fig. 1). Amiloride-sensitive Na+ currents were readily detected in oocytes expressing channels bearing mutations in the wrist domain (Table 1). The Na+ self-inhibition response and the LSS response were examined in oocytes expressing wild-type ENaC or mutant channels bearing a single Cys substitution. As shown in Fig. 2, the Na+ self-inhibition response was significantly reduced when Cys was introduced at αSer-136, αTyr-137, αPro-138, and αSer-568. Cys mutations of αPro-138 and αSer-568 had the greatest effects on the inhibitory effect of external Na+. The LSS response was also examined in each of these mutants. Significant changes in the LSS response were not observed with most mutants. However, αS568Cβγ exhibited an enhanced magnitude of channel activation by LSS as well as a slower rate of channel activation, as indicated by a higher τ value (Fig. 3).
TABLE 1.
Amiloride-sensitive whole cell Na+ currents of wild-type ENaC and channels with mutations in the wrist domain
Data for wild-type ENaC were collected from 21 batches of oocytes, whereas each mutant was examined in oocytes harvested from a minimum of two frogs. Both normalized amiloride-sensitive whole cell Na+ currents and actual currents are listed. Amiloride-sensitive Na+ currents for each mutant were normalized to the average current of wild-type ENaC from the same batch of oocytes assayed on the same day. p values were determined with Student's unpaired t test.
| Channel | Relative I ± S.E. | Iamiloride ± S.E. | No. of oocytes |
|---|---|---|---|
| μA | |||
| αβγ | 1.00 ± 0.03 | −2.21 ± 0.13 | 99 |
| αF135Cβγ | 1.19 ± 0.17 | −2.13 ± 0.35 | 12 |
| αS136Cβγ | 1.45 ± 0.20 | −2.60 ± 0.41 | 11 |
| αY137Cβγ | 1.21 ± 0.16 | −2.55 ± 0.38 | 17 |
| αP138Cβγ | 1.50 ± 0.24a | −2.94 ± 0.47a | 13 |
| αS568Cβγ | 1.35 ± 0.10b | −3.94 ± 0.67b | 16 |
| αV569Cβγ | 1.34 ± 0.22 | −2.95 ± 0.60 | 9 |
| αT570Cβγ | 1.17 ± 0.16 | −3.10 ± 0.47 | 13 |
| αM571Cβγ | 0.84 ± 0.13 | −1.93 ± 0.30 | 11 |
| αP138C,S568Cβγ | 1.53 ± 0.15b | −3.43 ± 0.31b | 20 |
| αP138Wβγ | 0.99 ± 0.06 | −1.85 ± 0.30 | 13 |
| αS568Wβγ | 1.19 ± 0.12 | −1.63 ± 0.21 | 10 |
| αP138W,S568Wβγ | 0.79 ± 0.08 | −1.42 ± 0.21 | 12 |
| αR205A,R231Aβγ | 0.30 ± 0.03c | −0.41 ± 0.04c | 17 |
| αR205A,R231A, P138Cβγ | 0.38 ± 0.04c | −0.47 ± 0.06c | 15 |
| αR205A,R231A, S568Cβγ | 0.47 ± 0.06c | −0.52 ± 0.06c | 18 |
| αR205A,R231A, P138C,S568Cβγ | 0.43 ± 0.04c | −0.48 ± 0.03c | 19 |
| αβW77Cγ | 1.04 ± 0.08 | −1.48 ± 0.11 | 12 |
| αβE78Cγ | 0.85 ± 0.09 | −1.22 ± 0.13 | 13 |
| αβV79Cγ | 0.74 ± 0.07a | −1.06 ± 0.11a | 15 |
| αβP509Cγ | 0.52 ± 0.07c | −1.76 ± 0.24c | 12 |
| αβA510Cγ | 0.90 ± 0.09 | −2.16 ± 0.24 | 26 |
| αβN511Cγ | 0.54 ± 0.03c | −2.03 ± 0.27c | 14 |
| αβγT80C | 0.71 ± 0.15 | −1.58 ± 0.18 | 10 |
| αβγV81C | 0.49 ± 0.04c | −1.76 ± 0.20c | 11 |
| αβγP526C | 0.58 ± 0.10b | −1.29 ± 0.10b | 12 |
| αβγA527C | 0.85 ± 0.13 | −2.10 ± 0.40 | 12 |
| αβγN528C | 0.83 ± 0.07 | −2.41 ± 0.40 | 12 |
| Wrist6C | 0.61 ± 0.07c | −1.06 ± 0.14c | 13 |
a p < 0.05 versus wild-type channels from the same batch assayed on the same day.
b p < 0.01.
c p < 0.001.
FIGURE 2.
Na+ self-inhibition is reduced by selected mutations in the α subunit wrist domain. The α subunit wrist domain residues with introduced Cys mutations are noted. The extent of Na+ self-inhibition (Iss/Ipeak values; A) and the time constants of current decay (τ values; B) were obtained as described previously (7). The average values of wild-type channels are from 31 observations (seven batches of oocytes), whereas mutant data are from 9 to 20 oocytes from a minimum of two batches of oocytes. The average amiloride-sensitive whole cell current measured in wild-type channels was 2.19 ± 0.28 μA. The responses of mutant and wild-type ENaCs were always examined within the same batch of oocytes to account for variability of the Na+ self-inhibition response within different batches of oocytes. Statistically significant differences between wild-type and mutant channels are indicated by asterisks (p < 0.05, determined by one-way ANOVA followed by the Bonferroni test). C, representative recordings of Na+ self-inhibition responses of the wild-type and selected mutant channels are shown. Oocytes were clamped at −60 mV, and whole cell currents were continuously recorded while the bath [Na+] was rapidly increased from 1 mm (black bar) to 110 mm (gray bar). D, representative recordings of wild-type and selected mutant channels illustrated in C are plotted on a relative scale, where the relative peak currents are the same value to illustrate differences in the Na+ self-inhibition responses.
FIGURE 3.
αS568Cβγ exhibits an enhanced LSS response. The Cys mutations introduced at sites in the α subunit wrist domain are noted. The LSS responses of mutant channels were examined in 8–17 oocytes from a minimum of two batches of oocytes. The average amiloride-sensitive whole cell current measured in wild-type channels was 2.83 ± 0.31 μA. A, relative response of whole cell Na+ currents to LSS. To assess the magnitude of the LSS response, the peak response of the benzamil-sensitive Na+ current (ILSS) following the initiation of LSS was normalized to the basal benzamil-sensitive current (Ibasal) prior to initiation of LSS. The ILSS/Ibasal values were then normalized to the average ILSS/Ibasal values of wild-type ENaCs from the same batch. The average ILSS/Ibasal value for wild-type channels was 2.18 ± 0.11 (n = 45). B, time constants of channel activation by LSS. τ values were determined by fitting the first 40 s of current increase following the initiation of LSS with a one-component exponential equation as described under “Experimental Procedures.” The response of mutant channels was compared with the response of wild-type channels within the same batch of oocytes to account for variability of the LSS response within different batches of oocytes. Significant changes in the LSS response of mutant αS568Cβγ compared with the wild-type channel are noted. *, p < 0.05 (determined by one-way ANOVA followed by the Bonferroni test). C, representative recordings of the LSS response of wild-type and αS568Cβγ channels. Oocytes expressing ENaCs were subjected to LSS generated through fluid-jet perfusion via a vertical pipette, which was initiated at t = 30 (light gray bar). At the end of the experiment, ENaC-specific Na+ currents were determined by adding 5 μm benzamil to the bath perfusion (dark gray bar).
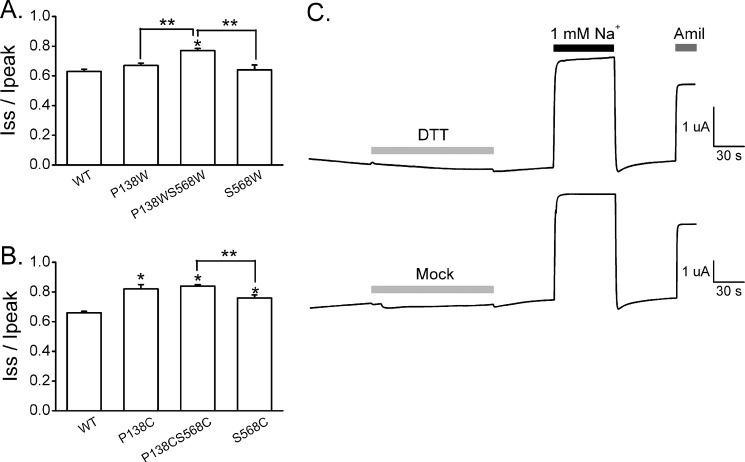
The resolved ASIC1 structure and a model of the ENaC α subunit (Fig. 1A) place αPro-138 and αSer-568 at the most distal sites from the transmembrane helices, connecting the wrist domain to the β1 and β12 strands in the palm domain, respectively (2, 3). In addition, αPro-138 and αSer-568 are predicted to be in close proximity to each other in the closed state (2, 3). To further explore the role of these two sites in the response of ENaC to external Na+ and LSS, we introduced a bulky residue (Trp) at these sites. The Na+ self-inhibition response was examined in oocytes expressing channels with Trp substitutions at either site (αP138W or αS568W single mutant) or both sites (αP138W,S568W double mutant). Compared with the wild-type channel, only αP138W,S568Wβγ exhibited a loss of Na+ self-inhibition, shown as an increased Iss/Ipeak value (Fig. 4A). Channels with Cys substitutions at both sites (αP138C,S568C mutant) also exhibited a reduced Na+ self-inhibition response compared with the wild-type channel. However, the average Iss/Ipeak value of αP138C,S568Cβγ was not different from that of αP138Cβγ (Fig. 4B), suggesting that the effects of Cys substitutions at these sites are not additive. As αPro-138 and αSer-568 are in close proximity in our predicted αENaC model, it is possible that the introduction of two Cys residues at these two sites forms a disulfide bridge that might limit conformational transitions that affect channel gating. To explore this possibility, we treated the oocytes with a reducing reagent, DTT, prior to examining the Na+ self-inhibition response of αP138C,S568Cβγ. DTT treatment did not alter the response of these channels to external Na+ (Fig. 4C). Furthermore, treatment with bifunctional MTS reagents (MTS-2-MTS or MTS-4-MTS) did not alter the Na+ self-inhibition response of αP138C,S568Cβγ channels (data not shown).
FIGURE 4.
Mutations at both sites adjacent to the palm domain alter the channel's Na+ self-inhibition response. A and B, amiloride-sensitive Iss/Ipeak ratios of mutant and wild-type channels. Ipeak was measured as the maximal inward current immediately after bath exchange. Iss was measured 40 s after Ipeak. The Cys or Trp mutations introduced at specific sites are noted. The average amiloride-sensitive whole cell current measured in wild-type channels was 2.02 ± 0.32 μA. Significant differences between wild-type and mutant channels are indicated by asterisks (p < 0.05). Significant differences between oocytes expressing channels with the two mutations in the α subunit and channels with the corresponding single mutations are indicated by double asterisks (p < 0.05). Statistical significance was determined by one-way ANOVA followed by the Bonferroni test. C, representative recordings of the Na+ self-inhibition response of αP138C,S568Cβγ channels in the presence or absence of DTT treatment. Oocytes were clamped at −60 mV, and whole cell currents were continuously recorded. After treating the oocytes with DTT or with the perfusion solution without DTT (Mock) for 2 min (light gray bars), Na+ self-inhibition was measured when the bath [Na+] was rapidly increased from 1 mm (black bar) to 110 mm. At the end of the experiment, amiloride (Amil) was added to the bath (dark gray bar).
ENaCs that lack proteolytic processing of the α subunit by furin (αR205A,R231A mutant) retain an intrinsic inhibitory tract and thus have enhanced Na+ self-inhibition and a very low Po in the presence of external Na+ (14, 15). As shown in Table 1, whole cell currents in oocytes expressing the α subunit furin mutant were significantly lower than those in oocytes expressing wild-type ENaC. Subsequent trypsin treatment resulted in large amiloride-sensitive Na+ currents in oocytes expressing αR205A,R231Aβγ channels or αR205A,R231A,P138C,S568Cβγ channels, which were similar in magnitude to amiloride-sensitive currents in oocytes expressing wild-type channels treated with trypsin (Fig. 5A). We examined whether mutations in the wrist domain relieve the enhanced Na+ self-inhibition observed with channels bearing the αR205A,R231A mutation. We observed that αR205A,R231Aβγ channels with Cys substitutions at both αPro-138 and αSer-568 elicited a reduced Na+ self-inhibition response compared with αR205A,R231Aβγ channels (Fig. 5B).
FIGURE 5.
Na+ self-inhibition response of ENaC lacking α subunit cleavage is altered by the αP138C,S568C mutation. A, amiloride-sensitive whole cell currents were measured in oocytes expressing wild-type ENaC or mutant channels that were voltage-clamped at −60 mV. The average amiloride-sensitive whole cell current measured in wild-type channels was 1.36 ± 0.17 μA. Trypsin (2 μg/ml) was subsequently added to the bath for 10 min before a second measurement was made. Data gathered from two batches of oocytes (n = 10–14 for each group) were normalized to the average current of wild-type channels before trypsin treatment. B, amiloride-sensitive Iss/Ipeak ratios of wild-type and mutant channels are shown. The mutations introduced at the different residues are noted. Significant differences between wild-type and mutant channels are indicated by asterisks (p < 0.05). Significant differences between oocytes expressing channels with the α subunit furin site mutations (αR205A,R231A) and the other mutant channels are indicated with double asterisks (p < 0.05). p values were determined by one-way ANOVA followed by the Bonferroni test.
Cys Substitutions at Sites in the Wrist Domains of the β and γ Subunits Alter the Na+ Self-inhibition Response
As substitutions in the α subunit wrist domain adjacent to the palm domain led to changes in the channel's response to external Na+ and LSS, we examined whether Cys substitutions at homologous sites in the β (βW77C, βE78C, βA510C, and βN511C) and γ (γT80C, γA527C, γN528C) subunit wrist domains altered the Na+ self-inhibition response (Fig. 6). In contrast to α subunit mutants, none of the mutations altered the extent of Na+ self-inhibition (no changes in Iss/Ipeak values), whereas one mutant (βE78C) enhanced the rate of Na+ self-inhibition (a lower τ value). As the wrist domain transitions to β strands (β1 and β12) in the palm domain, we examined whether the introduction of Cys at the start of β1 (βV79C and γV81C) or the end of β12 (βP509C and γP526C) altered the Na+ self-inhibition response. Two mutants, βV79C and γV81C, displayed enhance inhibition by extracellular Na+ (Fig. 6). It is not surprising that mutations at similar sites in the β and γ subunits resulted in enhanced Na+ self-inhibition, whereas reduced Na+ self-inhibition was seen with α subunit mutants. Asymmetry with regard to the inhibitory effects of extracellular Na+ on channels with mutations at analogous sites among the three ENaC subunits have been reported previously (7, 11).
FIGURE 6.
Na+ self-inhibition response is enhanced by selected mutations in the wrist domains of β and γ subunits. Cys residues introduced at the different sites within the wrist domains of the β (A) and γ (B) subunits are noted. The extent of inhibition by Na+ (Iss/Ipeak values; left) and the time constants of current decay (τ values; right) were obtained as described previously (7). The average amiloride-sensitive whole cell current measured in wild-type channels was 2.58 ± 0.26 μA. The response of mutant ENaCs was examined with wild-type channels expressed in the same batch of oocytes to account for variability of the Na+ self-inhibition response between different batches of oocytes. Statistically significant differences between oocytes expressing wild-type and mutant channels are indicated by asterisks (p < 0.05, determined by one-way ANOVA followed by the Bonferroni test).
Na+ Self-inhibition Response of Channels Containing Cys Substitutions in All Three Subunits Is Not Altered by Chemical Modification
Our results suggested that substitutions at sites in the wrist domain adjacent to the palm domain altered the channel's response to external Na+. The resolved ASIC1 structure suggests that these residues are in close proximity when the channel is in a desensitized state (3). We generated channels with Cys substitutions in the wrist domain at sites immediately adjacent to the palm domain in all three subunits (αP138C,αS568C,βE78C,βA510C,γT80C,γA527C (referred to as Wrist6C)) and asked whether cross-linking the introduced Cys residues would affect the channel's response to external Na+. Amiloride-sensitive currents were readily detected in oocytes expressing the Wrist6C mutant. Interestingly, the Na+ self-inhibition response of this mutant was not different from that of the wild-type channel (Fig. 7A). To examine whether cross-linking-introduced Cys residues lock the channel at a specific state that affects its response to external Na+, we measured the Na+ self-inhibition response prior to and following treatment of oocytes with Cu(Phen)3 or MTS-2-MTS. Treating the oocytes with Cu(Phen)3 had no effects on the inhibitory effect of extracellular Na+. Although MTS-2-MTS inhibited channel activity in a time-dependent manner, as we have reported previously (16), the Na+ self-inhibition response remained intact (Fig. 7). Furthermore, the Na+ self-inhibition response was not altered by treating oocytes expressing the Wrist6C mutant with either MTSET or MTSES (Fig. 7). In addition, DTT did not alter Wrist6C whole cell currents or Wrist6C inhibition by extracellular Na+ (data not shown). The Na+ self-inhibition response in oocytes expressing wild-type ENaC was not altered by any of these reagents (Fig. 7).
FIGURE 7.
Na+ self-inhibition response of Wrist6C channels was not altered by Cys-modifying reagents. A, the average Iss/Ipeak values and τ values of wild-type ENaC and the Wrist6C mutant. The average amiloride-sensitive whole cell current measured in wild-type channels was 1.74 ± 0.16 μA. B, representative recordings showing the effect of Cys-modifying reagents on the Na+ self-inhibition response in oocytes expressing wild-type (left) or Wrist6C (right) channels. After assessing Na+ self-inhibition and perfusing oocytes in the presence of the reagent for 2 min (light gray bars), Na+ self-inhibition was reassessed. At the end of the experiment, oocytes were perfused with 10 μm amiloride (Amil; dark gray bars). C, effects of different reagents on the Na+ self-inhibition response of WT channels (left) and the Wrist6C mutant (right). No significant changes in the Iss/Ipeak values following treatments were noted (determined by Student's unpaired t test). Experiments were performed with 8–14 oocytes expressing mutant channels from a minimum of two batches of oocytes.
DISCUSSION
Similar to other members of the ENaC/degenerin family, the Po of ENaC is regulated by a number of extracellular factors, including proteolytic cleavage, extracellular Na+, and LSS (1, 2, 17). Specific proteases have been implicated in the activation of ENaC through cleaving channel subunits at defined sites within their extracellular domains, releasing inhibitory tracts (2, 17). Mechanical forces, such as LSS, activate ENaC by increasing channel Po (10, 12, 13). Increases in flow rates within the distal segments of the nephron enhance the transepithelial transport of Na+ and K+ (13, 18–24). It has suggested that the flow-dependent activation of both ENaC and large conductance, Ca2+-activated K+ channels provides a mechanism to enhance renal K+ secretion and may reflect an adaptive response of animals to intermittent consumption of a protein- and K+-rich meal (25). ENaC activity is also regulated by both intracellular Na+ and extracellular Na+, phenomena described as Na+ feedback inhibition and Na+ self-inhibition, respectively. Although feedback inhibition is a slow process that affects both the number of channels at the plasma membrane and channel Po, Na+ self-inhibition is a more rapid response that reduces channel Po (8). Previous studies have shown a correlation between the magnitude of Na+ self-inhibition and channel Po (11). In this study, we measured the response of channels with mutations in the wrist domain to external Na+ and LSS to investigate the role of the wrist domain in modulating the channel's response to these external factors.
We observed that the extent of Na+ self-inhibition was reduced (higher Iss/Ipeak values) by Cys substitutions of selected sites (αSer-136, αTyr-137, and αPro-138 following TM1 and αSer-568 preceding TM2) within the wrist domain of the α subunit (Fig. 2A). A reduced Na+ self-inhibition response is often associated with an increased time constant of current decay (higher τ value), which is what we observed with the αY137C, αP138C, and αS568C mutants (Fig. 2B). Interestingly, two sites where mutations elicited the greatest changes in the channel's response to external Na+, αPro-138 and αSer-568, are at the transition sites between the wrist domain and the β strands in the palm domain (Fig. 1A). In addition, we also observed that ENaC activation in response to shear stress was enhanced with the αS568Cβγ mutant (Fig. 3). The reduction in Na+ self-inhibition and the enhanced LSS response with selected α subunit mutants are consistent with an increased channel Po in the presence of external Na+ or LSS.
Although selected α subunit wrist domain Cys mutants led to a loss of the Na+ self-inhibition response, this response was enhanced by selected β and γ subunit Cys mutants. Although our data support the hypothesis that the wrist domains of all three subunits participate in conformational changes in response to extracellular Na+, the effects of mutations are subunit-specific, as we have reported previously in studies of the thumb and finger domains (7, 8, 11, 26).
ENaC inhibition by external Na+ and LSS-mediated channel activation reflect changes in channel Po (8, 11–14). We have proposed that the base of the thumb has a role in transmitting structural changes within the periphery of the channel, which are induced by external factors (i.e. Na+ and shear stress), to the channel pore. In support of this notion, mutations at the base of the thumb domain of ENaC subunits altered the channel's response to both LSS and external Na+, and a significant correlation was observed between changes in Na+ self-inhibition and the channel's response to LSS (7). In contrast, we did not observe a correlation between changes in Na+ self-inhibition and the magnitude of the LSS response with mutations introduced within the peripheral finger domain (27, 28). As the wrist domain is in close proximity to the channel pore and the base of the thumb domain, it is likely that the wrist domain participates in transmitting movements within the channel periphery to the pore during channel gating in response to external cues. For the 10 α subunit mutants examined in this study, we observed a significant correlation between changes in the extent of Na+ self-inhibition and the channel's relative LSS response (Fig. 8). In general, mutants that exhibited a greater Iss/Ipeak also showed an enhanced response to LSS. Both changes reflect increased channel Po in the presence of external factors.
FIGURE 8.

Relationship between the Na+ self-inhibition response and the relative LSS response of mutant channels bearing Cys substitutions in the wrist domain of the α subunit. The Iss/Ipeak values are plotted against the relative LSS response values. Individual mutant channels are represented as closed circles, and the wild-type channel is represented as an open circle. The solid line was obtained from linear fitting of all data points (n = 11). The correlation coefficient (r = 0.86; r2 = 0.72) was statistically significant (p < 0.001).
Previous studies using voltage clamp fluorometry demonstrated that the region in ASIC1a preceding TM2 experiences a conformational rearrangement following extracellular acidification that is associated with activation of the channel (29). Moreover, studies using substituted Cys accessibility suggested that the region preceding TM2 of ASIC1a has different conformations in the closed and desensitized states (29, 30). These findings based on functional studies are supported by recently resolved structures of ASIC1 bound to psalmotoxin (5, 31). These sites are relatively permissive to amino acids substitutions, suggesting that there are no particular side chain requirements (29). This notion is supported by the relatively poor conservation of residues at these positions within members of the ENaC/degenerin family (Fig. 1). Our results and previous studies regarding the wrist domain suggest that bulky modifications in this region have minor effects on ENaC or ASIC gating in response to extracellular factors (29). The recently resolved structure of ASIC1 bound to psalmotoxin illustrates the profound structural changes that occur in the TMs and wrist region during channel opening (5, 31). In summary, our results support the hypothesis that external factors, such as Na+ and LSS, induce conformational changes within the extracellular region and that these movements are transmitted to the channel pore via the short wrist linkers.
This work was supported, in whole or in part by National Institutes of Health Grants R37 DK051391, R01 DK084060, and P30 DK079307.
- ENaC
- epithelial Na+ channel
- TM
- transmembrane domain
- ASIC
- acid-sensing ion channel
- LSS
- laminar shear stress
- MTS
- methanethiosulfonate
- MTSES
- 2-sulfonatoethyl methanethiosulfonate sodium salt
- MTSET
- (2-(trimethylammonium)ethyl methanethiosulfonate bromide
- MTS-2-MTS
- 1,2-ethanediyl bismethanethiosulfonate
- Cu(Phen)3
- copper(II) phenanthroline
- ANOVA
- analysis of variance
- Po
- open probability.
REFERENCES
- 1. Bhalla V., Hallows K. R. (2008) Mechanisms of ENaC regulation and clinical implications. J. Am. Soc. Nephrol. 19, 1845–1854 [DOI] [PubMed] [Google Scholar]
- 2. Kashlan O. B., Kleyman T. R. (2011) ENaC structure and function in the wake of a resolved structure of a family member. Am. J. Physiol. Renal Physiol. 301, F684–F696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449, 316–323 [DOI] [PubMed] [Google Scholar]
- 4. Gonzales E. B., Kawate T., Gouaux E. (2009) Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baconguis I., Gouaux E. (2012) Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature 489, 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li T., Yang Y., Canessa C. M. (2009) Interaction of the aromatics Tyr-72/Trp-288 in the interface of the extracellular and transmembrane domains is essential for proton gating of acid-sensing ion channels. J. Biol. Chem. 284, 4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi S., Ghosh D. D., Okumura S., Carattino M. D., Kashlan O. B., Sheng S., Kleyman T. R. (2011) Base of the thumb domain modulates epithelial sodium channel gating. J. Biol. Chem. 286, 14753–14761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheng S., Bruns J. B., Kleyman T. R. (2004) Extracellular histidine residues crucial for Na+ self-inhibition of epithelial Na+ channels. J. Biol. Chem. 279, 9743–9749 [DOI] [PubMed] [Google Scholar]
- 9. Collier D. M., Snyder P. M. (2009) Extracellular chloride regulates the epithelial sodium channel. J. Biol. Chem. 284, 29320–29325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carattino M. D., Sheng S., Kleyman T. R. (2004) Epithelial Na+ channels are activated by laminar shear stress. J. Biol. Chem. 279, 4120–4126 [DOI] [PubMed] [Google Scholar]
- 11. Maarouf A. B., Sheng N., Chen J., Winarski K. L., Okumura S., Carattino M. D., Boyd C. R., Kleyman T. R., Sheng S. (2009) Novel determinants of epithelial sodium channel gating within extracellular thumb domains. J. Biol. Chem. 284, 7756–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Althaus M., Bogdan R., Clauss W. G., Fronius M. (2007) Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J. 21, 2389–2399 [DOI] [PubMed] [Google Scholar]
- 13. Morimoto T., Liu W., Woda C., Carattino M. D., Wei Y., Hughey R. P., Apodaca G., Satlin L. M., Kleyman T. R. (2006) Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am. J. Physiol. Renal Physiol. 291, F663–F669 [DOI] [PubMed] [Google Scholar]
- 14. Sheng S., Carattino M. D., Bruns J. B., Hughey R. P., Kleyman T. R. (2006) Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am. J. Physiol. Renal Physiol. 290, F1488–F1496 [DOI] [PubMed] [Google Scholar]
- 15. Hughey R. P., Bruns J. B., Kinlough C. L., Harkleroad K. L., Tong Q., Carattino M. D., Johnson J. P., Stockand J. D., Kleyman T. R. (2004) Epithelial sodium channels are activated by furin-dependent proteolysis. J. Biol. Chem. 279, 18111–18114 [DOI] [PubMed] [Google Scholar]
- 16. Kashlan O. B., Blobner B. M., Zuzek Z., Carattino M. D., Kleyman T. R. (2012) Inhibitory tract traps the epithelial Na+ channel in a low activity conformation. J. Biol. Chem. 287, 20720–20726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleyman T. R., Carattino M. D., Hughey R. P. (2009) ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J. Biol. Chem. 284, 20447–20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kunau R. T., Jr., Webb H. L., Borman S. C. (1974) Characteristics of the relationship between the flow rate of tubular fluid and potassium transport in the distal tubule of the rat. J. Clin. Invest. 54, 1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khuri R. N., Strieder W. N., Giebisch G. (1975) Effects of flow rate and potassium intake on distal tubular potassium transfer. Am. J. Physiol. 228, 1249–1261 [DOI] [PubMed] [Google Scholar]
- 20. Good D. W., Wright F. S. (1979) Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am. J. Physiol. 236, F192–F205 [DOI] [PubMed] [Google Scholar]
- 21. Engbretson B. G., Stoner L. C. (1987) Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am. J. Physiol. 253, F896–F903 [DOI] [PubMed] [Google Scholar]
- 22. Malnic G., Berliner R. W., Giebisch G. (1989) Flow dependence of K+ secretion in cortical distal tubules of the rat. Am. J. Physiol. 256, F932–F941 [DOI] [PubMed] [Google Scholar]
- 23. Woda C. B., Bragin A., Kleyman T. R., Satlin L. M. (2001) Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am. J. Physiol. Renal Physiol. 280, F786–F793 [DOI] [PubMed] [Google Scholar]
- 24. Satlin L. M., Sheng S., Woda C. B., Kleyman T. R. (2001) Epithelial Na+ channels are regulated by flow. Am. J. Physiol. Renal Physiol. 280, F1010–F1018 [DOI] [PubMed] [Google Scholar]
- 25. Satlin L. M., Carattino M. D., Liu W., Kleyman T. R. (2006) Regulation of cation transport in the distal nephron by mechanical forces. Am. J. Physiol. Renal Physiol. 291, F923–F931 [DOI] [PubMed] [Google Scholar]
- 26. Carattino M. D., Hughey R. P., Kleyman T. R. (2008) Proteolytic processing of the epithelial sodium channel γ subunit has a dominant role in channel activation. J. Biol. Chem. 283, 25290–25295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kashlan O. B., Boyd C. R., Argyropoulos C., Okumura S., Hughey R. P., Grabe M., Kleyman T. R. (2010) Allosteric inhibition of the epithelial Na+ channel through peptide binding at peripheral finger and thumb domains. J. Biol. Chem. 285, 35216–35223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi S., Blobner B. M., Kashlan O. B., Kleyman T. R. (2012) Extracellular finger domain modulates the response of the epithelial sodium channel to shear stress. J. Biol. Chem. 287, 15439–15444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Passero C. J., Okumura S., Carattino M. D. (2009) Conformational changes associated with proton-dependent gating of ASIC1a. J. Biol. Chem. 284, 36473–36481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tolino L. A., Okumura S., Kashlan O. B., Carattino M. D. (2011) Insights into the mechanism of pore opening of acid-sensing ion channel 1a. J. Biol. Chem. 286, 16297–16307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dawson R. J., Benz J., Stohler P., Tetaz T., Joseph C., Huber S., Schmid G., Hügin D., Pflimlin P., Trube G., Rudolph M. G., Hennig M., Ruf A. (2012) Structure of the acid-sensing ion channel 1 in complex with the gating modifier Psalmotoxin 1. Nat. Commun. 3, 936. [DOI] [PubMed] [Google Scholar]