Background: Na+/Ca2+ exchangers were found in animals, but no Na+/Ca2+ exchanger had been reported in plants.
Results: AtNCL showed Na+/Ca2+ exchanger-like activity and regulated stress response.
Conclusion: Functional Na+/Ca2+ exchanger-like protein exists in plants.
Significance: Na+/Ca2+ exchange also play a role in Ca2+ homeostasis under abiotic stress in plants.
Keywords: Arabidopsis, Calcium-binding Proteins, Calcium Transport, Sodium/Calcium Exchange, Stress, AtNCL, Ca2+ Homeostasis, Salt Stress
Abstract
Calcium ions (Ca2+) play a crucial role in many key physiological processes; thus, the maintenance of Ca2+ homeostasis is of primary importance. Na+/Ca2+ exchangers (NCXs) play an important role in Ca2+ homeostasis in animal excitable cells. Bioinformatic analysis of the Arabidopsis genome suggested the existence of a putative NCX gene, Arabidopsis NCX-like (AtNCL), encoding a protein with an NCX-like structure and different from Ca2+/H+ exchangers and Na+/H+ exchangers previously identified in plant. AtNCL was identified to localize in the Arabidopsis cell membrane fraction, have the ability of binding Ca2+, and possess NCX-like activity in a heterologous expression system of cultured mammalian CHO-K1 cells. AtNCL is broadly expressed in Arabidopsis, and abiotic stresses stimulated its transcript expression. Loss-of-function atncl mutants were less sensitive to salt stress than wild-type or AtNCL transgenic overexpression lines. In addition, the total calcium content in whole atncl mutant seedlings was higher than that in wild type by atomic absorption spectroscopy. The level of free Ca2+ in the cytosol and Ca2+ flux at the root tips of atncl mutant plants, as detected using transgenic aequorin and a scanning ion-selective electrode, required a longer recovery time following NaCl stress compared with that in wild type. All of these data suggest that AtNCL encodes a Na+/Ca2+ exchanger-like protein that participates in the maintenance of Ca2+ homeostasis in Arabidopsis. AtNCL may represent a new type of Ca2+ transporter in higher plants.
Introduction
Calcium ions (Ca2+) are required for many physiological functions. Numerous stimulations produce change in the cytosolic concentration of Ca2+ [Ca2+]cyt3 (1–11). The mechanisms of production and elimination of [Ca2+]cyt have become a key topic in Ca2+ signaling and related functions. Various environmental stresses can stimulate the opening of Ca2+-permeable channels in the plasma or inner membrane, leading to an influx of Ca2+ along its electrochemical gradient (12, 13). After this influx, the cytosolic level of Ca2+ could be returned to its resting state through the activity of several types of Ca2+ transport proteins that extrude Ca2+ out of the cytosol.
In animal cells, Ca2+ transport proteins include Ca2+-ATPase pumps that use ATP directly and transporters that are driven indirectly by ATP using ion gradients (14–19). Na+/Ca2+ exchangers (NCXs) are due to the later Ca2+ transport proteins. Na+/Ca2+ exchange was first reported in guinea pig cardiac muscle (20). Since then, NCX proteins have been identified in the plasma membrane of many types of cells (21–27). NCX proteins, which consist of 9–11 transmembrane domains with a large intracellular hydrophilic loop, play important roles in adjusting the [Ca2+]cyt (28). For example, NCXs maintain the [Ca2+]cyt during the intrinsic excitation-contraction cycle in cardiac muscle cells (29). NCXs are bidirectional Ca2+ transporters, and the direction of Ca2+ flux is dependent on the electrochemical Na+ gradient (30–33). Thus, NCXs play important roles in Ca2+ signaling and homeostasis in animal cells.
Until recently, only Ca2+/H+ exchangers (CAXs) and Ca2+-ATPases were reported to transport Ca2+ through the membrane in plant cells (1,34–39), whereas Na+/H+ exchangers (NHXs) were reported to drive Na+ through the plasma membrane based on a H+ gradient (40, 41). Thus, we sought to address whether a protein similar to animal NCXs exists in planta. Wang et al. suggested the existence of Na+-dependent Ca2+ uptake activity in vacuolar membrane vesicles from wheat(42). Here one NCX-like gene in Arabidopsis (AtNCL) encoded a protein similar to animal NCXs in structure, had Na+/Ca2+ exchange activity, and was involved in Ca2+ homeostasis under salt stress in Arabidopsis.
EXPERIMENTAL PROCEDURES
Plant Materials and Growth Conditions
The Arabidopsis thaliana stocks described in this work were of the Columbia (Col) ecotype. atncl-1 and atncl-2 correspond to Syngenta Arabidopsis Insertion Library (SAIL)_791_D12 and SAIL_770_A10, respectively. For salt stress, 7-day-old seedlings were transferred to plates containing 1/2 MS medium or 1/2 MS medium supplemented with 150 mm NaCl. After 7 days, the seedlings were photographed, and the survival rate was determined based on the number of plants with two to four true leaves that had become completely white in color; the chlorophyll content was measured as reported previously (43). Freezing tolerance was assayed as described (44), 10-day-old seedlings were frozen at −6 °C for 3 h and thawed at 4 °C for 12 h. After a 5-day recovery, survival rate and chlorophyll contents were measured. Heat stress was performed as described previously (45). 7-day-old seedlings were exposed to 45 °C for 75 min and recovered at 22 °C for 7days.
Yeast Functional Complementation Test
A CAX function test was carried out in Saccharomyces cerevisiae strain K667 (MATa cnb1::LEU1 pmc1::TRP1 vcx1Δ) as described (36). The coding sequences of AtNCL, sAtNCL, and OsCAX3 were isolated and cloned into p416GPD then transformed into K667 cells using the LiAc/PEG method. Saturated liquid cultures of K667 containing the various plasmids were diluted to A600 = 1.0 then spotted onto selection medium (-Ura), yeast extract peptone dextrose (YPD) medium, or YPD containing 200 mm CaCl2. The dishes were incubated at 30 °C for 3 days before taking pictures.
NHX function test was carried out in S. cerevisiae strain AXT3 (Δena1–4::HIS3, Δnha1::LEU2, Δnhx1::TRP1) (46). AtNCL and AtNHX1 (46) were cloned into pDR195 and transformed into AXT3 cells. Cells were cultured in selection medium (-Ura). Saturated liquid cultures were diluted and spotted onto selection medium (-Ura, 20 g/liter galactose, 20 g/liter agar) with or without 200 mm NaCl. The dishes were incubated at 30 °C for 4 days before taking pictures.
Quantitative RT-PCR
qRT-PCR was employed to measure the transcript levels. The RNA samples were pretreated extensively with an RNase-free DNase to remove any contaminating genomic DNA prior to use. The PCR primers are listed in supplemental Table 1. qRT-PCRs were performed in 96-well blocks with an Applied Biosystems 7500 real-time PCR system using the SYBR Green I mix in a volume of 20 μl. The specificity of the PCRs was determined by melt curve analysis of the amplified products using the standard methods installed in the system. The comparative ΔΔCT method was employed to evaluate relative quantities of each product amplified from the samples. All qRT-PCRs were performed in biological triplicates using RNA samples extracted from three independent plant materials grown under identical growth conditions.
β-Glucuronidase (GUS) Assay
Histochemical staining and fluorometric assays were performed according to the method reported by Jefferson (66).
Bacterial Recombinant Protein Expression and 45Ca2+ Overlay Assays
The putative EF-hand Ca2+ binding domain (CaBD) sequence from AtNCL was cloned into pET-30(a) (Novagen) then transformed into Escherichia coli BL21 (DE3) cells (Novagen). His-tagged recombinant AtNCL was separated using Ni-affinity column (Novagen).The binding of 45Ca2+ to CaBD of AtNCL was assayed as described (47). The purified protein was resolved on a 12 or 18% SDS-polyacrylamide gel then transferred to PVDF membrane and incubated for 30 min with 100 mCi/ml 45CaC12. The membrane was then washed and exposed to a storage PhosphorImage screen for 12 h. Images were collected using a Typhoon 9210 imager (Amersham Biosciences).
Measurement of Reverse-mode NCX Activity
Full-length AtNCL CDS with an extra Kozak sequence (GCCACC) before the initiation codon was cloned into pDsRed1-N1 using NdeI and AgeI. CHO-K1 cells were transfected with the resulting construct by electroporation as described (48). Reverse-mode NCX activity was measured as described (49). The fluorescence intensity was calculated at each time point before and after the addition of Ca2+ containing loading buffer (Fbasal and F, respectively). The data for each cell were plotted as follows: ΔF/F = (F − Fbasal)/Fbasal.
Fluorescence Imaging
Fluorescence microscopy was performed using a Zeiss LSM 510 Meta microscope (Carl Zeiss Micro Imaging, Inc.). For yellow fluorescent protein (YFP) and propidium iodide (PI) imaging, an argon ion laser at 514 nm was used for excitation; emission was detected using a BP500–550 nm filter for YFP and LP615 nm for PI. Fluo-3 quantitative analysis was done as described (50, 51). Excitation of Fluo-3 was performed using an argon ion laser at 488 nm. Emitted light was detected through a BP505-530-nm filter from a 545-nm dichroic mirror. DsRed fluorescence was excited using a green helium-neon laser at 543 nm and was detected through a 545-nm dichroic mirror and LP560-nm filter. The images were processed using LSM 4.2 software (Carl Zeiss Micro Imaging, Inc.).
Plant Membrane Fractionation and Western Blotting
Protein extracts were prepared from the wild-type or transgenic seedlings and separated into soluble and membrane fractions by ultracentrifugation as described previously (52). The fractions were then analyzed by Western blotting using anti-GFP serum as the primary antibody.
Element Analysis by AAS
The pretreated or untreated seedlings were washed with deionized water, dried using a blast dryer, and weighed. The seedlings were then subjected to digestion by the aqueous method using the chloric acid/perchloric acid (4:1, v/v) method. Next, the digested samples were diluted with deionized water and analyzed by AAS (AA240FS+240Z, Varian).
[Ca2+]cyt Measurement Using Aequorin
atncl was crossed with a p35S::Aequorin transgenic line. The [Ca2+]cyt level in the homozygotes was then determined as described (53).
Noninvasive Ion Flux Measurement Using an Ion-selective Microelectrode
The net fluxes of Ca2+ and H+ in the root tips of 4-day-old seedlings grown on MS plates were measured noninvasively by SIET (54) using the BIO-001A SIET system (Xuyue (Beijing) Science and Technology Co., Ltd., Beijing, China). The SIET system measures static ionic/molecular concentrations and concentration gradients using ion-selective microelectrodes (55). The concentration gradient was measured by moving the electrode repeatedly between two positions along a predefined excursion (5–30 μm) at a fixed frequency in the range of 0.3–0.5 Hz.
RESULTS
AtNCL Is a Putative NCX
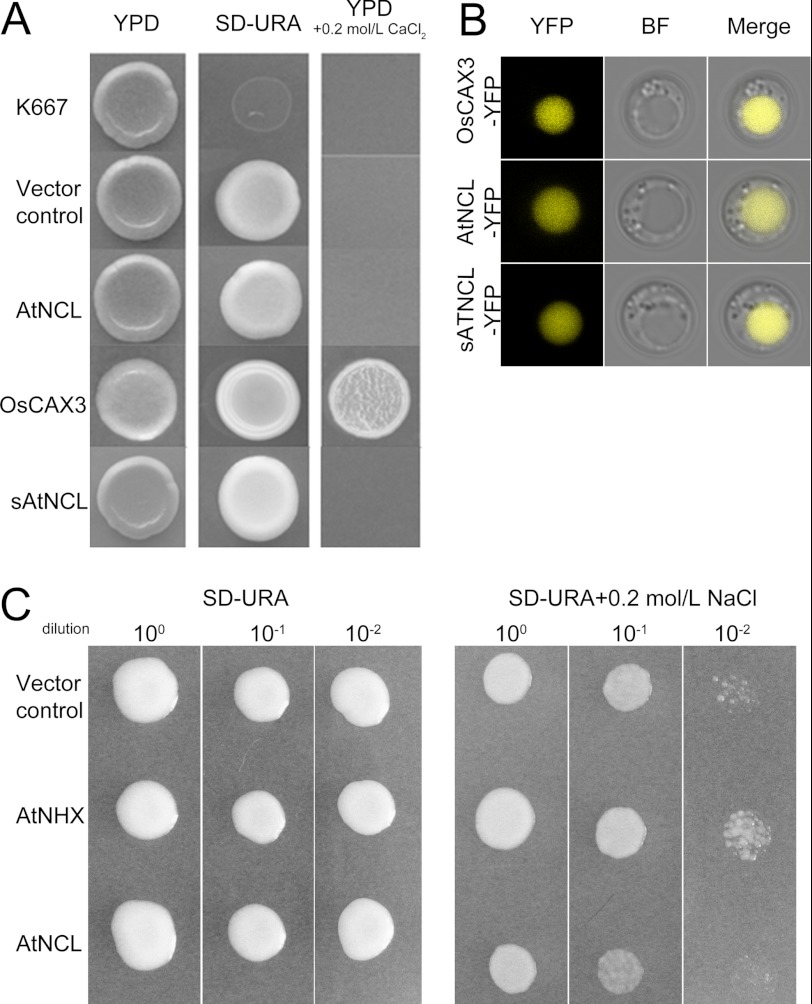
AtNCL (At1g53210) was predicted as a sodium/calcium exchanger family protein by the TAIR database. AtNCL comprises seven exons and six introns, encoding a putative protein with 585 amino acids. The N-terminal-most 22 amino acids in AtNCL comprise a putative signaling peptide (analyzed by SignalP). The protein had 10 putative transmembrane domains, with a hydrophilic loop between the fifth and sixth transmembrane domains containing two predicted EF-hand domains (analyzed by SMART). The deduced structure of AtNCL is similar to HsNCX1 from Homo sapiens (supplemental Fig. S1). AtNCL was considered a relative of CAXs (56), but it could not function as CAX in yeast, even if they have similar localization in yeast cells (Fig. 1). CAXs always have a conserved N-terminal autoinhibitory sequence (57–59); however, N-terminal deleted sAtNCL also cannot recover the yeast K667 phenotype (Fig. 1). On the other hand, AtNCL cannot work as a NHX in yeast (Fig. 1C), so we wanted to know whether it was a NCX-like protein.
FIGURE 1.
AtNCL shows no CAX/NHX-like activity in yeast. A, the expression of AtNCL and sAtNCL in K667 mutant yeast did not complement the Ca2+-hypersenstive phenotype. OsCAX3 was used as a positive control. B, the localization of AtNCL-YFP, sAtNCL-YFP, and OsCAX3-YFP in K667 cells was similar. YFP, YFP channel; BF, bright field channel; Merge, merged image of the YFP and BF channels. C, the expression of AtNCL in AXT3 mutant yeast did not complement the Na+-hypersensitive phenotype.
Ca2+ Binding Ability of AtNCL
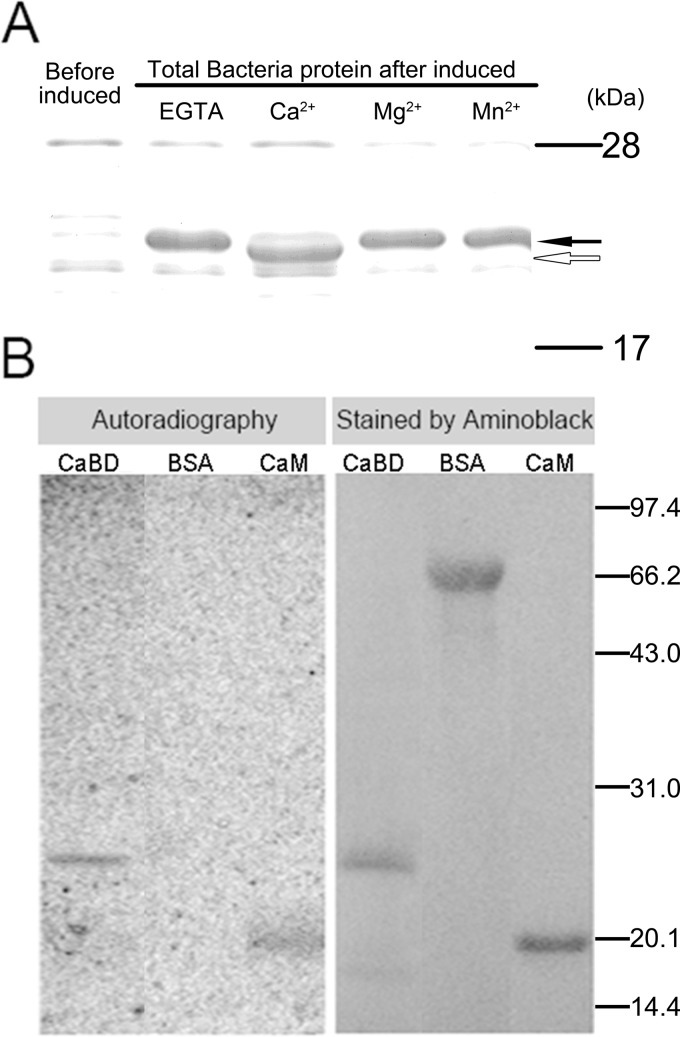
Because animal NCXs have intracellular loop with Ca2+ binding activity, we have tested the Ca2+ binding ability of AtNCL. Ca2+-dependent electrophoretic mobility shift and 45Ca2+ binding assays were used to examine the predicted the Ca2+ binding ability of the putative EF-hand domain in AtNCL (amino acids 262–430; CaBD). The vector pET-30(a)-CaBD was constructed as shown in supplemental Table S1, and recombinant CaBD tagged with six His residues at its N terminus was expressed in E. coli. Before loading the sample, EGTA or divalent ions were added to the sample. By SDS-PAGE, CaBD ran faster in the presence of 5 mm Ca2+ than it did in the presence of EGTA or the bivalent ions Mg2+ and Mn2+ (Fig. 2A). According to our 45Ca2+ binding assay results, recombinant CaBD bound Ca2+ as did our positive control, recombinant Arabidopsis CaM isoform 2 (AtCaM2), a known Ca2+-binding protein, whereas our negative control, bovine serum albumin (BSA), did not (Fig. 2B).
FIGURE 2.
The putative EF-hand domains of AtNCL can bind Ca2+. A, Ca2+-dependent electrophoretic mobility shift of the recombinant putative CaBD. EGTA, Mn2+, Ca2+, or Mg2+ was added at a final concentration of 5 mm to the bacterial proteins dissolved in 18% SDS-PAGE sample buffer. The hollow arrow indicates the CaBD band in the presence of Ca2+, which ran faster than in the presence of EGTA, Mn2+, or Mg2+ (filled arrow). B, purified CaBD, BSA, and CaM were separated by 12% SDS-PAGE and transferred to PVDF membranes for a 45Ca2+ binding assay and Amido Black staining. The molecular masses of the standards in kDa are shown at the right of the sections.
AtNCL Possesses NCX-like Activity in CHO-K1 cells
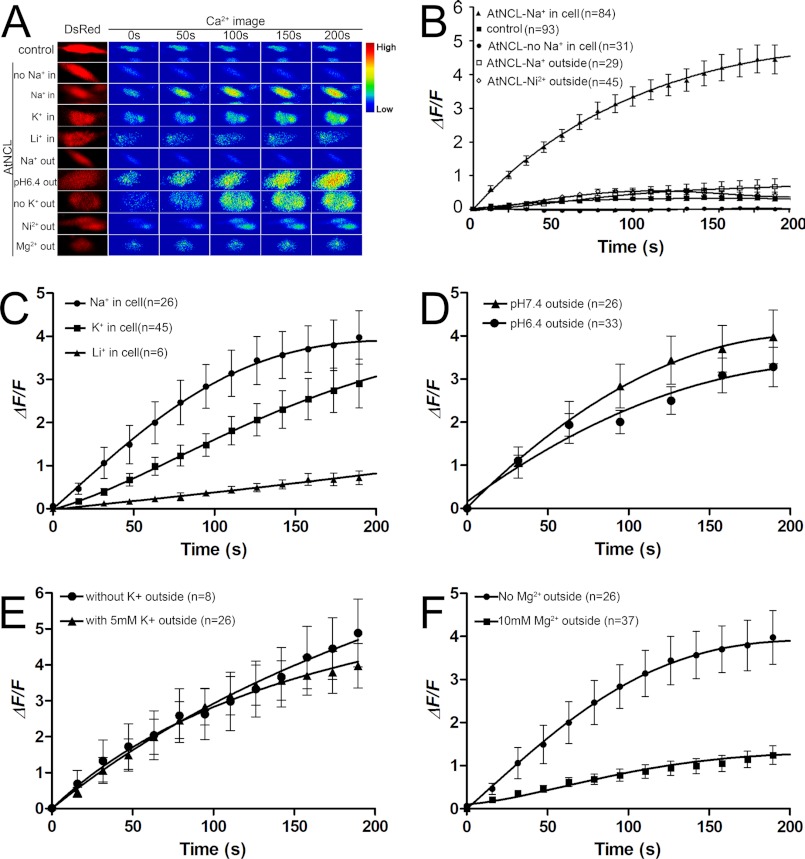
To assess whether AtNCL has NCX-like activity, a CHO-K1 cell-based heterologous expression system was used. CHO-K1 cells transfected with pAtNCL-DsRed were tested against CHO-K1 cells transfected with pDsRed1-N1 as a control. The [Ca2+]cyt was indicated by Fluo-3 fluorescence. In Na+-free buffer containing 2 mm Ca2+, the signal from the Na+-loaded AtNCL-expressing cells rose to saturation within 5 min whereas the vector control showed little signal (Fig. 3B). In contrast, the [Ca2+]cyt did not increase significantly if the cells were not loaded with Na+ (Fig. 3B). This suggests that the reverse NCX activity in the AtNCL-transfected cells (Fig. 3) was much higher than in the controls (Fig. 3). This activity was inhibited by 150 mm extracellular Na+ (the concentration was set approximately equal to the inner Na+ concentration to offset the Na+ gradient) or by 5 mm Ni2+, a NCX inhibitor used in animal cell (60) (Fig. 3B).
FIGURE 3.
Time course of Na+ gradient-dependent Ca2+ transport in CHO-K1 cells expressing AtNCL. A, pseudocolor images of CHO-K1 cells. The Ca2+ image was transformed from the Fluo-3 signal. B–F, statistical diagram of the CHO-K1 cells with treatments. The data shown are the mean values for the samples. Bar, the S.E. B, [Ca2+]cyt level in the AtNCL-expressing cells increased. In comparison, the vector control and unloaded cells showed little change, whereas outside 5 mm Ni2+ or 150 mm Na+ inhibited AtNCL activity. C, ion selectivity test of AtNCL. D, changing of outside pH from 7.4 to 6.4 having no effect on AtNCL-mediated Ca2+ uptake. E, outside K+ not necessary for Ca2+ uptake. F, outside 10 mm Mg2+ inhibition Ca2+ uptake.
To confirm the ion selectivity for the exchange function of AtNCL, we performed another set of Ca2+ flux assays using cells loaded with a buffer solution containing Na+, K+, or Li+. The result demonstrated that Na+ and K+ both can drive the Ca2+ flux and Na+ is more efficient than K+. However, Li+ had no effect (Fig. 3C). Proton concentration was increased outside the cell by changing the bath buffer pH from 7.4 to 6.4, and the Na+/Ca2+ exchange activity was not significantly affected (Fig. 3D).
To reveal whether the activity of AtNCL is dependent on K+-like Na+/Ca2+-K+ exchanger, we tested the bath buffer with K+ or without K+, respectively. The data indicated that outside K+ was not necessary for AtNCL activity (Fig. 3E).
Mg2+ was reported not affect NCX activity (61). Here, we tested the Mg2+ effect on AtNCL, by add 10 mm Mg2+ to bath buffer. The result suggested that Mg2+ can suppress the AtNCL activity (Fig. 3F). Overall, AtNCL has NCX-like Ca2+ uptake activity in CHO-K1 cells.
Membrane Localization of AtNCL in Planta
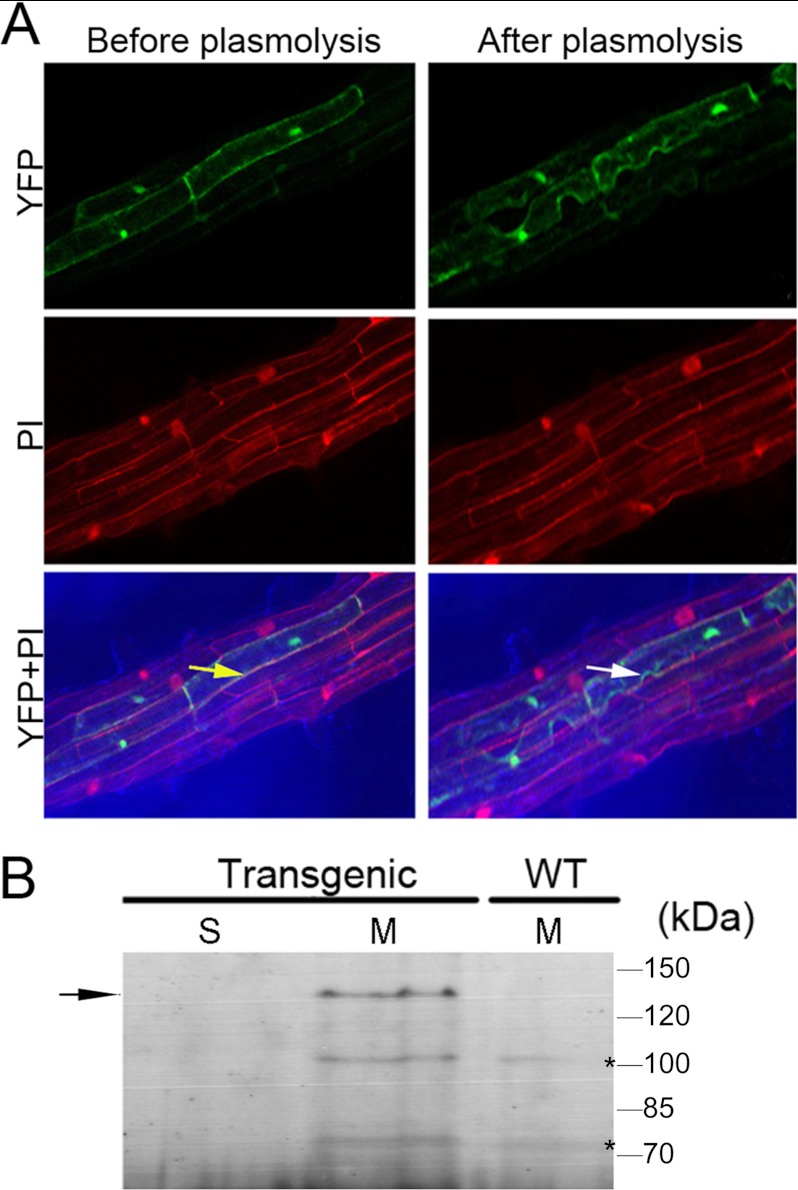
AtNCL was reported to be localized to the vacuole by prediction and proteomics strategy (62–65). In this study, p35S::AtNCL-YFP was constructed and transformed into Arabidopsis, the signal in the AtNCL-YFP transgenic seedlings was stronger near the plasma membrane after plasmolysis (Fig. 4A). Overlap detected between the PI and YFP signals indicated that AtNCL may localized to the plasma membrane in the transgenic root cells. However, AtNCL-YFP was also detected at the inner membrane.
FIGURE 4.
The membrane localization of AtNCL in Arabidopsis. A, laser-scanning confocal microscopy was used to determine the subcellular localization of an AtNCL-YFP fusion protein in transgenic root cells before and after plasmolysis with 30% sucrose. YFP, YFP channel; PI, PI channel; YFP-PI, merged image. The yellow arrow indicates the merged yellow signal from the cell membrane and cell wall, whereas the white arrow indicates the green color of the plasma membrane after plasmolysis. B, immunoblot analysis of various subcellular fractions with anti-GFP serum is shown. Transgenic, 3–4-week-old AtNCL-YFP-transgenic plants; WT, Col-0 seedlings; S, soluble protein; M, microsomal protein. The molecular masses of the standards are shown at the right of the sections. The AtNCL-YFP-specific band is indicated by an arrow on the left; the nonspecific bands are labeled by asterisks.
Next, microsomal proteins were extracted from the p35S::AtNCL-YFP transgenic lines and probed with anti-GFP antibodies (Fig. 4B). A specific band was detected in microsome fraction but not in the soluble proteins from the transgenic lines or the microsomal proteins from wild-type seedlings.
AtNCL Expression in Arabidopsis
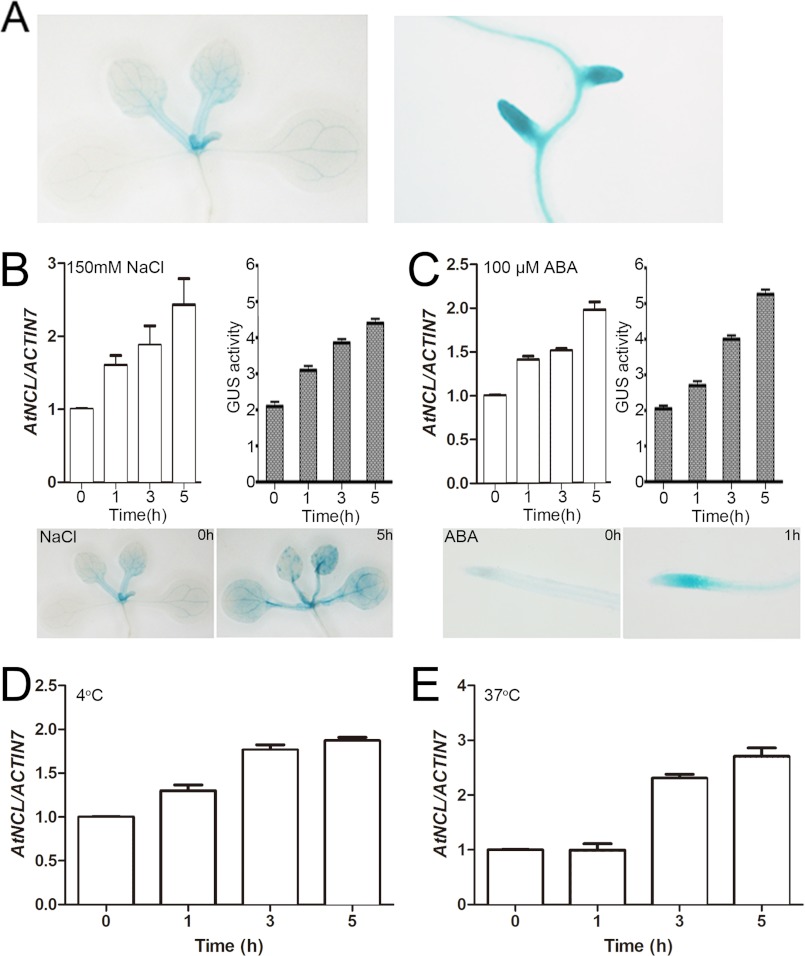
The expression pattern of AtNCL was examined using a GUS reporter gene fusion system (66). pAtNCL::AtNCL-GUS was constructed as described in supplemental Table S1 and transformed into Arabidopsis. GUS staining of 10 independent transgenic lines revealed that AtNCL was expressed broadly in the seedlings and flowers under normal growth conditions (Fig. 5A).
FIGURE 5.
AtNCL expression under normal conditions and in response to stress. A, GUS staining of AtNCL::AtNCL cDNA-GUS transgenic seedlings under normal growth conditions. B, qRT-PCR and GUS assay of seedling exposure to 150 mm NaCl. C, qRT-PCR and GUS assay of seedling exposure to 100 μm ABA. D, qRT-PCR assay of wild-type seedling exposure to 4 °C. E, qRT-PCR assay of wild-type seedling exposure to 37 °C.
AtNCL expression was increased during abiotic stress, such as salt, ABA, heat shock, and cold stress, as indicated by qRT-PCR and GUS assay (Fig. 5, B–E). AtNCL expression increased within 1 h of exposure to 150 mm NaCl and reached its peak after approximately 5 h (Fig. 5B). Expression of the gene was unchanged following exposure to the same concentration of LiCl or in a mock sample under salt stress (data not shown). Exogenous ABA treatment caused a similar change in AtNCL expression (Fig. 5C). Heat shock and cold stress also increased AtNCL expression; however, heat shock had a greater effect than cold stress (Fig. 5, D and E).
Phenotypic Analysis of atncl Mutant and Overexpression Lines
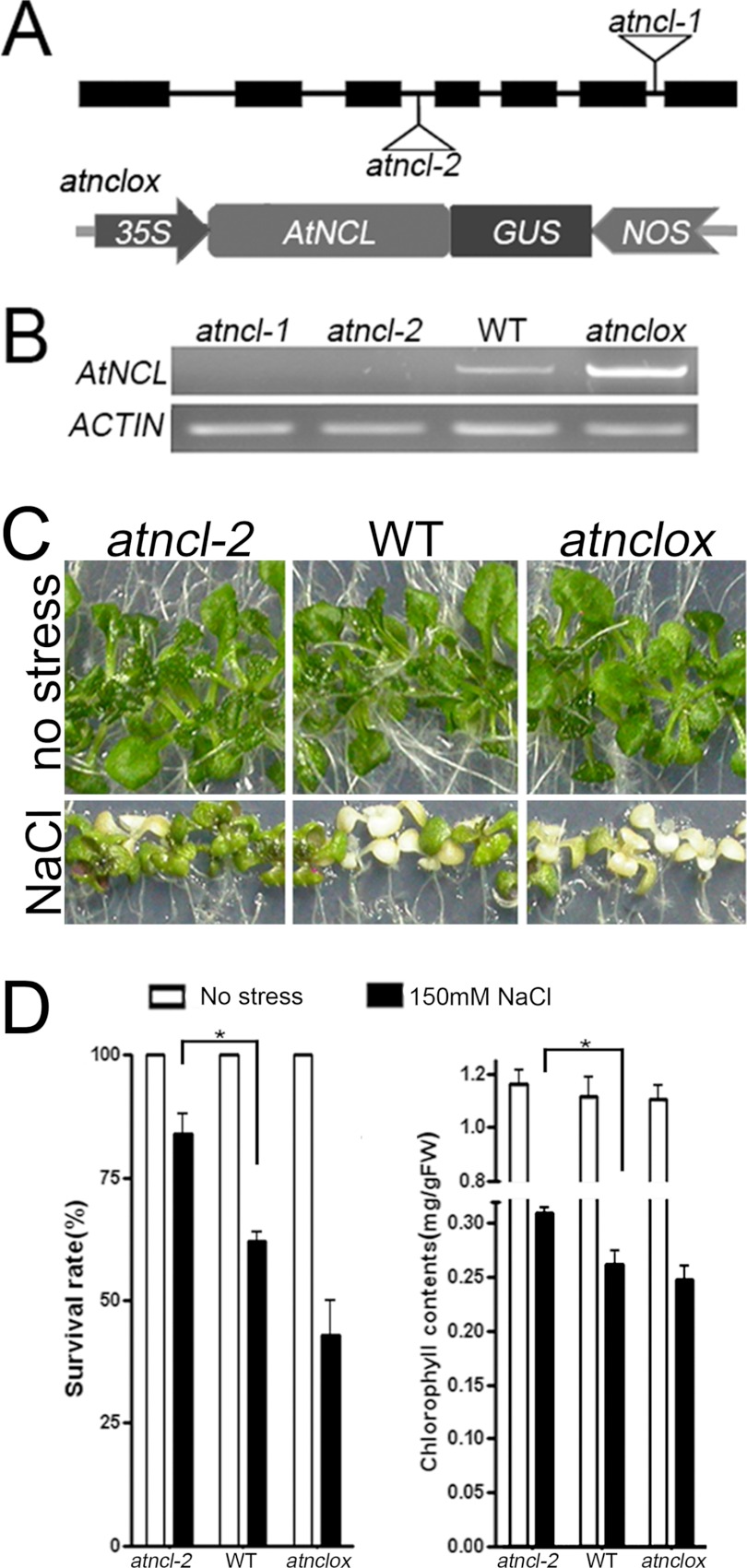
The phenotypes of several atncl loss-of-function mutants and overexpression lines under salt stress were investigated (Fig. 6). Two AtNCL T-DNA insertion lines, atncl-1 and atncl-2, were isolated from the SAIL collection. The T-DNA was inserted into the sixth and third introns of AtNCL in atncl-1 and atncl-2, respectively. Full-length AtNCL mRNA was not detected in either of the mutant lines (Fig. 6, A and B). Both the atncl-1 and atncl-2 alleles were backcrossed with wild-type Col-0. BASTA resistance was observed among the F2 progeny at a 3:1 ratio, indicating that both alleles may contain a single T-DNA insertion with a functional selection marker. Next, p35S::AtNCL-GUS was transformed into Arabidopsis ecotype Col-0 and selected using Hygromycin B. atnclox, a single-copy insertion line, was included in the experiment.
FIGURE 6.
Functional analysis of AtNCL in plant. A, plant materials. The upper panel shows a diagram of AtNCL with triangles representing the sites of the T-DNA insertions in atncl-1 and -2. The lower panel shows the construction of the overexpression vector. Full-length AtNCL cDNA was cloned into pCAMBIA1300 under control of the CaMV 35S-promoter with a NOS terminator and GUS as the reporter gene. B, RT-PCR analysis of total RNA extracted from the seedlings at 10 days after germination. ACTIN7 was amplified as an internal control. C, growth phenotypes of seedlings grown on 1/2 MS containing 150 mm NaCl for 7 days beginning at 7 days after germination. D, survival rate and chlorophyll content after 7 days of salt stress. Seedlings without salt stress were used as a control. The test was performed at least three times using Prism 4 (n = 30). The columns show the mean value plus the S.E. (error bars). Those pairs for which p < 0.05 are marked with an asterisk.
No differences were detected in atncl and atnclox in terms of seed germination, plant growth, and flowering time under normal growth conditions compared with wild-type Col-0. However, under salt stress conditions, the atncl mutant seedlings were less sensitive than wild-type or the overexpression lines. The survival rate and chlorophyll contents were measured after treatment with 150 mm NaCl (Fig. 6C). The survival rate for atncl-2 was 83.2% compared with 62.5 and 42.8% for wild-type and the overexpression lines, respectively (Fig. 6C). The average total chlorophyll content for atncl-2 after salt stress was 0.308 mg/g fresh weight, compared with 0.261 and 0.246 mg/g fresh weight for wild-type and the overexpression lines, respectively (Fig. 6C), whereas the length of roots of atncl-2 showed no difference with wild type and overexpression lines. The survival rate for atncl-1 was similar to that for atncl-2 (supplemental Fig. S4). The survival rate and chlorophyll contents of mutant seedlings were also slightly higher than wild type after heat shock and freezing stress (supplemental Fig. S5).
atncl Mutants Show an Altered Ca Content and Ca2+ Flux Activity during Salt Stress
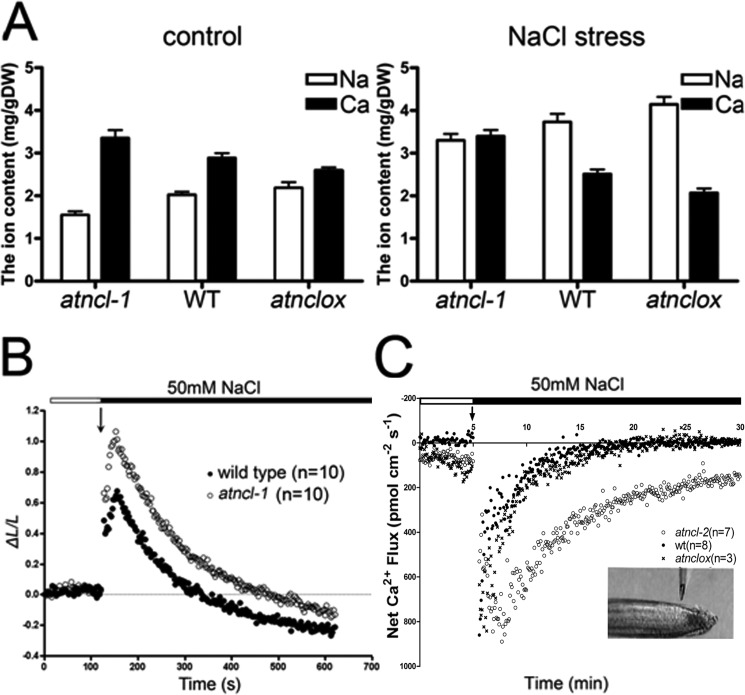
AAS was used to verify the total elemental Na and Ca contents in plants with or without NaCl stress (Fig. 7A). Under normal growth conditions, the total Na content in the atncl mutant seedlings was slightly lower than that in the wild-type or atnclox seedlings, whereas the level of Ca was higher. The ion content change in the atnclox lines was opposite that in the mutant lines. Under conditions of NaCl stress, the Na and Ca content change was different (Fig. 7A). The Ca content in the atncl loss-of-function mutants was largely unchanged, whereas that in wild-type and the overexpression lines was decreased. In comparison, the Na content in wild-type and the overexpression lines was much higher than that in atncl.
FIGURE 7.
AtNCL participates in Na+ and Ca2+ ion homeostasis. A, seedlings treated with or without 200 mm NaCl for 1 h were subjected to AAS. The experiment was repeated at least three times. The columns show the mean value plus the S.E. (error bars). B, kinetics of the [Ca2+]cyt level in the seedlings at 4 days after germination was determined using transgenic aequorin. C, Ca2+ flux in the roots was measured by SIET. The bath [Ca2+] was 0.1 mm. After approximately 5 min at the resting level, 50 mm NaCl was added. The Ca2+ flux was then measured for about 30 min in the root tips (shown in the inset). The number of samples is shown in parentheses. The data in B and C are the mean values for the samples.
Transgenic p35S::aequorin seedlings were used to monitor the [Ca2+]cyt in response to salt stress (Fig. 7B). Aequorin luminescence in the atncl and wild-type seedlings quickly increased approximately 2-fold following exposure to 50 mm NaCl. However, atncl showed stronger luminescence than wild type during their return to the resting level, which took about 600 s. This indicates that the [Ca2+]cyt is higher in the atncl seedlings compared with the wild-type seedlings.
To determine whether AtNCL participates in Na+/Ca2+ transport, SIET was used to monitor the flux of Ca2+ at the root tip during salt stress (Fig. 7C). After the addition of 50 mm NaCl to the test system, an outward Ca2+ flux exceeding 800 pmol·cm−2 s−1 was recorded in wild-type seedlings, atncl and atnclox. The Ca2+ flux reached the resting level within 10 min in wild type and atnclox, whereas in the atncl mutant seedlings, the flux lasted for approximately 30 min before returning to the resting level. In comparison, the H+ flux patterns in atncl and wild type were similar following treatment with 50 mm NaCl (supplemental Fig. S6). The H+ flux increased rapidly, reaching its peak within 5 min; thereafter, the flux decreased gradually, returning to the resting level within 20 min.
DISCUSSION
AtNCL May be a Novel Exchanger in Plant
AtNCL was once predicted to be a CAX-like protein (56); however AtNCL cannot work like CAXs in yeast to suppress the Ca2+-hypersensitive phenotype in K667, even if they share similar localization in yeast (Fig. 1). The AtNCL-transformed yeast growth was not affected by moderate change in pH (supplemental Fig. S3). By increasing the outside H+, the Ca2+ uptake of AtNCL-expressing cells was not affected. AtNCL also cannot work as a NHX in yeast (Fig. 1C), and AtNCL showed NCX-like activity in CHO-K1 cells (Fig. 3). By SIET, H+ flux patterns in atncl and wild type were similar during salt stress. These data suggested that AtNCL was probably a NCX-like protein rather than a CAX.
Recently, the structure of a NCX family member NCX_Mj had been resolved (27), and some critical site for ion binding and transport was identified. Sequence alignment analysis of NCXs and AtNCL showed that some critical amino acids that were conserved in NCXs also could be found in AtNCL, such as in α1 and α2 repeat, conservative glutamic acid or aspartic acid for bidentate Ca2+ coordination, conservative serine or threonine for extracellular Na+ binding site, and some Thr, Ser, or asparagine sites forming the Na+ site on the intracellular side were also found in AtNCL (supplemental Fig. S2). This suggests that AtNCL may share a similar ion exchange mechanism with NCXs.
However, there were also some differences between AtNCL and mammalian NCXs. For instance, unlike the NCXs (27,61), the Ca2+ uptake activity of AtNCL can be driven by the K+ gradient and inhibited by Mg2+ (Fig. 3), but outside K+ was not necessary for the Ca2+ uptake (Fig. 3). This suggested that AtNCL might not be a Na+/Ca2+-K+ exchanger-like protein.
AtNCL May Contribute to Ca2+ Homeostasis under Abiotic Stress
The results of a previous microarray analysis and expression level analysis indicate that salt stress, heat shock, cold stress, and ABA treatment can induce AtNCL expression (Fig. 5). In addition, it is known that Ca2+ signaling can be evoked by various stimuli. We found that salt and ABA stress induced greater AtNCL expression than temperature stimuli; however, we focused on the salt stress phenotype in our subsequent experiments to determine the function of AtNCL in plants. AtNCL was expressed broadly in Arabidopsis seedlings, flowers, and root tips (Fig. 5). Measurement of the elemental Ca content by AAS showed that under normal conditions, the atncl, wild-type, and overexpression seedlings all maintained a balance between Ca2+ and Na+ (Fig. 7A), whereas during salt stress the Ca2+ signal was ended by transfer of the Ca2+ from the cytoplasm to the apoplast or vacuole. During this process, AtNCL may be activated to extrude Ca2+; thus, atncl showed a higher [Ca2+]cyt and root surface Ca2+ flux by SIET than wild type (Fig. 7C). The enhanced Ca2+ signal may evoke a stronger response to salt stress, which may explain the improved growth of the atncl mutant lines compared with wild-type or the overexpression lines under conditions of salt stress.
To better understand the role of AtNCL, aequorin was used to monitor the [Ca2+]cyt level. We found that during salt stress, the atncl seedlings had higher [Ca2+]cyt than wild type. This apparent defect in Ca2+ extrusion was likely caused by the lack of AtNCL. Measurement of the root surface ion flux by SIET revealed a slower Ca2+ flux recovery process in the atncl mutant seedlings, which might be a consequence of the slower recovery of the [Ca2+]cyt.
It has been shown that the root surface ion flux might originate from ion exchange at the cell wall (67); however, the manner of the ion flux differed between the atncl and wild-type seedlings (Fig. 7C), possibly due to the difference in ion transport activity. In some cases, the detected ion flux might reflect a combination of ion transport across the cell wall and membrane. Even if AtNCL is absent, there are other transporters capable of terminating the Ca2+ signal, such as CAXs or Ca2+ pumps in the endomembrane and plasma membrane. Thus, the mutant seedlings showed no difference from wild type under normal conditions. AtNCL may behave like an animal NCX (68), regulating Ca2+ homeostasis under abnormal conditions. However, further experiments are needed to clarify how many Na+ ions AtNCL exchanges for one Ca2+ and the regulating mechanism of its exchanger activity in planta.
Acknowledgments
We thank Dr. M. R. Knight for the p35S::Aequorin transgenic Arabidopsis seeds, Dr. K. Cunningham and Dr. Baoshan Wang for the yeast strain and vector used for CAX complementation, Dr. José M. Pardo for the yeast strain and vector for NHX complementation, Dr. L. S. Kao for assistance during the NCX activity tests, Dr. Ying Sun and Ligeng Ma for helpful discussions, Junfeng Zhao for technical assistance with the laser scanning microscopy, and the Arabidopsis Biological Resource Center for the T-DNA insertion lines.
This work was supported by National Basic Research Program of China Grant 2006CB100101 and Research Program of the Ministry of Agriculture, China, Grant 2008ZX08009-003 (to D. S.); National Science Foundation of Hebei Province, China, Grant C2009001516, New Century Excellent Teacher Project of Education Department, China, Grant NCET-06-0256, and National Basic Research Program of China Grant 2006CB910600 (to S. C.).

This article contains supplemental Figs. S1–S6, Table S1, and additional references.
- [Ca2+]cyt
- cytosolic Ca2+ concentration
- AAS
- atomic absorption spectroscopy
- ABA
- abscissic acid
- AtNCL
- Arapidopsis NCX-like
- CaBD
- Ca2+ binding domain
- CaM
- calmodulin
- CAX
- cation/Ca2+ exchangers
- GUS
- β-glucuronidase
- NCX
- sodium/calcium exchanger
- NHX
- sodium/proton exchanger
- PI
- propidium iodide
- qRT-PCR
- quantitative RT-PCR
- SIET
- scanning ion-selective electrode technique.
REFERENCES
- 1. Spalding E. P., Harper J. F. (2011) The ins and outs of cellular Ca2+ transport. Curr. Opin. Plant Biol. 14, 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poovaiah B. W., Reddy A. S. (1993) Calcium and signal transduction in plants. Crit. Rev. Plant Sci. 12, 185–211 [DOI] [PubMed] [Google Scholar]
- 3. Monroy A. F., Dhindsa R. S. (1995) Low-temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25 ºC. Plant Cell 7, 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knight H., Trewavas A. J., Knight M. R. (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 12, 1067–1078 [DOI] [PubMed] [Google Scholar]
- 5. Knight H., Trewavas A. J., Knight M. R. (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knight H., Brandt S., Knight M. R. (1998) A history of stress alters drought calcium signalling pathways in Arabidopsis. Plant J. 16, 681–687 [DOI] [PubMed] [Google Scholar]
- 7. Dodd A. N., Kudla J., Sanders D. (2010) The language of calcium signaling. Annu. Rev. Plant Biol. 61, 593–620 [DOI] [PubMed] [Google Scholar]
- 8. Bush D. S. (1995) Calcium regulation in plant cells and its role in signaling. Plant Mol. Biol. 46, 95–122 [Google Scholar]
- 9. Paredes-Gamero E. J., Barbosa C. M., Ferreira A. T. (2012) Calcium signaling as a regulator of hematopoiesis. Front. Biosci. 4, 1375–1384 [DOI] [PubMed] [Google Scholar]
- 10. Baczyk D., Kingdom J. C., Uhlén P. (2011) Calcium signaling in placenta. Cell Calcium 49, 350–356 [DOI] [PubMed] [Google Scholar]
- 11. Baba Y., Kurosaki T. (2011) Impact of Ca2+ signaling on B cell function. Trends Immunol. 32, 589–594 [DOI] [PubMed] [Google Scholar]
- 12. Sanders D., Brownlee C., Harper J. F. (1999) Communicating with calcium. Plant Cell 11, 691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piñeros M., Tester M. (1997) Calcium channels in higher plant cells: selectivity, regulation and pharmacology. J. Exp. Bot. 48, 551–577 [DOI] [PubMed] [Google Scholar]
- 14. Axelsen K. B., Palmgren M. G. (2001) Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 126, 696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Axelsen K. B., Palmgren M. G. (1998) Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 46, 84–101 [DOI] [PubMed] [Google Scholar]
- 16. Briskin D. P. (1990) Ca2+-translocating ATPase of the plant plasma membrane. Plant Physiol. 94, 397–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrol N., Bennett A. B. (1996) A single gene may encode differentially localized Ca2+-ATPases in tomato. Plant Cell 8, 1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pfeiffer W., Hager A. (1993) A Ca2+-ATPase and a Mg2+/H+-antiporter are present on tonoplast membranes from roots of Zea mays L. Planta 191, 377–385 [Google Scholar]
- 19. Hong B., Ichida A., Wang Y., Gens J. S., Pickard B. G., Harper J. F. (1999) Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol. 119, 1165–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reuter H., Seitz N. (1968) The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J. Physiol. 195, 451–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicoll D. A., Longoni S., Philipson K. D. (1990) Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+ exchanger. Science 250, 562–565 [DOI] [PubMed] [Google Scholar]
- 22. Nakasaki Y., Iwamoto T., Hanada H., Imagawa T., Shigekawa M. (1993) Cloning of the rat aortic smooth muscle Na+/Ca2+ exchanger and tissue-specific expression of isoforms. J. Biochem. 114, 528–534 [DOI] [PubMed] [Google Scholar]
- 23. Tsuruya Y., Bersohn M. M., Li Z., Nicoll D. A., Philipson K. D. (1994) Molecular cloning and functional expression of the guinea pig cardiac Na+-Ca2+ exchanger. Biochim. Biophys. Acta 1196, 97–99 [DOI] [PubMed] [Google Scholar]
- 24. Ruknudin A., Valdivia C., Kofuji P., Lederer W. J., Schulze D. H. (1997) Na+/Ca2+ exchanger in Drosophila: cloning, expression, and transport differences. Am. J. Physiol. 273, C257–265 [DOI] [PubMed] [Google Scholar]
- 25. He Z., Tong Q., Quednau B. D., Philipson K. D., Hilgemann D. W. (1998) Cloning, expression, and characterization of the squid Na+–Ca2+ exchanger (NCX-SQ1). J. Gen. Physiol. 111, 857–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marshall C. R., Pan T. C., Le H. D., Omelchenko A., Hwang P. P., Hryshko L. V., Tibbits G. F. (2005) cDNA cloning and expression of the cardiac Na+/Ca2+ exchanger from Mozambique tilapia (Oreochromis mossambicus) reveal a teleost membrane transporter with mammalian temperature dependence. J. Biol. Chem. 280, 28903–28911 [DOI] [PubMed] [Google Scholar]
- 27. Liao J., Li H., Zeng W., Sauer D. B., Belmares R., Jiang Y. (2012) Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335, 686–690 [DOI] [PubMed] [Google Scholar]
- 28. Nicoll D. A., Ottolia M., Lu L., Lu Y., Philipson K. D. (1999) A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J. Biol. Chem. 274, 910–917 [DOI] [PubMed] [Google Scholar]
- 29. Nicoll D. A., Hryshko L. V., Matsuoka S., Frank J. S., Philipson K. D. (1996) Mutation of amino acid residues in the putative transmembrane segments of the cardiac sarcolemmal Na+-Ca2+ exchanger. J. Biol. Chem. 271, 13385–13391 [DOI] [PubMed] [Google Scholar]
- 30. Levitsky D. O., Nicoll D. A., Philipson K. D. (1994) Identification of the high affinity Ca2+-binding domain of the cardiac Na+-Ca2+ exchanger. J. Biol. Chem. 269, 22847–22852 [PubMed] [Google Scholar]
- 31. Philipson K. D., Nicoll D. A. (2000) Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 62, 111–133 [DOI] [PubMed] [Google Scholar]
- 32. Blaustein M. P., Lederer W. J. (1999) Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79, 763–854 [DOI] [PubMed] [Google Scholar]
- 33. Weber C. R., Piacentino V., 3rd, Ginsburg K. S., Houser S. R., Bers D. M. (2002) Na+-Ca2+ exchange current and submembrane [Ca2+] during the cardiac action potential. Circ. Res. 90, 182–189 [DOI] [PubMed] [Google Scholar]
- 34. Manohar M., Shigaki T., Hirschi K. D. (2011) Plant cation/H+ exchangers (CAXs): biological functions and genetic manipulations. Plant Biol. 13, 561–569 [DOI] [PubMed] [Google Scholar]
- 35. Zhao J., Connorton J. M., Guo Y., Li X., Shigaki T., Hirschi K. D., Pittman J. K. (2009) Functional studies of split Arabidopsis Ca2+/H+ exchangers. J. Biol. Chem. 284, 34075–34083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirschi K. D., Zhen R. G., Cunningham K. W., Rea P. A., Fink G. R. (1996) CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 93, 8782–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang L., Berkelman T., Franklin A. E., Hoffman N. E. (1993) Characterization of a gene encoding a Ca2+-ATPase-like protein in the plastid envelope. Proc. Natl. Acad. Sci. U.S.A. 90, 10066–10070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin D., Kuczera K., Squier T. C. (2000) The sensitivity of carboxyl-terminal methionines in calmodulin isoforms to oxidation by H2O2 modulates the ability to activate the plasma membrane Ca-ATPase. Chem. Res. Toxicol. 13, 103–110 [DOI] [PubMed] [Google Scholar]
- 39. Pittman J. K. (2011) Vacuolar Ca2+ uptake. Cell Calcium 50, 139–146 [DOI] [PubMed] [Google Scholar]
- 40. Kronzucker H. J., Britto D. T. (2011) Sodium transport in plants: a critical review. New Phytol. 189, 54–81 [DOI] [PubMed] [Google Scholar]
- 41. Rodríguez-Rosales M. P., Gálvez F. J., Huertas R., Aranda M. N., Baghour M., Cagnac O., Venema K. (2009) Plant NHX cation/proton antiporters. Plant Signal. Behav. 4, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y. Z., Li R. L., Xu X. Z., Zhu W. C. (1994) Ca2+ transport across the tonoplast of wheat roots. J. Wuhan Univ. (Nat. Sci. Ed) (in Chinese) 5, 111–115 [Google Scholar]
- 43. Arnon D. I. (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xin Z., Browse J. (1998) Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc. Natl. Acad. Sci. U.S.A. 95, 7799–7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zheng S. Z., Liu Y. L., Li B., Shang Z. L., Zhou R. G., Sun D. Y. (2012) Phosphoinositide-specific phospholipase C9 is involved in the thermotolerance of Arabidopsis. Plant J. 69, 689–700 [DOI] [PubMed] [Google Scholar]
- 46. Quintero F. J., Blatt M. R., Pardo J. M. (2000) Functional conservation between yeast and plant endosomal Na+/H+ antiporters. FEBS Lett. 471, 224–228 [DOI] [PubMed] [Google Scholar]
- 47. Maruyama K., Mikawa T., Ebashi S. (1984) Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J. Biochem. 95, 511–519 [DOI] [PubMed] [Google Scholar]
- 48. Sambrook J., Russell D. W. (2006) The Condensed Protocols from Molecular Cloning: A Laboratory Manual, pp. 599–621, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 49. Yang Y. C., Fann M. J., Chang W. H., Tai L. H., Jiang J. H., Kao L. S. (2010) Regulation of sodium-calcium exchanger activity by creatine kinase under energy-compromised conditions. J. Biol. Chem. 285, 28275–28285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Srikanth S., Banerjee S., Hasan G. (2006) Ectopic expression of a Drosophila InsP3R channel mutant has dominant-negative effects in vivo. Cell Calcium 39, 187–196 [DOI] [PubMed] [Google Scholar]
- 51. Calderón-Sánchez E. M., Ruiz-Hurtado G., Smani T., Delgado C., Benitah J. P., Gómez A. M., Ordóñez A. (2011) Cardioprotective action of urocortin in postconditioning involves recovery of intracellular calcium handling. Cell Calcium 50, 84–90 [DOI] [PubMed] [Google Scholar]
- 52. Tang W., Deng Z., Oses-Prieto J. A., Suzuki N., Zhu S., Zhang X., Burlingame A. L., Wang Z. Y. (2008) Proteomics studies of brassinosteroid signal transduction using prefractionation and two-dimensional DIGE. Mol. Cell Proteomics 7, 728–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Knight M. R., Campbell A. K., Smith S. M., Trewavas A. J. (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352, 524–526 [DOI] [PubMed] [Google Scholar]
- 54. Vincent P., Chua M., Nogue F., Fairbrother A., Mekeel H., Xu Y., Allen N., Bibikova T. N., Gilroy S., Bankaitis V. A. (2005) A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J. Cell Biol. 168, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kühtreiber W. M., Jaffe L. F. (1990) Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J. Cell Biol. 110, 1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shigaki T., Rees I., Nakhleh L., Hirschi K. D. (2006) Identification of three distinct phylogenetic groups of CAX cation/proton antiporters. J. Mol. Evol. 63, 815–825 [DOI] [PubMed] [Google Scholar]
- 57. Pittman J. K., Sreevidya C. S., Shigaki T., Ueoka-Nakanishi H., Hirschi K. D. (2002) Distinct N-terminal regulatory domains of Ca2+/H+ antiporters. Plant Physiol. 130, 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pittman J. K., Shigaki T., Cheng N. H., Hirschi K. D. (2002) Mechanism of N-terminal autoinhibition in the Arabidopsis Ca2+/H+ antiporter CAX1. J. Biol. Chem. 277, 26452–26459 [DOI] [PubMed] [Google Scholar]
- 59. Pittman J. K., Hirschi K. D. (2001) Regulation of CAX1, an Arabidopsis Ca2+/H+ antiporter: identification of an N-terminal autoinhibitory domain. Plant Physiol. 127, 1020–1029 [PMC free article] [PubMed] [Google Scholar]
- 60. Kimura J., Miyamae S., Noma A. (1987) Identification of sodium-calcium exchange current in single ventricular cells of guinea pig. J. Physiol. 384, 199–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kimura J. (1996) Effects of external Mg2+ on the Na-Ca exchange current in guinea pig cardiac myocytes. Ann. N.Y. Acad. Sci. 779, 515–520 [DOI] [PubMed] [Google Scholar]
- 62. Mitra S. K., Gantt J. A., Ruby J. F., Clouse S. D., Goshe M. B. (2007) Membrane proteomic analysis of Arabidopsis thaliana using alternative solubilization techniques. J. Proteome Res. 6, 1933–1950 [DOI] [PubMed] [Google Scholar]
- 63. Dunkley T. P., Hester S., Shadforth I. P., Runions J., Weimar T., Hanton S. L., Griffin J. L., Bessant C., Brandizzi F., Hawes C., Watson R. B., Dupree P., Lilley K. S. (2006) Mapping the Arabidopsis organelle proteome. Proc. Natl. Acad. Sci. U.S.A. 103, 6518–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Szponarski W., Sommerer N., Boyer J. C., Rossignol M., Gibrat R. (2004) Large-scale characterization of integral proteins from Arabidopsis vacuolar membrane by two-dimensional liquid chromatography. Proteomics 4, 397–406 [DOI] [PubMed] [Google Scholar]
- 65. Carter C., Pan S., Zouhar J., Avila E. L., Girke T., Raikhel N. V. (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16, 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jefferson R. (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5, 387–405 [Google Scholar]
- 67. Shabala S., Newman I. (2000) Salinity effects on the activity of plasma membrane H+ and Ca2+ transporters in bean leaf mesophyll: masking role of the cell wall. Ann. Bot. 85, 681–686 [Google Scholar]
- 68. Eisner D. A., Sipido K. R. (2004) Sodium calcium exchange in the heart: necessity or luxury? Circ. Res. 95, 549–551 [DOI] [PubMed] [Google Scholar]