Background: Activation of yeast trehalase has been a convenient read-out for nutrient signaling to PKA, but demonstration of phosphorylation in vivo is lacking.
Results: Nutrient activation is associated with phosphorylation, but phosphorylation is not enough for activation.
Conclusion: Nutrient activation of trehalase is a reliable read-out for nutrient activation of PKA in vivo.
Significance: Nutrient-sensing mechanisms can be identified using trehalase activation as a read-out.
Keywords: Enzyme Mechanisms, Phosphorylation, Protein Kinase A (PKA), Signaling, Yeast Metabolism, 14-3-3, Nutrient Activation, Nutrient Signaling, Protein Kinase Read-out, Trehalase
Abstract
The readdition of an essential nutrient to starved, fermenting cells of the yeast Saccharomyces cerevisiae triggers rapid activation of the protein kinase A (PKA) pathway. Trehalase is activated 5–10-fold within minutes and has been used as a convenient reporter for rapid activation of PKA in vivo. Although trehalase can be phosphorylated and activated by PKA in vitro, demonstration of phosphorylation during nutrient activation in vivo has been lacking. We now show, using phosphospecific antibodies, that glucose and nitrogen activation of trehalase in vivo is associated with phosphorylation of Ser21 and Ser83. Unexpectedly, mutants with reduced PKA activity show constitutive phosphorylation despite reduced trehalase activation. The same phenotype was observed upon deletion of the catalytic subunits of yeast protein phosphatase 2A, suggesting that lower PKA activity causes reduced trehalase dephosphorylation. Hence, phosphorylation of trehalase in vivo is not sufficient for activation. Deletion of the inhibitor Dcs1 causes constitutive trehalase activation and phosphorylation. It also enhances binding of trehalase to the 14-3-3 proteins Bmh1 and Bmh2, suggesting that Dcs1 inhibits by preventing 14-3-3 binding. Deletion of Bmh1 and Bmh2 eliminates both trehalase activation and phosphorylation. Our results reveal that trehalase activation in vivo is associated with phosphorylation of typical PKA sites and thus establish the enzyme as a reliable read-out for nutrient activation of PKA in vivo.
Introduction
Depending on the available nutrients, cells of the yeast Saccharomyces cerevisiae show dramatic changes in properties affected by the activity of protein kinase A (PKA) (1–5). During growth on glucose, storage carbohydrate levels are low, stress tolerance is low, cell wall composition is very sensitive to lyticase treatment, etc. During growth on respirative carbon sources, on the other hand, as well as upon starvation of glucose-fermenting cells for another essential nutrient, these properties are reversed. This has led to the concept that a complete fermentable growth medium is required to maintain high PKA activity. In addition, it has allowed the investigators to establish conditions under which glucose but also other essential nutrients, like nitrogen, phosphate and sulfate, trigger rapid activation of the PKA pathway (6).
The discovery that different essential nutrients can trigger rapid activation of the PKA pathway in appropriately starved fermenting cells has laid the basis for detailed studies on the nutrient-sensing and signaling systems involved (1, 6–11). This has required the use of reporter systems to follow rapid activation of the PKA pathway: trehalase activation; mobilization of trehalose and glycogen; loss of stress tolerance, repression of stress response (STRE-controlled) genes; induction of ribosomal protein genes; etc. The 5–10-fold increase in trehalase activity, which can be detected within 3–5 min after the addition of the agonist nutrient, has been a favorite reporter system in our studies on nutrient activation of the PKA pathway because of the rapidity and purely post-transcriptional character of the response.
Recently, we reported that the two main protein phosphatases of eukaryotic cells, PP2A2 and PP1, are also rapidly activated within a few min after the addition of glucose to cells growing on a non-fermentable carbon source. This activation is dependent on glucose activation of the cAMP-PKA pathway and thus suggests that both phosphatases are positively regulated by PKA (12).
The yeast S. cerevisiae has two enzymes for trehalose hydrolysis: neutral trehalase, encoded by NTH1 (13), and acid trehalase, encoded by ATH1 (14). Neutral trehalase is responsible for the rapid changes in trehalose content observed upon stimulation of the PKA pathway with glucose and other nutrients (15). Rapid glucose activation of neutral trehalase in glucose-deprived cells was first described by Van der Plaat in 1974 (16), whereas later also amino acid, phosphate, sulfate, and ammonium activation (6) were reported in appropriately starved cells. Van der Plaat (16, 17) provided evidence for the involvement of PKA, demonstrating a correlation with glucose-induced increase in cAMP and also in vitro activation of trehalase by incubation with cAMP and PKA. App and Holzer (18) demonstrated for the first time that in vitro activation of trehalase by PKA was correlated with phosphorylation. Extensive evidence indicates that rapid nutrient activation of trehalase in vivo is mediated by PKA. Mutants with reduced or constitutively high cAMP levels and mutants with reduced or constitutively high PKA activity show similarly reduced or constitutively elevated trehalase activity (6, 8, 19–22).
The precise phosphorylation site(s) responsible for activation of S. cerevisiae trehalase has remained enigmatic. The enzyme contains eight putative PKA phosphorylation sites: Ser20, Ser21, Ser60, Ser83, Ser475, Thr58, Thr135, and Thr149. Site-directed mutagenesis of individual sites did not reveal a specific site involved in activation, and mutagenesis of multiple sites led to a gradual loss of trehalase activity, preventing proper assessment of a role in the activation process (23). Recently, mass spectrometry evidence was reported for phosphorylation of purified trehalase by PKA in vitro on Ser20, Ser21, Ser60, and Ser83 (24). Schizosaccharomyces pombe neutral trehalase has a structure similar to that of S. cerevisiae trehalase and is rapidly activated under similar environmental conditions (25, 26). Mutagenesis of the putative PKA phosphorylation sites resulted in inactive trehalases unresponsive to environmental stimulation. Hence, also for S. pombe trehalase, it remains unclear what putative PKA phosphorylation sites are relevant for activation in vivo (27).
The extent of trehalase activation is always lower in vitro compared with in vivo, suggesting the existence of additional regulatory mechanisms. Two such mechanisms have been identified. Dcs1, an mRNA decapping enzyme, has been shown to interact with and act as a negative regulator of trehalase activity (28, 29). The yeast 14-3-3 proteins, encoded by Bmh1 and Bmh2, have recently been demonstrated to bind to phosphorylated residues in the N terminus of trehalase. Furthermore, the addition of recombinant Bmh1 protein to in vitro phosphorylated trehalase stimulated activation of the enzyme up to 7-fold (24, 30). The individual phosphorylation sites sustain no activation or only poor activation of trehalase in vivo, suggesting that phosphorylation on all or a majority of the sites is important for full 14-3-3 binding and thus for full activation (24). The possible importance of both mechanisms for in vivo regulation of trehalase has remained unclear.
In this paper, we have made use of custom-made phosphospecific antibodies against two phosphorylation sites in trehalase: Ser21 and Ser83. We show that glucose and nitrogen activation in vivo are associated with rapid phosphorylation of both sites. However, we also show that phosphorylation of these sites is not sufficient for activation of trehalase in vivo. Reduction of PKA activity surprisingly results in constitutive phosphorylation of trehalase. Furthermore, we show that the yeast 14-3-3 proteins are required for both activation of trehalase and phosphorylation of the two sites. Deletion of the trehalase inhibitor, Dcs1, causes constitutive activation and phosphorylation of trehalase and also results in stronger binding of 14-3-3 to trehalase, suggesting that Dcs1 inhibits by preventing 14-3-3 binding. Our results underscore the reliability of the rapid increase in trehalase catalytic activity as a valid marker for nutrient activation of the PKA pathway.
EXPERIMENTAL PROCEDURES
Yeast Strains
The strains used in this study are listed in Table 1. Strains JT21766 and JT21765 were constructed by Dr. T. Peeters (Leuven, Belgium). The tpk3::LEU2 construct was obtained by PCR amplification from vector pRS425 and transformed into BY4742. The resulting tpk3::LEU2 strain was crossed with either the tpk1::KanMX4 or tpk2::KanMX4 strains from the yeast deletion collection (31). Segregants carrying the double deletion were subsequently transformed with plasmid M4754 to swap the KanMX4 marker for HIS3 (32). Strain JT21721 was constructed by Dr. T. Peeters using crossing of the corresponding deletion mutants from the deletion collection followed by sporulation and segregant selection. The DC90 strain carrying the tpk2w3 allele was created by Dr. D. Castermans (Leuven, Belgium), following the procedure described by Cameron et al. (33). Briefly, a tpk1Δ TPK2 tpk3Δ bcy1Δ strain was plated on galactose-containing medium. The enhanced PKA activity in such a strain prevents growth on media with galactose as the carbon source, and spontaneous mutations in TPK2 arise that lower PKA activity and allow growth on galactose. Growing colonies were selected and mutations in TPK2 were identified by Sanger sequencing. The tpk2w3 allele has a G415C substitution in the ORF, which translates into D139H replacement in the protein. The allele was isolated by PCR, cloned into YIplac33, and integrated into the genome at the TPK2 locus. The plasmid was subsequently lost by plating the cells on 5-fluoroorotic acid medium, effectively replacing wild type TPK2 with the tpk2w3 allele. Strain DC127 was created by Dr. D. Castermans (Leuven, Belgium) by crossing BY4741 pph21Δ and BY4742 pph22Δ (12). Strains WS01, WS02, GV299, and GV300 were constructed as described (34). The GFP-KanMX6 cassette was isolated with PCR from plasmid pFA6a-GFP(S65T)-KanMX6 and transformed into BY4742 or b.1986. Strain WS10 was obtained by transforming the KanMx4 cassette isolated from b.1986 into PJ69-4A.
TABLE 1.
Yeast strains used in this study
| Strain | Relevant genotype | Source/Reference |
|---|---|---|
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Ref. 60 |
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Ref. 60 |
| BJ2168 | MATa leu2 trp1 ura3-52 prb1-1122 pep4-3 prc1407 gal2 | Ref. 61 |
| JT21766 | BY4741 tpk2::HIS3 tpk3::LEU2 | This study |
| JT21765 | BY4742 tpk1::HIS3 tpk3::LEU2 | This study |
| JT21721 | BY4742 tpk1::KanMX4 tpk2::KanMX4 | This study |
| DC90 | BY4742 tpk1::HIS3 tpk2w3 tpk3::LEU2 | This study |
| DC127 | BY4742 pph21::KanMX4 pph22::KanMX4 | Ref. 12 |
| WS01 | BY4742 BMH1-GFP::KanMX6 | This study |
| WS02 | BY4742 BMH2-GFP::KanMX6 | This study |
| b.1986 | BY4742 dcs1::KanMX4 | Ref. 31 |
| GV299 | BY4742 dcs1::KanMX4 BMH1-GFP::KanMX6 | This study |
| GV300 | BY4742 dcs1::KanMX4 BMH2-GFP::KanMX6 | This study |
| L6281 | MATa ura3-52 leu2::hisG | Ref. 48 |
| L6245 | L6281 his3::hisG bmh2::HIS3+ bmh1::HIS3+ | Ref. 48 |
| pJ69-4A | MATa leu2-312 ura3-52 trp1-901 his3-200 gal4Δ gal80 Δ GAL2-ADE2 lys2::GAL1-HIS3 met2::GAL7-LacZ | Ref. 62 |
| WS10 | pJ69-4A dcs1::KanMX4 | This study |
Plasmid Construction
Plasmid pTPI1-NTH1-HA-URA3 was constructed using 5′ BamHI PCR primer CGGGATCCATGAGTCAAGTTAATACAAGC and 3′ SmaI PCR primer TCCCCCCGGGTAGTCCATAGAGGTTTCTTTC. The PCR product was digested and ligated into the episomal vector pYX212, which contains the TPI promotor and the URA3 selection gene. Plasmid pTPI1-NTH1-HA-LEU2 was constructed by subcloning of the NTH1-HA allele, using BamHI and EcoRV restriction sites, into pYX242, which contains the same TPI promotor as pYX212. Plasmids pTPI1-BMH1-HA-URA3 and pTPI1-BMH2-HA-URA3 were constructed using 5′ EcoRI PCR primer CTACGGAATTCATGTCAACCAGTCGTGAAG or CTACGGAATTCATGTCCCAAACTCGTGAAG and 3′ BamHI PCR primer CGTAGGGATCCCTTTGGTGCTTCACCTTC or CGT-AGGGATCCTTTGGTTGGTTCACCTTGAG, respectively. PCR products were digested and ligated into pYX212. Plasmid pGEX-4T1-NTH1 used in the in vitro kinase assay was constructed using 5′ BamHI PCR primer CGGGATCCATGAGTCAAGTTAATACAAGC and 3′ NotI PCR primer TTTTCCTTTTGCGGCCGCCTATAGTCCATAGAGGTTTC. The PCR product was digested and ligated in frame with the GST tag into the pGEX-4T1 vector. Plasmid pADE-GFP-HA3-TPK1, used to express Tpk1 for the in vitro kinase assay, was constructed as described (35). Plasmids pGAD424-BMH1 and pGAD424-BMH2 used in the two-hybrid assay were constructed using 5′ BamHI PCR primers GAAGGATCCGGATGTCAACCAGTCGTGAAGATTC and 3′ PstI AAAACTGCAGTTACTTTGGTGCTTCACCTTC or 5′ GAAGGATCCGGATGTCCCAAACTCGTGAAGATTC and 3′ PstI AAAACTGCAGTTATTTGGTTGGTTCACCTTGAG. The PCR product was digested and ligated into the pGAD424 vector. Plasmid pGBT9-NTH1 used in the two-hybrid assay was constructed using 5′ SmaI TCCCCCGGGGATGAGTCAAGTTAATACAAGC and 3′ PstI AAAACTGCAGCTATAGTCCATAGAGGTTTC PCR primers. The PCR product was digested and ligated into the pGBT9 vector.
Growth Conditions
For the experiments involving glucose activation of trehalase, cells were grown in rich yeast peptone (YP) containing 3% glycerol and 0.1% glucose under continuous shaking until exponential phase. For the experiments involving nitrogen activation, cells were grown in SD medium with 2% glucose and appropriate amino acids into exponential phase. Exponential phase cells were subsequently harvested and transferred to nitrogen starvation medium (1.7 g/liter yeast nitrogen base without amino acids and without ammonium sulfate) with 4% glucose. Cells were starved for 6 h at 30 °C under continuous shaking.
In Vitro Kinase Assay
GST-Nth1 fusion proteins were expressed in Escherichia coli from a pGEX-4T1 vector. After a 3-h induction with 0.3 mm IPTG, a clarified bacterial lysate was prepared, and the fusion protein was bound to gluthatione-Sepharose (GE Healthcare) following standard procedures. Tpk1 was expressed in the partially protease-deficient yeast strain BJ2168 from plasmid pADE-GFP-HA3-TPK1 and immunoprecipitated as described below. Bead-bound Tpk1 and GST-Nth1 were washed and resuspended in 100 μl of kinase buffer (50 mm Tris, pH 8.0, 1 mm EGTA, 1 mm DTT, 5 mm MgCl2, 0.5 mm Na3VO4, 10 mm β-glycerol phosphate). 20 μl of bead-bound Tpk1 and GST-Nth1 were mixed, and the reactions were started by the addition of 50 μm ATP. Reactions were incubated for 45 min at 30 °C while shaking and terminated by the addition of 5× SDS sample buffer (250 mm Tris/HCl, pH 8, 50 mm 2-mercaptoethanol, 10% SDS, 0.5% bromphenol blue, and 50% glycerol). Samples were heated for 3 min at 95 °C and separated on SDS-PAGE. The gel was stained with Coomassie, and bands corresponding to Nth1 were analyzed by mass spectrometry.
Mass Spectrometry Analysis
Nth1 bands were excised from the gel, reduced and alkylated with dithiotreitol and iodoacetamide, and finally digested with sequencing grade trypsin in 25 mm ammonium bicarbonate, pH 8.0. Proteolytic digests were acidified by adding 5% formic acid (final concentration) and analyzed using a nanoAcquity ultrahigh pressure liquid chromatograph (Waters, Manchester, UK) directly coupled to a Q-TOF premier (Waters). The mass spectrometer was set in a data-dependent mode. Survey scans of 1 s were acquired in positive ion centroid mode from m/z 400 to 1500, and low energy collision-induced dissociation spectra were acquired in profile mode from m/z 50 to 2000 for 3 s. Peptides were identified by MS/MS ion search by using an in-house licensed MASCOT 2.0 server (available from the Matrix Science Web site). Searches were done in the SwissProt database (UniProt_SwissProt 50.8) using the following parameters: S. cerevisiae as taxonomy restriction, trypsin as proteolytic enzyme with two missed cleavages allowed, peptide tolerance 25 ppm, fragment tolerance 0.05 Da, carbamidomethylcysteine as fixed and phosphorylated serine, and phosphorylated threonine and oxidized methionine as variable modifications. Phosphorylated peptides identified by mascot were validated by manual interpretation of the fragmentation spectra.
Phosphospecific Antibody Production
Antibodies against phosphorylated Ser21 and Ser83 were generated by Eurogentec, using keyhole limpet hemocyanin-conjugated phosphopeptides RQRRLSpSLSEFND (flanking Ser21) and LQQTRRGpSEDDTY (flanking Ser83). Phosphospecific antibodies were purified by affinity chromatography using phosphopeptide-conjugated resin.
Trehalase Activity Assay
Appropriately nutrient-deprived cells were cooled on ice for 30 min, harvested by centrifugation, and washed with Mes/KOH buffer (25 mm, pH 6). Cells were resuspended in fresh medium (either YPglycerol or nitrogen starvation medium, depending on the deprivation condition) at a density of 25 mg of wet weight/ml. Trehalase activity was determined in crude cell extracts as described (36). The glucose liberated was assayed by the glucose oxidase/peroxidase method. Protein concentration was determined by the Lowry procedure. The specific activity of trehalase is expressed as nmol of glucose liberated/min/mg of protein.
Protein Extraction and Immunoprecipitation
∼100 mg of cells were lysed mechanically in 500 μl of extraction buffer containing 1× PBS, 10% glycerol, 0.1% Triton X-100, 2.5 mm MgCl2, 1 mm EDTA, phosphatase inhibitors (10 mm NaF, 0.1 mm β-glycerol phosphate, 0.4 mm Na3VO4), and 10 μl/ml Sigma protease inhibitor mixture for yeast extracts. Crude cell extracts were cleared twice by centrifugation. Protein concentration was measured using the Bradford assay (Bio-Rad) or Pierce 660-nm protein assay. Samples were diluted to match the protein concentration of the sample with the lowest concentration. HA-tagged trehalase was immunoprecipitated using protein G-conjugated Dynabeads (Invitrogen) and high affinity anti-HA antibody (clone 3F10, Roche Applied Science). Dynabeads were equilibrated in lysis buffer, and immunoprecipitation was carried out at 4 °C under continuous gentle agitation. Immunoprecipitates were washed three times with lysis buffer and subsequently boiled in 2.5× SDS sample buffer containing 125 mm Tris/HCl, pH 8, 25 mm 2-mercaptoethanol, 5% SDS, 0.25% bromphenol blue, and 25% glycerol.
Western Blotting
After SDS-PAGE, proteins were transferred to a nitrocellulose membrane (Hybond, GE Healthcare). Nonspecific antibody/reagent binding sites on the blots were blocked using 5% skimmed milk (anti-HA Ab and anti-GFP Ab) or 5% BSA (anti-phosphospecific Abs) for 1 h. Blots were incubated with antibody solutions overnight at 4 °C under continuous, gentle agitation, washed three times with 1× PBST, and incubated with secondary antibodies (anti-rabbit or anti-mouse Abs conjugated to horseradish peroxidase) (GE Healthcare). Proteins were visualized using Pierce ECL reagents and the ImageQuant LAS4000 mini CCD imaging system (Fujifilm).
λ-Phosphatase Treatment
Immunoprecipitates were obtained as described previously and washed three times with 1× PBST (0.05% Triton X-100) to remove all traces of phosphatase inhibitors. After removal of the last wash, beads were resuspended in 50 μl of 1× λ-phosphatase buffer (50 mm HEPES, 100 mm NaCl, 2 mm DTT, 0.01% Brij 35, pH 7.5) supplemented with 1 mm MnCl2 and 80 units of λ-phosphatase (New England Biolabs). Samples were incubated for 30 min at 30 °C; subsequently, supernatant was removed, and the beads were boiled in SDS sample buffer.
RESULTS
Trehalase Is a Phosphoprotein
Based on its amino acid sequence, two Pfam domains have been identified in trehalase: a small, 30-amino acid-long Ca2+ binding domain located near the N terminus and a much larger catalytic domain spanning residues 163–721 (Fig. 1A). Apart from these two domains, an N-terminal extension of about 100 amino acids is present. Over the last 5 years, several large scale phosphoproteomic studies have identified 11 phosphorylated serines or threonines in trehalase (37–41). Interestingly, all of the identified residues are located in the 100-amino acid-long N-terminal extension, which suggests that this region plays an important role in the regulation of the enzyme. In 2008, Panni et al. (30) mapped the binding site of the yeast 14-3-3 proteins to this region. They showed that phosphorylation at either Ser21 or Ser23 was necessary to promote binding of yeast 14-3-3 proteins to trehalase.
FIGURE 1.
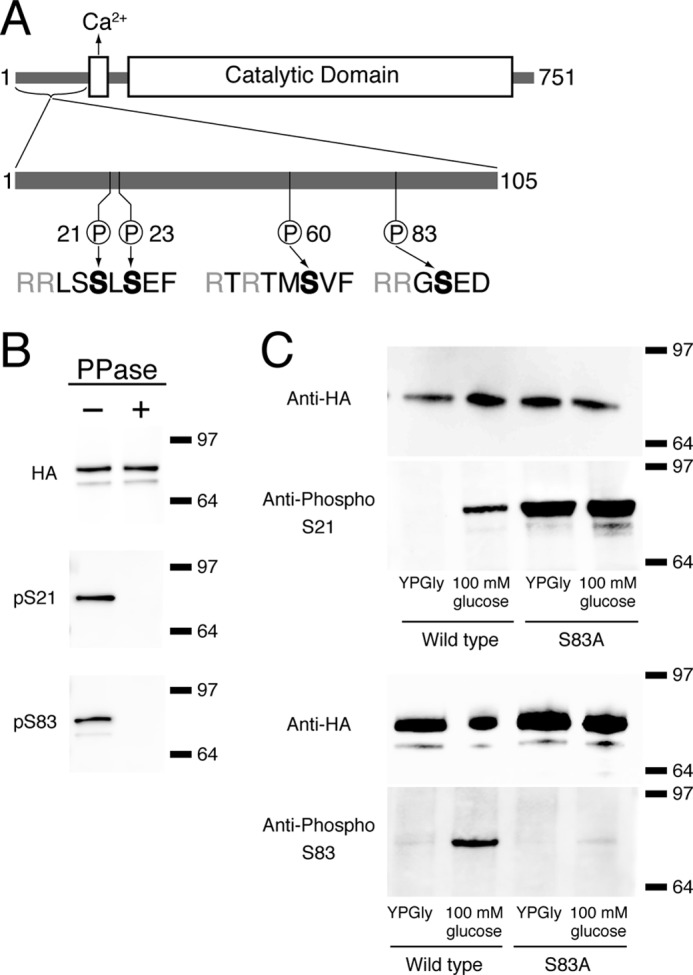
Structure of neutral trehalase and evaluation of the specificity of the custom-made phosphospecific Abs. A, trehalase consists of a catalytic domain, a Ca2+-binding domain, and an N-terminal extension. Phosphorylated serines and adjacent residues in the N-terminal extension are depicted to illustrate putative PKA phosphorylation sites. B, immunoprecipitation of trehalase expressed in BY4742 from plasmid pTPI1-NTH1-HA-URA3 treated with (+) or without (−) phosphatase. Equal amounts of immunoprecipitated Nth1-HA were loaded on all three Western blots. The upper blot was incubated with anti-HA Ab, the middle blot with anti-phospho-Ser21 Ab (pS21), and the bottom blot with anti-phospho-Ser83 Ab (pS83). C, phosphorylation status of Ser21 and Ser83 in trehalase after the addition of 100 mm glucose to respiring yeast cells. Strains were transformed with pYX212-NTH1S21A-HA or pYX212-NTH1S83A-HA.
To identify putative PKA-specific phosphorylation sites in trehalase, we performed an in vitro kinase assay. Recombinant GST-tagged trehalase purified from E. coli was phosphorylated with HA-tagged Tpk1 immunoprecipitated from yeast in the presence of ATP, cAMP, and MgCl2. The samples were separated with SDS-PAGE, and protein bands corresponding to trehalase were excised and digested with trypsin. The resulting tryptic peptides were analyzed, after TiO2 chromatography for phosphopeptide enrichment, by nano-ultrahigh pressure liquid chromatography electrospray ionization Q-TOF mass spectrometry. Two phosphorylated residues were identified, Ser60 and Ser83, both previously observed in some of the phosphoproteomic studies mentioned above. To evaluate the relevance of the N-terminal phosphorylations for nutrient-induced trehalase activation, we ordered phosphospecific Abs against peptides containing phospho-Ser21 and phospho-Ser23 (the residues designated by Panni et al. (30) as required for 14-3-3 binding to trehalase) and against phospho-Ser60 and phospho-Ser83 (the putative PKA phosphorylation sites discovered in our in vitro kinase assay). Upon validating the quality of the Abs, using alkaline phosphatase treatment of immunopurified trehalase, we found that only the anti-phospho-Ser21 and anti-phospho-Ser83 Abs could distinguish properly between the phosphorylated and unphosphorylated versions of the whole trehalase protein (Fig. 1B). The specificity of the antibodies was also tested using versions of Nth1 in which either Ser21 or Ser83 was mutated to alanine. In the case of phospho-Ser83, the signal in the Western blot was no longer observed, whereas the S21A mutant protein now showed a constitutive signal with the antibody (Fig. 1C). Apparently, mutagenesis of Ser21 to alanine causes a structural change in the domain, which allows the antibody to detect the site in both the phosphorylated and non-phosphorylated form. Hence, we do not think that this result compromises the conclusion that this antibody is also specific for the phosphorylated native Ser21 residue. This conclusion was supported by later results (see below). In our subsequent experiments, only these two Abs were used. It must be noted that both Ser21 and Ser83 fulfill the requirements of the consensus PKA phosphorylation site, RX2(S/T) and RRX(S/T), respectively (42).
Trehalase Is Phosphorylated in Vivo in Response to a Glucose Signal
Upon the addition of glucose to derepressed yeast cells, trehalase was rapidly activated (Fig. 2A). To evaluate the phosphorylation status of the enzyme during the activation process, we expressed a C-terminal HA-tagged version of trehalase in a wild type strain and took samples over a 30-min time period after the addition of glucose. The trehalase enzyme was isolated by immunoprecipitation using anti-HA antibodies, the samples were subsequently separated by SDS-PAGE and transferred by Western blotting. The presence or absence of phosphorylation was then examined with our custom-made phosphospecific Abs. SDS-PAGE and Western blotting were done in triplicate; one blot was used for detection of total trehalase with the anti-HA Ab, and the other two were used for detection with the phosphospecific Abs. The result can be seen in Fig. 2B; glucose-induced trehalase activation correlates with phosphorylation of Ser21 and Ser83.
FIGURE 2.
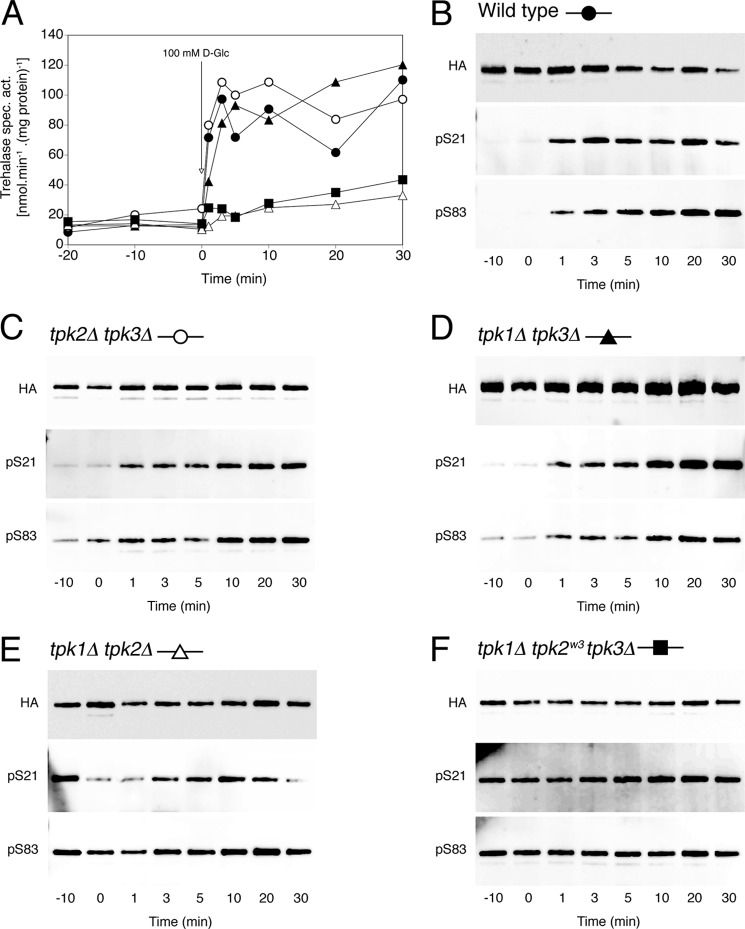
Glucose-induced trehalase activation and phosphorylation in strains with reduced PKA activity. Trehalase activity (A) and phosphorylation status of Ser21 and Ser83 in trehalase (B–F) after the addition of 100 mm glucose to respiring yeast cells. Strains were transformed with pTPI1-NTH1-HA-URA3. Shown are wild type (B, ●), tpk2Δ tpk3Δ (C, ○), tpk1Δ tpk3Δ (D, ▴), tpk1Δ tpk2Δ (E, ▵), and tpk1Δ tpk2w3 tpk3Δ (F, ■). B–F, top Western blot, detection of total Nth1-HA with anti-HA Ab; middle Western blot, detection with anti-phospho-Ser21 Ab (pS21); bottom Western blot, detection with anti-phospho-Ser83 Ab (pS83).
Aberrant Phosphorylation of Trehalase in Strains with Reduced PKA Activity
PKA is a holoenzyme consisting of two regulatory subunits encoded by BCY1 and two catalytic subunits redundantly encoded by three TPK genes. Because a triple TPK deletion is synthetically lethal, we examined the involvement of PKA in the in vivo phosphorylation of trehalase using a collection of strains expressing a single TPK as the sole source of PKA catalytic subunit. Yeast strains expressing only TPK1 or TPK2 displayed both a wild type trehalase activation and phosphorylation pattern (Fig. 2, A, C, and D). However, glucose-induced trehalase activation was severely affected in a strain expressing only TPK3 as the PKA catalytic subunit. Phosphorylation on Ser21 and Ser83 was constitutive in this strain and did not change upon the addition of glucose (Fig. 2E). A similar phenotype is observed in a yeast strain carrying an attenuated allele of TPK2 as the sole source of PKA catalytic subunit (Fig. 2F). These results clearly show that phosphorylation alone is not sufficient to promote activation of the trehalase enzyme.
Inactivation of the Protein Phosphatase PP2A Also Causes Constitutive Phosphorylation of Trehalase
Recently, we have provided evidence that PKA stimulates PP2A activity (12). Hence, we evaluated whether the constitutive phosphorylation of trehalase in strains with reduced PKA activity might be due to lowered dephosphorylation by PP2A. Determination of trehalase phosphorylation in a yeast strain in which both catalytic subunits of PP2A, Pph21 and Pph22, are deleted indeed also revealed constitutive phosphorylation (Fig. 3). This suggests that the constitutive phosphorylation in strains with reduced PKA activity could be due to reduced dephosphorylation by PP2A rather than by enhanced phosphorylation by an alternative protein kinase.
FIGURE 3.
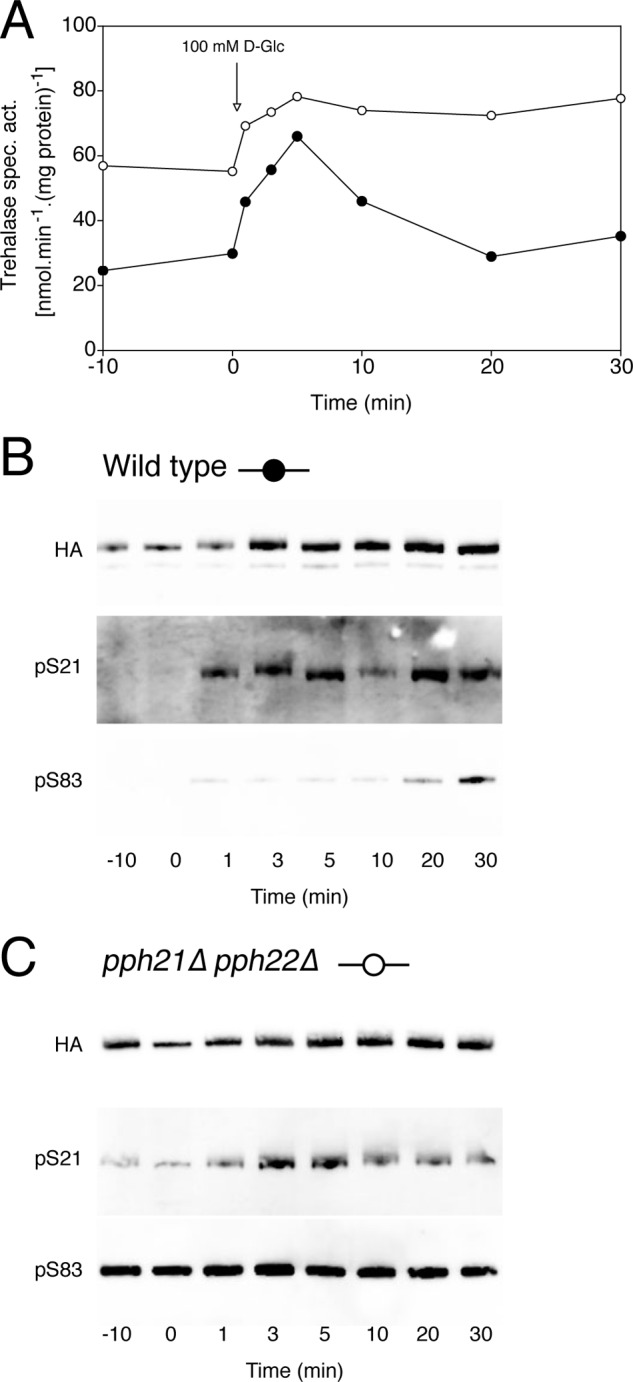
Glucose-induced trehalase activation and phosphorylation in PP2A deletion strains. Trehalase activity (A) and phosphorylation status of Ser21 and Ser83 in trehalase (B and C) after the addition of 100 mm glucose to respiring yeast cells. Strains were transformed with pTPI1-NTH1-HA-URA3. Shown are wild type (B, ●) and pph21Δ pph22Δ (C, ○). B and C, top Western blot, detection of total Nth1-HA with anti-HA Ab; middle Western blot, detection with anti-phospho-Ser21 Ab (pS21); bottom Western blot, detection with anti-phospho-Ser83 Ab (pS83).
The Yeast 14-3-3 Proteins Are Required for Activation of Trehalase in Vivo
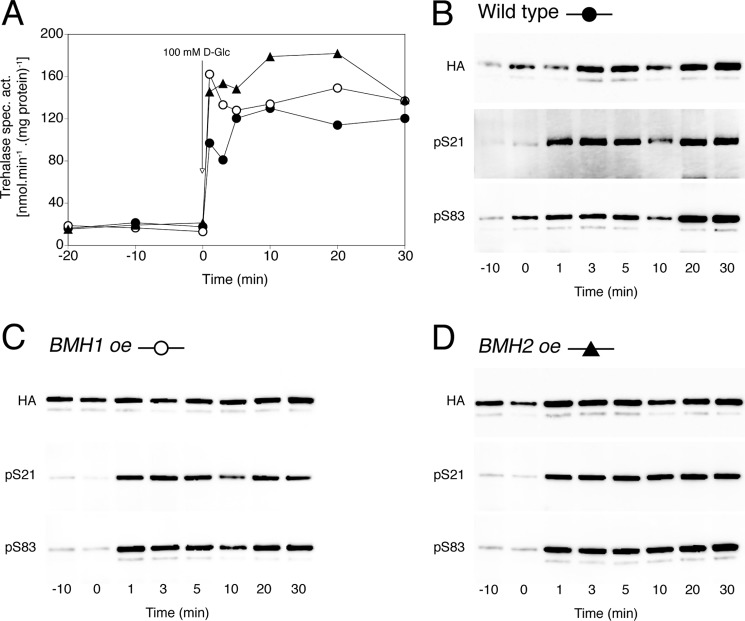
Several genome-wide interactome studies have found the yeast 14-3-3 proteins, Bmh1 and Bmh2, in complex with trehalase (43–47). Panni et al. (30) demonstrated that recombinant Bmh1 could stimulate the activity of PKA-phosphorylated trehalase in vitro. To further assess the role of 14-3-3 in the regulation of trehalase, we measured in vivo glucose-induced trehalase activation and phosphorylation in 14-3-3 overexpression and deletion mutants. Overexpression of BMH1 or BMH2 did not have any effect on glucose-induced trehalase activation or phosphorylation when compared with wild type (Fig. 4). Deletion of both 14-3-3 isoforms is lethal in the S288c strain background but not in the Σ1278B strain. The Fink laboratory kindly provided us with their bmh1Δ bmh2Δ strain (48) in which we determined glucose-induced trehalase activation and phosphorylation. As can be seen in Fig. 5, the bmh1Δ bmh2Δ strain failed to show trehalase activation. Moreover, phosphorylation of the two PKA sites in trehalase was absent in this strain. We conclude that the 14-3-3 proteins are required for glucose-induced activation of trehalase and for establishment or maintenance of its phosphorylation.
FIGURE 4.
Glucose-induced trehalase activation and phosphorylation in BMH1 and BMH2 overexpression strains. Trehalase activity (A) and phosphorylation status of Ser21 and Ser83 in trehalase (B–D) after the addition of 100 mm glucose to respiring yeast cells. Strains shown are as follows: wild type (B, ●), BMH1 overexpression (C, ○), and BMH2 overexpression (D, ▴). BY4742 was transformed with pTPI1-HA-URA3 (empty plasmid) and pTPI1-NTH1-HA-LEU2 for wild type; with pTPI1-BMH1-HA-URA3 and pTPI1-NTH1-HA-LEU2 for BMH1 overexpression; and with pTPI1-BMH1-HA-URA3 and pTPI1-NTH1-HA-LEU2 for BMH2 overexpression.
FIGURE 5.
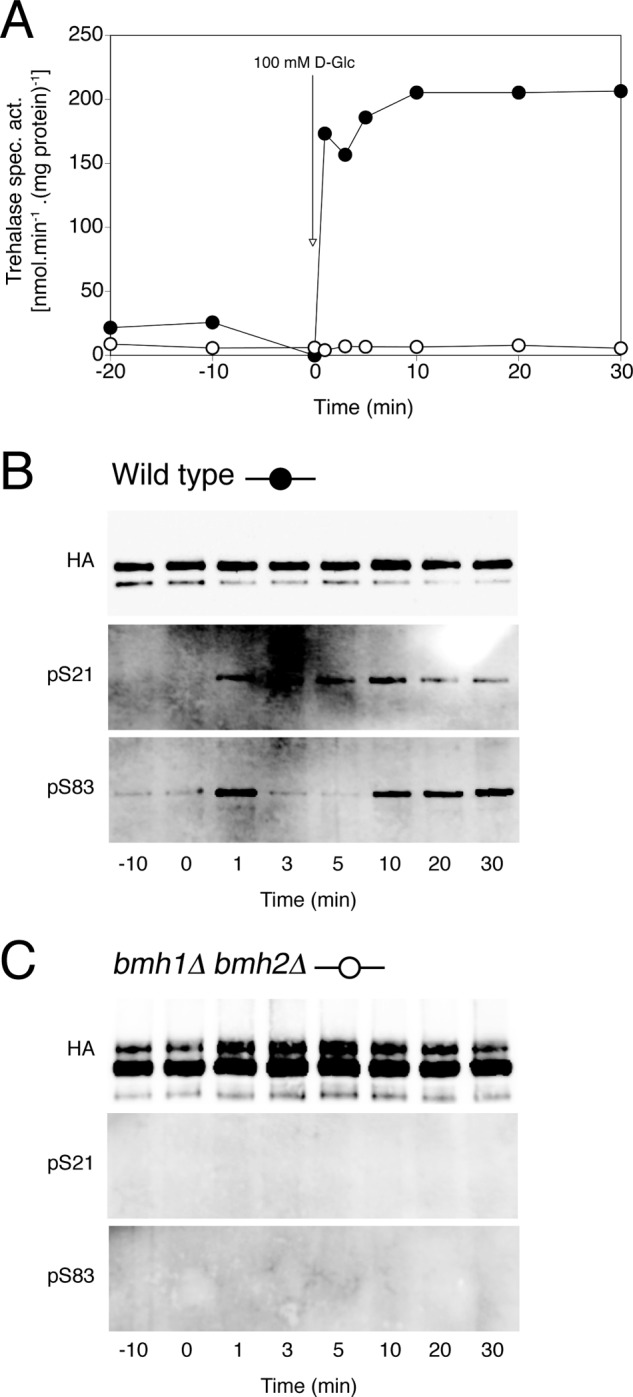
Glucose-induced trehalase activation and phosphorylation in the bmh1,2Δ strain. Trehalase activity (A) and phosphorylation status of Ser21 and Ser83 in trehalase (B and C) after the addition of 100 mm glucose to respiring yeast cells. Strains shown are as follows: wild type (B, ●) and bmh1Δ bmh2Δ strain (C, ○). Wild type and bmh1Δ bmh2Δ strains were transformed with pTPI1-NTH1-HA-URA3.
The Yeast 14-3-3 Proteins Interact with Phosphorylated Trehalase in Vivo
Because the 14-3-3 proteins are well known to bind specifically to phosphorylated proteins, we reasoned that trehalase phosphorylation could promote interaction with 14-3-3 in vivo. To examine this hypothesis, we transformed strains expressing GFP-tagged alleles of BMH1 and BMH2 with the plasmid carrying the HA-tagged NTH1 allele in order to perform co-immunoprecipitation. After immunoprecipitation of trehalase with anti-HA Abs, separation by SDS-PAGE, and transfer by Western blotting, we visualized the phosphorylation status with the phosphospecific Abs and any interacting 14-3-3 proteins with anti-GFP Ab (Fig. 6). In respirative yeast cells, only small amounts of both Bmh1 and Bmh2 can be co-immunoprecipitated with trehalase. However, upon activation of trehalase and phosphorylation of its Ser21 and Ser83 residues after the addition of glucose, significantly more 14-3-3 protein becomes bound to trehalase, suggesting that phosphorylation of trehalase enhances interaction with 14-3-3. This is in agreement with a recent report in which enhanced binding of Bmh1 and Bmh2 to phosphorylated trehalase was demonstrated in vitro (24).
FIGURE 6.
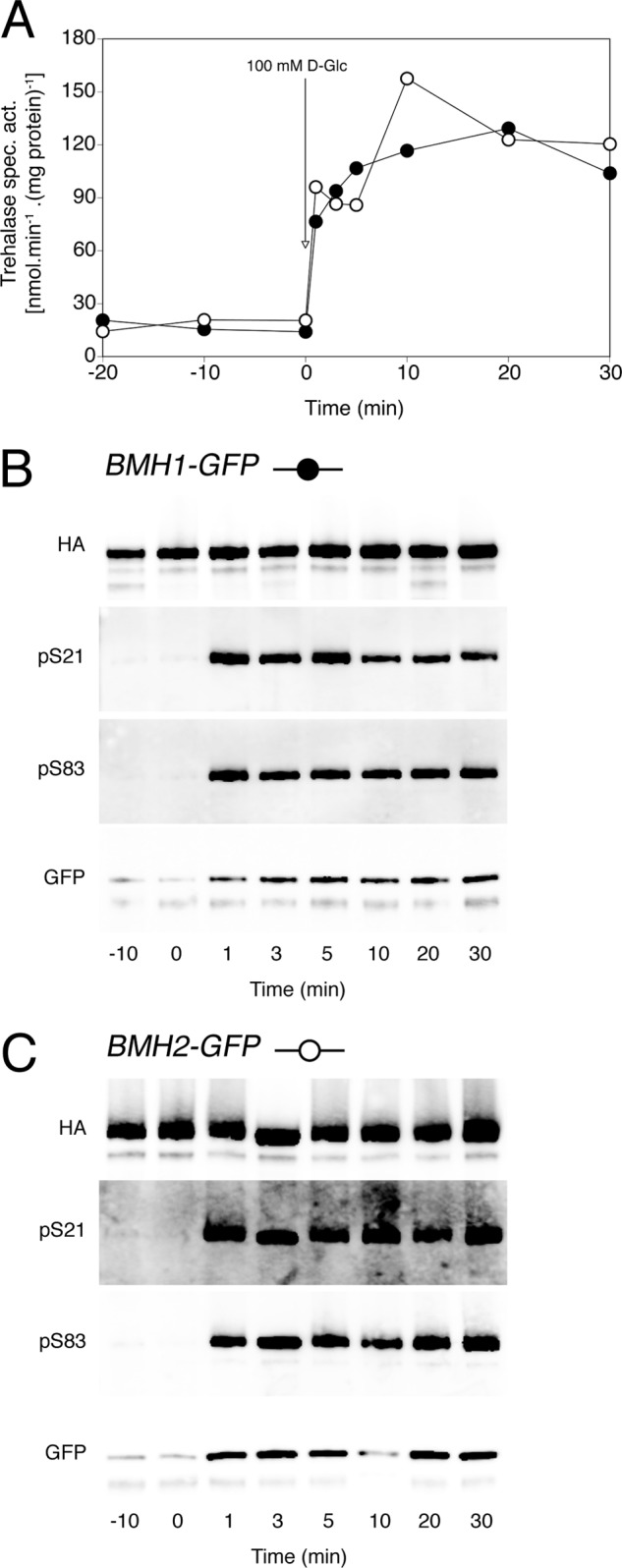
Glucose-induced trehalase activation, phosphorylation and interaction with the Bmh1 and Bmh2 proteins. Trehalase activity (A), phosphorylation status of Ser21 and Ser83 in trehalase (B and C), and interaction with Bmh1 (B, bottom panel) and Bmh2 (C, bottom panel) after the addition of 100 mm glucose to respiring yeast cells. The strains were transformed with plasmid pTPI1-NTH1-HA-URA3 and were expressing in addition to Nth1-HA either BMH1-GFP (B, ●) or BMH2-GFP (C, ○). Trehalase was immunoprecipitated, and phosphorylation of Ser21 and Ser83 was visualized with phosphospecific Abs (pS21 and pS83). In addition, anti-GFP Ab was used to visualize any interacting Bmh proteins (GFP).
Trehalase Is Phosphorylated in Vivo in Response to a Nitrogen Signal
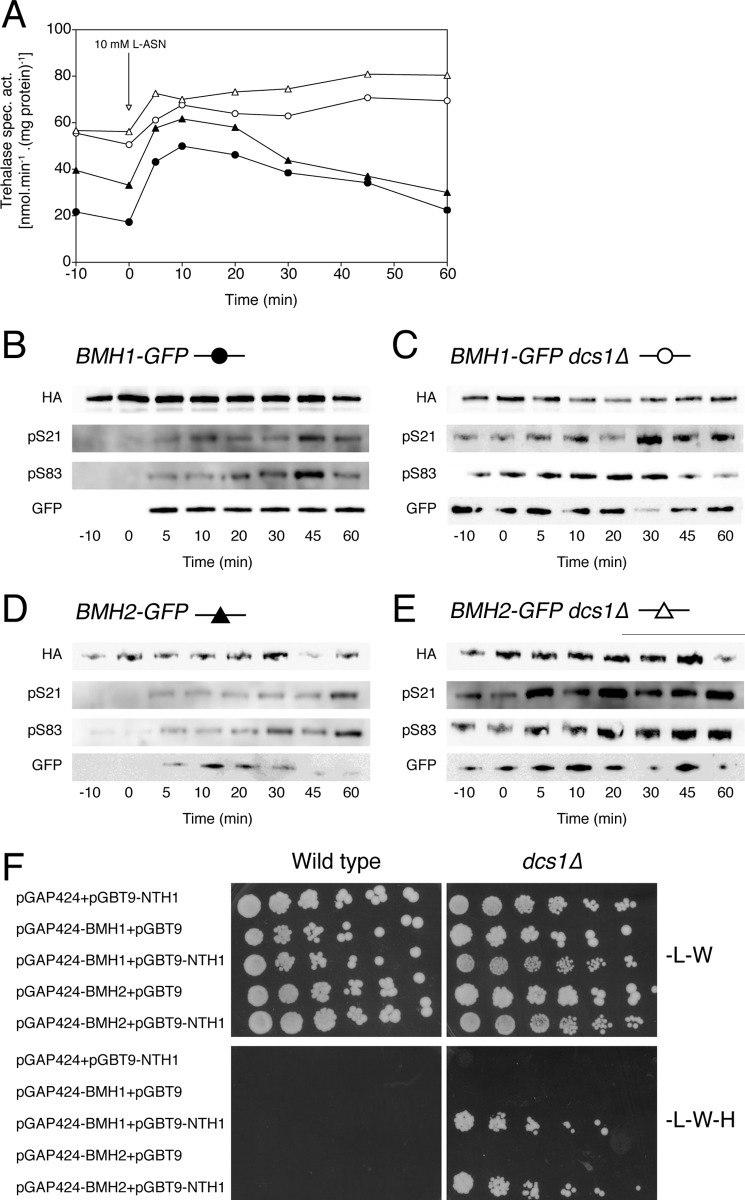
Like respirative cells, fermenting cells deprived of nitrogen are characterized by low trehalase activity. Upon the addition of a good nitrogen source, like asparagine, trehalase is rapidly activated (5–10 min). Using the phosphospecific Abs, we show that nitrogen-induced trehalase activation is also correlated with an increase in phosphorylation on Ser21 and Ser83, pointing toward a similar mechanism for both glucose- and nitrogen-induced trehalase activation (Fig. 7, A, B, and D). Hence, this result supports the previous conclusion that PKA is mediating nitrogen-induced activation of trehalase despite the absence of cAMP signaling after the addition of a nitrogen source (6, 8, 19, 22).
FIGURE 7.
Nitrogen-induced trehalase activation and phosphorylation in the dcs1Δ strain. Trehalase activity (A) and phosphorylation status of Ser21 (pS21) and Ser83 (pS83) in trehalase (B–E) after the addition of 10 mm l-asparagine to fermenting, nitrogen-starved yeast cells. Wild type and dcs1Δ cells, expressing Bmh1-GFP or Bmh2-GFP, were transformed with pTPI1-NTH1-HA-LEU2. Trehalase was immunoprecipitated, and phosphorylation of Ser21 and Ser83 was visualized with phosphospecific Abs (pS21 and pS83). In addition, anti-GFP Ab was used to visualize any interacting Bmh proteins (GFP). F, two-hybrid analysis of direct physical interaction between Nth1 and the 14-3-3 proteins, Bmh1 and Bmh2. Two-hybrid reporter strains PJ69-4A wild type and PJ69-4A dcs1Δ, transformed with the indicated plasmids, expressing Nth1, Bmh1, and/or Bmh2, were grown overnight in liquid selective medium (SD−Leu−Trp) and spotted on selective SD−Leu−Trp (−L−W) and SD−Leu−Trp−His (−L−W−H) media.
Dcs1 Is an Inhibitor of Trehalase Activity
Although originally described as the yeast orthologue of human DcpS, a scavenger mRNA decapping enzyme (29), the unnamed ORF YLR270w had already been implicated in trehalase regulation, before it was named Dcs1. A genome-wide yeast two-hybrid study had reported interaction between trehalase and YLR270w (49). Additional research by the Panek laboratory (28, 50) confirmed the role of Dcs1 as an inhibitor of trehalase activity. To gain more insight into the role of Dcs1 in trehalase regulation, we assessed nutrient-induced trehalase activation under a variety of conditions in a dcs1Δ strain. Because a dcs1Δ strain is unable to grow on non-fermentable carbon sources (51, 52), we tried several other methods of glucose deprivation but failed to obtain consistent, reproducible results for both activation and phosphorylation of trehalase (data not shown). Therefore, we focused on nitrogen-induced trehalase activation in nitrogen-deprived cells. The results showed that in a dcs1Δ strain, trehalase activity is constitutively high under nitrogen deprivation and fails to increase further upon the addition of asparagine. The phosphorylation pattern correlates well with enzyme activity: constitutive phosphorylation on both Ser21 and Ser83 under nitrogen deprivation and no further change after the addition of asparagine (Fig. 7, C and E). Moreover, trehalase isolated from the dcs1Δ strain was much more stable, suggesting that phosphorylation and/or activation of the enzyme increases its stability. We also show increased interaction between Nth1 and the 14-3-3 proteins as a function of time after the addition of nutrient and coinciding with the activation of trehalase. Deletion of DCS1 results in constitutive interaction (Fig. 7, C and E). Using the yeast two-hybrid system, we confirmed that there is stronger interaction between trehalase and the 14-3-3 proteins in a dcs1Δ strain (Fig. 7F). This suggests that Dcs1 may inhibit trehalase activity by prevention of 14-3-3 binding.
DISCUSSION
Nutrient Activation of Trehalase in Vivo Is Associated with Phosphorylation on Putative PKA Recognition Sites
Our results have shown for the first time that rapid nutrient activation of trehalase in vivo is associated with rapid phosphorylation of the enzyme on two putative PKA phosphorylation sites, Ser21 and Ser83, in its N terminus. Other sites (e.g. Ser23 and Ser60) may also become phosphorylated, but we were not able to obtain phosphospecific antibodies against these sites. The rapid phosphorylation in vivo is consistent with the view obtained from in vitro studies on trehalase activation (16–18) and from in vivo studies with mutants in the cAMP-PKA pathway (6, 8, 19–22) that PKA-mediated phosphorylation is an important mechanism underlying the rapid changes in trehalase activity observed in vivo. Nutrient stimulation of trehalase has not only been observed in vivo in the yeast S. cerevisiae but also in several other yeast and fungal species (26, 53, 54). Given the sequence conservation and similar predicted domains of the Nth1 neutral trehalase, this conclusion is probably valid also for neutral trehalases in other species.
Trehalase Activation as a Read-out for Activation of PKA in Vivo
Because of the regulation of PKA by allosteric cAMP-induced dissociation of the regulatory subunits from the catalytic subunits, the activity of PKA measured in cell extracts, either in the absence or in the presence of cAMP, does not reflect the actual activity in vivo. It only reflects the basal and maximal activity. Therefore, estimation of in vivo PKA activity is inferred from its effect on well established targets of the enzyme. Hence, the phosphorylation status of protein kinase substrates has been a popular read-out for inferring their in vivo activity. Also for PKA, phosphorylation of specific substrates has been proposed as a direct read-out for in vivo activity.
Although mutants with reduced activity of the PKA catalytic subunits always display reduced nutrient activation of trehalase in vivo (6, 8, 19–22) (Fig. 2), our current results unexpectedly showed that this reduced activation was not associated with reduced phosphorylation on the PKA consensus sites (Fig. 2). On the contrary, the Ser21 and Ser83 sites were constitutively phosphorylated. This allows several conclusions. First, it shows that trehalase activity is a more reliable indicator for in vivo PKA activity than trehalase phosphorylation and probably also phosphorylation of PKA sites in other target proteins. Our results question the use of specific phosphorylation sites in substrate proteins as read-out for the activity of protein kinases in vivo. Second, it shows that phosphorylation of trehalase in vivo is not sufficient for its activation. It also raises the question as to what system is responsible for the constitutive phosphorylation. Reduction of PKA activity could have caused up-regulation of one or more (related) protein kinases able to phosphorylate trehalase on the same PKA recognition sites. However, for technical reasons (i.e. instability of the Nth1 protein), we were unable to explore the identity of possible alternative kinases (Sch9 and Yak1) responsible for the constitutive phosphorylation of trehalase. On the other hand, a yeast strain lacking the catalytic subunits of PP2A also showed constitutive trehalase phosphorylation, in agreement with previous results suggesting that PKA stimulates PP2A activity (12). Hence, reduced dephosphorylation by PP2A could explain the constitutive phosphorylation of trehalase in mutants with reduced PKA activity but does not exclude the involvement of alternative protein kinases.
Phosphorylation of Trehalase Is Not Enough for Activation
The constitutive phosphorylation of the Ser21 and Ser83 sites in mutant strains with reduced PKA activity or lacking PP2A activity shows that trehalase phosphorylation is not enough for its activation. This discrepancy can be explained in at least two ways. First, Panni et al. (30) observed that, although Bmh1 and Bmh2 can bind to trehalase when Ser21 or Ser23 is phosphorylated, they are unable to bind if both residues are phosphorylated. Hence, it could be that the mutant strains have an aberrant trehalase phosphorylation pattern, which prevents proper binding of the 14-3-3 proteins and thus also activation of the enzyme. Second, more recently, Wang et al. (55) demonstrated that the yeast 14-3-3 proteins, like their mammalian counterparts, are phosphoproteins themselves. They showed that Tpk1 could phosphorylate Bmh1 in vitro on Ser238. If this phosphorylation were required to promote binding of 14-3-3 to phosphorylated substrate proteins like trehalase, this might explain why activation of trehalase is impaired in a strain with reduced PKA activity despite the phosphorylation of the PKA sites.
The Role of 14-3-3 Proteins in Trehalase Activation
The molecular anvil hypothesis, proposed by Yaffe (56) to explain the modus operandi of the 14-3-3 proteins, can be applied to the trehalase activation mechanism. The structural rigidity of the 14-3-3 dimer forces trehalase to alter its conformation upon recruitment of the 14-3-3 dimer to its phosphorylated sites. Currently, it is unclear which kinetic property, either substrate affinity or Vmax, of the trehalase enzyme is changed in vivo by the conformational change. Ortiz et al. (57) demonstrated that for in vitro activated trehalase, only Vmax is altered.
The absence of phosphorylation on the two sites of trehalase in the 14-3-3 null strain can be explained in different ways. A simple explanation is that the 14-3-3 proteins protect the phosphorylated sites in trehalase against dephosphorylation by protein phosphatases, like PP2A. On the other hand, the absence of the 14-3-3 proteins may have a more dramatic effect on the cell. Over 200 binding partners have been described for the yeast 14-3-3 proteins, and they have been implicated in many different cellular processes, including retrograde signaling, transcriptional regulation, nucleocytoplasmic shuttling, and enzyme activation (see Ref. 58 for a recent review). Hence, the possibility cannot be excluded that PKA itself or a component of the signaling pathway involved in nutrient-induced activation of PKA is negatively affected by the absence of the 14-3-3 proteins. Certain phenotypic features of the 14-3-3 null strain suggest that PKA activity may be affected in such a strain. The cells flocculate spontaneously on YPD medium, and under nitrogen starvation conditions, pseudohyphal growth is enhanced.3 Both of these properties are (partially) controlled by PKA. Moreover Gelperin et al. (59) described that BMH1 overexpression could partially suppress the temperature sensitivity of the cdc25-1 mutant, and TPK1 overexpression could rescue a 14-3-3 null strain, indicating genetic interaction between the PKA pathway and the yeast 14-3-3 proteins.
Dcs1 Inhibits Trehalase by Prevention of 14-3-3 Binding
The action mechanism of the Dcs1 inhibitor of trehalase has remained enigmatic. Our results now show that deletion of Dcs1 enhances binding of the 14-3-3 proteins, which provides a logical explanation for its inhibitory effect. Dcs1 and 14-3-3 may function as competitive modulators, with Dcs1 having a preference for the unphosphorylated and 14-3-3 for the phosphorylated N terminus. In itself, this does not explain the enhanced phosphorylation of trehalase before the addition of nutrient (Fig. 7, C and E). However, lack of competition with Dcs1 may allow enhanced stabilization and protection of phosphorylated trehalase against dephosphorylation by the 14-3-3 proteins, shifting the equilibrium between unphosphorylated and phosphorylated forms toward the latter. This interpretation is supported by the enhanced stability of trehalase and by the correlation between enhanced activity and phosphorylation in the dcs1Δ strain.
Acknowledgments
We thank D. Castermans, T. Peeters, and G. R. Fink for providing strains; P. Vandecruys, M. De Jonghe, S. Castermans, and R. Wicik for technical assistance; M. Versele for useful discussions; and N. Vangoethem for help with the figures.
This work was supported by a Ph.D. fellowship from the Agency for Innovation by Science and Technology (IWT-Flanders) (to W. S.); a postdoctoral fellowship from the Fonds Wetenschappelijk Onderzoek (to G. V. Z.); and grants from the Fund for Scientific Research-Flanders, Interuniversity Attraction Poles Networks (P6/14 and P7/40) and the Research Fund of the KU Leuven (Concerted Research Actions) (to J. M. T.).
W. Schepers, G. Van Zeebroeck, and J. M. Thevelein, unpublished observations.
- PP2A
- protein phosphatase 2A
- PP1
- protein phosphatase 1
- Ab
- antibody
- SD
- synthetic dextrose.
REFERENCES
- 1. Thevelein J. M., Bonini B. M., Castermans D., Haesendonckx S., Kriel J., Louwet W., Thayumanavan P., Popova Y., Rubio-Texeira M., Schepers W., Vandormael P., Van Zeebroeck G., Verhaert P., Versele M., Voordeckers K. (2008) Novel mechanisms in nutrient activation of the yeast protein kinase A pathway. Acta Microbiol. Immunol. Hung. 55, 75–89 [DOI] [PubMed] [Google Scholar]
- 2. Rolland F., Winderickx J., Thevelein J. M. (2002) Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2, 183–201 [DOI] [PubMed] [Google Scholar]
- 3. Thevelein J. M., de Winde J. H. (1999) Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33, 904–918 [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto K., Uno I., Ishikawa T. (1985) Genetic analysis of the role of cAMP in yeast. Yeast 1, 15–24 [DOI] [PubMed] [Google Scholar]
- 5. Broach J. R., Deschenes R. J. (1990) The function of ras genes in Saccharomyces cerevisiae. Adv. Cancer Res. 54, 79–139 [DOI] [PubMed] [Google Scholar]
- 6. Hirimburegama K., Durnez P., Keleman J., Oris E., Vergauwen R., Mergelsberg H., Thevelein J. M. (1992) Nutrient-induced activation of trehalase in nutrient-starved cells of the yeast Saccharomyces cerevisiae. cAMP is not involved as second messenger. J. Gen. Microbiol. 138, 2035–2043 [DOI] [PubMed] [Google Scholar]
- 7. Crauwels M., Donaton M. C., Pernambuco M. B., Winderickx J., de Winde J. H., Thevelein J. M. (1997) The Sch9 protein kinase in the yeast Saccharomyces cerevisiae controls cAPK activity and is required for nitrogen activation of the fermentable-growth-medium-induced (FGM) pathway. Microbiology 143, 2627–2637 [DOI] [PubMed] [Google Scholar]
- 8. Durnez P., Pernambuco M. B., Oris E., Argüelles J. C., Mergelsberg H., Thevelein J. M. (1994) Activation of trehalase during growth induction by nitrogen sources in the yeast Saccharomyces cerevisiae depends on the free catalytic subunits of cAMP-dependent protein kinase, but not on functional Ras proteins. Yeast 10, 1049–1064 [DOI] [PubMed] [Google Scholar]
- 9. Popova Y., Thayumanavan P., Lonati E., Agrochão M., Thevelein J. M. (2010) Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc. Natl. Acad. Sci. U.S.A. 107, 2890–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Zeebroeck G., Bonini B. M., Versele M., Thevelein J. M. (2009) Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor. Nat. Chem. Biol. 5, 45–52 [DOI] [PubMed] [Google Scholar]
- 11. Rubio-Texeira M., Van Zeebroeck G., Thevelein J. M. (2012) Peptides induce persistent signaling from endosomes by a nutrient transceptor. Nat. Chem. Biol. 8, 400–408 [DOI] [PubMed] [Google Scholar]
- 12. Castermans D., Somers I., Kriel J., Louwet W., Wera S., Versele M., Janssens V., Thevelein J. M. (2012) Glucose-induced posttranslational activation of protein phosphatases PP2A and PP1 in yeast. Cell Res. 22, 1058–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kopp M., Müller H., Holzer H. (1993) Molecular analysis of the neutral trehalase gene from Saccharomyces cerevisiae. J. Biol. Chem. 268, 4766–4774 [PubMed] [Google Scholar]
- 14. Alizadeh P., Klionsky D. J. (1996) Purification and biochemical characterization of the ATH1 gene product, vacuolar acid trehalase, from Saccharomyces cerevisiae. FEBS Lett. 391, 273–278 [DOI] [PubMed] [Google Scholar]
- 15. Thevelein J. M. (1984) Regulation of trehalose mobilization in fungi. Microbiol. Rev. 48, 42–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Plaat J. B. (1974) Cyclic 3′,5′-adenosine monophosphate stimulates trehalose degradation in baker's yeast. Biochem. Biophys. Res. Commun. 56, 580–587 [DOI] [PubMed] [Google Scholar]
- 17. van Solingen P., van der Plaat J. B. (1975) Partial purification of the protein system controlling the breakdown of trehalose in baker's yeast. Biochem. Biophys. Res. Commun. 62, 553–560 [DOI] [PubMed] [Google Scholar]
- 18. App H., Holzer H. (1989) Purification and characterization of neutral trehalase from the yeast ABYS1 mutant. J. Biol. Chem. 264, 17583–17588 [PubMed] [Google Scholar]
- 19. Giots F., Donaton M. C., Thevelein J. M. (2003) Inorganic phosphate is sensed by specific phosphate carriers and acts in concert with glucose as a nutrient signal for activation of the protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 47, 1163–1181 [DOI] [PubMed] [Google Scholar]
- 20. Mbonyi K., van Aelst L., Argüelles J. C., Jans A. W., Thevelein J. M. (1990) Glucose-induced hyperaccumulation of cyclic AMP and defective glucose repression in yeast strains with reduced activity of cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 10, 4518–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thevelein J. M., Beullens M. (1985) Cyclic AMP and the stimulation of trehalase activity in the yeast Saccharomyces cerevisiae by carbon sources, nitrogen sources and inhibitors of protein synthesis. J. Gen. Microbiol. 131, 3199–3209 [DOI] [PubMed] [Google Scholar]
- 22. Van Nuland A., Vandormael P., Donaton M., Alenquer M., Lourenço A., Quintino E., Versele M., Thevelein J. M. (2006) Ammonium permease-based sensing mechanism for rapid ammonium activation of the protein kinase A pathway in yeast. Mol. Microbiol. 59, 1485–1505 [DOI] [PubMed] [Google Scholar]
- 23. Wera S., De Schrijver E., Geyskens I., Nwaka S., Thevelein J. M. (1999) Opposite roles of trehalase activity in heat-shock recovery and heat-shock survival in Saccharomyces cerevisiae. Biochem. J. 343, 621–626 [PMC free article] [PubMed] [Google Scholar]
- 24. Veisova D., Macakova E., Rezabkova L., Sulc M., Vacha P., Sychrova H., Obsil T., Obsilova V. (2012) Role of individual phosphorylation sites for the 14-3-3-protein-dependent activation of yeast neutral trehalase Nth1. Biochem. J. 443, 663–670 [DOI] [PubMed] [Google Scholar]
- 25. Franco A., Soto T., Vicente-Soler J., Paredes V., Madrid M., Gacto M., Cansado J. (2003) A role for calcium in the regulation of neutral trehalase activity in the fission yeast Schizosaccharomyces pombe. Biochem. J. 376, 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soto T., Fernandez J., Cansado J., Vicente-Soler J., Gacto M. (1997) Protein kinase Sck1 is involved in trehalase activation by glucose and nitrogen source in the fission yeast Schizosaccharomyces pombe. Microbiology 143, 2457–2463 [DOI] [PubMed] [Google Scholar]
- 27. Franco A., Soto T., Madrid M., Vicente-Soler J., Gacto M., Cansado J. (2005) Functional characterization of Schizosaccharomyces pombe neutral trehalase altered in phosphorylatable serine residues. Arch Microbiol. 183, 394–400 [DOI] [PubMed] [Google Scholar]
- 28. De Mesquita J. F., Panek A. D., de Araujo P. S. (2003) In silico and in vivo analysis reveal a novel gene in Saccharomyces cerevisiae trehalose metabolism. BMC Genomics 4, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu H., Rodgers N. D., Jiao X., Kiledjian M. (2002) The scavenger mRNA decapping enzyme DcpS is a member of the HIT family of pyrophosphatases. EMBO J. 21, 4699–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Panni S., Landgraf C., Volkmer-Engert R., Cesareni G., Castagnoli L. (2008) Role of 14-3-3 proteins in the regulation of neutral trehalase in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 8, 53–63 [DOI] [PubMed] [Google Scholar]
- 31. Giaever G., Chu A. M., Ni L., Connelly C., Riles L., Véronneau S., Dow S., Lucau-Danila A., Anderson K., André B., Arkin A. P., Astromoff A., El-Bakkoury M., Bangham R., Benito R., Brachat S., Campanaro S., Curtiss M., Davis K., Deutschbauer A., Entian K. D., Flaherty P., Foury F., Garfinkel D. J., Gerstein M., Gotte D., Güldener U., Hegemann J. H., Hempel S., Herman Z., Jaramillo D. F., Kelly D. E., Kelly S. L., Kötter P., LaBonte D., Lamb D. C., Lan N., Liang H., Liao H., Liu L., Luo C., Lussier M., Mao R., Menard P., Ooi S. L., Revuelta J. L., Roberts C. J., Rose M., Ross-Macdonald P., Scherens B., Schimmack G., Shafer B., Shoemaker D. D., Sookhai-Mahadeo S., Storms R. K., Strathern J. N., Valle G., Voet M., Volckaert G., Wang C. Y., Ward T. R., Wilhelmy J., Winzeler E. A., Yang Y., Yen G., Youngman E., Yu K., Bussey H., Boeke J. D., Snyder M., Philippsen P., Davis R. W., Johnston M. (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418, 387–391 [DOI] [PubMed] [Google Scholar]
- 32. Voth W. P., Jiang Y. W., Stillman D. J. (2003) New “marker swap” plasmids for converting selectable markers on budding yeast gene disruptions and plasmids. Yeast 20, 985–993 [DOI] [PubMed] [Google Scholar]
- 33. Cameron S., Levin L., Zoller M., Wigler M. (1988) cAMP-independent control of sporulation, glycogen metabolism, and heat shock resistance in S. cerevisiae. Cell 53, 555–566 [DOI] [PubMed] [Google Scholar]
- 34. Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 35. Griffioen G., Anghileri P., Imre E., Baroni M. D., Ruis H. (2000) Nutritional control of nucleocytoplasmic localization of cAMP-dependent protein kinase catalytic and regulatory subunits in Saccharomyces cerevisiae. J. Biol. Chem. 275, 1449–1456 [DOI] [PubMed] [Google Scholar]
- 36. Pernambuco M. B., Winderickx J., Crauwels M., Griffioen G., Mager W. H., Thevelein J. M. (1996) Glucose-triggered signalling in Saccharomyces cerevisiae. Different requirements for sugar phosphorylation between cells grown on glucose and those grown on non-fermentable carbon sources. Microbiology 142, 1775–1782 [DOI] [PubMed] [Google Scholar]
- 37. Albuquerque C. P., Smolka M. B., Payne S. H., Bafna V., Eng J., Zhou H. (2008) A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol. Cell Proteomics 7, 1389–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chi A., Huttenhower C., Geer L. Y., Coon J. J., Syka J. E., Bai D. L., Shabanowitz J., Burke D. J., Troyanskaya O. G., Hunt D. F. (2007) Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 104, 2193–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gruhler A., Olsen J. V., Mohammed S., Mortensen P., Faergeman N. J., Mann M., Jensen O. N. (2005) Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway. Mol. Cell Proteomics 4, 310–327 [DOI] [PubMed] [Google Scholar]
- 40. Li X., Gerber S. A., Rudner A. D., Beausoleil S. A., Haas W., Villén J., Elias J. E., Gygi S. P. (2007) Large-scale phosphorylation analysis of α-factor-arrested Saccharomyces cerevisiae. J. Proteome Res. 6, 1190–1197 [DOI] [PubMed] [Google Scholar]
- 41. Smolka M. B., Albuquerque C. P., Chen S. H., Zhou H. (2007) Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl. Acad. Sci. U.S.A. 104, 10364–10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kennelly P. J., Krebs E. G. (1991) Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 266, 15555–15558 [PubMed] [Google Scholar]
- 43. Collins S. R., Kemmeren P., Zhao X. C., Greenblatt J. F., Spencer F., Holstege F. C., Weissman J. S., Krogan N. J. (2007) Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae. Mol. Cell Proteomics 6, 439–450 [DOI] [PubMed] [Google Scholar]
- 44. Gavin A. C., Aloy P., Grandi P., Krause R., Boesche M., Marzioch M., Rau C., Jensen L. J., Bastuck S., Dümpelfeld B., Edelmann A., Heurtier M. A., Hoffman V., Hoefert C., Klein K., Hudak M., Michon A. M., Schelder M., Schirle M., Remor M., Rudi T., Hooper S., Bauer A., Bouwmeester T., Casari G., Drewes G., Neubauer G., Rick J. M., Kuster B., Bork P., Russell R. B., Superti-Furga G. (2006) Proteome survey reveals modularity of the yeast cell machinery. Nature 440, 631–636 [DOI] [PubMed] [Google Scholar]
- 45. Gavin A. C., Bösche M., Krause R., Grandi P., Marzioch M., Bauer A., Schultz J., Rick J. M., Michon A. M., Cruciat C. M., Remor M., Hofert C., Schelder M., Brajenovic M., Ruffner H., Merino A., Klein K., Hudak M., Dickson D., Rudi T., Gnau V., Bauch A., Bastuck S., Huhse B., Leutwein C., Heurtier M. A., Copley R. R., Edelmann A., Querfurth E., Rybin V., Drewes G., Raida M., Bouwmeester T., Bork P., Seraphin B., Kuster B., Neubauer G., Superti-Furga G. (2002) Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 [DOI] [PubMed] [Google Scholar]
- 46. Ho Y., Gruhler A., Heilbut A., Bader G. D., Moore L., Adams S. L., Millar A., Taylor P., Bennett K., Boutilier K., Yang L., Wolting C., Donaldson I., Schandorff S., Shewnarane J., Vo M., Taggart J., Goudreault M., Muskat B., Alfarano C., Dewar D., Lin Z., Michalickova K., Willems A. R., Sassi H., Nielsen P. A., Rasmussen K. J., Andersen J. R., Johansen L. E., Hansen L. H., Jespersen H., Podtelejnikov A., Nielsen E., Crawford J., Poulsen V., Sørensen B. D., Matthiesen J., Hendrickson R. C., Gleeson F., Pawson T., Moran M. F., Durocher D., Mann M., Hogue C. W., Figeys D., Tyers M. (2002) Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415, 180–183 [DOI] [PubMed] [Google Scholar]
- 47. Krogan N. J., Cagney G., Yu H., Zhong G., Guo X., Ignatchenko A., Li J., Pu S., Datta N., Tikuisis A. P., Punna T., Peregrín-Alvarez J. M., Shales M., Zhang X., Davey M., Robinson M. D., Paccanaro A., Bray J. E., Sheung A., Beattie B., Richards D. P., Canadien V., Lalev A., Mena F., Wong P., Starostine A., Canete M. M., Vlasblom J., Wu S., Orsi C., Collins S. R., Chandran S., Haw R., Rilstone J. J., Gandi K., Thompson N. J., Musso G., St Onge P., Ghanny S., Lam M. H., Butland G., Altaf-Ul A. M., Kanaya S., Shilatifard A., O'Shea E., Weissman J. S., Ingles C. J., Hughes T. R., Parkinson J., Gerstein M., Wodak S. J., Emili A., Greenblatt J. F. (2006) Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 [DOI] [PubMed] [Google Scholar]
- 48. Roberts R. L., Mösch H. U., Fink G. R. (1997) 14-3-3 proteins are essential for RAS/MAPK cascade signaling during pseudohyphal development in S. cerevisiae. Cell 89, 1055–1065 [DOI] [PubMed] [Google Scholar]
- 49. Uetz P., Giot L., Cagney G., Mansfield T. A., Judson R. S., Knight J. R., Lockshon D., Narayan V., Srinivasan M., Pochart P., Qureshi-Emili A., Li Y., Godwin B., Conover D., Kalbfleisch T., Vijayadamodar G., Yang M., Johnston M., Fields S., Rothberg J. M. (2000) A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403, 623–627 [DOI] [PubMed] [Google Scholar]
- 50. Souza A. C., De Mesquita J. F., Panek A. D., Silva J. T., Paschoalin V. M. (2002) Evidence for a modulation of neutral trehalase activity by Ca2+ and cAMP signaling pathways in Saccharomyces cerevisiae. Braz. J. Med. Biol. Res. 35, 11–16 [DOI] [PubMed] [Google Scholar]
- 51. Dimmer K. S., Fritz S., Fuchs F., Messerschmitt M., Weinbach N., Neupert W., Westermann B. (2002) Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Merz S., Westermann B. (2009) Genome-wide deletion mutant analysis reveals genes required for respiratory growth, mitochondrial genome maintenance and mitochondrial protein synthesis in Saccharomyces cerevisiae. Genome Biol. 10, R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carrillo D., Vicente-Soler J., Fernandez J., Soto T., Cansado J., Gacto M. (1995) Activation of cytoplasmic trehalase by cyclic-AMP-dependent and cyclic-AMP-independent signalling pathways in the yeast Candida utilis. Microbiology 141, 679–686 [DOI] [PubMed] [Google Scholar]
- 54. d'Enfert C., Bonini B. M., Zapella P. D., Fontaine T., da Silva A. M., Terenzi H. F. (1999) Neutral trehalases catalyse intracellular trehalose breakdown in the filamentous fungi Aspergillus nidulans and Neurospora crassa. Mol. Microbiol. 32, 471–483 [DOI] [PubMed] [Google Scholar]
- 55. Wang C., Skinner C., Easlon E., Lin S. J. (2009) Deleting the 14-3-3 protein Bmh1 extends life span in Saccharomyces cerevisiae by increasing stress response. Genetics 183, 1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yaffe M. B. (2002) How do 14-3-3 proteins work? Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 513, 53–57 [DOI] [PubMed] [Google Scholar]
- 57. Ortiz C. H., Maia J. C., Tenan M. N., Braz-Padrão G. R., Mattoon J. R., Panek A. D. (1983) Regulation of yeast trehalase by a monocyclic, cyclic AMP-dependent phosphorylation-dephosphorylation cascade system. J. Bacteriol. 153, 644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Heusden G. P. (2009) 14-3-3 proteins. Insights from genome-wide studies in yeast. Genomics 94, 287–293 [DOI] [PubMed] [Google Scholar]
- 59. Gelperin D., Weigle J., Nelson K., Roseboom P., Irie K., Matsumoto K., Lemmon S. (1995) 14-3-3 proteins. Potential roles in vesicular transport and Ras signaling in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 92, 11539–11543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., Hieter P., Boeke J. D. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C. A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132 [DOI] [PubMed] [Google Scholar]
- 61. Jones E. W. (1991) Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 194, 428–453 [DOI] [PubMed] [Google Scholar]
- 62. James P., Halladay J., Craig E. A. (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]