Background: Synoviolin, a ubiquitin ligase, modulates the generation of Aβ produced by γ-secretase.
Results: Rer1, a negative regulator of γ-secretase activity, is ubiquitinated and degraded by synoviolin.
Conclusion: Synoviolin modulates the generation of Aβ by inducing the degradation of Rer1, thereby regulating assembly of the γ-secretase complex.
Significance: Rer1 is a key factor in modulating the generation of Aβ by synoviolin, which is associated with ERAD.
Keywords: Alzheimer's Disease, Amyloid, ER-associated Degradation, Secretases, Ubiquitin-dependent Protease, Rer1, γ-Secretase, Nicastrin, Synoviolin
Abstract
Alzheimer's disease is characterized by the deposition of Aβ, which is generated from the amyloid precursor protein through its cleavage by β- and γ-secretases. The γ-secretase complex component nicastrin (NCT) plays significant roles in the assembly and proper trafficking of the γ-secretase complex and in the recognition of amyloid precursor protein. NCT is incorporated into the γ-secretase complex in the endoplasmic reticulum (ER) and glycosylated in the Golgi. In contrast, unassembled NCT is retrieved or retained in the ER by the protein Retention in endoplasmic reticulum 1 (Rer1). We reported previously that synoviolin (Syvn), an E3 ubiquitin ligase, degrades NCT and affects the generation of Aβ. Here, we examined in more detail the effect of Syvn on the generation of Aβ. We found that overexpression of a dominant negative form of Syvn (C307A mutant) and a Syvn-RNAi decreased the generation of Aβ. These results indicate that the ubiquitin ligase activity of Syvn up-regulates the generation of Aβ. We hypothesized, therefore, that Syvn regulates the assembly or localization of the γ-secretase complex by ubiquitinating Rer1, resulting in its subsequent degradation. Our findings that the level of Rer1 was increased in Syvn knockout fibroblasts because of inhibition of its degradation support this hypothesis. Moreover, we found that Rer1 interacts with Syvn in the ER, is ubiquitinated by Syvn, and is then degraded via the proteasome or lysosomal pathways. Finally, we showed that localization of mature NCT to the plasma membrane as well as γ-secretase complex levels are decreased in fibroblasts of Syvn knockout mice. Thus, it is likely that Syvn regulates the assembly of the γ-secretase complex via the degradation of Rer1, which results in the generation of Aβ.
Introduction
Alzheimer disease (AD)4 is characterized by the formation of senile plaques, which are composed of amyloid β-peptide (Aβ) (1). Aβ is produced by cleavage of amyloid precursor protein (APP) by β- and γ-secretases. γ-Secretase is a multiprotein complex consisting of presenilin (PS), nicastrin (NCT), anterior pharynx defective-1 (Aph-1), and presenilin enhancer-2 (Pen-2) (2). The assembly of this complex is initiated by the formation of an NCT·Aph-1 subcomplex and completed by transmembrane domain (TMD)-mediated association of additional components (3, 4). The protein designated Retention in endoplasmic reticulum 1 (Rer1) is a limiting factor in this process that negatively regulates γ-secretase complex assembly by binding to the TMD of NCT and competing with Aph-1 (5). Rer1 is also involved in retention in the ER of Pen2 by interacting with its TMD (6).
NCT, the only glycoprotein in this complex, plays a significant role in recruiting one of the substrates, APP, into the γ-secretase complex (7). NCT exists as 3 forms as follows: unglycosylated form (∼80 kDa), an immature N-linked glycosylated form (immature NCT, ∼110 kDa), and a mature N-linked isoform (mature NCT, ∼150 kDa) (8). Immature NCT is incorporated into the γ-secretase complex in the ER and glycosylated in the Golgi and is converted to the mature form, which activates γ-secretase (8–10), although an unassembled NCT is retrieved or retained to the ER by Rer1 (5).
Synoviolin (Syvn), also called Hrd1, is named after the rheumatoid synovial cells in which it was discovered and is a causative factor for arthropathy because of its antiapoptotic effects (11). Syvn/Hrd1 is a mammalian homologue of yeast Hrd1p/Del3p, which is a member of the E3 ubiquitin ligase family. These enzymes are centrally involved in ER-associated degradation (ERAD) and contribute to ER quality control, which is a process that insures correct protein degradation by the proteasome pathway (12–14). Our previous research demonstrates that Syvn is involved in the degradation of immature NCT and that the overexpression of Syvn enhances the generation of Aβ (15). However, the precise mechanisms responsible for up-regulating Aβ levels and influencing the effect of Syvn on intracellular trafficking of NCT are unknown.
Here, we focused on Rer1, which modulates the trafficking and assembly of γ-secretase (5), and determined the effect of Syvn on Rer1. We show that Syvn is involved in the degradation of Rer1 by ubiquitination of the latter, thereby modulating the generation of Aβ.
EXPERIMENTAL PROCEDURES
Plasmids and Retrovirus-mediated Infection
The plasmid vectors pMX-Syvn-FLAG and pMX-Syvn C307A-FLAG were generated as described (11, 15). The human sequence encoding Rer1 (hRer1) was excised from hRer1 pcDNA3.1 Zeo(+) (Invitrogen) (6) by double digestion with HindIII and XhoI and inserted into the pMX vector. Retrovirus-mediated infection was carried out according to published methods (16).
Antibodies and Reagents
The commercial antibodies used in this study are as follows: anti-HRD1 (Syvn) C-terminal antibody (Abgent); monoclonal antibody against mono- and polyubiquitin (Biomol); anti-Aph-1aL (C terminus) polyclonal antibody (Covance); anti-APP (N terminus) monoclonal antibody, anti-PS1 loop monoclonal antibody, and anti-NCT monoclonal antibody (Chemicon International); anti-FLAG M2 monoclonal antibody, anti-NCT polyclonal antibody, anti-Rer1 polyclonal antibody, and anti-β-Actin monoclonal antibody (Sigma-Aldrich); anti-integrin α3 antibody, anti-integrin β1 antibody, and anti-syntaxin 6 antibody (BD Biosciences); anti-calnexin antibody (Santa Cruz Biotechnology, Inc.); and HRP-conjugated anti-mouse IgG and anti-rabbit IgG (Cell Signaling Technology). The commercial reagents used in this study are as follows: lactacystin (Peptide Institute), chloroquine diphosphate salt (Sigma-Aldrich), and cycloheximide (Wako Pure Chemical Industries).
Cell Culture
Synoviolin-null (Syvn−/−) murine fibroblasts (17) and murine fibroblasts overexpressing human APP (APP fibroblasts) were cultured in DMEM (Wako Pure Chemical Industries) supplemented with 10% FBS (Sigma). Cells were maintained at 37 °C in an atmosphere of 5% CO2 in a tissue culture incubator.
Treatment of Cells with Cycloheximide
WT or Syvn−/− fibroblasts (1 × 105 cells) overexpressing hRer1 were seeded in 60-mm tissue culture dishes, incubated overnight at 37 °C, and then incubated with 50 μg/ml cycloheximide at 37 °C for 0, 6, or 12 h and collected.
Western Blotting
Cells were lysed on ice in lysis buffer (20 mm HEPES (pH 7.5), 150 mm NaCl, 1% Triton X-100) containing a complete protease inhibitor mixture (Roche Applied Science). Protein concentrations were determined using a BCA protein assay kit (Thermo Fisher Scientific). Protein samples were subjected to 5–20% SDS-PAGE and electrophoretically transferred to PVDF membranes (Millipore). The membranes were soaked in 5% nonfat dry milk (BD Biosciences) in TBST (10 mm Tris-HCl (pH 7.5), 150 mm NaCl, and 0.05% Tween 20) for 1 h and then incubated with primary antibody in TBST containing 0.1% BSA, and 1 mm NaN3 overnight at 4 °C. After washing with TBST, the membranes were incubated with HRP-conjugated secondary antibodies for 1 h. The antigen-antibody complexes were detected by enhanced chemiluminescence using a Luminescent Image Analyzer LAS-3000 (Fujifilm). The magnitude of the signal was digitized using Multi Gauge Ver. 2.3 software (Fujifilm).
Immunoprecipitation
Syvn−/− fibroblasts coinfected with pMX-hRer1 and pMX, pMX-Syvn-FLAG, or pMX-C307A-FLAG were collected and solubilized on ice in radioimmune precipitation assay buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing complete protease inhibitor mixture. To detect ubiquitinated Rer1, cells were treated with 10 μm lactacystin for 10 h or 50 μm chloroquine for 24 h before cell collection. Lysates containing equal amounts of protein were incubated with protein G-Sepharose beads (GE Healthcare) for 1 h at 4 °C. Proteins were immunoprecipitated overnight with primary antibody or normal IgG (Santa Cruz Biotechnology, Inc.). The antibody-bound complexes were isolated by incubation with protein G-Sepharose beads for 4 h and then washed in radioimmune precipitation assay buffer. The protein complexes were eluted in 2× SDS sample buffer and analyzed by Western blotting.
Subcellular Fractionation Using Iodixanol-gradient Centrifugation
WT fibroblasts or Syvn−/− fibroblasts were grown in eight 10-cm tissue culture dishes, and subcellular fractionation was performed as described previously (18). Cells were homogenized in a buffer (10 mm HEPES (pH 7.4), 1 mm EDTA, and 0.25 m sucrose containing complete protease inhibitor mixture). The postnuclear supernatant was centrifuged at 65,000 × g for 1 h at 4 °C. The resultant vesicle pellets were rehomogenized in 0.8 ml of the homogenization buffer and layered on an iodixanol (Cosmo Bio) step gradient (1 ml of 2.5%, 2 ml of 5%, 2 ml of 7.5%, 2 ml of 10%, 0.5 ml of 12.5%, 2 ml of 15%, 0.5 ml of 17.5%, 0.5 ml of 20%, and 0.3 ml of 30% (v/v)). After centrifugation at 90,000 × g for 2.5 h at 4 °C, 11 fractions were collected from the top of the gradient. Equal volumes of each fraction were then analyzed by Western blotting.
Biotinylation and Isolation of Cell Surface Proteins
WT fibroblasts or Syvn−/− fibroblasts grown in two 10-cm culture dishes were washed with cold PBS and incubated at room temperature for 15 min with solutions containing 0.25 mg/ml of EZ-Link Sulfo-NHS-SS-Biotin (Thermo Fisher Scientific) or PBS (non-biotinylated control). This solution was removed, and the cells were treated with a quenching solution (Thermo Fisher Scientific), washed with PBS, and lysed with radioimmune precipitation assay buffer containing a complete protease inhibitor mixture. The biotinylated proteins were coimmunoprecipitated overnight with streptavidin-agarose beads (Solulink) and analyzed by Western blotting.
Blue Native PAGE
WT fibroblasts or Syvn−/− fibroblasts were prepared with a native sample buffer (Invitrogen) containing 1% digitonin and a protease inhibitor mixture and subjected to blue native PAGE using the Novex Bis-Tris gel system (Invitrogen) according to the instructions of the manufacturer.
RNA Interference and ELISA
APP fibroblasts (6 × 105 cells) were seeded in a 24-well plate and transfected with synoviolin siRNA or negative control RNA using HiPerfect transfection reagent (Qiagen). The target sequence used in this study was 5′-CTGGAGAGTTTCAGATGATTA-3′. AllStars negative control siRNA labeled with Alexa Fluor 488 (RNAi human/mouse starter kit, Qiagen) was used as a negative control. After 3 days of incubation, conditioned media were collected, and the Aβ levels were measured using human/rat βamyloid ELISA kits for Aβ40 and Aβ42 (Wako Pure Chemical Industries).
Quantitative Real-time PCR
The expression levels of Rer1 in Syvn−/− fibroblasts or WT fibroblasts were compared using quantitative real-time PCR. RNA was extracted using ISOGEN (Nippon Gene) according to the instructions of the manufacturer and reverse-transcribed with ImProm-II reverse transcriptase (Promega). Real-time PCR was performed using FastStart Universal SYBR Green Master (Roche Applied Science) and specific oligonucleotide primer pairs for mouse Rer1 (QT00146580, Qiagen) according to the instructions of the manufacturer. Amplification and detection were performed using a 7500 fast real-time PCR system (Applied Biosystems) under the following conditions: 1 cycle each at 50 °C for 2 min and 95 °C for 10 min, 40 cycles each at 95 °C for 15 s and 60 °C for 1 min. All data were normalized to that of β-actin.
RESULTS
Decrease in Aβ Secretion by Knockdown of Syvn or Overexpression of Syvn C307A
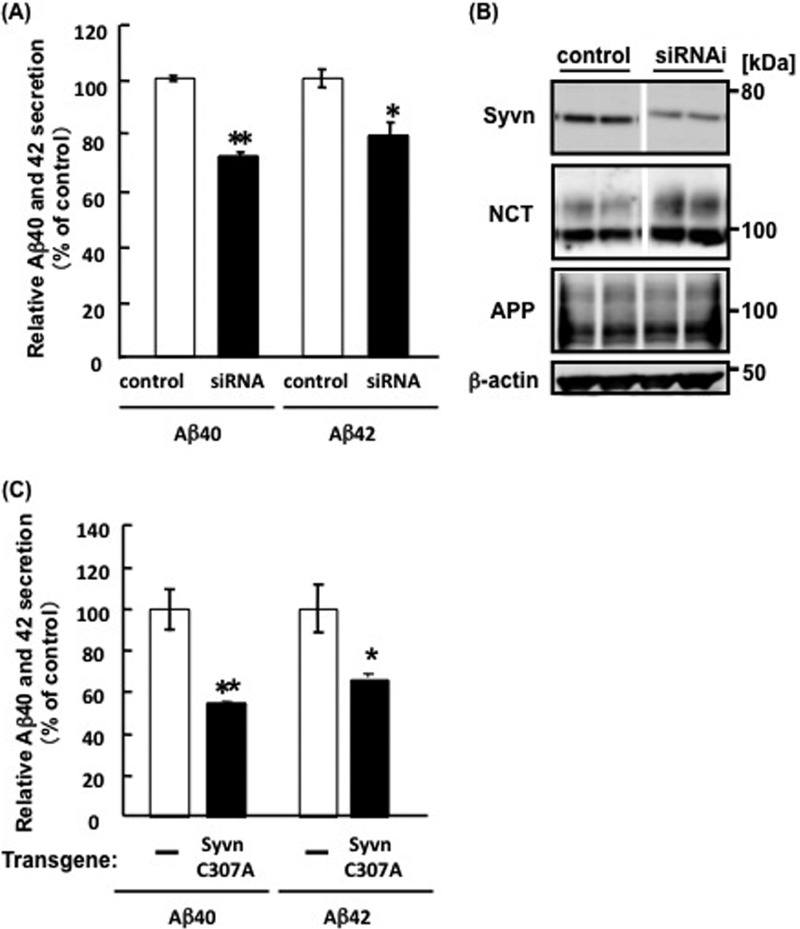
Because overexpression of Syvn increased the secretion of Aβ from APP fibroblasts (15), we analyzed the effect of Syvn on Aβ secretion by transfecting Syvn siRNA into APP fibroblasts and measuring Aβ levels in the medium by ELISA. The levels of Aβ 40 and Aβ 42 were decreased to ∼72.1 ± 1.4% (n = 4, p < 0.001) and 79.6 ± 4.7% (n = 4, p < 0.05), respectively, compared with the control (Fig. 1A). The suppression of Syvn expression was confirmed by Western blotting (Fig. 1B). The level of NCT expression was increased in cells transfected with Syvn RNAi, whereas that of APP was not changed (Fig. 1B). These results agreed with our previous observations (15). Moreover, we also determined whether ubiquitin ligase activity is required for the elevation of Aβ secretion in cells overexpressing Syvn. We compared the levels of Aβ secretion by APP fibroblasts that overexpressed a dominant-negative mutant of Syvn, (Syvn C307A), which lacks ubiquitin ligase activity, with the control. The levels of Aβ 40 and Aβ 42 decreased to ∼54.6 ± 0.75% (n = 4, p < 0.001) and 65.6 ± 1.9% (n = 4, p < 0.05) (Fig. 1C). These results indicate that the ubiquitin ligase activity of Syvn significantly contributed to the increased levels of secreted Aβ.
FIGURE 1.
The effect of Syvn siRNA or a Syvn dominant-negative mutant (C307A) on secretion of Aβ. A, APP fibroblasts transfected with Syvn or control siRNAs. After 3 days of incubation, Aβ40 and Aβ42 levels in the conditioned media were measured by ELISA as described under “Experimental Procedures.” Values are expressed as mean ± S.E. from four independent experiments. *, p < 0.05; **, p < 0.001 (two-tailed Student's t test). B, the down-regulation of Syvn by its cognate RNAi was confirmed by Western blotting with an anti-Syvn antibody. Endogenous NCT levels were assessed with an anti-NCT antibody. The upper and lower bands represent mature and immature NCT, respectively. The space between the blots indicates that they were assembled from different areas of the same blot. Exogenous APP and endogenous β-actin served as a loading control were blotted with an anti-APP monoclonal antibody and anti-β-actin antibody, respectively. C, Aβ40 and Aβ42 levels in the conditioned media harvested from APP fibroblasts overexpressing Syvn C307A or from control cells (-) were measured by ELISA. Values are expressed as mean ± S.E. from four independent experiments. *, p < 0.05; **, p < 0.001 (two-tailed Student's t test).
Accumulation of Rer1 in Syvn−/− Fibroblasts
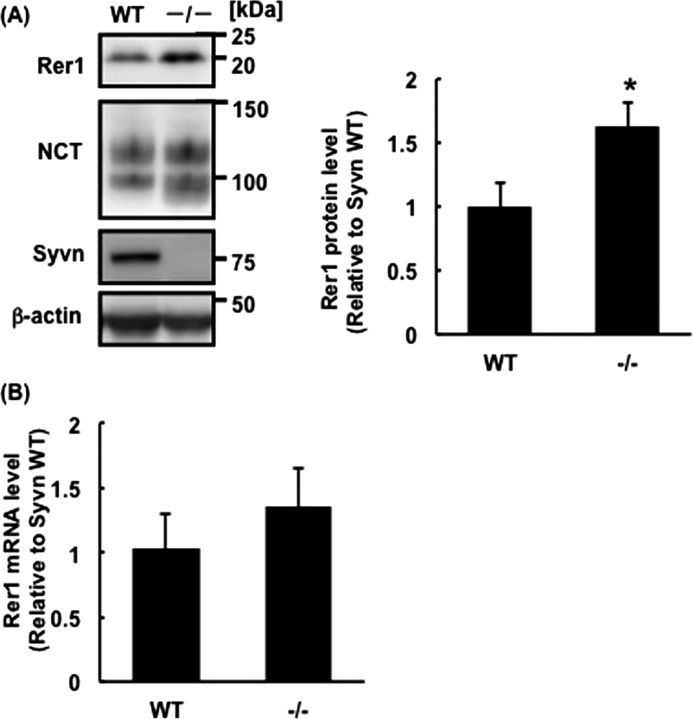
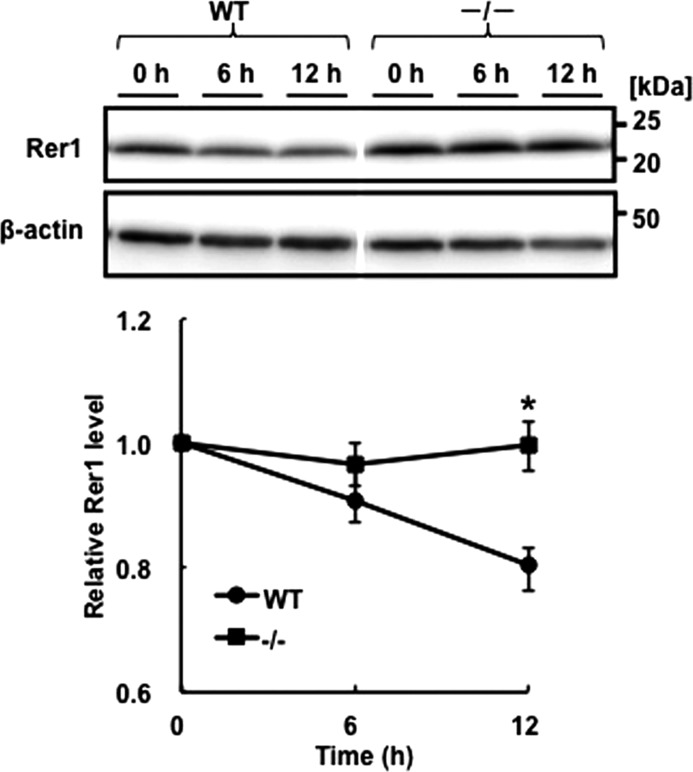
Immature NCT accumulates in Syvn−/− fibroblasts compared with WT (15). Although NCT was ubiquitinated and degraded in cells overexpressing Syvn, the level of Aβ increased (15). These results suggest that Syvn-mediated ubiquitination modulates the ability of the γ-secretase complex to process APP. To better understand why the Aβ level increased, we focused on Rer1, which is the only component known, to our knowledge, to modulate trafficking and assembly of γ-secretase (5). First, we analyzed Rer1 levels in WT and Syvn−/− fibroblasts by Western blotting. The results show that the level of Rer1 in Syvn−/− fibroblasts was ∼1.6-times higher than that in WT (Fig. 2A). To determine whether this increase was caused by protein synthesis or by diminished degradation of Rer1, the expression of Rer1 mRNA was determined by quantitative real-time PCR. There was no significant change in the levels of Rer1 mRNA between WT and Syvn−/− fibroblasts (Fig. 2B), strongly suggesting that the accumulation of Rer1 was caused by a delay in protein degradation. Moreover, we examined the stability of Rer1 in WT and Syvn−/− fibroblasts overexpressing Rer1 by treatment with cycloheximide, a protein synthesis inhibitor. The degradation of Rer1 was delayed in Syvn−/− fibroblasts compared with WT (Fig. 3). These results indicate that Syvn is involved in the degradation of Rer1.
FIGURE 2.
Rer1 levels are elevated in Syvn−/− fibroblasts. A, Western blot analysis of lysates prepared from WT and Syvn−/− fibroblasts with the indicated antibodies. Twenty micrograms of protein was loaded in each lane, and β-actin served as a loading control (left panel). Quantitation of Rer1 levels from three independent experiments as shown in the left panel (right graph). Values are expressed as mean ± S.D. *, p < 0.05 (two-tailed Student's t test). B, quantitative real-time PCR analysis of Rer1 mRNA levels in WT and Syvn−/− fibroblasts. Values are expressed as means ± S.D. from four independent experiments. p = 0.24.
FIGURE 3.
The stability of Rer1 in WT or Syvn−/− fibroblasts after cycloheximide treatment. WT or Syvn−/− fibroblasts overexpressing hRer1 were treated with 50 μg/ml cycloheximide for 0, 6, or 12 h. Rer1 levels in the lysates were determined by Western blotting with an anti-Rer1 antibody (upper panel). Five micrograms of protein was loaded in each lane, and β-actin served as a loading control (lower panel). Quantitation of Rer1 levels from four independent experiments shown in the upper panel is presented by the graph below the blots. Values are expressed as means ± S.E. *, indicates statistical significance of the differences between the cell line data on the basis of a two-tailed Student's t test and p < 0.05. The space between the blots indicates that they were assembled from different areas of the same blot.
The Degradation of Rer1 Mediated by Syvn
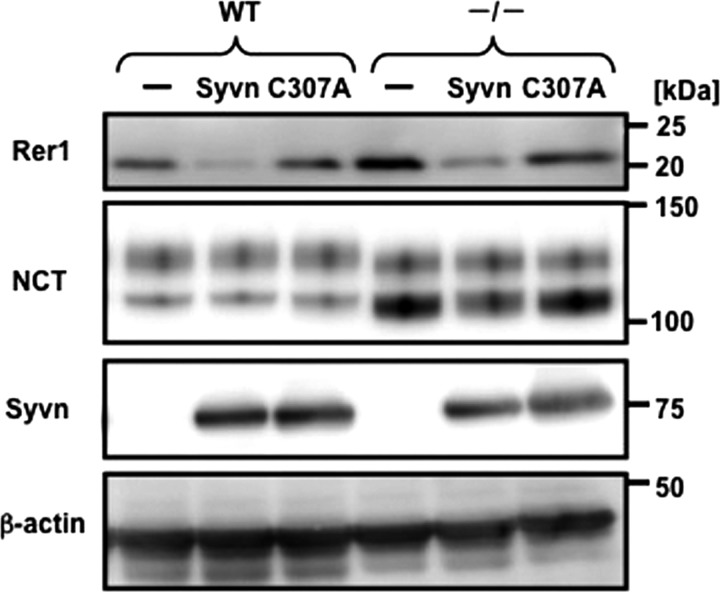
We next determined whether the ubiquitin ligase activity of Syvn is required for the degradation of Rer1. We compared endogenous Rer1 levels in WT and Syvn−/− fibroblasts overexpressing either Syvn or Syvn C307A and found that Rer1 levels were decreased by overexpressing Syvn but not Syvn C307A in both cell lines (Fig. 4). Thus, Rer1 degradation depends on the ubiquitin ligase activity of Syvn. The levels of immature NCT were dramatically increased in Syvn−/− fibroblasts (Fig. 4) (15). Moreover, an increase in the levels of immature NCT was also observed when Syvn C307A was overexpressed (15).
FIGURE 4.
The ubiquitin ligase activity of Syvn is required for the degradation of Rer1. Western blot analysis of cell lysates from WT and Syvn−/− fibroblasts overexpressing pMX (-), pMX-Syvn (Syvn), or pMX-Syvn C307A (C307A). Antibodies are indicated, and 20 μg of protein was loaded in each lane, and β-actin served as a loading control.
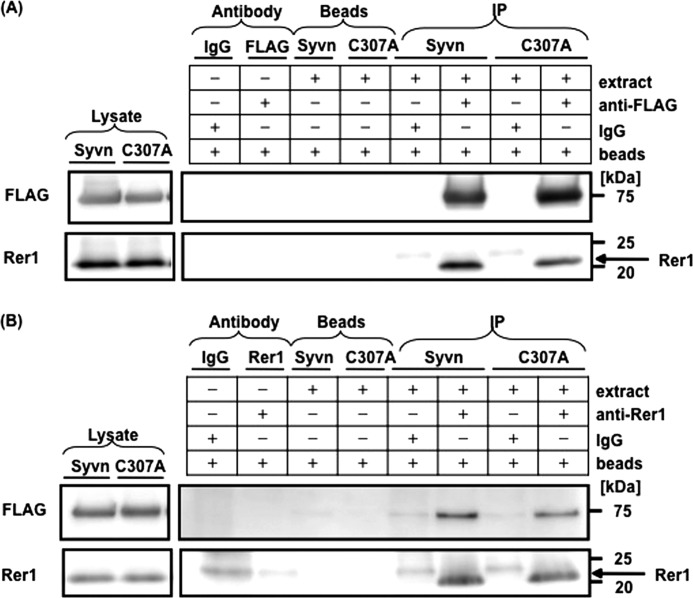
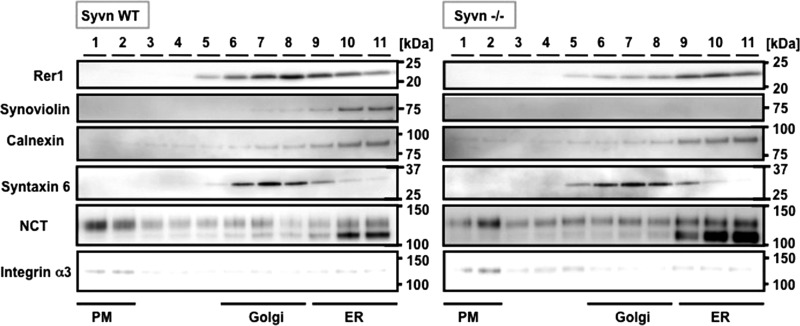
E3 ubiquitin ligases confer specificity upon the ubiquitin system by directly interacting with substrates. If Rer1 is a substrate for Syvn, these two proteins might interact strongly enough to be detected. We therefore attempted to determine whether we could immunoprecipitate these putative complexes from Syvn−/− fibroblasts overexpressing Syvn-FLAG or C307A-FLAG together with Rer1 using an anti-FLAG antibody to precipitate. An anti-Rer1 antibody was used to probe Western blot analyses of the putative complexes. As shown in Fig. 5A, Rer1 was coimmunoprecipitated with Syvn or Syvn C307A. The interactions between Rer1 and Syvn or Syvn C307A were also demonstrated by immunoprecipitation with an anti-Rer1 antibody (Fig. 5B) and then by probing with anti-FLAG. These results show that Syvn can interact with Rer1 independently of the ubiquitin ligase activity of Syvn. Moreover, subcellular fractionation confirmed that Rer1 colocalized with Syvn in the ER, although Rer1 was mainly distributed in the Golgi in WT fibroblasts (Fig. 6). Interestingly, Rer1 accumulated in the ER in Syvn−/− fibroblasts in contrast to WT (Fig. 6), strongly suggesting that Rer1 in the ER undergoes degradation mediated by Syvn.
FIGURE 5.
Role of the ubiquitin ligase activity of Syvn on its interaction with Rer1. A, Syvn−/− fibroblasts overexpressing Syvn-FLAG or Syvn C307A-FLAG together with hRer1 were immunoprecipitated with an anti-FLAG antibody (FLAG) or normal mouse IgG (IgG), followed by Western blot analysis of the immunoprecipitated (IP) fraction using an anti-Rer1 antibody (right panels). The expression levels of Syvn-FLAG or Syvn C307A-FLAG and Rer1 were confirmed in each lysate fraction (left panels). The antibody fraction and beads fraction served as controls and demonstrated the specificity of the immunoprecipitated bands. B, Syvn−/− fibroblasts overexpressing Syvn-FLAG or Syvn C307A-FLAG together with hRer1 were immunoprecipitated with an anti-Rer1 antibody (Rer1) or normal rabbit IgG followed by Western blot analysis of the IP fraction using an anti-FLAG antibody (right panels). The expression levels of Syvn-FLAG or Syvn C307A-FLAG and Rer1 were confirmed in the lysate fraction (left panels). The antibody fraction and beads fraction served as controls and demonstrated the specificity of the immunoprecipitated bands.
FIGURE 6.
Subcellular fractionation of Rer1, Syvn, and NCT in WT and Syvn−/− fibroblasts. The numbers above the lanes represent the fractions of the gradient separation from the top to the bottom of the tube. Equal volumes of each fraction were analyzed by Western blotting with the indicated antibodies. Calnexin, syntaxin 6, and integrin α3 are the ER, Golgi, and PM markers, respectively.
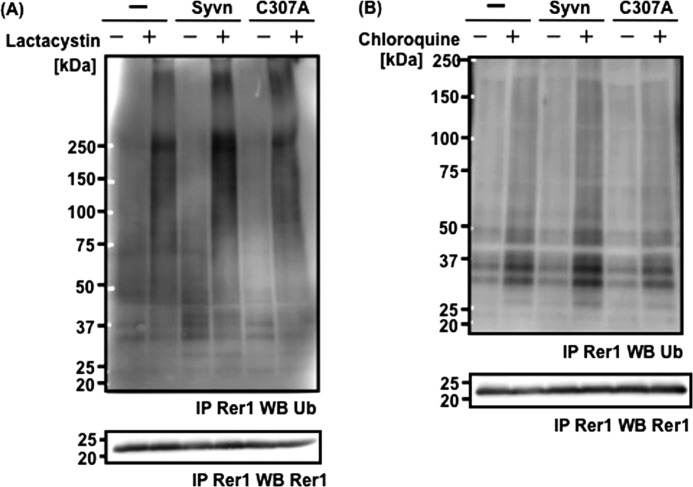
We next determined whether Syvn is involved in the ubiquitination of Rer1. Rer1 was immunoprecipitated from Syvn−/− fibroblasts overexpressing Syvn-FLAG or C307A-FLAG together with Rer1 after treatment with lactacystin, a proteasome inhibitor. Levels of ubiquitinated Rer1 were compared by Western blotting with mono- and polyubiquitin antibody, which can detect both types of ubiquitination. The levels of ubiquitinated Rer1 (high-molecular-weight smear bands, ∼250 kDa) were elevated in cells that overexpressed Syvn but not Syvn C307A (Fig. 7A). We also examined the effect of Syvn on the ubiquitination of endogenous Rer1 using Syvn−/− fibroblasts overexpressing Syvn-FLAG or C307A-FLAG, and obtained similar results (data not shown). These results suggest that Rer1 can be ubiquitinated by Syvn and then degraded via the proteasome pathway. However, we cannot completely exclude the possibility that the immunoprecipitated high-molecular-weight bands (∼250 kDa) were generated by other ubiquitinated proteins associated with Rer1. Interestingly, we found that the level of Syvn-dependent Rer1 ubiquitination (∼30 kDa) is increased after treatment with chloroquine, a lysosomal enzyme inhibitor (Fig. 7B). Polyubiquitinated Rer1 (∼250 kDa) and monoubiquitinated Rer1 (∼30 kDa) might therefore be degraded by a proteasome pathway and a lysosomal pathway, respectively. Thus, Rer1 is ubiquitinated by Syvn and degraded by the proteasome and, possibly, by a lysosomal pathway.
FIGURE 7.
Rer1 is ubiquitinated by Syvn. A, Rer1 immunoprecipitation from the following cell lines treated with 10 μm lactacystin for 10 h (lower panel): Syvn−/− fibroblasts infected with pMX (-), pMX-Syvn-FLAG (Syvn), or pMX-C307A-FLAG (C307A) together with pMX-hRer1. Ubiquitinated Rer1 precipitated by the anti-Rer1 antibody was detected by Western blotting with anti-mono- and polyubiquitin antibody (upper panel). B, Rer1 was immunoprecipitated from Syvn−/− fibroblasts treated with 50 μm chloroquine for 24 h (lower panel) and infected as shown in Fig. 7A. Ubiquitinated Rer1 precipitated with the anti-Rer1 antibody was detected by Western blotting with anti- mono- and polyubiquitin antibody (upper panel).
The Levels of the NCT and γ-Secretase Complex on the Cell Surface Were Decreased in Syvn Knockout Fibroblasts
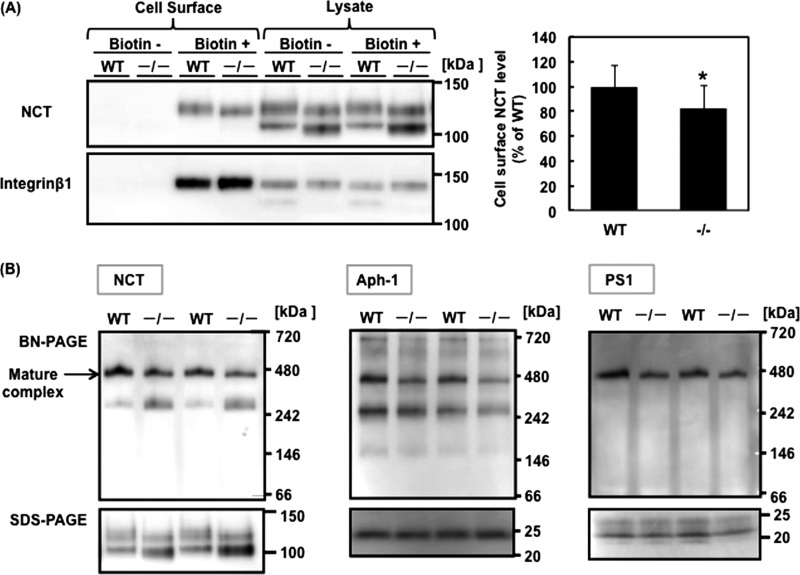
We observed here that Syvn is involved in the degradation of Rer1, which modulates the trafficking and assembly of γ-secretase. To determine whether Aβ secretion is modulated by Syvn via the localization or assembly of γ-secretase, we investigated the localization of mature NCT in the plasma membrane (PM), where γ-secretase recognizes the APP CTF generated by cleavage with α- or β-secretase (19). We found that localization of mature NCT in the PM fractions from Syvn−/− fibroblasts was significantly lower than that in WT fibroblasts (the percentage of mature NCT level in PM fractions relative to that in total fractions was 27 ± 12% (mean ± S.D.) (Syvn−/− fibroblasts) versus 46 ± 13% (WT), n = 4, p < 0.05) (Fig. 6). We also determined the levels of NCT on the cell surface using a biotinylation assay. Previous studies examining biotinylation of the cell surface show that immature NCT is also present on the plasma membrane and that the levels of mature NCT on the plasma membrane increases in Syvn−/− fibroblasts (15). These results differ from our results reported here (Fig. 6). However, we found that the previously employed method also recovered intracellular proteins, such as mature NCT and immature NCT residing in the ER of Syvn−/− cells within the eluate. The reason for this is unknown (data not shown). Therefore, we employed a modified cell surface biotinylation protocol here. The results show that mature NCT (and not immature NCT) is present on the cell surface in Syvn−/− cells as reported by others (19) and decreased to 82.3 ± 18.5% (p < 0.01) in Syvn−/− fibroblasts (Fig. 8A). This supports the results obtained from the subcellular fractionation experiment (Fig. 6) and strongly suggests that Syvn facilitates the trafficking of mature NCT to the cell surface. Further, we analyzed the effect of Syvn on the assembly of the γ-secretase complex using Blue Native PAGE. Consistent with published data, we could detect the mature γ-secretase complex at ∼440 kDa (5) by Western blotting using antibodies against NCT, Aph-1, and the C terminus of PS1 (Fig. 8B). The results show that the level of the mature complex was decreased in Syvn−/− fibroblasts (Fig. 8B). Interestingly, we also detected a subcomplex (∼250 kDa) by Western blotting using anti-NCT and anti-Aph-1 antibodies but not an anti-PS1 antibody. We consider this to represent a complex between immature NCT and the Aph-1 subcomplex because the level of NCT in the subcomplex was increased in Syvn−/− fibroblasts and was accompanied by an increase in the level of immature NCT in Syvn−/− fibroblasts (Fig. 8B). Thus, we conclude that it is likely that Syvn regulates the assembly of the γ-secretase complex via the degradation of Rer1, which causes an increase in the generation of Aβ.
FIGURE 8.
Syvn facilitates the trafficking of mature NCT and the assembly of the γ-secretase complex. A, Western blot analysis of cell surface proteins and lysates prepared from WT and Syvn−/− fibroblasts with the indicated antibodies. Cell surface proteins were biotinylated (Biotin+) and isolated as described under “Experimental Procedures.” Non-biotinylated cells (Biotin-) were used as a negative control. The same volume of eluted sample (Cell Surface) and the same amount of protein (Lysate) were loaded in each lane. Integrin β1 served as a loading control (left panel). Quantitation of cell-surface NCT levels from four independent experiments is shown in the left panel (right graph). Values are expressed as mean ± S.D. *, p < 0.01 (two-tailed Student's t test). B, Blue Native PAGE (BN-PAGE) and SDS-PAGE analysis of lysates prepared from WT and Syvn−/− fibroblasts as described under “Experimental Procedures” and blotted with anti-NCT (left panel), anti-Aph1 (middle panel), and anti-PS1 antibodies (right panel).
DISCUSSION
We reported that Syvn regulates the maturation of NCT and generation of Aβ without changing the levels of APP and other γ-secretase components, such as PS1, Aph-1, and Pen-2 (15). We show here that the suppression of Syvn expression led to a decrease in the level of Aβ and the magnitude of the ratio of mature to immature NCT (Figs. 1, A and B, and 2A). Moreover, the effect of overexpressing a dominant negative form of Syvn (Syvn C307A) on the generation of Aβ was consistent with this result (Fig. 1C). These results strongly suggest that the ubiquitin ligase activity of Syvn is involved in the generation of Aβ, possibly by modulating the maturation of NCT.
To better understand the precise mechanisms underlying the modulation of the generation of Aβ mediated by Syvn, we focused here on Rer1. Rer1 is a limiting factor that negatively regulates the assembly of the γ-secretase complex by binding to the TMD of NCT and competing with Aph-1 (5). Overexpression of Rer1 leads to a decrease in the generation of Aβ (5). Here, we show that Syvn is involved in the degradation of Rer1, as demonstrated by our findings that the levels of Rer1 increased in Syvn−/− fibroblasts because of the inhibition of its degradation (Figs. 2 and 3). This was abolished by the overexpression of Syvn (Fig. 4). Moreover, our immunoprecipitation and subcellular fractionation analyses suggest strongly that Syvn interacts with Rer1 in the ER (Figs. 5 and 6). We also demonstrated that the level of ubiquitinated Rer1 is increased depending on the ubiquitin ligase activity of Syvn (Fig. 7). Therefore, it is likely that Rer1 is a substrate for Syvn. In addition, the localization of mature NCT in the plasma membrane was decreased in Syvn−/− fibroblasts (Figs. 6 and 8A), perhaps because NCT in Syvn−/− cells is retrieved to the ER to a greater extent than that in WT cells because the level of Rer1 in Syvn−/− cells is higher than that in WT cells.
The level of the γ-secretase complex on the cell surface was also likely to be decreased in Syvn−/− fibroblasts because mature glycosylation of NCT is necessary for trafficking the γ-secretase complex to the post-Golgi compartments (19). Indeed, using Blue Native PAGE, we showed that the γ-secretase complex was decreased in Syvn−/− fibroblasts (Fig. 8B). These findings agree with data from the original studies showing that Rer1 negatively regulates the γ-secretase complex assembly and γ-secretase activity (5, 20, 21). This study now shows that Syvn is involved in the degradation of Rer1. When we consider published data along with those generated here, we propose that the ubiquitination of Rer1 by Syvn decreases the level of Rer1, facilitating the incorporation of NCT into the γ-secretase complex. However, Syvn deficiency increases the level of Rer1. This inhibits the incorporation of NCT into the γ-secretase complex, thereby reducing the level of the γ-secretase complex and the generation of Aβ (Fig. 9).
FIGURE 9.
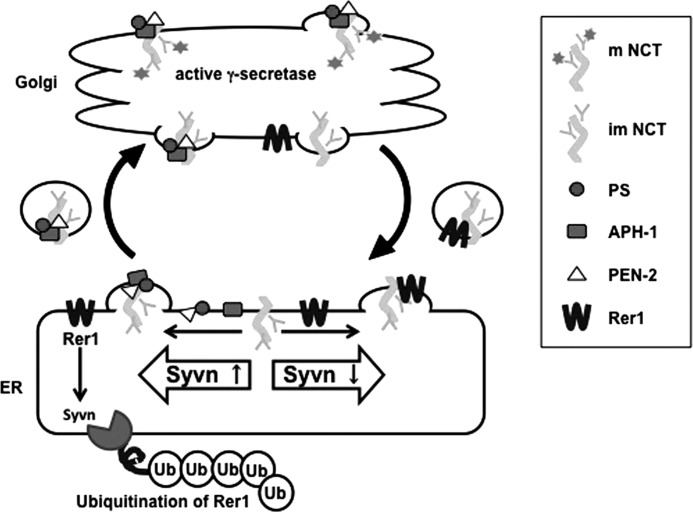
Model for modulation of γ-secretase complex assembly by Syvn-mediated by Rer1 degradation. NCT is incorporated into the γ-secretase complex in the ER and glycosylated in the Golgi, thereby activating γ-secretase activity (left panel). In contrast, an unassembled NCT in the Golgi is translocated to the ER by Rer1 (right panel). Rer1 is also a limiting factor that negatively regulates γ-secretase complex assembly by binding to NCT, which competes with Aph-1 (bottom panel). Syvn is involved in the degradation of Rer1, thereby facilitating the formation of the γ-secretase complex and generation of Aβ (bottom panel).
Syvn is an E3 ubiquitin ligase involved in ERAD, a highly complex process that ensures the integrity of the protein degradation pathway and contributes to ER quality control (14). Syvn is associated with the degradation of proteins that accumulate in the ER by ERAD and prevents ER stress (unfolded protein response) (12, 13). Further, Syvn expression is induced by ER stress and protects against ER stress-induced apoptosis (17, 22, 23). These results suggest that Rer1 was ubiquitinated by Syvn and degraded through ERAD. Therefore, Rer1 might also play a role in ER-Golgi quality control. Rer1 recognizes polar amino acids in TMDs of some membrane proteins (24) and modulates the localization of unassembled subunits in the ER (5, 6, 25, 26). A decrease in the levels of Rer1 could allow its target membrane proteins to be transported via the secretory pathway. Thus, an important issue that must be addressed is the possible involvement of Rer1 in ER-Golgi quality control.
We show here that ubiquitinated Rer1 was degraded by the proteasome and possibly by the lysosomal pathways (Fig. 7). Although Rer1 resides mainly in the cis-Golgi and not in post-Golgi in both yeast and mammalian cells (27, 28), a small fraction of ubiquitinated Rer1 might pass through the Golgi complex and be delivered through the trans-Golgi network to the lysosome for degradation. Ubiquitinated proteins are usually degraded by the proteasome pathway. However, there are only a few reports, to our knowledge, on the lysosomal pathway (29). We believe that this study provides evidence to support the conclusion that ubiquitinated protein can be degraded by the lysosomal pathway. Alternatively, autophagy, which is involved in the degradation of ER (reticulophagy) (30), might mediate degradation. It is important to note that autophagy acts as a compensatory degradation system when the ubiquitin-proteasome system is impaired and is linked to this process by histone deacetylase 6 (HDAC6), which interacts with polyubiquitinated proteins (31).
In conclusion, this study reveals that Syvn is involved in the degradation of Rer1, which modulates the maturation and localization of NCT and the generation of Aβ. Moreover, our data strongly suggest that ubiquitinated Rer1 was degraded by the proteasome and lysosomal pathways. Thus, we conclude that Rer1, a negative regulator of the assembly of the γ-secretase complex, appears to be a key factor that modulates the generation of Aβ by synoviolin associated with ERAD. These findings better illuminate the link between ERAD and the generation of Aβ and may contribute to the development of new diagnostic and therapeutic tools for AD.
Acknowledgments
We thank Dr. Christoph Kaether (Leibniz Institute for Age Research-Fritz Lipmann Institute) for providing the hRer1 pcDNA3.1 Zeo(+) plasmid and helpful comments.
This work was supported by Grant-in-Aid for Young Scientists (B) 23700425 (to C. T.); Grant-in-Aid for Scientific Research (C) 23590110 (to T. M.); by a Grant-in-Aid for Strategic Medical Science Research Center (The MIAST Project) (to H. K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and a grant from the Takeda Science Foundation (to T. M.).
- AD
- Alzheimer disease
- Aβ
- amyloid β peptide
- APP
- amyloid precursor protein
- PS
- presenilin
- NCT
- nicastrin
- TMD
- transmembrane domain
- ER
- endoplasmic reticulum
- Syvn
- synoviolin
- ERAD
- ER-associated degradation
- PM
- plasma membrane.
REFERENCES
- 1. Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 2. Kimberly W. T., LaVoie M. J., Ostaszewski B. L., Ye W., Wolfe M. S., Selkoe D. J. (2003) γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc. Natl. Acad. Sci. U.S.A. 100, 6382–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dries D. R., Yu G. (2008) Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer's disease. Curr. Alzheimer Res. 5, 132–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capell A., Kaether C., Edbauer D., Shirotani K., Merkl S., Steiner H., Haass C. (2003) Nicastrin interacts with γ-secretase complex components via the N-terminal part of its transmembrane domain. J. Biol. Chem. 278, 52519–52523 [DOI] [PubMed] [Google Scholar]
- 5. Spasic D., Raemaekers T., Dillen K., Declerck I., Baert V., Serneels L., Füllekrug J., Annaert W. (2007) Rer1p competes with APH-1 for binding to nicastrin and regulates γ-secretase complex assembly in the early secretory pathway. J. Cell Biol. 176, 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaether C., Scheuermann J., Fassler M., Zilow S., Shirotani K., Valkova C., Novak B., Kacmar S., Steiner H., Haass C. (2007) Endoplasmic reticulum retention of the γ-secretase complex component Pen2 by Rer1. EMBO Rep. 8, 743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah S., Lee S. F., Tabuchi K., Hao Y. H., Yu C., LaPlant Q., Ball H., Dann C. E., 3rd, Südhof T., Yu G. (2005) Nicastrin functions as a γ-secretase-substrate receptor. Cell 122, 435–447 [DOI] [PubMed] [Google Scholar]
- 8. Kimberly W. T., LaVoie M. J., Ostaszewski B. L., Ye W., Wolfe M. S., Selkoe D. J. (2002) Complex N-linked glycosylated nicastrin associates with active γ-secretase and undergoes tight cellular regulation. J. Biol. Chem. 277, 35113–35117 [DOI] [PubMed] [Google Scholar]
- 9. Leem J. Y., Vijayan S., Han P., Cai D., Machura M., Lopes K. O., Veselits M. L., Xu H., Thinakaran G. (2002) Presenilin 1 is required for maturation and cell surface accumulation of nicastrin. J. Biol. Chem. 277, 19236–19240 [DOI] [PubMed] [Google Scholar]
- 10. Tomita T., Katayama R., Takikawa R., Iwatsubo T. (2002) Complex N-glycosylated form of nicastrin is stabilized and selectively bound to presenilin fragments. FEBS Lett. 520, 117–121 [DOI] [PubMed] [Google Scholar]
- 11. Amano T., Yamasaki S., Yagishita N., Tsuchimochi K., Shin H., Kawahara K., Aratani S., Fujita H., Zhang L., Ikeda R., Fujii R., Miura N., Komiya S., Nishioka K., Maruyama I., Fukamizu A., Nakajima T. (2003) Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev. 17, 2436–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bays N. W., Gardner R. G., Seelig L. P., Joazeiro C. A., Hampton R. Y. (2001) Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat. Cell Biol. 3, 24–29 [DOI] [PubMed] [Google Scholar]
- 13. Bordallo J., Plemper R. K., Finger A., Wolf D. H. (1998) Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol. Biol. Cell 9, 209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christianson J. C., Olzmann J. A., Shaler T. A., Sowa M. E., Bennett E. J., Richter C. M., Tyler R. E., Greenblatt E. J., Harper J. W., Kopito R. R. (2012) Defining human ERAD networks through an integrative mapping strategy. Nat. Cell Biol. 14, 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maeda T., Marutani T., Zou K., Araki W., Tanabe C., Yagishita N., Yamano Y., Amano T., Michikawa M., Nakajima T., Komano H. (2009) An E3 ubiquitin ligase, Synoviolin, is involved in the degradation of immature nicastrin, and regulates the production of amyloid β-protein. FEBS J. 276, 5832–5840 [DOI] [PubMed] [Google Scholar]
- 16. Komano H., Shiraishi H., Kawamura Y., Sai X., Suzuki R., Serneels L., Kawaichi M., Kitamura T., Yanagisawa K. (2002) A new functional screening system for identification of regulators for the generation of amyloid β-protein. J. Biol. Chem. 277, 39627–39633 [DOI] [PubMed] [Google Scholar]
- 17. Yagishita N., Ohneda K., Amano T., Yamasaki S., Sugiura A., Tsuchimochi K., Shin H., Kawahara K., Ohneda O., Ohta T., Tanaka S., Yamamoto M., Maruyama I., Nishioka K., Fukamizu A., Nakajima T. (2005) Essential role of synoviolin in embryogenesis. J. Biol. Chem. 280, 7909–7916 [DOI] [PubMed] [Google Scholar]
- 18. Zou K., Hosono T., Nakamura T., Shiraishi H., Maeda T., Komano H., Yanagisawa K., Michikawa M. (2008) Novel role of presenilins in maturation and transport of integrin β 1. Biochemistry 47, 3370–3378 [DOI] [PubMed] [Google Scholar]
- 19. Kaether C., Lammich S., Edbauer D., Ertl M., Rietdorf J., Capell A., Steiner H., Haass C. (2002) Presenilin-1 affects trafficking and processing of βAPP and is targeted in a complex with nicastrin to the plasma membrane. J. Cell Biol. 158, 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spasic D., Annaert W. (2008) Building γ-secretase: The bits and pieces. J. Cell Sci. 121, 413–420 [DOI] [PubMed] [Google Scholar]
- 21. De Strooper B., Annaert W. (2010) Novel research horizons for presenilins and γ-secretases in cell biology and disease. Annu. Rev. Cell Dev. Biol. 26, 235–260 [DOI] [PubMed] [Google Scholar]
- 22. Kaneko M., Ishiguro M., Niinuma Y., Uesugi M., Nomura Y. (2002) Human HRD1 protects against ER stress-induced apoptosis through ER-associated degradation. FEBS Lett. 532, 147–152 [DOI] [PubMed] [Google Scholar]
- 23. Yamasaki S., Yagishita N., Sasaki T., Nakazawa M., Kato Y., Yamadera T., Bae E., Toriyama S., Ikeda R., Zhang L., Fujitani K., Yoo E., Tsuchimochi K., Ohta T., Araya N., Fujita H., Aratani S., Eguchi K., Komiya S., Maruyama I., Higashi N., Sato M., Senoo H., Ochi T., Yokoyama S., Amano T., Kim J., Gay S., Fukamizu A., Nishioka K., Tanaka K., Nakajima T. (2007) Cytoplasmic destruction of p53 by the endoplasmic reticulum-resident ubiquitin ligase 'Synoviolin'. EMBO J. 26, 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sato K., Sato M., Nakano A. (2003) Rer1p, a retrieval receptor for ER membrane proteins, recognizes transmembrane domains in multiple modes. Mol. Biol. Cell 14, 3605–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwappach B. (2008) An overview of trafficking and assembly of neurotransmitter receptors and ion channels (Review). Mol. Membr. Biol. 25, 270–278 [DOI] [PubMed] [Google Scholar]
- 26. Valkova C., Albrizio M., Röder I. V., Schwake M., Betto R., Rudolf R., Kaether C. (2011) Sorting receptor Rer1 controls surface expression of muscle acetylcholine receptors by ER retention of unassembled α-subunits. Proc. Natl. Acad. Sci. U.S.A. 108, 621–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sato K., Sato M., Nakano A. (2001) Rer1p, a retrieval receptor for endoplasmic reticulum membrane proteins, is dynamically localized to the Golgi apparatus by coatomer. J. Cell Biol. 152, 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Füllekrug J., Boehm J., Röttger S., Nilsson T., Mieskes G., Schmitt H. D. (1997) Human Rer1 is localized to the Golgi apparatus and complements the deletion of the homologous Rer1 protein of Saccharomyces cerevisiae. Eur. J. Cell Biol. 74, 31–40 [PubMed] [Google Scholar]
- 29. Fujita E., Kouroku Y., Isoai A., Kumagai H., Misutani A., Matsuda C., Hayashi Y. K., Momoi T. (2007) Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: Ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum. Mol. Genet. 16, 618–629 [DOI] [PubMed] [Google Scholar]
- 30. Kaminskyy V., Zhivotovsky B. (2012) Proteases in autophagy. Biochim. Biophys. Acta 1824, 44–50 [DOI] [PubMed] [Google Scholar]
- 31. Pandey U. B., Nie Z., Batlevi Y., McCray B. A., Ritson G. P., Nedlsky N. B., Schwartz S. L., DiProspero N. A., Knight M. A., Schuldiner O., Padmanabhan R., Hild M., Berry D. L., Garza D., Hubbert C. C., Yao T. P., Baehrecke E. H., Taylor J. P. (2007) HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447, 859–863 [DOI] [PubMed] [Google Scholar]