Background: G protein-coupled receptors (GPCR) can form heteromers and thereby alter their signaling properties.
Results: GPR55 and cannabinoid 1 (CB1) receptor signaling is modulated if receptors are co-expressed.
Conclusion: GPR55 signaling is inhibited in the presence of CB1 receptors; in contrast, CB1 receptor-mediated signaling is enhanced if GPR55 is co-expressed.
Significance: Cross-regulation of CB1 receptor and GPR55 may affect cell function when endogenously co-expressed.
Keywords: Cannabinoid Receptors, G Protein-coupled Receptors (GPCR), Protein-Protein Interactions, Receptor Endocytosis, Signal Transduction
Abstract
The G protein-coupled receptor (GPCR) 55 (GPR55) and the cannabinoid receptor 1 (CB1R) are co-expressed in many tissues, predominantly in the central nervous system. Seven transmembrane spanning (7TM) receptors/GPCRs can form homo- and heteromers and initiate distinct signaling pathways. Recently, several synthetic CB1 receptor inverse agonists/antagonists, such as SR141716A, AM251, and AM281, were reported to activate GPR55. Of these, SR141716A was marketed as a promising anti-obesity drug, but was withdrawn from the market because of severe side effects. Here, we tested whether GPR55 and CB1 receptors are capable of (i) forming heteromers and (ii) whether such heteromers could exhibit novel signaling patterns. We show that GPR55 and CB1 receptors alter each others signaling properties in human embryonic kidney (HEK293) cells. We demonstrate that the co-expression of FLAG-CB1 receptors in cells stably expressing HA-GPR55 specifically inhibits GPR55-mediated transcription factor activation, such as nuclear factor of activated T-cells and serum response element, as well as extracellular signal-regulated kinases (ERK1/2) activation. GPR55 and CB1 receptors can form heteromers, but the internalization of both receptors is not affected. In addition, we observe that the presence of GPR55 enhances CB1R-mediated ERK1/2 and nuclear factor of activated T-cell activation. Our data provide the first evidence that GPR55 can form heteromers with another 7TM/GPCR and that this interaction with the CB1 receptor has functional consequences in vitro. The GPR55-CB1R heteromer may play an important physiological and/or pathophysiological role in tissues endogenously co-expressing both receptors.
Introduction
The cannabinoid 1 and 2 receptors (CB1R and CB2R)3 are seven-transmembrane spanning (7TM)/G protein-coupled receptors (GPCRs) belonging to the endocannabinoid system (1, 2). Additional receptors have been described to be activated by cannabinoid ligands, such as G protein-coupled receptor 55 (GPR55), GPR18, GPR119, and others, but their classification as novel cannabinoid receptors is still under debate (3). The CB1R is highly abundant in the central nervous system (CNS) (e.g. cerebral cortex, hippocampus, and striatum) (1, 4). In contrast, CB2Rs are mainly found on immune cells (2, 5). The lipid receptor GPR55 is highly expressed in the CNS, e.g. putamen, striatum, and hippocampus, as well as in intestine, bone marrow, spleen, immune cells, and endothelial cells (6–11). GPR55 was also detected in cancer tissues and cancer cell lines (12–15).
CB1Rs predominantly couple to Gαi/o proteins and thereby inhibit adenylyl cyclase, activate mitogen-activated protein kinases (MAPKs), and further activate numerous transcription factors. In addition, CB1Rs have been described to mediate the activation of several potassium channels (16, 17). Multiple GPR55-mediated signaling pathways have been described (6, 7, 18–20), whereby the most consistent reports suggest that GPR55 couples to Gα13 in recombinant HEK cells transiently or stably expressing GPR55 (9, 18, 19, 21–23). GPR55 signaling can be mediated via small GTPases (6, 21, 22) and the mobilization of intracellular calcium stores (21, 22, 24, 25). This leads to the activation of several transcription factors, such as nuclear factor of activated T-cells (NFAT), nuclear factor κ-light chain-enhancer of activated B cells (NF-κB), cyclic AMP response-element binding protein, and activating transcription factor 2 (21, 22, 26). In addition, the activation of MAP kinases, such as p38 and ERK1/2 MAPKs were described to be induced after GPR55 stimulation (22, 26). Furthermore, the formation of filamentous actin (F-actin) upon GPR55 activation was reported in HEK293 cells and human neutrophils and this process is mediated by Gα13 and RhoA (6). The formation of F-actin is related to the induction of serum response elements (SRE) and is under control of the Gα13-mediated RhoA signaling (27, 28).
The CB1R is activated by numerous endogenous cannabinoid ligands, such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), as well as the psychoactive phytocannabinoid Δ(9)-tetrahydrocannabinol, and synthetic compounds such as WIN55,212-2 or CP55940 ((2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-5-(2-methyloctan-2-yl)phenol) (3, 29). In addition, numerous synthetic CB1R inverse agonists/antagonists have been described (30, 31), among which SR141716A (rimonabant) recently gained attention as a promising treatment for obesity and smoking cessation (32). Interestingly, the CB1R inverse agonists/antagonists, SR141716A, AM251, and AM281, act as agonists on GPR55 (9, 22, 24, 33). So far, the most potent and endogenous GPR55 ligand is l-α-lysophosphatidylinositol (LPI) (22, 25, 26, 33). In addition, three groups recently described several potent and selective GPR55 agonists and antagonists (24, 34, 35). These new compounds are reported to be inactive at CB1 and CB2 receptors and are promising tools to elucidate the pharmacology and (patho-) physiological functions of GPR55.
7TM/GPCRs can form homomers and heteromers and thereby alter the biochemical properties of the receptors involved (36, 37). These interactions modulate the potential signaling pathways and have been shown to have functional relevance in vitro (38) and in vivo (39–41). The existence of CB1R homomers was detected by using antibodies specifically recognizing CB1R dimers (42). In addition, it was reported that CB1Rs can form heteromers (3). The G protein coupling is altered in CB1R-dopamine D2R heteromers (43), CB1R-orexin-1 receptor heteromers show different trafficking and signaling properties (44) and the signaling pathways are modulated in CB1R-adenosine A2A receptor heteromers (45).
To date, it is unknown whether GPR55 can form functional heteromers with other 7TM/GPCRs. Here we show that GPR55 and CB1Rs can physically interact and modulate each others signaling properties. Our data show that GPR55 signaling is specifically inhibited in the presence of the CB1 receptor.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfections, and Stable Cell Lines
HEK293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) at 37 °C in a 5% CO2, humidified atmosphere. HEK293 cells stably expressing the human 3×HA-GPR55 (HEK-GPR55) were previously described (21) and maintained in G418 (PAA) containing medium (0.4 mg/ml). To generate HEK293 cells stably expressing human FLAG-CB1 alone (HEK-CB1) or 3×HA-GPR55 and FLAG-CB1 receptor (HEK-GPR55+CB1), HEK293 or HEK-GPR55 cells were transfected with pcDNA3.1 encoding the FLAG-CB1 receptor using Lipofectamine 2000 (Invitrogen). Cells were generated in selection media (0.5 mg/ml of Zeocin for HEK-CB1 or 0.8 mg/ml of G418 and 0.5 mg/ml of Zeocin for HEK-GPR55+CB1) and single colonies were propagated. HEK-CB1 cells were cultured in DMEM containing 0.2 mg/ml of Zeocin, and HEK-GPR55+CB1 cells were maintained in DMEM containing 0.4 mg/ml of G418 and 0.2 mg/ml of Zeocin. All cells were serum starved in Opti-MEM (Invitrogen) prior to all experiments. Transient transfections were performed using Lipofectamine 2000 following the manufacturer's instructions.
Specific GPR55 Agonist GSK319197A
GSK319197A is [4-(3,4-dichloro-phenyl)-piperazin-1-yl]-(4′-fluoro-4-methanesulfonyl-biphenyl-2-yl)-methanone (example 13 from Ref. 46), a structural analog of GSK494581A (24) and CID2440433 (35). It is one of a series of benzoylpiperazines originally identified as inhibitors of the glycine transporter subtype 1 (GlyT1) and shown also to be selective ligands of GPR55 (24, 35). As expected for examples in this series, GSK319197A activates human GPR55 (pEC50 = 6.4 ± 0.29 (mean ± S.D.); n = 6) in the yeast reporter gene assay described by Brown et al. (24) but does not activate rat GPR55 expressed in the same host. GSK319197A also activates human GPR55 stably expressed in HEK293 cells in combination with apoaequorin and the promiscuous Gα subunit, Gα16z49 (24), causing mobilization of intracellular calcium (pEC50 = 5.2 ± 0.16 (mean ± S.D.), n = 6). In yeast and mammalian HEK293 expression systems, GSK319197A behaves as a full agonist at human GPR55. GSK319197A also inhibits binding of [3H]glycine to HEK293 cells stably expressing GlyT1 in a Scintillation Proximity Assay (pIC50 = 6.4 ± 0.02 (mean ± S.D.), n = 2) (24).
Reporter Gene Assay
Transcription factor luciferase assays were carried out as previously described (21). Briefly, HEK293, HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells were seeded in 96-well plates (40,000 cells/well) and transiently transfected with the cis-reporter plasmids (PathDetect; Stratagene) for NFAT-luc (100–200 ng) or SRE-luc (50 ng/well) (kindly provided by Silvio Gutkind, National Institutes of Health, Bethesda, MD) using Lipofectamine 2000. For gene-dose experiments, HEK-GPR55 cells were additionally transfected with pcDNA FLAG-CB1, pcDNA FLAG-CCR5, or pcDNA 3.1 (25–100 ng). For β-arrestin overexpression studies, cells were co-transfected with 100 ng of control pcDNA-EGFP or 100 ng of β-arrestin 2-EGFP plasmid. 24 to 48 h post-transfection, cells were incubated with the indicated ligand concentrations for 4 h in serum-free media at 37 °C. The cell number was determined in a FlexStation II (Molecular Devices) and luciferase activity was visualized using the Steadylite Plus Kit (Packard Instrument Company, Meriden, CT) and was measured in a TopCounter (Top Count NXT; Packard) for 5 s. Luminescence values are given as relative light units (RLU). For reporter gene experiments, RLU were normalized to the cell number.
Preparation of HEK-Cell Membranes
HEK-GPR55, HEK-CB1 and HEK-GPR55+CB1 cells were grown to confluence in 10-cm dishes, washed with ice-cold PBS, and scraped off with 1 ml of HME buffer (25 mm Hepes-NaOH, pH 7.5, 2 mm MgCl2, 1 mm EDTA and protease inhibitors (Roche Applied Science)). Cells were centrifuged at 14,000 × g for 15 min at 4 °C, the supernatant was aspirated and the pellet was resuspended in 500 μl of 1× HME buffer and snap frozen in liquid nitrogen. Thawed pellets were sonicated on ice in HME buffer and centrifuged at 14,000 × g for 15 min at 4 °C. The membranes were then resuspended and protein concentration was measured using a Bradford assay.
Homologous and Heterologous Competition Binding Assay
Radioligand binding on HEK293, HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cell membranes was performed essentially as reported in Ref. 47. In brief, 20 μg of membranes were diluted in assay buffer (50 mm Tris-HCl, 3 mm MgCl2, 1 mm EDTA, 0,5% BSA, pH 7.4) and preincubated with DMSO or increasing concentrations (10 pm to 10 μm) of SR141716A or GSK319197A. [3H]SR141716A (PerkinElmer Life Sciences) was added in a final concentration of 1.25 nm and incubated at 30 °C for 2 h. The reaction was terminated with ice-cold wash buffer (50 mm Tris-HCl and 0.1% BSA, pH 7.4), followed by rapid filtration through glass fiber filters (Whatman). Bound radioactivity was determined by liquid scintillation counting. The determination of bound [3H]SR141716A was performed in duplicates. IC50 values were determined by nonlinear regression from competition binding experiments. Kd values were calculated from the homologous binding experiments using the equations Kd = IC50 − L and Ki = IC50/(1 + L/Kd) (GraphPad Software, San Diego, CA).
Western Blot
ERK1/2 phosphorylation was detected as previously described (22). In brief, HEK293, HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells were seeded in 6-well plates and confluent wells were serum starved overnight. Then cells were incubated with pre-warmed Opti-MEM containing vehicle (DMSO or EtOH, final concentration: 0.025%, Merck, Lactan), SR141716A (rimonabant, Sanofi-Synthélabo Recherche, Montpellier, France), GSK319197A (GlaxoSmithKline), WIN55-212,2 (Tocris), anandamide (Tocris), or combinations thereof for 25 min at 37 °C. Cells were washed once with ice-cold PBS (Invitrogen), snap-frozen in liquid nitrogen, and lysed in IPB (10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 25 mm KCl, 1 mm CaCl2, 0.3% Triton X-100, 92 mg/ml of sucrose and protease inhibitors (Roche Applied Science)). Lysates were centrifuged and supernatants were resolved by SDS-PAGE (Invitrogen) and transferred to a PVDF membrane (Millipore). Membranes were blocked in TBST buffer (1 mm CaCl2, 136 mm NaCl, 2.5 mm KCl, 25 mm Tris-HCl, 0.1% (v/v) Tween 20) containing 5% milk, washed in TBST without milk and incubated with rabbit anti-pERK1/2 (1:1000) or rabbit anti-tERK1/2 (1:1000) antibodies overnight at 4 °C (New England Biolabs). Membranes were immunoblotted with HRP-conjugated goat anti-rabbit antibody (1:4000, Jackson ImmunoResearch) for 2 h at room temperature and protein bands were visualized with ECL Western blotting substrate (Thermo Fisher Scientific). At least three independent blots were analyzed for quantification of phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (tERK1/2) levels using ImageJ software (NIH) and pERK1/2 was normalized to tERK1/2 levels.
FLAG-CB1 and FLAG-CCR5 Western blots were performed as described above. HEK-GPR55 cells were transfected with increasing concentrations of FLAG-CB1 or FLAG-CCR5 plasmid and 48 h post-transfection cells were lysed, incubated with peptide:N-glycosidase (New England Biolabs) for 1 h at 37 °C, and transferred to a PVDF membrane. Membranes were blocked in TBST buffer containing 5% milk and incubated with anti-FLAG M2 (1:500, Sigma) or β-actin (1:1000, Sigma) antibodies and immonoblotted with HRP-conjugated goat anti-mouse antibody (1:4000, Jackson ImmunoResearch).
ELISA
Enzyme-linked immunosorbent assay was carried out as previously described (48). Experiments were performed in parallel to the reporter gene assays. Cells were fixed with 3.7% formaldehyde, blocked, and permeabilized in blotto (50 mm Tris-HCl, pH 7.5, 1 mm CaCl2, 0.1% Triton X-100, and 3% milk) for 1 h. The expression of β-arrestin 2 was determined by incubating cells with β-arrestin 2 antibody (1:100, Santa Cruz) overnight at 4 °C, followed by incubation with an HRP-conjugated anti-mouse antibody (1:2500, Jackson ImmunoResearch) for 2 h at room temperature. Cells were washed with TBS (25 mm Tris base, 135 mm NaCl, 2.5 mm KCl, 1 mm CaCl2·2H2O, pH 7.4) and cell numbers were determined by means of optical density using a FlexStation II device. 3,3′,5,5′-Tetramethylbenzidine (Sigma) substrate was added and the coloring reaction was stopped by the addition of 0.5 m sulfuric acid after 2 min at room temperature. Color intensity was measured at 450 nm in a Bio-Rad xMark Microplate Spectrophotometer.
Co-immunoprecipitation
HA-GPR55 was immunoprecipitated with the FLAG-CB1 receptor using HEK-GPR55+CB1 cells. In brief, cells were washed twice with ice-cold PBS and lysed in IPB (10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 25 mm KCl, 1 mm CaCl2, 0.3% Triton X-100 and protease inhibitors (Roche Applied Science)). Cell lysates were centrifuged and the supernatant was incubated with 20 μl of anti-FLAG M2 monoclonal antibody affinity matrix (Sigma) for 1 h at 4 °C. 30 μl of lysate was kept for FLAG, HA, and β-actin control blots. Samples were washed, deglycosylated with peptide:N-glycosidase for 1 h at 37 °C, and incubated with reducing sample buffer (100 mm Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 0.2% bromphenol blue, 200 mm dithiothreitol) for 5 min at 95 °C. Proteins were resolved by SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked, probed with anti-FLAG M2 antibody (1:500), anti-HA-11 antibody (1:1000, Covance), or β-actin monoclonal antibody (1:1000) for 2 h, and immunoblotted with HRP-conjugated anti-mouse antibody (1:4000) for 2 h. Blots were visualized with ECL Western blotting substrate. HEK293, HEK-GPR55, and HEK-CB1 cells were used as control.
Immunocytochemistry and Confocal Microscopy
Cells were grown on poly-d-lysine (Sigma)-coated coverslips to 50% confluence, starved in Opti-MEM overnight, and antibody feeding experiments were performed essentially as described in Ref. 49. In brief, living cells were fed anti-FLAG M1 antibody (1:1000, Sigma) and/or anti-HA-11 (1:1000) for 30 min at 37 °C. Subsequently, cells were stimulated with selective agonists or DMSO (control, final concentration: 0.025%) for 45 min. Then cells were fixed in 3.7% formaldehyde, permeabilized in blotto (50 mm Tris-HCl, pH 7.5, 1 mm CaCl2, 0.3% Triton X-100, and 3% milk), and labeled with secondary antibodies: Alexa Fluor 488 nm-conjugated IgG2b against the FLAG tag (1:1000, Invitrogen) and/or Alexa Fluor 594-conjugated IgG1 against the HA tag (1:1000, Invitrogen) for 20 min. Immunolabeled receptors were visualized by using a laser-scanning confocal imaging system (Zeiss LSM510).
Statistical Analysis
Statistical analyses were performed using analysis of variance for comparisons between multiple groups, followed by a Bonferroni's post hoc analysis and t tests using GraphPad Prism software (GraphPad Inc., San Diego, CA). A p value of <0.05 was considered statistically significant.
RESULTS
The CB1 Receptor Modulates GPR55 Transcription Factor Activation
It has previously been reported that CB1 receptors can form heteromers with other 7TM/GPCRs and thereby modulate the signaling properties of the receptors involved (3). In contrast, no reports exist to date that GPR55 is able to form functional heteromers with other 7TM/GPCRs. However, we have recently described that GPR55 modulates the signaling capacities of the CB2 receptor in human neutrophils, where both receptors are naturally co-expressed (6). Likewise, GPR55 and CB1 receptors are found to be co-expressed in several cell types (11, 18) and, importantly, some cannabinoid ligands have been reported to modulate both receptors in opposite ways (e.g. SR141716A is an inverse agonist/antagonist on the CB1 receptor and an agonist on GPR55 (22, 24, 30, 31, 33)). Hence, we were interested whether GPR55 and CB1 receptors heteromerize and/or modulate each others signaling properties. In addition, we set out to elucidate the properties of the “dual acting” ligand SR141716A in a cell system where both GPR55 and CB1 receptors are co-expressed.
We engineered HEK293 cells stably co-expressing HA-tagged GPR55 and FLAG-tagged CB1 receptor (referred to as HEK-GPR55+CB1) or control cells expressing either a FLAG-CB1 receptor (HEK-CB1) or a HA-GPR55 receptor (HEK-GPR55) alone. Expression of GPR55 and CB1 receptors in HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells was determined by flow cytometry with anti-HA and Alexa 488-conjugated IgG1 antibodies as well as anti-FLAG and Alexa 488-conjugated IgG2b antibodies under nonpermeabilizing conditions. Expression levels of receptors were similar in single and double expressing cell lines (data not shown). In earlier studies we have shown that various transcription factors, i.e. NF-κB, cyclic AMP response-element binding protein, and NFAT can be activated by GPR55 (21, 22). Here we show for the first time that the SRE can be activated by GPR55 (Fig. 1, B, D, and F). Several previously described GPR55 agonists, such as LPI, SR141716A, AM251, and AM281 (9, 21, 22, 24, 33) induced SRE in a dose-dependent manner (data not shown, Table 1).
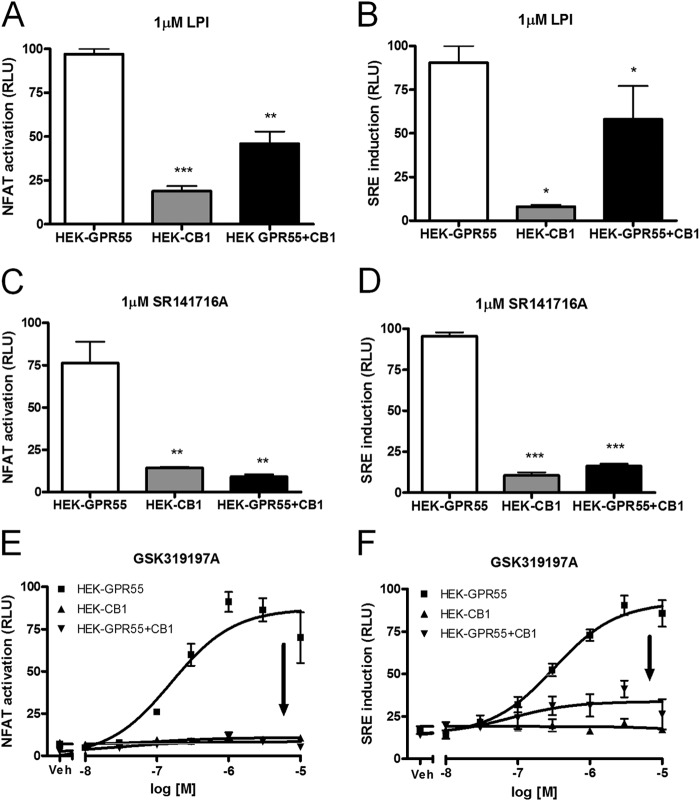
FIGURE 1.
The CB1 receptor modulates GPR55 transcription factor activation in HEK-GPR55+CB1 cells. HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells were transfected with NFAT (A, C, and E) or SRE (B, D, and F) transcription factor-luciferase-reporter plasmids. 24 h post-transfection, cells were stimulated with 1 μm LPI (A and B), 1 μm SR141716A (C and D), or with increasing concentrations of the selective GPR55 agonist GSK319197A (E and F) for 4 h in serum-free medium. NFAT activation (A, C, and E) and SRE induction (B, D, and F) was observed in HEK-GPR55 (white bars or ■), but not in HEK-CB1 (gray bars or ▴) cells. NFAT activation and SRE induction were reduced (A and B) or abolished (C–F) in HEK-GPR55+CB1 cells (black bars or ▾). Data are mean ± S.E. from one of four independent experiments performed in triplicates. Data were normalized and expressed as percent of maximum activation which was set as 100%, relative light units (RLU). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
TABLE 1.
Ligand potencies for SRE induction in HEK-GPR55 cells
Data are mean ± S.E. (n = 3).
| Ligand | EC50 |
|---|---|
| μm | |
| LPI | 0.095 ± 0.0013 |
| AM251 | 0.187 ± 0.0034 |
| SR141716A | 0.553 ± 0.0018 |
| AM281 | 4.338 ± 0.0014 |
We first tested whether NFAT and SRE induction via GPR55 was modulated by co-expressing the CB1 receptor. We observed NFAT activation and SRE induction in HEK-GPR55 (white bars), but not in HEK-CB1 (gray bars) cells after stimulation with the GPR55 agonists LPI or SR141716A (Fig. 1, A–D). In the presence of the CB1R, GPR55-mediated transcription factor activation was reduced by ∼50% after LPI treatment when compared with HEK cells expressing the GPR55 receptor alone (Fig. 1, A and B, compare white versus black bars). Interestingly, no activation of NFAT and SRE was observed in HEK-GPR55 + CB1 cells, following treatment with 1 μm SR141716A (Fig. 1, C and D, compare white versus black bars). Likewise, the GPR55-specific agonist GSK319197A failed to activate NFAT and SRE-luciferase in HEK-GPR55+CB1 cells (Fig. 1, E and F, ▾). Baseline NFAT activation and SRE induction of all three cell lines was similar after vehicle (DMSO) treatment (Fig. 1, E and F, veh). HEK293 cells did not show NFAT activation and SRE induction following GPR55 agonist treatment (data not shown).
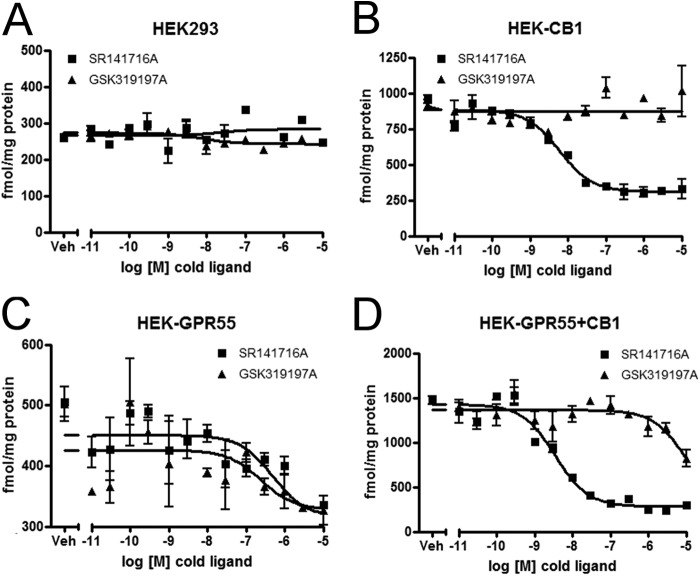
The binding affinities of GSK319197A were determined in heterologous competition binding experiments using [3H]SR141716A as a tracer for both, GPR55 and CB1 receptors (Fig. 2 and Table 2). No CB1R and GPR55 binding was observed in HEK293 cells in homologous and heterologous competition binding experiments (Fig. 2A). Competition of [3H]SR141716A tracer by SR141716A (Ki = 4.81 nm), but not by GSK319197A, was observed in HEK-CB1 cells (Fig. 2B). The binding affinity of SR141716A on HEK-GPR55 membranes was ∼100-fold lower (Ki = 492.65 nm) than on CB1 membranes. This, however, is in line with previous studies, where the potency of SR141716A in activating various GPR55-mediated signaling pathways was in the 1 μm range (22). GSK319197A was slightly more potent in displacing [3H]SR141716A from HEK-GPR55 membranes with a Ki value of 242.78 nm. On HEK-GPR55+CB1 membranes, the Ki value for SR141716A was 2.17 nm and 6263 nm for GSK319197A (Fig. 2, C and D). These data demonstrate that GSK319197A does not bind to the CB1 receptor, but it is a ligand for GPR55 with a Ki value of ∼200 nm.
FIGURE 2.
GSK319197A binds to GPR55, but not to the CB1 receptor. Competition binding experiments were performed with 1.25 nm [3H]SR141716A as a tracer on HEK293 (A), HEK-CB1 (B), HEK-GPR55 (C), and HEK-GPR55+CB1 (D) membranes in the presence of increasing concentrations of SR141716A (■) or GSK319197A (▴). Experiments were performed in duplicates, data are mean ± S.E.
TABLE 2.
GSK319197A potencies and affinities
Reporter gene assay and competition binding data are means ± S.E. (n = 2–5).
| HEK-GPR55 | HEK-CB1 | HEK-GPR55+CB1 | |
|---|---|---|---|
| NFAT activation (EC50 in μm) | 0.033 ± 0.013 | ||
| SRE induction (EC50 in μm) | 0.269 ± 0.048 | ||
| Competition binding (Ki in μm) | |||
| SR141716A | 0.4927 ± 0.2555 | 0.0048 ± 0.0012 | 0.0022 ± 0.0001 |
| GSK319197A | 0.2428 ± 0.0494 | 6.2630 ± 4.1452 |
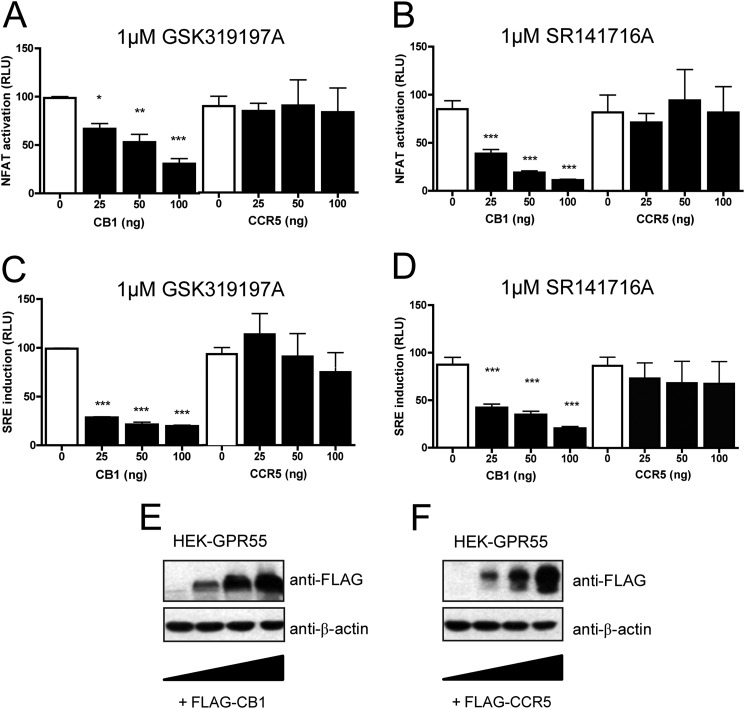
To further investigate whether activation of transcription factors by GPR55 is specifically altered in the presence of the CB1 receptor, we performed gene dose experiments. HEK-GPR55 cells were transfected with increasing concentrations of either CB1 or CCR5 receptor DNA. Cells were stimulated with either 1 μm GSK319197A (Fig. 3, A and C) or 1 μm SR141716A (Fig. 3, B and D). Increasing CB1R expression results in a loss of GPR55-mediated NFAT (Fig. 3, A and B) or SRE (Fig. 3, C and D) induction in a dose-dependent manner (Fig. 3, A–D, left panels). To control whether the presence of an unrelated Gαi-coupled 7TM/GPCR would likewise modulate GPR55-mediated signaling, HEK-GPR55 cells were transfected with increasing amounts of the chemokine receptor CCR5. In the presence of CCR5 no changes in NFAT (Fig. 3, A and B, right panels) or SRE (Fig. 3, C and D, right panels) induction were observed. Gene dose-dependent expression of CB1R (Fig. 3E) and CCR5 (Fig. 3F) was confirmed by Western blot analysis. In summary, these data show that the presence of the CB1 receptor inhibits GPR55-induced NFAT and SRE transcription factor activity, whereas an unrelated 7TM/GPCR, i.e. CCR5, had no effect on GPR55-mediated signaling.
FIGURE 3.
Increasing CB1 receptor levels inhibit GPR55 transcription factor activation. HEK-GPR55 cells were transfected with NFAT (A and B) or SRE (C and D) transcription factor-luciferase-reporter plasmids and increasing amounts of either FLAG-CB1 pcDNA (A--D, left panels) or FLAG-CCR5 pcDNA (A–D, right panels). DNA content in each well was kept constant by co-transfecting with empty pcDNA vector. 48 h post-transfection, cells were stimulated with 1 μm GSK319197A (A and C) or 1 μm SR141716A (B and D) for 4 h in serum-free medium. NFAT activation (A and B) and SRE induction (C and D) were reduced in the presence of increasing amounts of CB1 receptor (A-D, left panels), but remained unaffected by increasing amounts of CCR5 receptor (A–D, right panels). Data are mean ± S.E. from one of three independent experiments performed in triplicates. Data were normalized and expressed as percent of maximum activation of control (white bars), which was set as 100%, RLU, *, p < 0.05; **, p < 0.01; ***, p < 0.001. In parallel, FLAG-CB1 (E) and FLAG-CCR5 (F) receptor expression levels were analyzed by SDS-PAGE and immunoblotted for the receptor FLAG epitope (upper panels, anti-FLAG) and β-actin as protein control (lower panels).
ERK1/2 Phosphorylation Is Altered in HEK-GPR55+CB1 Cells Compared with HEK-GPR55 and HEK-CB1 Cells
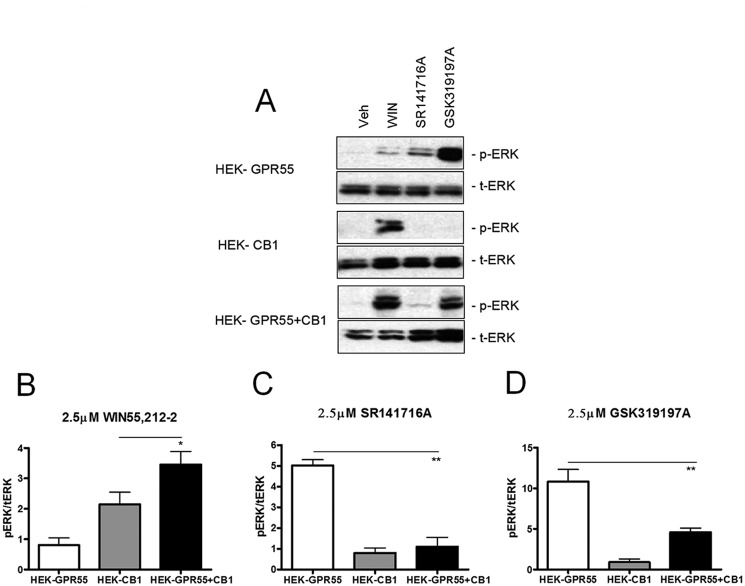
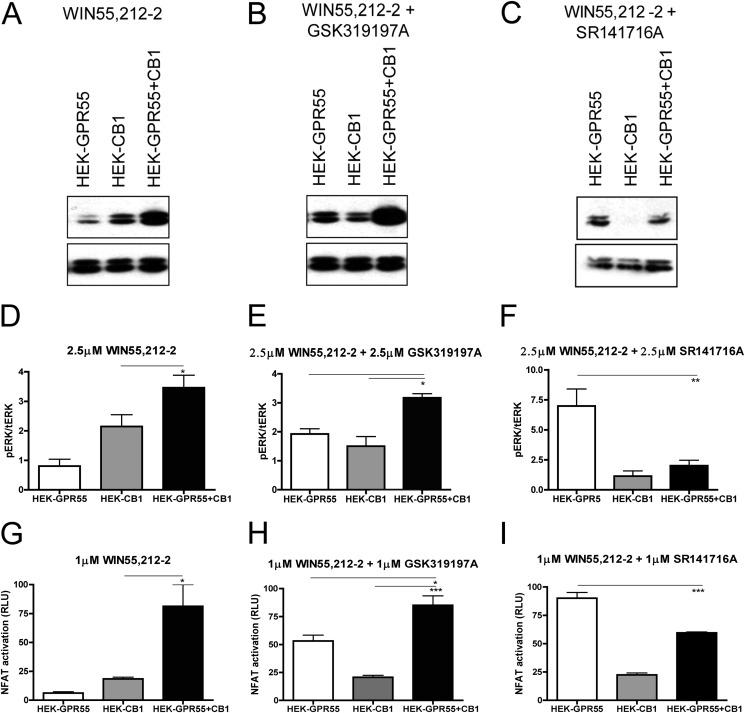
We and others have previously shown that ERK1/2 MAPKs are activated upon stimulation of GPR55 in a variety of cellular backgrounds (12, 22, 26). To test whether the presence of the CB1R interferes with GPR55-mediated signaling at a more upstream level than transcription factors, we tested MAPK activation in HEK293, HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells. As expected, ERK1/2 phosphorylation was significantly increased in HEK-GPR55 cells after stimulation with 2.5 μm of the GPR55 agonists SR141716A and GSK319197A for 25 min (Fig. 4, A, upper panel, C and D, white bars), but not after treatment with the CB1 agonist WIN55,212-2 (Fig. 4, A, upper panel, and B, white panel). ERK1/2 activation was observed in HEK-CB1 cells only after stimulation with 2.5 μm WIN55,212-2 (Fig. 4, A, middle panel, and B, gray bar). In HEK-GPR55+CB1 cells, stimulation with 2.5 μm of the respective GPR55 agonists GSK319197A and SR141716A resulted in only marginal ERK1/2 phosphorylation (Fig. 4, A, upper and lower panels, C and D, white versus black bars). Interestingly, the treatment of HEK-GPR55+CB1 with 2.5 μm WIN55,212-2 resulted in a significantly higher ERK1/2 phosphorylation when compared with HEK-CB1 cells (Fig. 4, A, compare middle and lower panels, B, gray versus black bars). No ERK1/2 activation was observed in all three cell lines after vehicle (DMSO) treatment (Fig. 3A). Likewise, WIN55,212-2, GSK319197A, and SR141716A treatment did not induce ERK1/2 phosphorylation in HEK293 cells (data not shown).
FIGURE 4.
ERK1/2 phosphorylation state is altered in HEK-GPR55+CB1 cells. HEK-GPR55 (white bars), HEK-CB1 (gray bars), and HEK-GPR55+CB1 cells (black bars) were serum starved overnight and stimulated with vehicle, 2.5 μm WIN55,212-2 (A and B), 2.5 μm SR141716A (A and C), or 2.5 μm GSK319197A (A and D) for 25 min. Cell lysates were resolved on a 12% SDS gel followed by antibody staining. Control stimulation with vehicle shows baseline pERK1/2 levels in single and double expression cell lines and the corresponding total ERK levels (t-ERK) are presented below the phospho-ERK (pERK) bands (A). WIN55,212-2 (A and B) induces ERK1/2 phosphorylation in HEK-CB1 and HEK-GPR55+CB1, but not in HEK-GPR55 cells. pERK1/2 levels were significantly increased in HEK-GPR55+CB1 cells compared with HEK-CB1 cells. The stimulation with 2.5 μm SR141716A (A and C) or 2.5 μm GSK319197A (A and D) mediates ERK1/2 phosphorylation in HEK-GPR55, but ERK1/2 phosphorylation is significantly reduced in HEK-GPR55+CB1 cells. A representative blot of three independent experiments is shown A. Blots show the mean ± S.E. from three independent experiments, whereby pERK1/2 bands were normalized to total ERK1/2 levels using densiometric analysis. *, p < 0.05; **, p < 0.01.
These data suggest that the CB1 receptor impairs GPR55-mediated signaling at the level of ERK1/2 MAP kinases. In contrast, the presence of GPR55 seems to enhance CB1R-mediated ERK phosphorylation.
To further investigate the cross-regulation of GPR55 and CB1 receptors on ERK1/2 MAP kinase phosphorylation and NFAT activity, we stimulated the respective receptors in single or co-expressing cells with the following ligand combinations: HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells were stimulated with WIN55,212-2, WIN55,212-2 + GSK319197A or WIN55,212-2 + SR141716A (2.5 μm for ERK1/2 activation and 1 μm for reporter gene experiments). Stimulation of HEK-CB1 and HEK-GPR55+CB1 cells with WIN55,212-2 lead to the phosphorylation of ERK1/2 and activation of NFAT in both cell lines, but signals were significantly increased in the double expressing cell line (Figs. 4, A and B, and 5, A, D, and G). Concomitant activation of both receptors with WIN55,212-2 and GSK319197A resulted in a significant increase in both ERK1/2 phosphorylation (Fig. 5, B and E, black bar) and NFAT activity (Fig. 5H, black bar) when compared with HEK-GPR55 (white bars) or HEK-CB1 (gray bars) cells. This observation suggests that the inhibitory effect of the CB1 receptor on GPR55 signaling may be abolished when the CB1 receptor is activated.
FIGURE 5.
Combinatorial effects of CB1 and GPR55 ligands on ERK1/2 phosphorylation and NFAT activation in HEK-GPR55+CB1 cells. Immunoblots showing ERK1/2 phosphorylation in response to 2.5 μm WIN55,212-2 (A), 2.5 μm WIN55,212-2 + 2.5 μm GSK319197A (B), or 2.5 μm WIN55,212-2 + 2.5 μm SR141716A (C) in HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells. Individual stimulation with 2.5 μm WIN55,212-2 leads to ERK1/2 (A and D) and NFAT (G) activation in HEK-CB1 cells when compared with HEK-GPR55 cells, whereby the strongest activation was detected in HEK-GPR55+CB1 cells. Co-stimulation with 2.5 μm of each, WIN55,212-2 and GSK319197A (B, E, and H), elevates ERK1/2 phosphorylation and NFAT activation in all tested cell lines, whereby the strongest activation is seen in HEK-GPR55+CB1 cells. In contrast, ERK1/2 (C and F) and NFAT (I) activation was significantly reduced by co-stimulating HEK-CB1 and HEK-GPR55+CB1 cells with 2.5 μm WIN55,212-2 and 2.5 μm SR141716A, when compared with HEK-GPR55 cells. Representative blots from 3 independent experiments are shown (A, B, and C). pERK1/2 was normalized to total ERK1/2 and data are means of three independent experiments ± S.E. Reporter gene assay data are mean ± S.E. from one of three independent experiments performed in triplicates. Data were normalized and expressed as percent of maximum activation, which was set as 100%, *, p < 0.05; **, p < 0.01; ***, p < 0.001.
A different picture arises when HEK-GPR55+CB1 cells were co-treated with WIN55,212-2 and the GPR55 agonist SR141716A instead of GSK319197A. Because SR141716A is both an inverse agonist/antagonist on CB1 and an agonist on GPR55, it was not surprising to see a significant decrease in both ERK1/2 (Fig. 5, C and F, black bar) and NFAT activity (Fig. 5I, black bar) when compared with HEK-GPR55. Importantly, these results indicate that only “inactive” CB1 receptors block GPR55-mediated signaling in HEK-GPR55+CB1 cells.
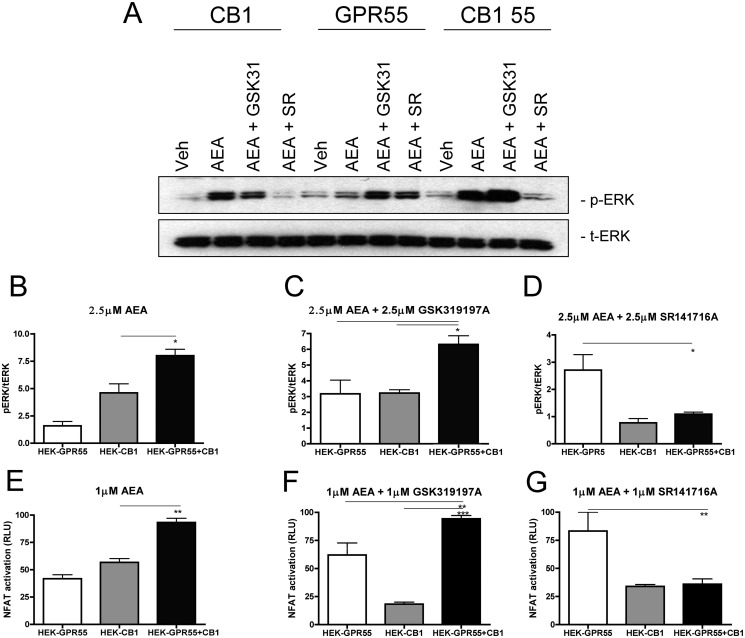
In addition, we tested whether endogenous cannabinoid ligands, such as AEA, could equally regulate GPR55 signaling in HEK-GPR55+CB1 cells. In fact, we found a very similar profile of ERK1/2 phosphorylation and NFAT activity when HEK-GPR55+CB1 cells were stimulated with AEA instead of WIN55,212-2 in all combinations described above. Although stimulation with AEA had no effect on ERK1/2 phosphorylation and NFAT activity in HEK-GPR55 cells (Fig. 6, A, B, and E), a significant increase was observed in HEK-GPR55+CB1 cells (Fig. 6, A, B, and E), when compared with HEK-CB1 cells. Concomitant stimulation of cells with AEA and GSK319197A in the double expressing cell line led to an increase in pERK1/2 and NFAT activation (Fig. 6, A, C, and F), whereas the combination of AEA and SR141716A showed significantly reduced pERK1/2 and NFAT levels (Fig. 6, A, D, and G). Taken together, these data suggest that both, synthetic (WIN55,212-2) and endogenous (AEA) CB1R agonists are capable of restoring GPR55-mediated signaling properties in cells co-expressing these receptors.
FIGURE 6.
Combined administration of the endogenous CB1 agonist anandamide and GPR55 ligands restore GPR55-mediated ERK1/2 phosphorylation and NFAT activation in HEK-GPR55+CB1 cells. Representative immunoblot showing ERK1/2 phosphorylation in response to vehicle, 2.5 μm AEA, 2.5 μm AEA + 2.5 μm GSK319197A and 2.5 μm AEA + 2.5 μm SR141716A in HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells (A). Activation of ERK1/2 (A-D) and NFAT (E–G) were altered in HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells after individual stimulation with 2.5 μm AEA (A, B, and E) or co-stimulation with 2.5 μm AEA + 2.5 μm GSK319197A (A, C, and F) or 2.5 μm AEA + 2.5 μm SR141716A (A, D, and G). Stimulation with 2.5 μm AEA leads to a significantly higher level of ERK1/2 (A and B) and NFAT (E) activation in HEK-GPR55+CB1 compared with HEK-CB1 cells. No activation over baseline was observed in HEK-GPR55 cells. Increased ERK1/2 phosphorylation (A and C) and NFAT (F) activation occurred in all three cell lines after co-stimulation with 2.5 μm AEA and 2.5 μm GSK319197A. HEK-GPR55+CB1 cells show significantly higher activation levels compared with single cell lines. pERK1/2 (A and D) and NFAT (G) activation is inhibited by co-stimulation of HEK-CB1 and HEK-GPR55+CB1 cells with 2.5 μm AEA and 2.5 μm SR141716A, but induced in HEK-GPR55 cells. pERK1/2 was normalized to total ERK1/2 and data are means of three independent experiments ± S.E. Reporter gene assay data are mean ± S.E. from one of four independent experiments performed in triplicates. Data were normalized and expressed as percent of maximum activation, which was set as 100%, *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Gαi Signaling and β-Arrestin 2 Are Not Involved in the Cross-talk between CB1R and GPR55
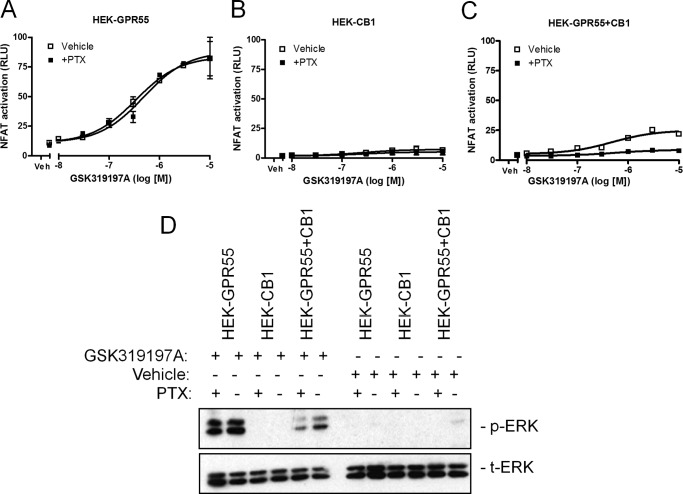
CB1 receptors have been reported to couple to Gαi proteins (16, 17). Here, we wanted to test whether Gαi signaling is involved in the CB1R-mediated inhibition of GPR55 signaling. We tested whether treatment of cells with pertussis toxin (PTX) was able to restore GPR55-mediated NFAT and ERK1/2 activation in HEK-GPR55+CB1 cells. PTX treatment did not alter GPR55-mediated NFAT or ERK activation in HEK-GPR55 cells (Fig. 7, A, compare □ versus ■, and D) or HEK-CB1 cells (Fig. 7, B, compare □ versus ■, and D) when stimulated with the GPR55 agonist GSK319197A. Control experiments on HEK-CB1 cells showed that PTX was active and could block ERK1/2 phosphorylation in WIN55,212-2-stimulated cells (data not shown). In contrast, we detected a decrease in both NFAT and ERK1/2 activity in GSK319197A-stimulated HEK-GPR55+CB1 cells after PTX treatment (Fig. 7, C, compare □ versus ■, and D), pointing toward an involvement of Gαi proteins in the signaling capacity of the GPR55-CB1 heteromer when activated by a GPR55 selective ligand.
FIGURE 7.
CB1R-mediated Gαi activation is not responsible for the loss of GPR55 signal in HEK-GPR55+CB1 cells. HEK-GPR55 (A), HEK-CB1 (B), or HEK-GPR55+CB1 (C) cells were transfected with the NFAT transcription factor plasmid. 24 h post-transfection cells were preincubated for 4 h with either vehicle (□) or 100 ng/ml of PTX (■) and stimulated with increasing concentrations of GSK319197A. NFAT activation was not altered by PTX in HEK-GPR55 cells (A). No NFAT activation was measured in HEK-CB1 cells after stimulation with the GPR55 agonist GSK319197A (B). In HEK-GPR55+CB1 cells (C), NFAT signaling was impaired in PTX-treated cells (■) when compared with cells treated with vehicle (□). For ERK1/2 phosphorylation (D) determination in the presence or absence of PTX, HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells were serum starved overnight, preincubated with vehicle or 100 ng/ml of PTX for 4 h, and stimulated with vehicle or 2.5 μm GSK319197A for 25 min. ERK1/2 phosphorylation was not altered by PTX in HEK-GPR55 cells. No ERK1/2 activity was observed after vehicle treatment in all cell lines and stimulation with the GPR55 agonist GSK319197A in HEK-CB1 cells. HEK-GPR55+CB1 cells were preincubated with PTX showed decreased pERK1/2 when compared with vehicle preincubated double expressing cell line. Reporter gene assay data are mean ± S.E. from one of three independent experiments performed in duplicates. Data were normalized and expressed as percent of maximum activation, which was set as 100% (A–C). Representative ERK1/2 blot from three independent experiments is shown.
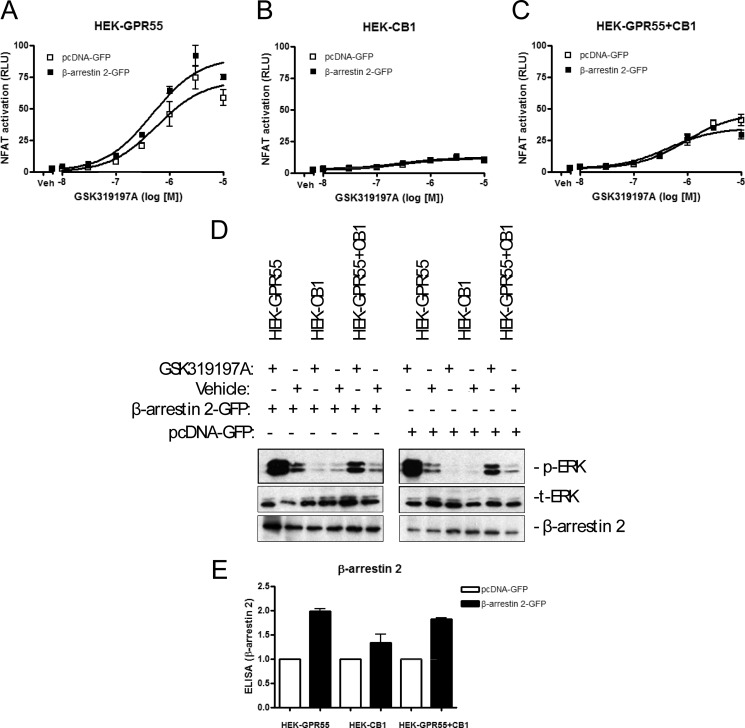
It is well accepted that β-arrestins can also serve as signal adaptors and/or transducers for 7TM/GPCRs (50, 51). In cells overexpressing two 7TM/GPCRs, it is hence possible that the endogenous β-arrestin levels are not high enough to serve as signaling partners for both receptors. We therefore tested whether overexpression of β-arrestin 2 could possibly restore GPR55-mediated transcription factor activation and ERK1/2 phosphorylation in HEK-GPR55+CB1 cells. However, we could observe no difference in both, NFAT (Fig. 8, A–C) or ERK phosphorylation (Fig. 8D) assays when all three cell lines were overexpressing β-arrestin 2. β-Arrestin 2 overexpression was verified by SDS-PAGE and ELISA experiments (Fig. 8, D and E).
FIGURE 8.
Overexpression of β-arrestin 2 does not restore GPR55-mediated signaling in HEK-GPR55+CB1 cells. HEK-GPR55 (A), HEK-CB1 (B), or HEK-GPR55+CB1 (C) cells were transfected with 100 ng of NFAT transcription factor plasmid and 100 ng of control pcDNA-GFP (□) or 100 ng of β-arrestin 2-GFP (■) plasmid. 48 h post-transfection cells were stimulated with increasing concentrations of GSK319197A. NFAT activation was not altered by β-arrestin 2 overexpression in HEK-GPR55 cells (A). No NFAT activation was detected in HEK-CB1 cells after stimulation with the GPR55 agonist GSK319197A (B). In HEK-GPR55+CB1 cells (C), NFAT signaling was not changed in β-arrestin 2-GFP cotransfected cells when compared with control pcDNA-GFP-transfected cells (□). Reporter gene assay data are mean ± S.E. from one of three independent experiments performed in triplicates. Data were normalized and expressed as percent of maximum activation, which was set as 100% (A–C). ERK1/2 phosphorylation was not altered by overexpression of β-arrestin 2 (D). ERK1/2 phosphorylation in the presence or absence of β-arrestin 2 was determined in HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells that were serum starved overnight. Cells were then stimulated with vehicle or 2.5 μm GSK319197A for 25 min. A representative ERK1/2 blot out of three independent experiments is shown. In parallel to reporter gene assays, overexpression of β-arrestin 2 was controlled by ELISA (E). ELISA data were normalized and are mean ± S.E.
Ligand-independent Heteromerization of GPR55 and CB1R
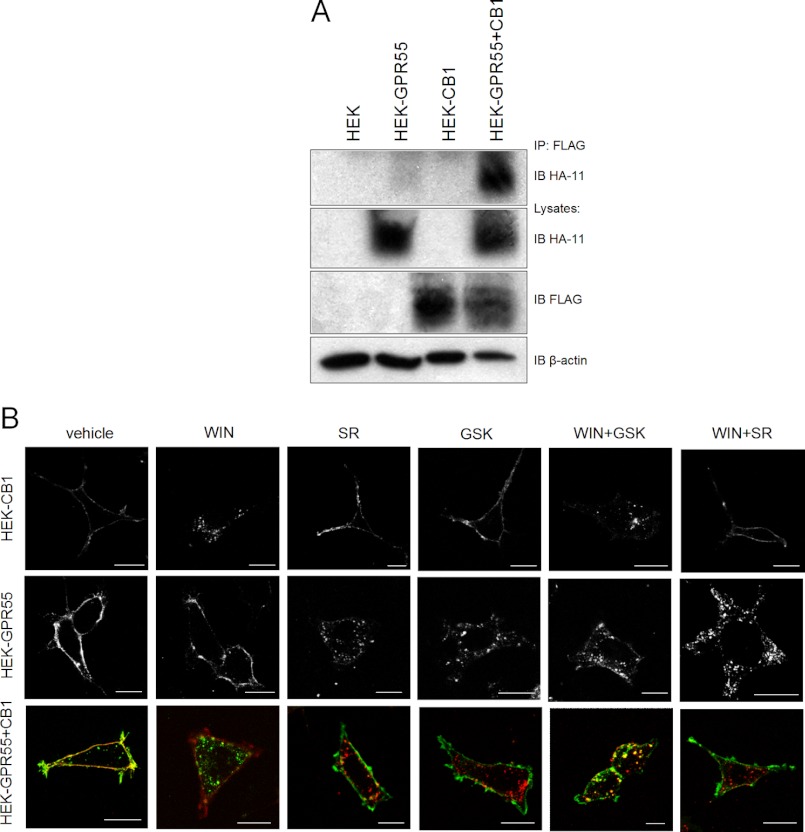
Many 7TM/GPCRs form heteromers and these can function as novel signaling entities (36, 37). Therefore, we next tested a possible heteromerization of GPR55 and CB1Rs by co-immunoprecipitation of the respective receptors from cell lysates derived from the HEK-GPR55+CB1 cell line (Fig. 9A). Indeed, as seen in Fig. 9A, 4th lane, GPR55 co-immunoprecipitated with the CB1 receptor. Control lysates were immunoblotted for HA-GPR55 (Fig. 9A, 2nd panel, lanes 2 and 4), FLAG-CB1R (Fig. 9A, 3rd panel, lanes 3 and 4), and β-actin (Fig. 9A, 4th panel). HEK293 served as negative controls (Fig. 9A, 1st lane). These data suggest that GPR55 and CB1 receptors form heteromers.
FIGURE 9.
Co-immunoprecipitation and internalization of GPR55 and CB1R in HEK293 cells. HEK293, HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cell lysates (A) were co-immunoprecipitated (IP) with anti-FLAG affinity matrix (IP) and immunoblotted (IB) for HA-GPR55 (1st panel). Lysates were probed for HA-GPR55 (2nd panel), FLAG-CB1 receptor (3rd panel), and β-actin (4th panel). GPR55 strongly interacts with CB1 receptors in the double expressing cell line. The blot is representative of three independent experiments. HEK-GPR55, HEK-CB1, and HEK-GPR55+CB1 cells were stimulated with 2.5 μm agonist for 45 min and receptor internalization was monitored (B). GPR55 and CB1 receptors are located on the cell surface without agonist treatment and internalize following agonist stimulation in single and double expressing cell lines. GPR55 and CB1 receptor interaction under unstimulated conditions does not alter receptor internalization. Scale bar = 20 μm.
Internalization Properties of Both, GPR55 and CB1Rs, Are Not Altered in HEK-GPR55+CB1 Cells
Both GPR55 and CB1 receptors have been described to rapidly internalize following agonist stimulation (6, 21, 22, 49). Interestingly, it has been reported that the μ-opioid receptor can ”co-internalize“ with an activated δ-opioid receptor, i.e. internalizes as a heteromer (52). Therefore, we were interested whether GPR55 and CB1R could likewise internalize as a heteromer upon stimulation with one of the respective agonists. Antibody feeding experiments in live cells revealed that upon treatment of cells with selective CB1R or GPR55 agonists for 45 min, GPR55 and CB1 receptors do not co-internalize (Fig. 9B, 3rd panels, WIN, SR, and GSK). Unstimulated GPR55 and CB1 receptors were detected on the cell surface (Fig. 9B, 1st lane). GPR55 was internalized following treatment with 2.5 μm SR141716A, 2.5 μm GSK319197A, 2.5 μm WIN55,212-2 + 2.5 μm GSK319197A, and 2.5 μm WIN55,212-2 + 2.5 μm SR141716A in HEK-GPR55 and HEK-GPR55+CB1 cells (Fig. 9B, 2nd and 3rd panels, red). Stimulation with 2.5 μm WIN55,212-2 and 2.5 μm WIN55,212-2 + 2.5 μm GSK319197A induced CB1 receptor internalization in single and double-expressing cells (Fig. 9B, 1st and 3rd panels, green). CB1 receptor internalization was inhibited by co-stimulation of 2.5 μm WIN55,212–2 + 2.5 μm SR141716A in HEK-CB1 and HEK-GPR55+CB1 cells (Fig. 9B, last lane). In summary, we could not observe any changes in the internalization properties of either GPR55 or CB1R in single receptor-expressing versus double receptor-expressing cell lines, suggesting that GPR55 and CB1R do not internalize as a heteromer.
DISCUSSION
GPR55 pharmacology and signaling properties were extensively studied in the past years, using many different cell systems and signaling readouts (7, 53). Nevertheless, the pharmacology of GPR55 is still rather controversial and seems to be highly cell system dependent (3, 53). It is well known that the co-expression of 7TM/GPCRs in specific tissues can lead to altered binding and/or signaling properties via the respective receptors (54–59). For instance, modulation of G protein activation was observed in the μ-opioid receptor (MOR)/δ-opioid receptor (DOR) (57) and the MOR/β2-AR heteromers (60), respectively. Although the MOR activity was decreased in the MOR/DOR heteromer, its G protein coupling was enhanced in the MOR/β2-AR heteromer. These data suggest that heteromers exhibit distinct and unique signaling profiles. Both GPR55 and CB1 receptors are highly expressed at similar levels in several brain regions, such as the striatum, hypothalamus, and brain stem (9). In light of these studies, we set out to investigate whether co-expression of CB1 and GPR55 in a recombinant HEK293 cell line could explain some of the controversial pharmacology reported for GPR55.
Here we demonstrate that GPR55 and CB1 receptors form heteromers and influence each others signaling properties in HEK293 cells co-expressing both receptors. Specifically, we show that GPR55-mediated signaling is inhibited in the presence of the CB1 receptor. Interestingly, this effect is only apparent when the CB1 receptor is inactive. In contrast, we show that the signaling capacity of the CB1R is enhanced in the presence of GPR55.
GPR55-mediated signaling is lowered or inhibited in the presence of unstimulated CB1 receptor at the level of MAP kinases (Fig. 4) and transcription factors, such as NFAT and SRE (Figs. 1 and 3). The signaling capacity of the CB1 receptor is enhanced in the presence of GPR55 (Figs. 4–6). Upon stimulation of the CB1 receptor with the synthetic ligand WIN55,212-2 (Figs. 4 and 5) or the endogenous ligand anandamide (Fig. 6), we observed elevated ERK1/2 signals and NFAT activation in HEK-GPR55+CB1 cells compared with HEK-CB1 cells. Interestingly, signaling via GPR55 is restored in the presence of activated CB1 receptors (Figs. 5 and 6), because co-stimulation of HEK-GPR55+CB1 cells with both GSK319197A and WIN55,212-2 or AEA resulted in a dramatic increase of pERK and NFAT activity (Figs. 5, B, E, and H, and 6, A, C, and F). In line with these data, the GPR55 signal was effectively blocked when HEK-GPR55+CB1 cells were co-stimulated with WIN55,212-2 or AEA and SR141716A (Figs. 5, C, F, and I, and 6, A, D, and G). SR141716A is both an inverse agonist/antagonist on CB1R and an agonist on GPR55 (Figs. 4, A and C; 5, C, F, and I; and 6, A, D, and G). Hence, when the CB1 receptor is inactivated in the presence of SR141716A, GPR55 is incapable of inducing ERK1/2 and/or NFAT activation (Figs. 5, C, F, and I, and 6, A, D, and G), despite the fact that SR141716A is a potent agonist on GPR55. These data further substantiate the hypothesis that only inactive CB1 receptors are able to inhibit GPR55-mediated signaling.
We next observed that the blockade of CB1 receptor-coupling to Gαi proteins with PTX does not restore GPR55-mediated transcription factor activation or pERK1/2 activation in HEK-GPR55+CB1 cells. Interestingly, we noticed a further decrease in GPR55-mediated NFAT and ERK1/2 activation after PTX treatment in HEK-GPR55+CB1 cells (Fig. 7). It has been reported that CB1 receptors are able to constitutively activate Gαi proteins (16, 17, 61). PTX irreversibly interacts with the Gαi subunits and thereby inhibits the interaction with 7TM/GPCRs. We hypothesize that the inhibition of the CB1 receptor-Gαi-subunit interaction keeps the CB1 receptor in its inactive state and thereby further renders GPR55 inactive when both receptors are co-expressed.
Most 7TM/GPCRs recruit β-arrestins following agonist stimulation (62, 63). Not only do β-arrestins play an important role in 7TM/GPCR internalization and desensitization, but also often serve as signaling partners (50, 51). One possible explanation for the lack of signaling response in cells overexpressing both CB1 and GPR55 receptors could be a lack of sufficient β-arrestin levels in our heterologous cell system. However, overexpression of β-arrestin 2 did not restore GPR55-mediated transcription factor activation or ERK1/2 phosphorylation in HEK-GPR55+CB1 cells (Fig. 8).
We next show that GPR55 and CB1 receptors physically interact in HEK-GPR55+CB1 cells in the absence of any ligand (Fig. 9A). However, we did not observe any altered internalization patterns following agonist activation of both receptors in double-expressing cells when compared with single-expressing cells (Fig. 9B).
Hence, when co-expressed, the GPR55-CB1 receptors alter each others signaling properties at the level of MAPKs and ensue transcription factor activation, i.e. when unstimulated, the CB1 receptor prevents the activation/signaling of GPR55. A similar mechanism has been reported for the opioid receptor system. The presence of the DOR decreases the activity of the MOR upon stimulation with selective MOR agonists (57). However, once activated, the CB1 receptor internalizes (Fig. 9B, WIN), thereby restoring GPR55-mediated signaling when co-stimulated with the specific GPR55 agonist GSK319197A (Fig. 5, B, E, and H). Another interesting finding of our study is that CB1 receptor-mediated signaling is greatly amplified in the presence of GPR55.
A recent report on the CRTH2 and DP receptor heteromers (64) describes a similar cross-talk mechanism, i.e. the DP receptor is able to amplify a CRTH2-induced Ca2+ release from intracellular stores and, coincidentally, loses its own signaling capacity. In addition, a recent study by Rozenfeld et al. (65) showed that heteromerization of CB1Rs and DOR affects receptor signaling. In fact, activation of the CB1 receptor results in a decrease in receptor signaling in the presence of DOR. Moreover, this decrease in signaling is associated with increased phospholipase C-dependent recruitment of β-arrestin 2 to the heteromer (65). Another study describes a cross-talk of the CB2 receptor and GPR55 at the level of small GTPases in neutrophils (6). Here, co-stimulation of both, CB2R and GPR55, leads to a synergistic effect of neutrophil migration and polarization via the small GTPase cdc42. In contrast, the GPR55-mediated inhibition of Rac2 activation results in reduced CB2R-mediated reactive oxygen species production and myeloperoxidase release. GPR55 activation in neutrophils enhances migration to the site of inflammation, but prevents exaggerated tissue injury mediated by CB2 receptor activation (6). Another study in a hybridoma endothelial cell line suggested that integrin clustering was a prerequisite for the inhibition of GPR55-mediated signaling in the presence of the CB1 receptor (11).
In summary, GPR55 plays an important role in several physiological and pathophysiological processes (7), such as the development of cancer (12–15), bone formation (20), pain and inflammation (6, 8, 66). GPR55 activation and function is still controversial and seems to be highly cell system dependent. Our data provide evidence for a cross-talk of GPR55 and CB1 receptors. Because both CB1 and GPR55 receptors are endogenously co-expressed in several tissues, a potential cross-regulation between the two receptor systems will have to be taken into consideration.
This work was supported by Austrian Science Fund Grants P18723 (to M. W.) and P22521 (to A. H.), Jubilaumsfonds of the Austrian National Bank Grant 12552 (to M. W.), grants form the Lanyar Stiftung (to M. W.), and the Ph.D. program Molecular Medicine from the Medical University of Graz, a research fellowship from the Austrian Government, the START-Funding Program (MUG), and the BA/CA visiting scientist program (to J. K.).
- CB1R
- cannabinoid 1 receptor
- 7TM/GPCR
- seven-transmembrane spanning/G protein-coupled receptor
- AM251
- 1-(2, 4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(1-piperidyl)pyrazole-3- carboxamide
- AM281
- 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide
- CB2R
- cannabinoid 2 receptor
- DMSO
- dimethyl sulfoxide
- ERK
- extracellular signal-regulated kinase
- GPR55
- G protein-coupled receptor 55
- LPI
- l-α-lysophosphatidylinositol
- MAPK
- mitogen-activated protein kinase
- SR141716A
- 5-(4-chlorophenyl)- 1-(2,4-dichlorophenyl)-4-methyl-N-(piperidine-1-yl)-1H-pyrazole-3-car- boxamide
- TBS
- Tris-buffered saline
- WIN55,212-2
- (R)-(−)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone
- AEA
- anandamide
- SRE
- serum response element
- RLU
- relative light unit
- PTX
- pertussis toxin
- NFAT
- nuclear factor of activated T-cells
- MOR
- μ-opioid receptor
- DOR
- δ-opioid receptor.
REFERENCES
- 1. Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 [DOI] [PubMed] [Google Scholar]
- 2. Munro S., Thomas K. L., Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65 [DOI] [PubMed] [Google Scholar]
- 3. Pertwee R. G., Howlett A. C., Abood M. E., Alexander S. P., Di Marzo V., Elphick M. R., Greasley P. J., Hansen H. S., Kunos G., Mackie K., Mechoulam R., Ross R. A. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands. Beyond CB1 and CB2. Pharmacol. Rev. 62, 588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsou K., Brown S., Sañudo-Peña M. C., Mackie K., Walker J. M. (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411 [DOI] [PubMed] [Google Scholar]
- 5. Buckley N. E. (2008) The peripheral cannabinoid receptor knockout mice. An update. Br. J. Pharmacol. 153, 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balenga N. A., Aflaki E., Kargl J., Platzer W., Schröder R., Blättermann S., Kostenis E., Brown A. J., Heinemann A., Waldhoer M. (2011) GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. 21, 1452–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henstridge C. M., Balenga N. A., Kargl J., Andradas C., Brown A. J., Irving A., Sanchez C., Waldhoer M. (2011) Minireview. Recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol. Endocrinol. 25, 1835–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pietr M., Kozela E., Levy R., Rimmerman N., Lin Y. H., Stella N., Vogel Z., Juknat A. (2009) Differential changes in GPR55 during microglial cell activation. FEBS Lett. 583, 2071–2076 [DOI] [PubMed] [Google Scholar]
- 9. Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N. O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P. J. (2007) The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152, 1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sawzdargo M., Nguyen T., Lee D. K., Lynch K. R., Cheng R., Heng H. H., George S. R., O'Dowd B. F. (1999) Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53, and GPR55. GPR55 is extensively expressed in human brain. Brain Res. Mol. Brain Res. 64, 193–198 [DOI] [PubMed] [Google Scholar]
- 11. Waldeck-Weiermair M., Zoratti C., Osibow K., Balenga N., Goessnitzer E., Waldhoer M., Malli R., Graier W. F. (2008) Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J. Cell Sci. 121, 1704–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andradas C., Caffarel M. M., Pérez-Gómez E., Salazar M., Lorente M., Velasco G., Guzmán M., Sánchez C. (2011) The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 30, 245–252 [DOI] [PubMed] [Google Scholar]
- 13. Ford L. A., Roelofs A. J., Anavi-Goffer S., Mowat L., Simpson D. G., Irving A. J., Rogers M. J., Rajnicek A. M., Ross R. A. (2010) A role for l-α-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br. J. Pharmacol. 160, 762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang L., Ramirez J. C., Frampton G. A., Golden L. E., Quinn M. A., Pae H. Y., Horvat D., Liang L. J., DeMorrow S. (2011) Anandamide exerts its antiproliferative actions on cholangiocarcinoma by activation of the GPR55 receptor. Lab. Invest. 91, 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piñeiro R., Maffucci T., Falasca M. (2011) The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 30, 142–152 [DOI] [PubMed] [Google Scholar]
- 16. Howlett A. C., Barth F., Bonner T. I., Cabral G., Casellas P., Devane W. A., Felder C. C., Herkenham M., Mackie K., Martin B. R., Mechoulam R., Pertwee R. G. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 54, 161–202 [DOI] [PubMed] [Google Scholar]
- 17. Howlett A. C. (2005) Cannabinoid receptor signaling. Handb. Exp. Pharmacol. 168, 53–79 [DOI] [PubMed] [Google Scholar]
- 18. Lauckner J. E., Jensen J. B., Chen H. Y., Lu H. C., Hille B., Mackie K. (2008) GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. U.S.A. 105, 2699–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharir H., Abood M. E. (2010) Pharmacological characterization of GPR55, a putative cannabinoid receptor. Pharmacol. Ther. 126, 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whyte L. S., Ryberg E., Sims N. A., Ridge S. A., Mackie K., Greasley P. J., Ross R. A., Rogers M. J. (2009) The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. U.S.A. 106, 16511–16516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henstridge C. M., Balenga N. A., Ford L. A., Ross R. A., Waldhoer M., Irving A. J. (2009) The GPR55 ligand l-α-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 23, 183–193 [DOI] [PubMed] [Google Scholar]
- 22. Henstridge C. M., Balenga N. A., Schröder R., Kargl J. K., Platzer W., Martini L., Arthur S., Penman J., Whistler J. L., Kostenis E., Waldhoer M., Irving A. J. (2010) GPR55 ligands promote receptor coupling to multiple signalling pathways. Br. J. Pharmacol. 160, 604–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schröder R., Schmidt J., Blättermann S., Peters L., Janssen N., Grundmann M., Seemann W., Kaufel D., Merten N., Drewke C., Gomeza J., Milligan G., Mohr K., Kostenis E. (2011) Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nat. Protoc. 6, 1748–1760 [DOI] [PubMed] [Google Scholar]
- 24. Brown A. J., Daniels D. A., Kassim M., Brown S., Haslam C. P., Terrell V. R., Brown J., Nichols P. L., Staton P. C., Wise A., Dowell S. J. (2011) Pharmacology of GPR55 in yeast and identification of GSK494581A as a mixed-activity glycine transporter subtype 1 inhibitor and GPR55 agonist. J. Pharmacol. Exp. Ther. 337, 236–246 [DOI] [PubMed] [Google Scholar]
- 25. Oka S., Nakajima K., Yamashita A., Kishimoto S., Sugiura T. (2007) Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 362, 928–934 [DOI] [PubMed] [Google Scholar]
- 26. Oka S., Kimura S., Toshida T., Ota R., Yamashita A., Sugiura T. (2010) Lysophosphatidylinositol induces rapid phosphorylation of p38 mitogen-activated protein kinase and activating transcription factor 2 in HEK293 cells expressing GPR55 and IM-9 lymphoblastoid cells. J. Biochem. 147, 671–678 [DOI] [PubMed] [Google Scholar]
- 27. Buhl A. M., Johnson N. L., Dhanasekaran N., Johnson G. L. (1995) Gα12 and Gα13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 270, 24631–24634 [DOI] [PubMed] [Google Scholar]
- 28. Kelly P., Casey P. J., Meigs T. E. (2007) Biologic functions of the G12 subfamily of heterotrimeric G proteins. Growth, migration, and metastasis. Biochemistry 46, 6677–6687 [DOI] [PubMed] [Google Scholar]
- 29. Pertwee R. G. (1997) Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 74, 129–180 [DOI] [PubMed] [Google Scholar]
- 30. Milligan G., Bond R. A., Lee M. (1995) Inverse agonism. Pharmacological curiosity or potential therapeutic strategy? Trends Pharmacol. Sci. 16, 10–13 [DOI] [PubMed] [Google Scholar]
- 31. Rinaldi-Carmona M., Barth F., Héaulme M., Shire D., Calandra B., Congy C., Martinez S., Maruani J., Néliat G., Caput D. (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 350, 240–244 [DOI] [PubMed] [Google Scholar]
- 32. Christensen R., Kristensen P. K., Bartels E. M., Bliddal H., Astrup A. (2007) Efficacy and safety of the weight-loss drug rimonabant. A meta-analysis of randomized trials. Lancet 370, 1706–1713 [DOI] [PubMed] [Google Scholar]
- 33. Kapur A., Zhao P., Sharir H., Bai Y., Caron M. G., Barak L. S., Abood M. E. (2009) Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J. Biol. Chem. 284, 29817–29827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heynen-Genel S., Dahl R., Shi S., Milan L., Hariharan S., Sergienko E., Hedrick M., Dad S., Stonich D., Su Y., Vicchiarelli M., Mangravita-Novo A., Smith L. H., Chung T. D. Y., Sharir H., Caron M. G., Barak L. S., Abood M. E. (2010) Probe Reports from the NIH Molecular Libraries Program [Internet]. National Center for Biotechnology Information, Bethesda, MD: [PubMed] [Google Scholar]
- 35. Kotsikorou E., Madrigal K. E., Hurst D. P., Sharir H., Lynch D. L., Heynen-Genel S., Milan L. B., Chung T. D., Seltzman H. H., Bai Y., Caron M. G., Barak L., Abood M. E., Reggio P. H. (2011) Identification of the GPR55 agonist binding site using a novel set of high-potency GPR55 selective ligands. Biochemistry 50, 5633–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferré S., Baler R., Bouvier M., Caron M. G., Devi L. A., Durroux T., Fuxe K., George S. R., Javitch J. A., Lohse M. J., Mackie K., Milligan G., Pfleger K. D., Pin J. P., Volkow N. D., Waldhoer M., Woods A. S., Franco R. (2009) Building a new conceptual framework for receptor heteromers. Nat. Chem. Biol. 5, 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferré S., Navarro G., Casadó V., Cortés A., Mallol J., Canela E. I., Lluís C., Franco R. (2010) G protein-coupled receptor heteromers as new targets for drug development. Prog. Mol. Biol. Transl. Sci. 91, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bouvier M. (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat. Rev. Neurosci. 2, 274–286 [DOI] [PubMed] [Google Scholar]
- 39. Prinster S. C., Hague C., Hall R. A. (2005) Heterodimerization of G protein-coupled receptors. Specificity and functional significance. Pharmacol. Rev. 57, 289–298 [DOI] [PubMed] [Google Scholar]
- 40. Waldhoer M., Bofill-Cardona E., Milligan G., Freissmuth M., Nanoff C. (1998) Differential uncoupling of A1 adenosine and D2 dopamine receptors by suramin and didemethylated suramin (NF037). Mol. Pharmacol. 53, 808–818 [PubMed] [Google Scholar]
- 41. Waldhoer M., Fong J., Jones R. M., Lunzer M. M., Sharma S. K., Kostenis E., Portoghese P. S., Whistler J. L. (2005) A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. U.S.A. 102, 9050–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wager-Miller J., Westenbroek R., Mackie K. (2002) Dimerization of G protein-coupled receptors. CB1 cannabinoid receptors as an example. Chem. Phys. Lipids 121, 83–89 [DOI] [PubMed] [Google Scholar]
- 43. Kearn C. S., Blake-Palmer K., Daniel E., Mackie K., Glass M. (2005) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation. A mechanism for receptor cross-talk? Mol. Pharmacol. 67, 1697–1704 [DOI] [PubMed] [Google Scholar]
- 44. Ellis J., Pediani J. D., Canals M., Milasta S., Milligan G. (2006) Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J. Biol. Chem. 281, 38812–38824 [DOI] [PubMed] [Google Scholar]
- 45. Carriba P., Ortiz O., Patkar K., Justinova Z., Stroik J., Themann A., Müller C., Woods A. S., Hope B. T., Ciruela F., Casadó V., Canela E. I., Lluis C., Goldberg S. R., Moratalla R., Franco R., Ferré S. (2007) Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 32, 2249–2259 [DOI] [PubMed] [Google Scholar]
- 46. Bradley D. M., Branch C. L., Brown B. J., Chan W. N., Coulton S., Dean A. W., Doyle P. M., Evans B., Gilpin M. L., Gough S. L., Macritchie J. A., Marshall H. R., Nash D. J., Porter R. A., Stasi L. P. (2006) World patent application WO2006094843 A1
- 47. Martini L., Thompson D., Kharazia V., Whistler J. L. (2010) Differential regulation of behavioral tolerance to WIN55,212-2 by GASP1. Neuropsychopharmacology 35, 1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tschische P., Moser E., Thompson D., Vischer H. F., Parzmair G. P., Pommer V., Platzer W., Schwarzbraun T., Schaider H., Smit M. J., Martini L., Whistler J. L., Waldhoer M. (2010) The G-protein coupled receptor associated sorting protein GASP-1 regulates the signalling and trafficking of the viral chemokine receptor US28. Traffic 11, 660–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kargl J., Balenga N. A., Platzer W., Martini L., Whistler J. L., Waldhoer M. (2012) The GPCR-associated sorting protein 1 regulates ligand-induced down-regulation of GPR55. Br. J. Pharmacol. 165, 2611–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lefkowitz R. J., Shenoy S. K. (2005) Transduction of receptor signals by β-arrestins. Science 308, 512–517 [DOI] [PubMed] [Google Scholar]
- 51. Reiter E., Lefkowitz R. J. (2006) GRKs and β-arrestins. Roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 17, 159–165 [DOI] [PubMed] [Google Scholar]
- 52. Milan-Lobo L., Whistler J. L. (2011) Heteromerization of the μ- and δ-opioid receptors produces ligand-biased antagonism and alters μ-receptor trafficking. J. Pharmacol. Exp. Ther. 337, 868–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balenga N. A., Henstridge C. M., Kargl J., Waldhoer M. (2011) Pharmacology, signaling and physiological relevance of the G protein-coupled receptor 55. Adv. Pharmacol. 62, 251–277 [DOI] [PubMed] [Google Scholar]
- 54. Jordan B. A., Devi L. A. (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399, 697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. AbdAlla S., Lother H., Quitterer U. (2000) AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 407, 94–98 [DOI] [PubMed] [Google Scholar]
- 56. George S. R., Fan T., Xie Z., Tse R., Tam V., Varghese G., O'Dowd B. F. (2000) Oligomerization of μ- and δ-opioid receptors. Generation of novel functional properties. J. Biol. Chem. 275, 26128–26135 [DOI] [PubMed] [Google Scholar]
- 57. Gomes I., Jordan B. A., Gupta A., Trapaidze N., Nagy V., Devi L. A. (2000) Heterodimerization of μ and δ opioid receptors. A role in opiate synergy. J. Neurosci. 20, RC110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rocheville M., Lange D. C., Kumar U., Patel S. C., Patel R. C., Patel Y. C. (2000) Receptors for dopamine and somatostatin. Formation of hetero-oligomers with enhanced functional activity. Science 288, 154–157 [DOI] [PubMed] [Google Scholar]
- 59. González-Maeso J., Ang R. L., Yuen T., Chan P., Weisstaub N. V., López-Giménez J. F., Zhou M., Okawa Y., Callado L. F., Milligan G., Gingrich J. A., Filizola M., Meana J. J., Sealfon S. C. (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jordan B. A., Gomes I., Rios C., Filipovska J., Devi L. A. (2003) Functional interactions between μ opioid and α2A-adrenergic receptors. Mol. Pharmacol. 64, 1317–1324 [DOI] [PubMed] [Google Scholar]
- 61. Seifert R., Wenzel-Seifert K. (2002) Constitutive activity of G-protein-coupled receptors. Cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 366, 381–416 [DOI] [PubMed] [Google Scholar]
- 62. Hinrichsen L., Meyerholz A., Groos S., Ungewickell E. J. (2006) Bending a membrane. How clathrin affects budding. Proc. Natl. Acad. Sci. U.S.A. 103, 8715–8720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wolfe B. L., Trejo J. (2007) Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 8, 462–470 [DOI] [PubMed] [Google Scholar]
- 64. Sedej M., Schröder R., Bell K., Platzer W., Vukoja A., Kostenis E., Heinemann A., Waldhoer M. (2012) D-type prostanoid receptor enhances the signaling of chemoattractant receptor-homologous molecule expressed on T(H)2 cells. J. Allergy Clin. Immunol. 129, 492–500 [DOI] [PubMed] [Google Scholar]
- 65. Rozenfeld R., Bushlin I., Gomes I., Tzavaras N., Gupta A., Neves S., Battini L., Gusella G. L., Lachmann A., Ma'ayan A., Blitzer R. D., Devi L. A. (2012) Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons. PLoS One 7, e29239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Staton P. C., Hatcher J. P., Walker D. J., Morrison A. D., Shapland E. M., Hughes J. P., Chong E., Mander P. K., Green P. J., Billinton A., Fulleylove M., Lancaster H. C., Smith J. C., Bailey L. T., Wise A., Brown A. J., Richardson J. C., Chessell I. P. (2008) The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 139, 225–236 [DOI] [PubMed] [Google Scholar]