Background: Understanding the mechanisms of cell wall biogenesis is important for development of antifungal strategies.
Results: Biosynthesis of the fungal polysaccharide galactomannan requires import of GDP-mannose into the Golgi apparatus.
Conclusion: It differs from the biosynthesis of other fungal cell wall polysaccharides.
Significance: This provides a new paradigm for cell wall polysaccharide biosynthesis.
Keywords: Carbohydrate Biosynthesis, Cell Wall, Fungi, Glycobiology, Glycosyl Phosphatidylinositol Anchors, Polysaccharide, Nucleotide Sugar Transporters
Abstract
Fungal cell walls frequently contain a polymer of mannose and galactose called galactomannan. In the pathogenic filamentous fungus Aspergillus fumigatus, this polysaccharide is made of a linear mannan backbone with side chains of galactofuran and is anchored to the plasma membrane via a glycosylphosphatidylinositol or is covalently linked to the cell wall. To date, the biosynthesis and significance of this polysaccharide are unknown. The present data demonstrate that deletion of the Golgi UDP-galactofuranose transporter GlfB or the GDP-mannose transporter GmtA leads to the absence of galactofuran or galactomannan, respectively. This indicates that the biosynthesis of galactomannan probably occurs in the lumen of the Golgi apparatus and thus contrasts with the biosynthesis of other fungal cell wall polysaccharides studied to date that takes place at the plasma membrane. Transglycosylation of galactomannan from the membrane to the cell wall is hypothesized because both the cell wall-bound and membrane-bound polysaccharide forms are affected in the generated mutants. Considering the severe growth defect of the A. fumigatus GmtA-deficient mutant, proving this paradigm might provide new targets for antifungal therapy.
Introduction
The fungal cell wall is a complex and dynamic entity essential for growth and development of fungi. It consists of an alkali-insoluble polysaccharide network, with which glycoproteins are covalently or non-covalently associated (1–3). A common trait is the presence of a β-1,6-branched β-1,3-glucan that forms a continuous, three-dimensional scaffold to which other components of the alkali-insoluble fraction are covalently linked. Most commonly, these are chitin, a β-1,4-N-acetylglucosamine polymer occurring in all fungi with the sole exception of the fission yeast Schizosaccharomyces pombe (4), and other glucans, such as the β-1,6-glucans in Saccharomyces cerevisiae (5) or the mixed β-1,3/β-1,4-glucans in Aspergillus fumigatus (6). In the latter fungus, galactomannan, a linear α-1,2/α-1,6-mannan with short side chains of β-1,5-galactofuran, also substitutes the cell wall β1,3/1,6-glucan (6, 7). This polysaccharide can additionally be anchored to the plasma membrane by a glycosylphosphatidylinositol (GPI)4 (8) or be part of the alkali-soluble fraction of the cell wall, containing the non-covalently associated polysaccharides α-1,3-glucan and galactosaminogalactan as well as secreted glycoproteins (3). Finally, circulating galactomannan is also present in patient blood, where it can be detected by the monoclonal antibody EB-A2 for clinical diagnostic of invasive aspergillosis (9, 10). Galactofuranose (Galf) is widely distributed among fungi (11), and galactomannan itself has been reported in many fungi, although structural variations exist.
The polysaccharides β-glucan and chitin are both synthesized at the plasma membrane by integral membrane complexes that use cytosolic nucleotide sugars and extrude the growing polysaccharide chains into the extracellular space (12–14). Biosynthesis of β-glucan is essential for fungal viability, and the likely catalytic subunit of the glucan synthase complex, FKS1, is the target of a recent class of antifungals, the echinocandins (15). In contrast, galactomannan biosynthesis as well as its biological role and importance for fungal viability are currently undefined. However, the recent characterization of a Golgi-localized UDP-Galf transporter and its subsequent deletion in A. fumigatus (16) and Aspergillus nidulans (17) suggested its implication in galactomannan galactofuranosylation. Indeed, a soluble cell extract of the transporter-deficient mutant was unreactive to the anti-galactofuran antibody EB-A2 (16).
Nucleotide sugar transporters form a family of structurally conserved integral membrane proteins that supply the endoplasmic reticulum or Golgi apparatus of eukaryotic cells with donor substrates of glycosyltransferases (18, 19). GDP-mannose (GDP-Man) transporters have been studied in various organisms, such as the protozoan parasite Leishmania (20), S. cerevisiae (21), plants (22), and various fungi (23–25), including two Aspergilli (26, 27). Mammalian cells do not possess any GDP-Man transporter because mannosylation is restricted to the conserved core glycosylation events in the endoplasmic reticulum that use lipid-activated mannose or cytosolic GDP-Man. In fungi, GDP-Man transport is indispensable for modification of protein N- and O-glycans (28–30), glycolipid biosynthesis (31), and GPI anchor substitution (32, 33). It has been reported to be essential in Saccharomyces (21), Candida (23), and Aspergillus niger (26).
Here, we provide genetic and biochemical evidence for the implication of nucleotide sugar transporters in cell wall-bound and membrane-bound galactomannan biosynthesis and in fungal growth through characterization of A. fumigatus mutants deficient in UDP-Galf or GDP-Man transporters.
EXPERIMENTAL PROCEDURES
A. fumigatus Strains
A derivate of the A. fumigatus clinical isolate D141 deficient in non-homologous end-joining (AfS35) was used (34). A. fumigatus gmtA was replaced by a pgpdA::ble/tk::trpCt cassette (35), using electroporation of conidia (36). Generation of the replacement cassette is described in the supplemental material and primers used are listed in Table S1. The resulting A. fumigatus ΔgmtA was grown in minimal medium containing 30 mg/liter phleomycin. For carbohydrate analysis, strains were grown in Sabouraud medium supplemented with 1.2 m sorbitol as osmotic stabilizer.
Extraction and Analysis of Glycosylinositolphosphorylceramides by MALDI-TOF Mass Spectrometry
Approximately 500 mg of mycelium (wet weight) was homogenized in 500 μl of TE buffer using a Precellys 24 tissue homogenizer (Peqlab) with 1.4-mm ceramic beads (3 × 20 s at 6800 rpm with 60-s breaks). Chloroform and methanol were added to obtain a 10:10:3 (v/v/v) CHCl3/MeOH/H2O ratio. Samples were sonicated and centrifuged (10 min, 4500 × g), and supernatants were collected. Extraction was repeated with CHCl3/MeOH/H2O (30:60:8) and CHCl3/MeOH (1:1). Supernatants were pooled, dried, and dissolved in CHCl3/MeOH/H2O (8:4:3), and phases were separated by centrifugation. The aqueous phase was purified using a C18 SepPak cartridge (Waters) as described previously (37). The organic phase was purified by butanol partitioning (38). Samples were dried and dissolved in ∼200 μl of CHCl3/MeOH/H2O 30:60:8. For mass spectrometry, 1 μl of 6-aza-2-thiothymine matrix (5 μg/ml), 2 μl of sample, and 1 μl of matrix were successively deposited on a metal target plate under a hot air stream. Measurements were carried out on a Voyager DE Pro work station (Applied Biosystems). Spectra were acquired in negative reflection mode, averaging about 2000 laser shots/spectrum. Mass spectra were calibrated to IPC peaks at m/z 952.7 using the Applied Biosystems Data Explorer® version 4.8 software.
Cell Wall Fractionation
Cell walls were prepared from ∼500 mg of mycelia as described previously (39). Alkali-insoluble fractions were obtained as described previously (6) with minor modifications. Cell walls were suspended in 2 ml of 1 m NaOH, heated at 65 °C for 1 h, and then centrifuged (5 min at 4500 × g). Supernatants were discarded, and extraction was repeated two more times. Subsequently, pellets were washed five times by successive centrifugation and resuspension in 2 ml of H2O using ultrasonication. Final suspensions were frozen at −80 °C.
Digestion of Alkali-insoluble Fraction by β-Glucanase
For digestion with β-glucanase (Quantazyme ylgTM, Qbiogene), 1 ml of cell wall suspensions was incubated with 200 units/ml β-glucanase in 50 mm triethylamine/acetate buffer, pH 7.4, 5 mm sodium azide at 37 °C for 5 days. Samples were centrifuged for 30 min at 13,000 × g to remove residual cell wall material. Sugar quantification was performed as described previously (40).
Galactomannan Quantification
Ten μg of the β-glucanase-released material were dissolved in 100 μl of TBS, and 10× dilution series of this stock were applied to a Platelia Aspergillus enzyme immunoassay plate (Bio-Rad). Galactomannan was quantified from reactivity to EB-A2 antibody according to the kit manual.
Determination of the Monosaccharide Composition
Defined amounts of freeze-dried alkali-insoluble cell walls (from 500 μl of suspension, yielding 2–4 mg of dry mass) were hydrolyzed with sulfuric acid as described previously (39). Alternatively, for hydrolysis of the β-glucanase-released material, 10-μg aliquots were dissolved in 130 μl of 4 m TFA and incubated at 100 °C for 4 h. Internal standard (25 μl of a 1 mm l-fucose solution) was added, and the hydrolysate was dried in a centrifugal vacuum concentrator. Labeling with anthranilic acid and separation by RP-HPLC (Prominence UFLC XR, Shimadzu with a LiChroCART® 250-4 Superspher® 100 RP-18 end-capped cartridge; Merck) was as described (41).
Purification and Analysis of Lipogalactomannan
Purification of lipogalactomannan and fractionation on octyl-Sepharose was essentially as described (8). Details are provided in the supplemental material. Reactivity to EB-A2 was tested using 1:500 dilutions of the fractions with the Platelia Aspergillus enzyme immunoassay kit (Bio-Rad) according to the kit manual.
RESULTS
A. fumigatus GmtA Is an Active GDP-Man Transporter
The A. fumigatus genome contains a single ortholog of the S. cerevisiae GDP-Man transporter Vrg4p. The gene AFUA_5G05740, named here gmtA, encodes a 382-amino acid protein displaying 50% identity with S. cerevisiae Vrg4p on the amino acid sequence level and clustering with other characterized GDP-Man transporters in phylogenetic analyses (26). The ability of GmtA to complement the lethal phenotype of a conditional S. cerevisiae VRG4 knock-out strain, generated by replacing the endogenous VRG4 promoter region with a galactose-inducible promoter, demonstrates that it is an active GDP-Man transporter (supplemental Fig. S1).
Deletion of A. fumigatus GmtA Results in a Severe Growth Defect
In order to investigate the importance of GDP-Man transport for cell wall biogenesis, we generated an A. fumigatus null mutant called ΔgmtA by replacement of the gmtA ORF with the phleomycin resistance gene ble (35), using swollen conidia electroporation. Correct replacement of the ΔgmtA genomic locus was confirmed by Southern blot analysis (supplemental Fig. S2).
At the macroscopic level, the ΔgmtA mutant is severely impaired in growth and sporulation, both on minimal medium and Sabouraud medium. It forms tiny, compact colonies fundamentally different from the aerial appearance of the wild type and is unable to sporulate or spread (Fig. 1, A and B), even after several weeks of incubation at 37 °C. Sporulation and growth can be slightly improved by the addition of 1.2 m sorbitol to the medium (Fig. 1, C and D). This requirement for osmotic stabilization suggests a weakened cell wall in the ΔgmtA mutant.
FIGURE 1.
Colony morphology of A. fumigatus ΔgmtA. Conidia of the A. fumigatus ΔgmtA mutant and the mother strain were plated on minimal medium (A and C) or Sabouraud medium (B and D), supplemented or not with 1.2 m sorbitol as indicated. Plates were incubated at 37 °C for 56 h.
Cell wall weakening of ΔgmtA was further indicated by time lapse recordings of swelling and germinating spores. Resting conidia were transferred to Sabouraud medium at 37 °C, and bright field microscopy pictures were captured every 15 min. Fig. 2 shows a representative germination of a ΔgmtA spore. The conidia swelled up to 8-fold the volume of swollen wild type conidia and frequently burst before or shortly after the emergence of the germ tube.
FIGURE 2.
Swelling and burst of ΔgmtA conidia. Swelling and germination of conidia were observed in bright field time lapse recordings at 37 °C in Sabouraud medium. Four representative snapshots with time indicated are depicted (bar, 10 μm).
Deletion of the A. fumigatus GDP-Man Transporter Arrests Glycosylinositolphosphorylceramide (GIPC) Biosynthesis
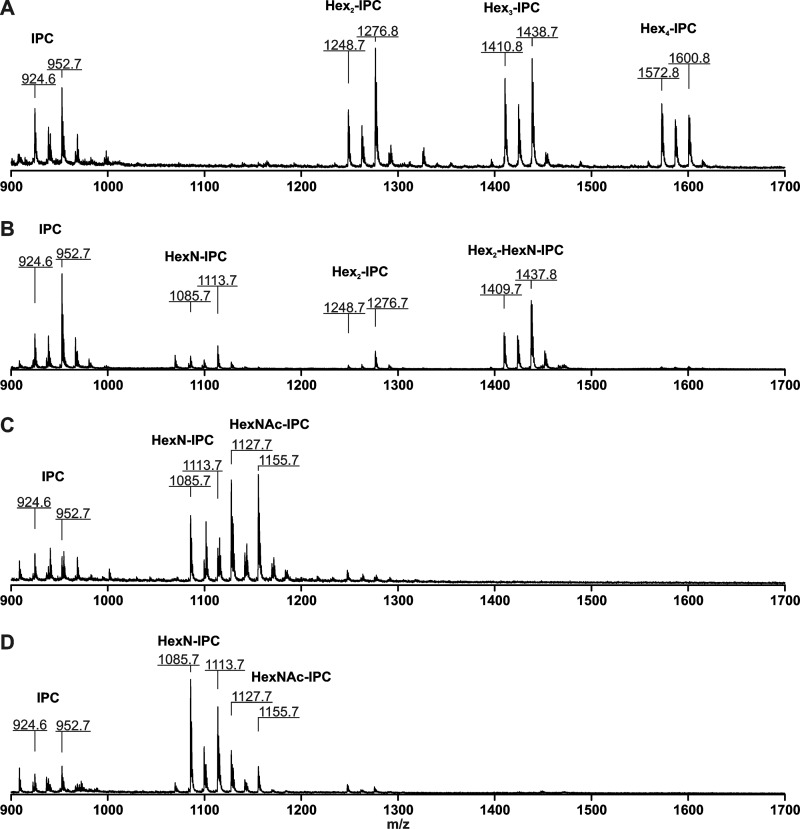
GDP-Man transporters have been localized to the Golgi apparatus in several organisms, including A. niger (26). Deletion of gmtA is thus expected to abrogate transport of GDP-Man in the Golgi apparatus and interfere with biosynthesis of glycoconjugates. GIPCs, whose structures have been comprehensively described (42, 43), have thus been analyzed by MALDI-TOF mass spectrometry. The core structure Manα1–3Manα1–2IPC (Hex2-IPC) can be modified by mannose and/or galactofuranose, yielding GIPCs with three or four hexoses (Hex3- and Hex4-IPCs). These acidic GIPCs, contained in the aqueous phase after solvent partitioning, were observed in the mother strain (Fig. 3A) but were absent from the ΔgmtA mass spectra (Fig. 3C). Similarly, the zwitterionic glycolipid Manα1–3Manα1–6GlcNα1–2IPC (Hex2-HexN-IPC) that associates with the organic phase during partitioning is only visible in the wild type strain (Fig. 3, B and D). Spectra obtained from the ΔgmtA mutant display intense peaks corresponding to the biosynthetic precursors of GIPCs: IPC, hexosamine-IPC, and N-acetylhexosamine-IPC (Fig. 3, C and D). It should be noted that in the conditions used, the desorption/ionization efficiency is much higher for acidic glycolipids than zwitterionic glycolipids. The relative intensity of HexNAc-IPC molecular ions observed in Fig. 3C reflects the properties of this glycolipid rather than its accumulation. In conclusion, deletion of the gmtA gene results in an arrest of glycolipid biosynthesis consistent with the absence of GDP-mannose in the Golgi lumen.
FIGURE 3.
Negative ion matrix-assisted laser desorption ionization spectra of GIPCs isolated from wild type and ΔgmtA mutant. GIPCs were extracted by solvent partitioning and recovered in the aqueous phase (A and C) or organic phase (B and D) from the wild type strain (A and B) or ΔgmtA mutant (C and D). Each mono- or oligosaccharide is linked to IPC containing C18:0 or C20:0 phytosphingosine conjugated to a fatty acid varying in chain length and hydroxylation state, giving rise to peak series. The masses of the main species containing monohydroxylated lignoceric acid are indicated. Hex, hexose; HexN, hexosamine; HexNAc, N-acetylhexosamine.
Biosynthesis of Galactomannan Is Blocked in A. fumigatus ΔgmtA and ΔglfB Mutants
The generation and partial characterization of an A. fumigatus mutant deficient in the UDP-Galf transporter GlfB, named ΔglfB, has been reported previously (16). To determine if galactomannan biosynthesis requires GlfB or the GDP-Man transporter GmtA, we first investigated the monosaccharide composition of alkali-insoluble cell wall fractions from the ΔgmtA, ΔglfB, and wild type strains of A. fumigatus. The complex polysaccharide network of the cell wall alkali-insoluble fraction is essentially made of four monosaccharides: glucose and N-acetylglucosamine, the monomers of β-glucan and chitin, respectively, as well as mannose and galactose, the monosaccharide units of galactomannan. Hydrolysis of the cell wall fractions was achieved using sulfuric acid (39), whereby the chitin monomer N-acetylglucosamine is deacetylated to glucosamine. The analyses revealed that the cell wall of the ΔglfB mutant lacked galactose, whereas mannose was still present (Fig. 4A). This result is consistent with the expected absence of galactofuran side chains and the presence of the underlying mannan. Other monosaccharides were essentially unchanged when compared with the parental strain, reflecting unaltered β-glucan and chitin levels in this mutant. As anticipated, the alkali-insoluble fraction of ΔgmtA was virtually devoid of mannose (Fig. 4A), indicating galactomannan deficiency in the cell wall of this mutant. Interestingly, ΔgmtA cell wall fractions contained high levels of galactose and galactosamine, although amounts were unsteady and therefore not statistically significant as compared with wild type. This might be due to increased levels of precipitated galactosaminogalactan, which is a known contaminant of the alkali-insoluble fraction (6). In addition, glucosamine was approximately twice as abundant as compared with wild type, whereas the glucose content was reduced by ∼65%. This strongly suggests alterations in β-glucan and chitin content.
FIGURE 4.
Monosaccharide composition of the cell wall of A. fumigatus wild type and ΔglfB and ΔgmtA mutants. Cell wall fragments of A. fumigatus wild type and ΔglfB and ΔgmtA mutants were extracted by hot alkali, and the monosaccharide composition of the alkali-insoluble material was determined before (A) and after solubilization by β-glucanase (B). After acid hydrolysis, monosaccharides were fluorescently labeled with anthranilic acid and separated by reverse-phase HPLC. l-Fucose was used as an internal standard for accurate quantification. Peak areas were normalized to the dry mass of cell wall material initially applied for analysis (A). After β-glucanase treatment, equal amounts of total sugar as determined by the phenol-sulfuric acid method were applied for analysis (B).
To confirm absence of galactomannan in the ΔgmtA cell wall, we also analyzed the monosaccharide composition of the fractions after β-glucanase digestion, a treatment that releases galactomannan covalently linked to β-glucan and enables a more precise quantification of its components. Mannose, but no galactose, was present in solubilized oligosaccharides from ΔglfB, whereas ΔgmtA contained neither mannose nor galactose (Fig. 4B). Finally, the presence of galactomannan was also assessed by a highly sensitive ELISA using the anti-galactofuran antibody EB-A2 (9) (Fig. 5). When using solubilized oligosaccharides from ΔgmtA, only the highest concentration of 100 μg/ml triggered a moderate signal, whereas wild type material reached saturation already at concentrations of 0.1 μg/ml. Concentrations yielding the half-maximal signal (V50) were calculated based on non-linear curve fitting (R2 > 0.98) and were ∼3000 times higher for ΔgmtA (111.7 μg/ml) than for wild type (0.039 μg/ml). Samples from ΔglfB were negative at all concentrations. The very low signal observed with fractions from the ΔgmtA mutant may reflect an extremely limited synthesis of galactomannan (less than 0.1%) due to the “leakiness” of the Golgi toward GDP-Man or be due to contamination by galactofuranosylated glycoproteins that are also recognized by the EB-A2 antibody (44). Together, these data clearly establish a galactofuran deficiency or galactomannan deficiency in the cell walls of ΔglfB or ΔgmtA, respectively.
FIGURE 5.
Detection of cell wall galactomannan from A. fumigatus wild type and ΔglfB and ΔgmtA mutants by quantitative ELISA. The alkali-insoluble cell wall fractions from A. fumigatus wild type and ΔglfB and ΔgmtA mutants were solubilized by β-glucanase digestion, and the sugar concentration was adjusted to 100 μg/ml. 10× dilutions were applied to ELISA and detected with the anti-galactofuran antibody EB-A2. Data points represent mean values of two biological replicates.
At last, we investigated the presence of membrane-bound galactomannan in the ΔgmtA mutant. Using established conditions that allowed purification of lipogalactomannan from the wild type strain, we were not able to isolate any galactomannan from the ΔgmtA mutant (Fig. 6). Thus, both membrane-bound and cell wall-bound galactomannan are essentially absent from the GDP-Man transporter-deficient strain of A. fumigatus.
FIGURE 6.
Purification of GPI-anchored galactomannan from A. fumigatus wild type and ΔgmtA mutant. GPI-anchored galactomannan was prepared from membranes of A. fumigatus wild type (diamonds) and ΔgmtA mutant (inverted triangles) and purified by hydrophobic interaction chromatography on octyl-Sepharose. The presence of galactomannan in the 2-ml fractions was investigated by ELISA using the anti-galactofuran antibody EB-A2.
DISCUSSION
The GDP-Man transporter of the protozoan parasite Leishmania donovani was one of the first nucleotide sugar transporters to be characterized (20). The authors demonstrated that the gene LPG2 is required for uptake of GDP-Man into the Golgi lumen and for synthesis of the GPI-anchored polysaccharide lipophosphoglycan. The biosynthesis of this unique polysaccharide has been partially elucidated. The GPI anchor biosynthesis is initiated in the endoplasmic reticulum and completed in the Golgi apparatus, where the polysaccharide repeating units are then assembled (45). Together with Leishmania lipophosphoglycan and Crithidia fasciculata lipoarabinogalactan (46), A. fumigatus lipogalactomannan constitutes one of the rare examples of GPI-anchored polysaccharide (8) and may also be synthesized along the secretory pathway. An absence of reactivity of the anti-galactofuran antibody EB-A2 toward cell extract of the Golgi UDP-Galf transporter-deficient mutant ΔglfB, which suggested an impaired biosynthesis of this polysaccharide, supported this assumption (16). The present data clearly establishes that the Galf side chains of galactomannan are also absent from the cell wall of this mutant. Similarly, both the GPI-linked galactomannan and cell wall-bound galactomannan are absent from an A. fumigatus GDP-Man transporter-deficient mutant named ΔgmtA. A compensatory chitin up-regulation and decreased glucan content seem to be associated with the lack of galactomannan. These cell wall alterations generated by unavailability of GDP-Man in intraluminal vesicles contrast with the limited changes in the ΔglfB cell wall composition. The latter is deficient in galactose but retains wild type levels of other monosaccharides and thus probably only lacks galactofuran. Modification of the cell wall composition of the ΔgmtA mutant might indicate that the mannan backbone of galactomannan, in contrast to the galactofuran side chains, plays an essential role in cell wall architecture. Moreover, the presence of GDP-Man in the Golgi apparatus is required for elongation of N- and O-glycans, biosynthesis of GIPCs, and “decoration” of protein GPI anchors. Therefore, the possibility that defective mannosylation in the Golgi leads to mislocalization or impaired trafficking of cell wall-remodeling enzymes cannot be excluded. Remodeling of β-glucan, for example, is mediated by extracellular GPI-anchored glucanosyltransferases of the GAS/GEL family containing several potential glycosylation sites (47, 48).
In contrast to filamentous fungi, ascomycetous yeasts express abundant cell wall mannoproteins carrying N-glycans with up to 200 mannose residues. A pool of intraluminal GDP-Man is required for their biosynthesis and may be responsible for the essentiality of the GDP-Man transporter in S. cerevisiae (21, 28) and the pathogenic yeast Candida albicans (23). Despite its very severe growth and sporulation defect, an A. fumigatus ΔgmtA mutant was obtained by spore electroporation. It represents the first A. fumigatus strain deficient in galactomannan. It is, however, difficult to ascribe the dramatic growth phenotype of the ΔgmtA mutant to a specific carbohydrate, because the absence of the transporter has pleiotropic effects on glycosylation. The addition of mannose to N-glycans may occur in the Golgi apparatus of filamentous fungi, but this maturation step seems limited and dispensable for growth (49, 50). Likewise, mannosylation of IPC is not required for growth (51). In contrast, the initiation of O-glycans biosynthesis by dolichol-phosphomannose-dependent mannosyltransferases is essential for yeast and fungal growth, but, at least in yeast, their elongation in the Golgi apparatus is dispensable (52, 53). It remains thus possible that the manifest cell wall weakening observed in the ΔgmtA mutant results from the sole absence of galactomannan and is responsible for the severe growth phenotype.
A critical question raised by the present work is the site of assembly of galactomannan. So far, fungal cell wall polysaccharides (such as glucan and chitin) have been shown to be synthesized at the cell surface by type III integral membrane synthases (54). This study demonstrates, however, that both the backbone and side chain assembly of galactomannan require nucleotide sugar transporters and thus provide indirect evidence of the involvement of the Golgi apparatus in the biosynthesis of a fungal cell wall polysaccharide. In plants, the contribution of the Golgi apparatus to the elaboration of hemicellulosic and pectic polysaccharides has long been recognized, and it has recently been demonstrated that type II glycosyltransferases can elaborate the polysaccharide backbone (55, 56). Similarly, biosynthesis of the unique polysaccharide capsule of the basiodiomycete Cryptococcus neoformans requires intraluminal GDP-mannose, and capsule material has even been observed within intracellular vesicles (25, 57, 58). The particularity of A. fumigatus galactomannan is its occurrence as membrane GPI-anchored polysaccharide, in addition to the cell wall-bound polysaccharide (8). Both forms require the GDP-Man and UDP-Galf transporters for their synthesis. These observations support a biosynthetic model according to which galactomannan is assembled on a GPI anchor in the Golgi apparatus and secreted to the plasma membrane before being cross-linked to cell wall β-glucan by extracellular transglycosidases. Transglycosidases mediating the remodeling of the cell wall β-glucan scaffold have already been characterized in A. fumigatus and S. cerevisiae (47, 48, 59, 60). Others are thought to be involved in the transfer of GPI-anchored mannoproteins to the cell wall of ascomycetous yeasts but remain elusive (1). Hitherto, very little attention has been given to GPI-anchored polysaccharides. This paradigm, however, may not be limited to Aspergillus galactomannan, and future studies will determine if it may also apply to other extracellular polysaccharides and organisms.
Acknowledgment
We thank Dr. Falk Büttner for help with mass spectrometry measurements.
This work was supported by the Deutsche Forschungsgemeinschaft.

This article contains supplemental Table S1 and Figs. S1 and S2.
- GPI
- glycosylphosphatidylinositol
- Galf
- galactofuranose
- Man
- mannose
- IPC
- inositolphosphorylceramide
- GIPC
- glycosylinositolphosphorylceramide.
REFERENCES
- 1. De Groot P. W., Ram A. F., Klis F. M. (2005) Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet. Biol. 42, 657–675 [DOI] [PubMed] [Google Scholar]
- 2. Ecker M., Deutzmann R., Lehle L., Mrsa V., Tanner W. (2006) Pir proteins of Saccharomyces cerevisiae are attached to β-1,3-glucan by a new protein-carbohydrate linkage. J. Biol. Chem. 281, 11523–11529 [DOI] [PubMed] [Google Scholar]
- 3. Bernard M., Latgé J. P. (2001) Aspergillus fumigatus cell wall. Composition and biosynthesis. Med. Mycol. 39, Suppl. 1, 9–17 [PubMed] [Google Scholar]
- 4. Kollár R., Petráková E., Ashwell G., Robbins P. W., Cabib E. (1995) Architecture of the yeast cell wall. The linkage between chitin and β(1→3)-glucan. J. Biol. Chem. 270, 1170–1178 [DOI] [PubMed] [Google Scholar]
- 5. Kollár R., Reinhold B. B., Petráková E., Yeh H. J., Ashwell G., Drgonová J., Kapteyn J. C., Klis F. M., Cabib E. (1997) Architecture of the yeast cell wall. β(1→6)-glucan interconnects mannoprotein, β(1→)3-glucan, and chitin. J. Biol. Chem. 272, 17762–17775 [DOI] [PubMed] [Google Scholar]
- 6. Fontaine T., Simenel C., Dubreucq G., Adam O., Delepierre M., Lemoine J., Vorgias C. E., Diaquin M., Latgé J. P. (2000) Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J. Biol. Chem. 275, 27594–27607 [DOI] [PubMed] [Google Scholar]
- 7. Latgé J. P., Kobayashi H., Debeaupuis J. P., Diaquin M., Sarfati J., Wieruszeski J. M., Parra E., Bouchara J. P., Fournet B. (1994) Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect. Immun. 62, 5424–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Costachel C., Coddeville B., Latgé J. P., Fontaine T. (2005) Glycosylphosphatidylinositol-anchored fungal polysaccharide in Aspergillus fumigatus. J. Biol. Chem. 280, 39835–39842 [DOI] [PubMed] [Google Scholar]
- 9. Stynen D., Goris A., Sarfati J., Latgé J. P. (1995) A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33, 497–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maertens J., Verhaegen J., Lagrou K., Van Eldere J., Boogaerts M. (2001) Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients. A prospective validation. Blood 97, 1604–1610 [DOI] [PubMed] [Google Scholar]
- 11. Tefsen B., Ram A. F., van Die I., Routier F. H. (2012) Galactofuranose in eukaryotes. Aspects of biosynthesis and functional impact. Glycobiology 22, 456–469 [DOI] [PubMed] [Google Scholar]
- 12. Shematek E. M., Braatz J. A., Cabib E. (1980) Biosynthesis of the yeast cell wall. I. Preparation and properties of β-(1→3)glucan synthetase. J. Biol. Chem. 255, 888–894 [PubMed] [Google Scholar]
- 13. Douglas C. M. (2001) Fungal β-(1,3)-d-glucan synthesis. Med. Mycol. 39, Suppl. 1, 55–66 [DOI] [PubMed] [Google Scholar]
- 14. Beauvais A., Bruneau J. M., Mol P. C., Buitrago M. J., Legrand R., Latgé J. P. (2001) Glucan synthase complex of Aspergillus fumigatus. J. Bacteriol. 183, 2273–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Douglas C. M., D'Ippolito J. A., Shei G. J., Meinz M., Onishi J., Marrinan J. A., Li W., Abruzzo G. K., Flattery A., Bartizal K., Mitchell A., Kurtz M. B. (1997) Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41, 2471–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Engel J., Schmalhorst P. S., Dörk-Bousset T., Ferrières V., Routier F. H. (2009) A single UDP-galactofuranose transporter is required for galactofuranosylation in Aspergillus fumigatus. J. Biol. Chem. 284, 33859–33868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Afroz S., El-Ganiny A. M., Sanders D. A., Kaminskyj S. G. (2011) Roles of the Aspergillus nidulans UDP-galactofuranose transporter, UgtA in hyphal morphogenesis, cell wall architecture, conidiation, and drug sensitivity. Fungal. Genet. Biol. 48, 896–903 [DOI] [PubMed] [Google Scholar]
- 18. Gerardy-Schahn R., Oelmann S., Bakker H. (2001) Nucleotide sugar transporters. Biological and functional aspects. Biochimie 83, 775–782 [DOI] [PubMed] [Google Scholar]
- 19. Handford M., Rodriguez-Furlán C., Orellana A. (2006) Nucleotide-sugar transporters. Structure, function and roles in vivo. Braz. J. Med. Biol. Res. 39, 1149–1158 [DOI] [PubMed] [Google Scholar]
- 20. Ma D., Russell D. G., Beverley S. M., Turco S. J. (1997) Golgi GDP-mannose uptake requires Leishmania LPG2. A member of a eukaryotic family of putative nucleotide-sugar transporters. J. Biol. Chem. 272, 3799–3805 [PubMed] [Google Scholar]
- 21. Dean N., Zhang Y. B., Poster J. B. (1997) The VRG4 gene is required for GDP-mannose transport into the lumen of the Golgi in the yeast, Saccharomyces cerevisiae. J. Biol. Chem. 272, 31908–31914 [DOI] [PubMed] [Google Scholar]
- 22. Baldwin T. C., Handford M. G., Yuseff M. I., Orellana A., Dupree P. (2001) Identification and characterization of GONST1, a Golgi-localized GDP-mannose transporter in Arabidopsis. Plant Cell 13, 2283–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishikawa A., Poster J. B., Jigami Y., Dean N. (2002) Molecular and phenotypic analysis of CaVRG4, encoding an essential Golgi apparatus GDP-mannose transporter. J. Bacteriol. 184, 29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arakawa K., Abe M., Noda Y., Adachi H., Yoda K. (2006) Molecular cloning and characterization of a Pichia pastoris ortholog of the yeast Golgi GDP-mannose transporter gene. J. Gen. Appl. Microbiol. 52, 137–145 [DOI] [PubMed] [Google Scholar]
- 25. Cottrell T. R., Griffith C. L., Liu H., Nenninger A. A., Doering T. L. (2007) The pathogenic fungus Cryptococcus neoformans expresses two functional GDP-mannose transporters with distinct expression patterns and roles in capsule synthesis. Eukaryot. Cell 6, 776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carvalho N. D., Arentshorst M., Weenink X. O., Punt P. J., van den Hondel C. A., Ram A. F. (2011) Functional YFP-tagging of the essential GDP-mannose transporter reveals an important role for the secretion related small GTPase SrgC protein in maintenance of Golgi bodies in Aspergillus niger. Fungal Biol. 115, 253–264 [DOI] [PubMed] [Google Scholar]
- 27. Jackson-Hayes L., Hill T. W., Loprete D. M., Fay L. M., Gordon B. S., Nkashama S. A., Patel R. K., Sartain C. V. (2008) Two GDP-mannose transporters contribute to hyphal form and cell wall integrity in Aspergillus nidulans. Microbiology 154, 2037–2047 [DOI] [PubMed] [Google Scholar]
- 28. Dean N. (1999) Asparagine-linked glycosylation in the yeast Golgi. Biochim. Biophys. Acta 1426, 309–322 [DOI] [PubMed] [Google Scholar]
- 29. Goto M. (2007) Protein O-glycosylation in fungi. Diverse structures and multiple functions. Biosci. Biotechnol. Biochem. 71, 1415–1427 [DOI] [PubMed] [Google Scholar]
- 30. Deshpande N., Wilkins M. R., Packer N., Nevalainen H. (2008) Protein glycosylation pathways in filamentous fungi. Glycobiology 18, 626–637 [DOI] [PubMed] [Google Scholar]
- 31. Takahashi H. K., Toledo M. S., Suzuki E., Tagliari L., Straus A. H. (2009) Current relevance of fungal and trypanosomatid glycolipids and sphingolipids. Studies defining structures conspicuously absent in mammals. An. Acad. Bras. Cienc. 81, 477–488 [DOI] [PubMed] [Google Scholar]
- 32. Fankhauser C., Homans S. W., Thomas-Oates J. E., McConville M. J., Desponds C., Conzelmann A., Ferguson M. A. (1993) Structures of glycosylphosphatidylinositol membrane anchors from Saccharomyces cerevisiae. J. Biol. Chem. 268, 26365–26374 [PubMed] [Google Scholar]
- 33. Fontaine T., Magnin T., Melhert A., Lamont D., Latge J. P., Ferguson M. A. (2003) Structures of the glycosylphosphatidylinositol membrane anchors from Aspergillus fumigatus membrane proteins. Glycobiology 13, 169–177 [DOI] [PubMed] [Google Scholar]
- 34. Krappmann S., Sasse C., Braus G. H. (2006) Gene targeting in Aspergillus fumigatus by homologous recombination is facilitated in a nonhomologous end- joining-deficient genetic background. Eukaryot. Cell 5, 212–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krappmann S., Bayram O., Braus G. H. (2005) Deletion and allelic exchange of the Aspergillus fumigatus veA locus via a novel recyclable marker module. Eukaryot. Cell 4, 1298–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weidner G., d'Enfert C., Koch A., Mol P. C., Brakhage A. A. (1998) Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5′-monophosphate decarboxylase. Curr. Genet. 33, 378–385 [DOI] [PubMed] [Google Scholar]
- 37. Schmalhorst P. S., Krappmann S., Vervecken W., Rohde M., Müller M., Braus G. H., Contreras R., Braun A., Bakker H., Routier F. H. (2008) Contribution of galactofuranose to the virulence of the opportunistic pathogen Aspergillus fumigatus. Eukaryot. Cell 7, 1268–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krakow J. L., Hereld D., Bangs J. D., Hart G. W., Englund P. T. (1986) Identification of a glycolipid precursor of the Trypanosoma brucei variant surface glycoprotein. J. Biol. Chem. 261, 12147–12153 [PubMed] [Google Scholar]
- 39. François J. M. (2006) A simple method for quantitative determination of polysaccharides in fungal cell walls. Nat. Protoc. 1, 2995–3000 [DOI] [PubMed] [Google Scholar]
- 40. Dubois M., Gilles K., Hamilton J. K., Rebers P. A., Smith F. (1951) A colorimetric method for the determination of sugars. Nature 168, 167. [DOI] [PubMed] [Google Scholar]
- 41. Stepan H., Staudacher E. (2011) Optimization of monosaccharide determination using anthranilic acid and 1-phenyl-3-methyl-5-pyrazolone for gastropod analysis. Anal. Biochem. 418, 24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toledo M. S., Levery S. B., Bennion B., Guimaraes L. L., Castle S. A., Lindsey R., Momany M., Park C., Straus A. H., Takahashi H. K. (2007) Analysis of glycosylinositol phosphorylceramides expressed by the opportunistic mycopathogen Aspergillus fumigatus. J. Lipid Res. 48, 1801–1824 [DOI] [PubMed] [Google Scholar]
- 43. Simenel C., Coddeville B., Delepierre M., Latgé J. P., Fontaine T. (2008) Glycosylinositolphosphoceramides in Aspergillus fumigatus. Glycobiology 18, 84–96 [DOI] [PubMed] [Google Scholar]
- 44. Morelle W., Bernard M., Debeaupuis J. P., Buitrago M., Tabouret M., Latgé J. P. (2005) Galactomannoproteins of Aspergillus fumigatus. Eukaryot. Cell 4, 1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McConville M. J., Mullin K. A., Ilgoutz S. C., Teasdale R. D. (2002) Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev. 66, 122–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schneider P., Treumann A., Milne K. G., McConville M. J., Zitzmann N., Ferguson M. A. (1996) Structural studies on a lipoarabinogalactan of Crithidia fasciculata. Biochem. J. 313, 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Popolo L., Vai M. (1999) The Gas1 glycoprotein, a putative wall polymer cross-linker. Biochim. Biophys. Acta 1426, 385–400 [DOI] [PubMed] [Google Scholar]
- 48. Mouyna I., Fontaine T., Vai M., Monod M., Fonzi W. A., Diaquin M., Popolo L., Hartland R. P., Latgé J. P. (2000) Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J. Biol. Chem. 275, 14882–14889 [DOI] [PubMed] [Google Scholar]
- 49. Wallis G. L., Easton R. L., Jolly K., Hemming F. W., Peberdy J. F. (2001) Galactofuranoic-oligomannose N-linked glycans of α-galactosidase A from Aspergillus niger. Eur. J. Biochem. 268, 4134–4143 [DOI] [PubMed] [Google Scholar]
- 50. Kotz A., Wagener J., Engel J., Routier F. H., Echtenacher B., Jacobsen I., Heesemann J., Ebel F. (2010) Approaching the secrets of N-glycosylation in Aspergillus fumigatus. Characterization of the AfOch1 protein. PLoS One 5, e15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kotz A., Wagener J., Engel J., Routier F., Echtenacher B., Pich A., Rohde M., Hoffmann P., Heesemann J., Ebel F. (2010) The mitA gene of Aspergillus fumigatus is required for mannosylation of inositol-phosphorylceramide, but is dispensable for pathogenicity. Fungal Genet. Biol. 47, 169–178 [DOI] [PubMed] [Google Scholar]
- 52. Mouyna I., Kniemeyer O., Jank T., Loussert C., Mellado E., Aimanianda V., Beauvais A., Wartenberg D., Sarfati J., Bayry J., Prévost M. C., Brakhage A. A., Strahl S., Huerre M., Latgé J. P. (2010) Members of protein O-mannosyltransferase family in Aspergillus fumigatus differentially affect growth, morphogenesis and viability. Mol. Microbiol. 76, 1205–1221 [DOI] [PubMed] [Google Scholar]
- 53. Lussier M., Sdicu A. M., Bussereau F., Jacquet M., Bussey H. (1997) The Ktr1p, Ktr3p, and Kre2p/Mnt1p mannosyltransferases participate in the elaboration of yeast O- and N-linked carbohydrate chains. J. Biol. Chem. 272, 15527–15531 [DOI] [PubMed] [Google Scholar]
- 54. Roncero C. (2002) The genetic complexity of chitin synthesis in fungi. Curr. Genet. 41, 367–378 [DOI] [PubMed] [Google Scholar]
- 55. Sandhu A. P., Randhawa G. S., Dhugga K. S. (2009) Plant cell wall matrix polysaccharide biosynthesis. Mol. Plant 2, 840–850 [DOI] [PubMed] [Google Scholar]
- 56. Atmodjo M. A., Sakuragi Y., Zhu X., Burrell A. J., Mohanty S. S., Atwood J. A., 3rd, Orlando R., Scheller H. V., Mohnen D. (2011) Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan:galacturonosyltransferase complex. Proc. Natl. Acad. Sci. U.S.A. 108, 20225–20230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yoneda A., Doering T. L. (2006) A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol. Biol. Cell 17, 5131–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Doering T. L. (2009) How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu. Rev. Microbiol. 63, 223–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mouyna I., Morelle W., Vai M., Monod M., Léchenne B., Fontaine T., Beauvais A., Sarfati J., Prévost M. C., Henry C., Latgé J. P. (2005) Deletion of GEL2 encoding for a β(1–3)glucanosyltransferase affects morphogenesis and virulence in Aspergillus fumigatus. Mol. Microbiol. 56, 1675–1688 [DOI] [PubMed] [Google Scholar]
- 60. Gastebois A., Mouyna I., Simenel C., Clavaud C., Coddeville B., Delepierre M., Latgé J. P., Fontaine T. (2010) Characterization of a new β(1–3)-glucan branching activity of Aspergillus fumigatus. J. Biol. Chem. 285, 2386–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]