Background: Aberrant glycosylation is a common feature of breast cancer.
Results: Cyclooxygenase-2 (COX-2), an enzyme associated with tumorigenesis in breast cancer, up-regulated the sialyltransferase ST3Gal-I.
Conclusion: COX-2 can regulate the expression of ST3Gal-I in breast cancer
Significance: Some of the tumorigenic properties associated with COX-2 may be the result of up-regulating ST3Gal-I that can alter the O-linked glycosylation of tumors.
Keywords: Breast Cancer, Cyclooxygenase (COX) Pathway, Gene Expression, Glycosylation, Sialyltransferase, Control of Transcription, ST3Gal-I
Abstract
Aberrant glycosylation is a common feature of malignant change. Changes in mucin-type O-linked glycosylation in breast cancer can result in the expression of truncated core 1-based sialylated glycans rather than the core 2-based glycans observed in normal mammary epithelium cells. This has been shown, in part, to be due to changes in the expression of glycosyltransferases, including the up-regulation of some sialyltransferases. Using the breast cancer cell line T47D, we have shown that PGE2, one of the final products of the cyclooxygenase-2 (COX-2) pathway, can induce the mRNA expression of the sialyltransferase α-2,3-sialyltransferase-3 (ST3Gal-I), resulting in increased sialyltransferase activity, demonstrated by a reduction in PNA lectin staining. Induction of COX-2 in the MDA-MB-231 breast cancer cell line also results in the increased expression of ST3Gal-I, leading to increased sialylation of the substrate of ST3Gal-I, core 1 Galβ1,3GalNAc. This effect on sialylation could be reversed by the selective COX-2 inhibitor celecoxib. The use of siRNA to knock down COX-2 and overexpression of COX-2 in MDA-MD-231 cells confirmed the involvement of COX-2 in the up-regulation of ST3Gal-I. Moreover, analysis of the expression of ST3Gal-I and COX-2 by 74 primary breast cancers showed a significant correlation between the two enzymes. COX-2 expression has been associated with a number of tumors, including breast cancer, where its expression is associated with poor prognoses. Thus, these results suggest the intriguing possibility that some of the malignant characteristics associated with COX-2 expression may be via the influence that COX-2 exerts on the glycosylation of tumor cells.
Introduction
Glycosylation is one of the most frequent forms of posttranslation modifications and is essential for many protein and cellular functions. Changes in the composition of glycans added to glycoproteins and glycolipids are common events in malignancy (1, 2), and these changes can affect the course of the disease (3–5). Dramatic changes occur in glycans attached to serine and threonines in mucin-type O-linkage, and this can be attributed, at least in part, to changes in the expression of key glycosyltransferases involved in the synthesis of O-glycans (2, 6).
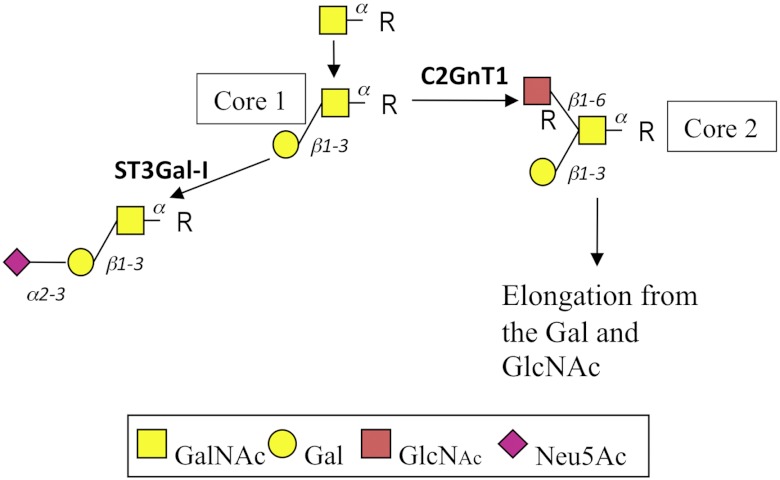
O-linked glycosylation has been extensively studied in breast cancer, where a number of tumor-associated glycans are observed (2, 7). One change involves the expression of sialylated core 1 (NeuAcα2,3Galβ13GalNAc) chains that are barely expressed by normal mammary epithelial cells. The presence of sialylated core 1 chains has been attributed to the overexpression of the sialyltransferase ST3Gal-I3 by breast cancer compared with normal mammary epithelial cells, which was originally shown by us (6) and has since been confirmed by others (8). Increased expression of ST3Gal-I is sometimes accompanied by the down-regulation of the glycosyltransferase C2GnT1, which competes for the same substrate as ST3Gal-I (9) and is responsible for the initiation of core 2 chains in the mammary gland (Fig. 1). Thus, in the normal mammary gland, the mucin-type O-linked glycans are core 2-based, whereas in breast cancer, sialylated core 1 glycans are found (Fig. 1). We recently demonstrated the importance of ST3Gal-I in mammary tumor development using a murine model. In this model, spontaneous Polyoma middle T (PyMT)-induced mammary tumors developed earlier when human ST3Gal-I was expressed as a transgene driven by the MUC1 promoter to ensure expression of the sialyltransferase in the mammary gland (4).
FIGURE 1.
Simplified pathway of the initial stages of mucin-type O-linked glycosylation in mammary cells. Core 1 (Galβ1,3GalNAc) is converted to core 2 (Galβ1,3[GlcNAcβ1,6]GalNAc) by the action of C2GnT1, which can then be extended, as seen in the normal mammary gland, or to sialyl core 1 by the action of ST3Gal-I, as observed in breast cancer. R, serine or threonine residues of the protein backbone.
Although sialylated core 1 glycans are not found attached to mucins in the normal mammary gland, they are found on proteins expressed by leukocytes. Moreover, switching from core 1- to core 2-based glycans is often observed at different stages of cell activation. Thus, resting T cells carry sialylated core 1-based glycans but, upon activation, switch to expressing core 2-based glycans (10, 11). Previous work by ourselves and others (12, 13) has shown that dendritic cells change their O-linked glycosylation when induced to mature in vitro. Immature dendritic cells carry core 2 glycans, but upon maturation, stimuli down-regulate C2GnT1 and up-regulate ST3Gal-I, resulting in a change from core 2- to core 1-based glycans (12). One method used for the maturation of dendritic cells in vitro is the addition of tumor necrosis factor α and PGE2 (14, 15), and we demonstrated that PGE2 alone was sufficient for the rapid down-regulation of C2GnT1 and up-regulation of ST3Gal-I (12).
PGE2 is the principle metabolic product of the enzyme cyclooxygenase-2 (COX-2), and dysregulation of COX-2 expression results in increased amounts of PGE2. Aberrant expression of COX-2 occurs in cancers, and its association with tumorigenesis is particularly strong for colon and breast cancer (16, 17). Indeed, elevated levels of COX-2 in breast cancers are associated with a poor prognosis and ductal carcinoma in situ recurrence (18). Studies show that COX-2 is involved in breast cancer cell proliferation, migration, and invasion (19, 21), and when the enzyme is knocked out, mice develop fewer mammary tumors (22), whereas overexpression of COX-2 in the mammary gland induces tumors (23). Moreover, although sometimes contradictory, epidemiological evidence suggests a correlation of intake of non-steroidal anti-inflammatory drugs (inhibitors of the COX enzymes) with decreased risk of breast cancer (24). A phase III clinical trial is now underway to assess the adjuvant benefit of a selective COX-2 inhibitor, celecoxib, for the treatment of primary breast cancer (25).
Given these data, we investigated the role of COX-2 in the control of the expression of the sialyltransferase ST3Gal-I in breast cancer. Here we show that the expression of ST3Gal-I can be up-regulated by COX-2 via PGE2 in both estrogen receptor α-positive and -negative cell lines. Moreover, we show that the expression of the two enzymes is positively correlated in primary breast cancers. To our knowledge, this is the first report of the control of ST3Gal-I expression in breast cancer.
EXPERIMENTAL PROCEDURES
Cell Line and Cell Culture
The breast cancer cell lines MDA-MB-231 and T47D were routinely grown in DMEM (Lonza) supplemented with 10% FCS (Invitrogen), penicillin (100 μg/ml), and streptomycin (100 UI/ml). For experimental procedures, cells were detached from culture flasks using EDTA (2 mm) in sterile phosphate-buffered saline.
RNA Extraction, Reverse Transcription, and Quantitative Real-time PCR (qRT-PCR)
Cell samples were snap-frozen using RNA Later reagent (Qiagen). Total RNA was extracted from cells using the Nucleospin RNA II kit (Macherey Nagel) according to the instructions of the manufacturer. RNA from primary breast cancers was prepared as described in Julien et al. (26). The tumors were obtained from the Guy's and St. Thomas' Research Breast Tissue Bank. DNased RNA (1–2 μg) was reverse-transcribed using random hexamer primers and Superscript III reverse transcriptase (Invitrogen). qRT-PCR was performed using the Opticon qRT-PCR analysis system (MJ Research), a hot-start PCR that contained the double strand-specific DNA-binding dye SYBR Green I (Sigma-Aldrich), and 10 pm of the forward and reverse primers (see below). After 5 min at 95 °C, 40 cycles were performed: 15 s of denaturation at 94 °C, 30 s of annealing at 60 °C, 30 s of extension at 72 °C, and fluorescence detection at 78 °C. A melting curve fluorescence analysis was performed on each sample once the amplification cycles were completed to verify that a single product had been amplified. Each sample was normalized to the housekeeping gene PUM1 (homolog of Pumilio, Drosophila) (27). The fold difference was calculated by subtracting the Ct of the test sample from the control sample to give ΔΔCt and then the fold difference 2−ΔΔCt. The following primers were used: PUM1, 5′GATTATTCAGGCACGCAGGT 3′ (forward) and 5′AGCAGCGCTGATGATGTATG3′ (reverse); COX-2, 5′TGAGCATCTACGGTTTGCTG3′ (forward) and 5′TGCTTGTCTGGAACAACTGC3′ (reverse); ST3Gal-I, 5′ATGAGAGGTTCAACCAGACC3′ (forward) and 5′ATGGTGTCATTCAAGTTATTGG3′ (reverse); and C2GnT1, 5′AGAAGGATACACAAAACGTACC3′ (forward) and 5′ACCTTTCTAGCTAACTGTGCTC3′ (reverse).
Electrophoresis and Western Blotting
Cell pellets (10–20 × 106 cells) were lysed in 150 mm sodium chloride, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mm Tris (pH 8.0). The protein concentration was measured by Pierce BCA protein assay (Thermo Scientific). 15 μg of proteins was fractionated by size on SDS 7.5% polyacrylamide gels, and proteins were transferred onto nitrocellulose sheet paper (Pall Corp.) in 1× SDS transfer buffer. Nitrocellulose membranes were blocked in blocking buffer (PBS, 0.1% Tween 20, and 5% skimmed powder milk) and incubated with polyclonal rabbit anti-human prostaglandin receptor 1,2,3, or 4 antibodies purchased from Cayman Chemicals (Ann Arbor, MI). Mouse anti-β-actin monoclonal antibody (Sigma Aldrich) was used to identify beta actin as a loading control. The membrane was then washed three times for 10 min in wash buffer, and an HRP-conjugated secondary antibody was added at the appropriate concentration. The protein bands were visualized with an ECL system (Amersham Biosciences) according to the instructions of the manufacturer.
Flow Cytometry Analysis
Cell monolayers were detached using 5 mmol/liter EDTA in PBS. When necessary, cells were treated with 500 mU of type V neuraminidase from Clostridium perfringens (Sigma-Aldrich) in serum-free medium for 1 h at 37 °C. Cells (5 × 105 cells) were stained with PNA (peanut agglutinin) for 1 h. After three washes, samples were incubated with FITC streptavidin antibody (BD Biosciences) for 30 min on ice in PBS containing 0.5% BSA. Cells were then fixed in 1% formaldehyde and analyzed by flow cytometry using a Coulter EPICS XL cytometer with Expo32 ADC software or a FACScalibur cytometer with Cellquest Pro software.
Transient Transfections
MDA-MB-231 cells were transiently cotransfected with a pECFP-C1 plasmid containing GFP cDNA (Clontech) and the COX-2 plasmid (pCMV6-AC, purchased from Origene) or with the same empty vector (pCMV6-AC without the insert mock-transfected cells). Briefly, cells were seeded in T80 flasks (LAB-TEK Nalge Nunc International) and cultured in standard conditions until they reached 60–70% of confluence. They were transfected with 9 μg of pECF-C1 plasmid and 9 μg pCMV6-AC using Lipofectamine LTX (Invitrogen). After transfection, cells were cultured in FCS-containing fresh medium, and after 72 h cells were harvested and gene expression-analyzed by RT-qPCR.
COX-2 siRNA Transfection
The siRNA COX-2 oligos were as described in Stasinopoulos et al. (20): Sense, AACAUUCCCUUCCUUCGAAAU and antisense, UUUUGUAAGGGAAGGAAGCUUUA; sense, AACUGCUCAACACCGGAAUUUUU and antisense, UUUUGACGAGUUGUGGCCUUAAAAA. MDA-MB-231 (1 × 106) were seeded in 6-well plates 18 h before treatment in complete DMEM without penicillin and streptomycin. Cells were transfected with COX-2 siRNA using transfection reagent as suggested by the manufacturer (Santa Cruz Biotechnology). 24 h after transfection, cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA) for 18 h.
Statistical Analysis
Statistical analysis was carried out using Graphpad software using the Student's t test calculator. A value of p < 0.05 was considered significant. For the correlation of ST3Gal-I and COX-2 expression in primary breast cancers, Spearman's rank correlation and Pearson's correlation tests were applied.
RESULTS
PGE2 Can Induce the Expression of ST3Gal-I in the Breast Cancer Cell Line T47D
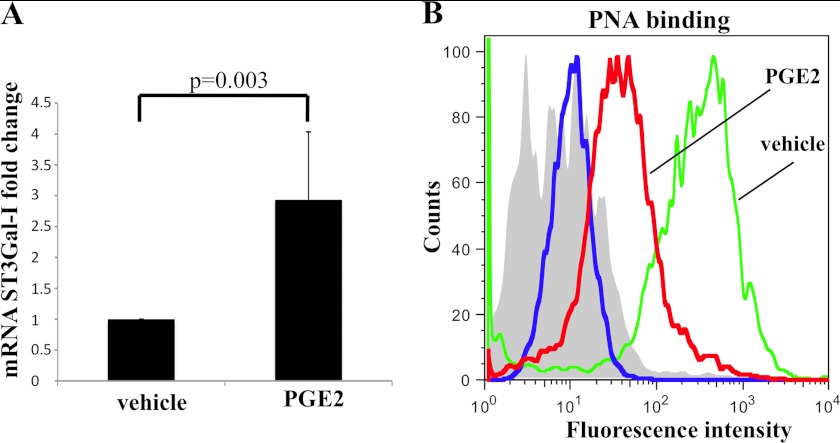
The estrogen receptor-positive breast cancer cell line T47D expresses the ST3Gal-I enzyme but not C2GnT1 (9), and so the glycans attached to proteins that carry mucin-type O-linked glycans in this cell line are core 1-based (Fig. 1), many being sialylated core 1 (28). To determine whether ST3Gal-I expression can be induced in T47D by PGE2, the cells were incubated with exogenous PGE2 (0.1 μm) for 4 h, and the expression of ST3Gal-I was measured by qRT-PCR. Fig. 2A shows that exogenous PGE2 could increase the expression of ST3Gal-I RNA at least 3-fold. To examine whether this increase in ST3Gal-I RNA expression translated to increased protein expression and so increased enzyme activity, the presence of ST3Gal-I substrate (core 1, Galβ1,3GalNAc) was measured by PNA binding. PNA is a lectin that binds core 1, but the binding is inhibited when sialic acid is added in an α 2,3 linkage to the galactose. As can be seen in Fig. 2B, the binding of PNA to the surface of T47D cells was decreased when the cells were incubated with PGE2. These data indicate that PGE2 induction of ST3Gal-I mRNA leads to an increase in ST3Gal-I activity.
FIGURE 2.
PGE2 can induce the expression and activity of ST3Gal-I in the breast cancer cell line T47D. T47D cells were incubated with 100 nm PGE2 or vehicle. A, after 4 h, cells were taken for RNA extraction, and mRNA for ST3Gal-I was measured by RT-qPCR. The results shown are the average of three independent biological experiments. B, after 72 h of incubation with PGE2, cells were analyzed by flow cytometry for PNA lectin staining. Green line, cells incubated with vehicle; red line, cells incubated with 0.1 μm PGE2; blue line and filled gray area, cells incubated with PGE2 or vehicle, respectively, but not incubated with PNA. The results show one representative of two independent biological experiments.
TPA Can Induce ST3Gal-I mRNA Expression and Reduce C2GnT1 mRNA
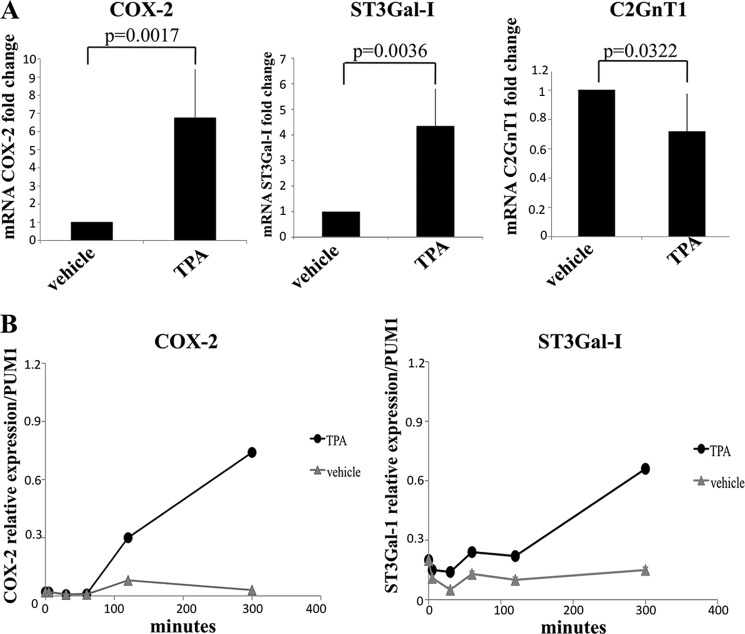
COX-2 expression in breast cancer has been associated with both ER+ and ER- breast cancers (17, 30). Therefore, to extend this observation to breast cancers of different phenotypes, we used the estrogen receptor-negative breast cancer cell line MDA-MB-231. This cell line also has the advantage that it expresses the core 2-initiating enzyme C2GnT1, which competes with ST3Gal-I for the same substrate. However, in MDA-MB-231 cells, the addition of exogenous PGE2 did not affect the expression of ST3Gal-I or C2GnT1 (data not shown). Analysis of the expression by MDA-MB-231 and T47D of the two PGE2 receptors most associated with carcinogenesis, EP2 and EP4 (30, 32), showed that although T47D expressed EP2 and EP4, MDA-MB-231 expressed the EP4 receptor but extremely low levels of EP2 (data not shown). EP2 and EP4 differ in their sensitivity to PGE2. EP4 is desensitized after short-term exposure, and this, coupled with the rapid metabolism of PGE2 (33, 34), may explain the lack of response to exogenous PGE2 of MDA-MB-231 cells. We therefore investigated the effect of an inducer of COX-2 on this cell line. MDA-MB-231 was incubated with the phorbol ester TPA, which, among its many effects, has been shown to induce COX-2 expression (35). qRT-PCR showed an increase in COX-2 mRNA after 18-h exposure to TPA (Fig. 3A). In parallel, ST3Gal-I expression was increased and C2GnT1 expression decreased (Fig. 3A). Fig. 3B shows a time course of treatment of MDA-MB-231 with 10 nm TPA. COX-2 mRNA expression started to rise between 50 and 120 min after the addition of TPA, whereas ST3Gal-I mRNA expression did not start to increase until some time after 120 min following the addition of TPA. The data from the 120-min time point is in agreement with the induction of ST3Gal-I mRNA being indirect through COX-2.
FIGURE 3.
TPA induces the expression of COX-2 and ST3Gal-I in MDA-MB-231 cells. A, MDA-MB-231 cells were incubated with vehicle or 10 nm TPA for 18 h. mRNA was extracted, and the expression of COX-2, ST3Gal-I, and C2GnT1 was determined by qRT-PCR. Results shown are the average of four independent biological experiments. B, time course of COX-2 (left panel) and ST3Gal-I (right panel) mRNA induction after incubation with TPA for the times indicated.
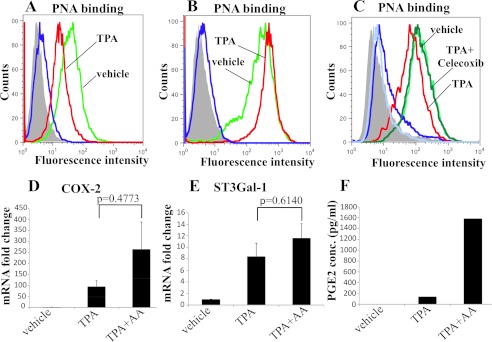
Analysis of PNA binding to the cells 72 h after TPA stimulation showed a reduction in PNA binding to TPA-treated MDA-MB-231 cells compared with vehicle-treated, indicating an increase in sialylation of core 1 (Fig. 4A). To confirm that it was sialic acid inhibiting the binding of PNA, cells were treated with neuraminidase. Fig. 4B shows an increased binding to vehicle-incubated cells because of the removal of the basal level of sialic acid on core 1 glycans. As expected, the binding of PNA to TPA-treated cells now overlaps with the vehicle-incubated cells. This confirms that the decrease in PNA binding observed upon TPA treatment was due to sialic acid masking of core 1 and not due to a decrease in the pool of core 1-based structures.
FIGURE 4.
TPA increases ST3Gal-I activity through COX-2. MDA-MB-231 cells were incubated with 10 nm TPA for 72 h and then analyzed for PNA binding by flow cytometry. A, PNA binding to MDA-MB-231 after incubation with TPA or vehicle. B, cells were treated with neuraminidase for 30 min at 37 °C before analyzing for PNA binding. C, cells were treated with TPA in the presence or absence of the COX-2 inhibitor celecoxib and then analyzed for PNA binding. Green line, cells incubated with vehicle; red lines, cells incubated with TPA; pale green lines, cells incubated with TPA and celecoxib; blue lines and dark gray filled areas are control cells incubated without PNA. D–F, MDA-MB-231 were incubated with TPA and 10 μm arachidonic acid for 18 hours after which the expression of COX-2 (D) and the expression of ST3Gal-I (E) were determined by qRT-PCR. F, the secretion of PGE2 was measured by ELISA.
To determine whether the increase in ST3Gal-I activity mediated by TPA is working through COX-2, the cells were incubated with the specific inhibitor of COX-2, celecoxib, during the stimulation with TPA. Fig. 4C shows again the decreased binding obtained with PNA when the cells were treated with TPA, but incubation with TPA in the presence of celecoxib returned the PNA binding to that observed with vehicle alone, strongly suggesting that TPA was increasing ST3Gal-I mRNA and activity via COX-2 induction.
COX-2 Can Induce the Expression of ST3Gal-I
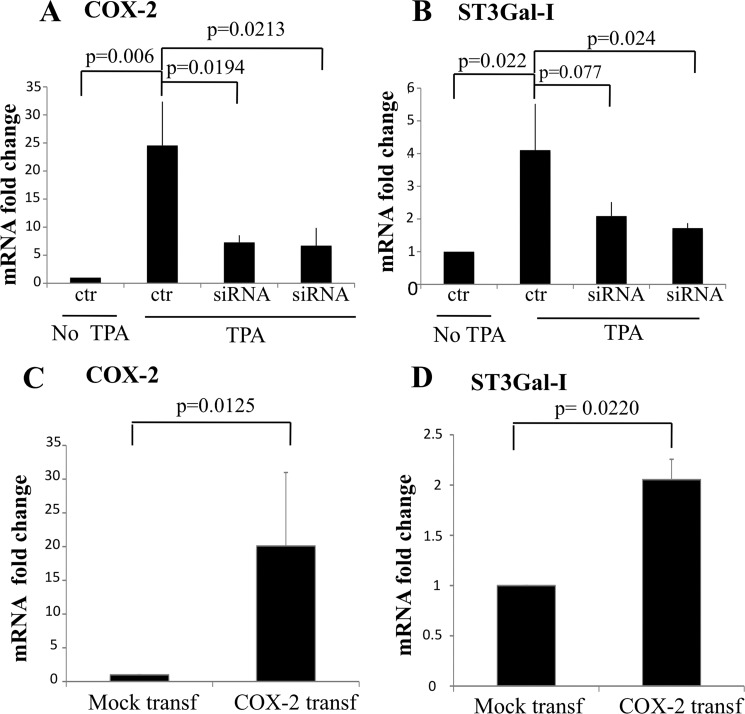
To further confirm that COX-2 was inducing the expression of ST3Gal-I, COX-2 was silenced by siRNA in MDA-MD-231 cells during incubation with TPA. Fig. 5A shows that COX-2 expression could be inhibited by 75% by two siRNAs and that this resulted in a 50% inhibition of ST3Gal-I mRNA expression (B). Moreover, overexpression of COX-2 in MDA-MB-231 by transfection of COX-2 cDNA resulted in increased COX-2 expression and increase ST3Gal-I expression (Fig. 5, C and D).
FIGURE 5.
COX-2 induces the increase expression of ST3Gal-I mRNA. A and B, MDA-MB-231 cells were incubated with TPA after the COX-2 expression was knocked down by siRNA. A, COX-2 mRNA expression was determined by qRT-PCR. B, ST3Gal-I expression was determined by qRT-PCR. C and D, COX-2 expression was increased in MDA-MB-231 cells by transfection of COX-2 cDNA. C, COX-2 mRNA expression was determined by qRT-PCR. D, ST3Gal-I expression was determined by qRT-PCR.
Correlation of COX-2 with ST3Gal-I Expression in Breast Cancers
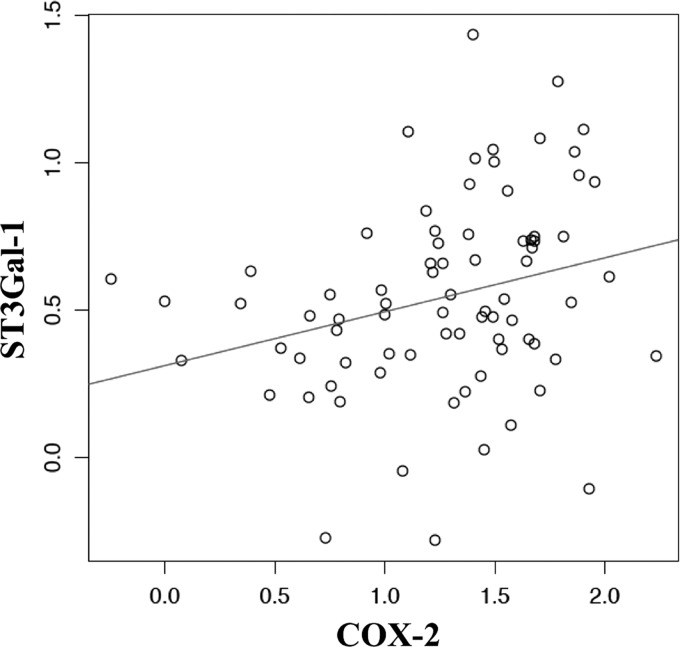
To investigate the clinical significance of our findings, we determined the expression of COX-2 and ST3Gal-I mRNA by RT-PCR in 74 primary breast cancers obtained from the Guy's and St. Thomas' Research Breast Tissue Bank. Fig. 6 shows a plot of the results and the line drawn from the least squares regression to graphically represent the correlation. We used the Pearson's product-moment correlation coefficient to measure the significance of the correlation, which gave a coefficient of 0.274 with a p value of 0.015. We also applied the Spearman's rank correlation test to the data, which gave a correlation of 0.309 with a p value of 0.00593. Thus, both tests confirmed the presence of a moderate but statistically significant correlation in the expression of the two enzymes.
FIGURE 6.
The expression of COX-2 and ST3Gal-I are correlated in primary breast cancers. RNA was extracted from 74 primary breast cancers, and the expression of COX-2 and ST3Gal-I was determined by RT-PCR. The -log of the ΔCt was plotted, and the line was drawn from the least squares regression. To measure the significance of the correlation of COX-2 and ST3Gal-I expression, the Pearson's correlation coefficient and Spearman's rank correlation was used. The former gave a correlation coefficient of 0.274, p = 0.0150. The Spearman's test gave a correlation 0.309, p = 0.00593.
DISCUSSION
Increased expression, relative to normal tissue, of ST3Gal-I is observed in many carcinomas, especially colon (36), bladder (37), and breast cancer (6, 8). This suggests that the transcriptional regulation of this sialyltransferase is altered during malignant transformation. Here we have shown that in two breast cancer cell lines, transcription of ST3Gal-I is increased by COX-2 or its product, PGE2. This increase in mRNA expression leads to increased activity of ST3Gal-I, as demonstrated by decreased binding of the lectin PNA, which binds to the substrate of ST3Gal-I (Galβ1,3GalNAc, also known as the Thomsen–Friedenreich (TF) antigen). Moreover, we have shown that there is a significant correlation between COX-2 expression and ST3Gal-I expression in primary breast cancers, indicating that our findings have clinical significance.
Nearly all proteins that are expressed on the cell membrane or are secreted carry glycans that are involved in cell adhesion, recognition, molecular trafficking, clearance, and signaling (38). Aberrant glycosylation occurs in essentially all types of human cancer and appears to be an early event as well as playing a key role in the induction of invasion and metastases (1–5, 26). Changes in glycosylation that occur in cancer can also alter molecular interactions with the immune system (39) and receptor signaling. Thus, increased expression of ST3Gal-I, which can result in the expression of truncated sialylated glycans, could play an important role in the promotion and progression of cancer. In support of this, we have shown previously that overexpressing ST3Gal-I in the mammary gland can promote the early development of spontaneous mammary tumors in a murine model (4).
Until now, very little was known about the control of expression of ST3Gal-I in breast cancer cells. Analysis of the promoter region of ST3Gal-I has identified binding sites for the ubiquitous transcription factors SP1 and USF. However, mutational studies confirm that other factors must play a role (40). In porcine kidney cells, transcriptional activation of this sialyltransferase can be induced by TGF-β1 (41), and in the colon carcinoma cell line HT-29, tumor necrosis factor α can enhance ST3Gal-I expression through NFκB binding sites (31). We now show that increased expression of ST3Gal-I in breast cancer cells can be induced by COX-2 working through the prostanoid PGE2. Further studies will be required to demonstrate the transcript factors involved.
COX-2 is undetectable in most normal tissues but is induced by inflammation when activated macrophages and other cells at sites of inflammation express it. However, COX-2 expression is also turned on in many cancers, and increased expression has been reported in colon (16), bladder (29), and breast cancers (17). Interestingly, these are all cancers that show increased expression of ST3Gal-I (6, 8, 36, 37). COX-2 expression has been associated with cell motility and invasion (19), angiogenesis (20), and lymph node metastasis (21), all processes that involve glycan interactions (3). Clinical trials investigating the efficacy of the selective COX-2 inhibitor celecoxib are now underway in colon, breast, and bladder cancer, and it would be interesting to measure the expression of ST3Gal-I in tumors from these patients. The results described here suggest the intriguing possibility that some of the malignant characteristics associated with COX-2 expression may be via the influence that COX-2 exerts on the glycosylation of tumor cells.
Acknowledgments
We thank Professor Anne Ridley for helpful discussions.
This work was supported by Grant 2008NovPhD28 from the Breast Cancer Campaign and supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' National Health Service Foundation Trust and King's College London. This work was also supported by the Experimental Cancer Medicine Centre at King's College London.
- ST3Gal-I
- α-2,3-sialyltransferase-3
- COX-2
- cyclooxygenase 2
- qRT-PCR
- quantitative real-time PCR
- PNA
- peanut agglutinin
- TPA
- 12-O-tetradecanoylphorbol-13-acetate.
REFERENCES
- 1. Hakomori S. (2002) Glycosylation defining cancer malignancy. New wine in an old bottle. Proc. Natl. Acad. Sci. U.S.A. 99, 10231–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burchell J. M., Mungul A., Taylor-Papadimitriou J. (2001) O-linked glycosylation in the mammary gland. Changes that occur during malignancy. J. Mammary Gland Biol. Neoplasia 6, 355–364 [DOI] [PubMed] [Google Scholar]
- 3. Ono M., Hakomori S. (2004) Glycosylation defining cancer cell motility and invasiveness. Glycoconj. J. 20, 71–78 [DOI] [PubMed] [Google Scholar]
- 4. Picco G., Julien S., Brockhausen I., Beatson R., Antonopoulos A., Haslam S., Mandel U., Dell A., Pinder S., Taylor-Papadimitriou J., Burchell J. (2010) Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 20, 1241–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mungul A., Cooper L., Brockhausen I., Ryder K., Mandel U., Clausen H., Rughetti A., Miles D. W., Taylor-Papadimitriou J., Burchell J. M. (2004) Sialylated core 1 based O-linked glycans enhance the growth rate of mammary carcinoma cells in MUC1 transgenic mice. Int. J. Oncol. 25, 937–943 [PubMed] [Google Scholar]
- 6. Burchell J., Poulsom R., Hanby A., Whitehouse C., Cooper L., Clausen H., Miles D., Taylor-Papadimitriou J. (1999) An α2,3 sialyltransferases (ST3Gal I) is elevated in primary breast carcinoma. Glycobiology 9, 1307–1311 [DOI] [PubMed] [Google Scholar]
- 7. Cazet A., Julien S., Bobowski M., Burchell J., Delannoy P. (2010) Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 12, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Potapenko I. O., Haakensen V. D., Lüders T., Helland A., Bukholm I., Sørlie T., Kristensen V. N., Lingjaerde O. C., Børresen-Dale A. L. (2010) Glycan gene expression signatures in normal and malignant breast tissue. Possible role in diagnosis and progression. Mol. Oncol. 4, 98–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalziel M., Whitehouse C., McFarlane I., Brockhausen I., Gschmeissner S., Schwientek T., Clausen H., Burchell J. M., Taylor-Papadimitriou J. (2001) The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumour-associated epitope on MUC1. J. Biol. Chem. 276, 11007–11015 [DOI] [PubMed] [Google Scholar]
- 10. Priatel J. J., Chui D., Hiraoka N., Simmons C. J., Richardson K. B., Page D. M., Fukuda M., Varki N. M., Marth J. D. (2000) The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity 12, 273–283 [DOI] [PubMed] [Google Scholar]
- 11. Tsuboi S., Fukuda M. (2001) Roles of O-linked oligosaccharides in immune responses. BioEssays 23, 46–53 [DOI] [PubMed] [Google Scholar]
- 12. Julien S., Grimshaw M. J., Sutton-Smith M., Coleman J., Morris H. R., Dell A., Taylor-Papadimitriou J., Burchell J. M. (2007) O-linked glycosylation is regulated during maturation of dendritic cells and has an impact on their migration. J. Immunol. 179, 5701–5710 [DOI] [PubMed] [Google Scholar]
- 13. Bax M., García-Vallejo J. J., Jang-Lee J., North S. J., Gilmartin T. J., Hernández G., Crocker P. R., Leffler H., Head S. R., Haslam S. M., Dell A., van Kooyk Y. (2007) Dendritic cell maturation results in pronounced changes in glycan expression affecting recognition by siglecs and galectins. J. Immunol. 179, 8216–8224 [DOI] [PubMed] [Google Scholar]
- 14. Legler D. F., Krause P., Scandella E., Singer E., Groettrup M. (2006) Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J. Immunol. 176, 966–973 [DOI] [PubMed] [Google Scholar]
- 15. Brunner C., Seiderer J., Schlamp A., Bidlingmaier M., Eigler A., Haimerl W., Lehr H. A., Krieg A. M., Hartmann G., Endres S. (2000) Enhanced dendritic cell maturation by TNF-α or cytidine-phosphate-guanosine DNA drives T cell activation in vitro and therapeutic anti-tumor immune responses in vivo. J. Immunol. 165, 6278–6286 [DOI] [PubMed] [Google Scholar]
- 16. Eberhart C. E., Coffey R. J., Radhika A., Giardiello F. M., Ferrenbach S., DuBois R. N. (1994) Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107, 1183–1188 [DOI] [PubMed] [Google Scholar]
- 17. Ristimäki A., Sivula A., Lundin J., Lundin M., Salminen T., Haglund C., Joensuu H., Isola J. (2002) Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res 62, 632–635 [PubMed] [Google Scholar]
- 18. Boland G. P., Butt I. S., Prasad R., Knox W. F., Bundred N. J. (2004) COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br. J. Cancer 90, 423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larkins T. L., Nowell M., Singh S., Sanford G. L. (2006) Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer 6, 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davies G., Salter J., Hills M., Martin L.-A., Sacks N., Dowsett M. (2003) Correlation between cyclooxygenase-2 expression and angiogenesis in human breast cancer. Clin. Cancer Res. 9, 2651–2656 [PubMed] [Google Scholar]
- 21. Costa C., Soares R., Reis-Filho J. S., Leitão D., Amendoeira I., Schmitt F. C. (2002) Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J. Clin. Pathol. 55, 429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stasinopoulos I., O'Brien D. R., Wildes F., Glunde K., Bhujwalla Z. M. (2007) Silencing of cyclooxygenase-2 inhibits metastasis and delays tumor onset of poorly differentiated metastatic breast cancer cells. Mol. Cancer Res. 5, 435–442 [DOI] [PubMed] [Google Scholar]
- 23. Liu C. H., Chang S. H., Narko K., Trifan O. C., Wu M. T., Smith E., Haudenschild C., Lane T. F., Hla T. (2001) Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J. Biol. Chem. 276, 18563–18569 [DOI] [PubMed] [Google Scholar]
- 24. Takkouche B., Regueira-Méndez C., Etminan M. (2008) Breast cancer and use of nonsteroidal anti- inflammatory drugs: A meta-analysis. J. Natl. Cancer Inst. 100, 1439–1447 [DOI] [PubMed] [Google Scholar]
- 25.Deleted in proof [Google Scholar]
- 26. Julien S., Ivetic A., Grigoriadis A., QiZe D., Burford B., Sproviero D., Picco G., Gillett C., Papp S. L., Schaffer L., Tutt A., Taylor-Papadimitriou J., Pinder S. E., Burchell J. M. (2011) Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 71, 7683–7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lyng M. B., Laenkholm. A. V., Pallisgaard. N., Ditzel H. J. (2008) Identification of genes for normalization of real-time RT-PCR data in breast carcinomas. BMC Cancer 2008 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brockhausen I., Yang J. M., Burchell J., Whitehouse C., Taylor-Papadimitriou J. (1995) Mechanisms underlying aberrant glycosylation of MUCl mucin in breast cancer cells. Eur. J. Biochem. 233, 607–617 [DOI] [PubMed] [Google Scholar]
- 29. Eltze E., Wülfing C., Von Struensee D., Piechota H., Buerger H., Hertle L. (2005) Cox-2 and Her2/neu co-expression in invasive bladder cancer. Int. J. Oncol. 26, 1525–1531 [PubMed] [Google Scholar]
- 30. Pan M.-R., Hou M.-F., Chang H.-C., Hung W.-C. (2008) Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signalling pathways to enhance lymphatic invasion of breast cancer cells. J. Biol. Chem. 283, 11155–11163 [DOI] [PubMed] [Google Scholar]
- 31. Higai K., Ishihara S., Matsumoto K. (2006) NF-κB-p65-dependent transcriptional regulation of glycosyltransferases in human colon adenocarcinoma HT-29 by stimulation with tumor necrosis factor α. Biol. Pharm. Bull. 29, 2372–2377 [DOI] [PubMed] [Google Scholar]
- 32. Subbaramaiah K., Hudis C., Chang S. H., Hla T., Dannenberg A. J. (2008) EP2 and EP4 receptors regulate aromatase expression in human adipocytes and breast cancer cells. Evidence of a BRCA1 and p300 exchange. J. Biol. Chem. 283, 3433–3444 [DOI] [PubMed] [Google Scholar]
- 33. Nishigaki N., Negishi M., Ichikawa A. (1996) Two Gs-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol. Pharmacol. 50, 1031–1037 [PubMed] [Google Scholar]
- 34. Fitzpatrick F. A., Aguirre R., Pike J. E., Lincoln F. H. (1980) The stability of 13,14-dihydro-15 keto-PGE2. Prostaglandins 19, 917–931 [DOI] [PubMed] [Google Scholar]
- 35. Bourin M. C., Delescluse C., Fürstenberger G., Marks F., Schweizer J., Klein-Szanto A. J., Prunieras M. (1982) Effect of phorbol esters on guinea pig skin in vivo. Carcinogenesis 3, 671–676 [DOI] [PubMed] [Google Scholar]
- 36. Kudo T., Ikehara Y., Togayachi A., Morozumi K., Watanabe M., Nakamura M., Nishihara S., Narimatsu H. (1998) Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab. Invest. 78, 797–811 [PubMed] [Google Scholar]
- 37. Videira P. A., Correia M., Malagolini N., Crespo H. J., Ligeiro D., Calais F. M., Trindade H., Dall'Olio F. (2009) ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer 9, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohtsubo K., Marth J. D. (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 [DOI] [PubMed] [Google Scholar]
- 39. Napoletano C., Rughetti A., Agervig Tarp M. P., Coleman J., Bennett E. P., Picco G., Sale P., Denda-Nagai K., Irimura T., Mandel U., Clausen H., Frati L., Taylor-Papadimitriou J., Burchell J., Nuti M. (2007) Tumor associated Tn-MUC1 glycoform is internalised through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells. Cancer Res. 67, 8358–8367 [DOI] [PubMed] [Google Scholar]
- 40. Taniguchi A., Yoshikawa I., Matsumoto K. (2001) Genomic structure and transcriptional regulation of human Galβ1, 3GalNAc α2,3-sialyltransferase (hST3Gal I) gene. Glycobiology 11, 241–247 [DOI] [PubMed] [Google Scholar]
- 41. Son S. W., Song K. H., Kwon H. Y., Kim K. S., Kim C. H., Oh K. B., Choo Y. K., Lee Y. C. (2011) Transcriptional activation of pig Galβ1,3GalNAc α2,3-sialyltransferase (pST3Gal I) gene by TGF-β1 in porcine kidney PK-15 cells. Biochem. Biophys. Res. Commun. 414, 159–164 [DOI] [PubMed] [Google Scholar]