Background: NASH is a disease characterized by fat accumulation and chronic inflammation in the liver.
Results: Pin1 expression was increased in NASH model mouse livers. Pin1 KO mice were resistant to NASH development.
Conclusion: Pin1 plays critical roles in NASH development.
Significance: A Pin1 inhibitor might be a novel agent for treating NASH.
Keywords: Bone Marrow, Fibrosis, Inflammation, Lipid Metabolism, Prolyl Isomerase, Nonalcoholic Steatohepatitis
Abstract
Nonalcoholic steatohepatitis (NASH) is a disorder characterized by simultaneous fat accumulation and chronic inflammation in the liver. In this study, Pin1 expression was revealed to be markedly increased in the livers of mice with methionine choline-deficient (MCD) diet-induced NASH, a rodent model of NASH. In addition, Pin1 KO mice were highly resistant to MCD-induced NASH, based on a series of data showing simultaneous fat accumulation, chronic inflammation, and fibrosis in the liver. In terms of Pin1-induced fat accumulation, it was revealed that the expression levels of peroxisome proliferator-activated receptor α and its target genes were higher in the livers of Pin1 KO mice than in controls. Thus, resistance of Pin1 KO mice to hepatic steatosis is partially attributable to the lack of Pin1-induced down-regulation of peroxisome proliferator-activated receptor α, although multiple other mechanisms are apparently involved. Another mechanism involves the enhancing effect of hematopoietic Pin1 on the expressions of inflammatory cytokines such as tumor necrosis factor and monocyte chemoattractant protein 1 through NF-κB activation, eventually leading to hepatic fibrosis. Finally, to distinguish the roles of hematopoietic or nonhematopoietic Pin1 in NASH development, mice lacking Pin1 in either nonhematopoietic or hematopoietic cells were produced by bone marrow transplantation between wild-type and Pin1 KO mice. The mice having nonhematopoietic Pin1 exhibited fat accumulation without liver fibrosis on the MCD diet. Thus, hepatic Pin1 appears to be directly involved in the fat accumulation in hepatocytes, whereas Pin1 in hematopoietic cells contributes to inflammation and fibrosis. In summary, this is the first study to demonstrate that Pin1 plays critical roles in NASH development. This report also raises the possibility that hepatic Pin1 inhibition to the appropriate level might provide a novel therapeutic strategy for NASH.
Introduction
Proline residues in proteins exist in completely cis and trans peptide bond conformations, and prolyl cis-trans isomerases (PPIases)3 control intrinsic conformational changes. At present, PPIases can be divided into four families as follows: cyclophilins, FK506-binding proteins, parvulins, and the recently identified protein phosphatase 2A (PP2A) activator (1–4). The parvulins consist of Pin1 and PAR14 (also termed Pin4), and Pin1 was initially cloned as an NIMA kinase-interacting protein (5). Since its discovery, numerous proteins have been identified as Pin1 substrates (6–9). For example, Pin1 inhibits phosphorylated Cdc25 (10), as well as regulating the protein stability and activity of tumor suppressor p53, and also plays an important role in cell cycle progression (11, 12). Indeed, the expression of Pin1 is up-regulated in most cancers, and increased expression of Pin1 has thus been implicated in malignant transformation (6, 13–16). Pin1 also plays a critical role in the brain and immune responses (8, 9, 17–19).
We previously reported that Pin1 binds to Crtc2 and suppresses cAMP-response element transcriptional and gluconeogenic activity in the liver (20). In addition, Pin1 associates with IRS-1, a major insulin receptor substrate, and enhances insulin-induced metabolic actions (21). The associations of Pin1 with IRS-1 and Crtc2 ultimately contribute to the incorporation of excess nutrients into tissues, based on a data series obtained using in vitro and in vivo overexpressions of Pin1, gene silencing, a specific inhibitor, and Pin1 knock-out (KO) mice. Pin1 is also indispensable for differentiation of pre-adipocytes into mature adipocytes, although the molecular mechanism underlying this maturation-inducing function has not been fully elucidated (21). Taking the increased expression of Pin1 in the fed state into account, we can reasonably speculate that altered expressions of Pin1 in accordance with nutrient conditions may be physiologically beneficial. However, Pin1 is also reportedly involved in activations of NF-κB and IRAK1, key regulators of cytokine production (19, 22–25), suggesting the involvement of Pin1 in some inflammatory diseases.
In this study, we investigated the role of Pin1 in the pathogenesis of nonalcoholic steatohepatitis (NASH), using Pin1 KO mice. NASH is a disease characterized by the coexistence of fat accumulation and inflammation, eventually leading to liver cirrhosis and hepatic cancer (26–28), and its incidence is increasing in many developed countries. Unfortunately, however, there are currently no treatment options specific for NASH, with this disease usually being managed only with lifestyle changes such as diet, exercise, and weight reduction (29, 30),
The involvement of Pin1 in the pathogenesis of NASH was strongly suggested by markedly elevated Pin1 expression in NASH model mouse livers, as well as the fact that Pin1 KO mice were highly resistant to the development of NASH. Here, we present evidence indicating a contribution of Pin1 to NASH development. Our present observations also raise the possibility of a Pin1 inhibitor being a novel agent for treating NASH.
EXPERIMENTAL PROCEDURES
Materials
Each antibody was purchased from Santa Cruz Biotechnology (Pin1 and PPARα), Sigma (actin), and Abcam (collagen1, α-SMA, and ACO, Tokyo, Japan). Anti-rabbit horseradish peroxidase (HRP) antibodies were obtained from Amersham Biosciences. RPMI medium was purchased from Nissui (Tokyo, Japan), Sepasol RNA I Super G from Nacalai Tesque (Kyoto, Japan), and SYBR Green from Takara (Tokyo, Japan).
Animals and Experimental Protocols
Pin1 KO mice were generated and genotyped as reported previously (31). They were maintained on a C57BL/6 background, and Pin1−/− and Pin1+/+ mice were prepared by mating the Pin1+/− mice. Mice were maintained in a temperature-and light-controlled facility. Eight-week-old mice were used for the methionine choline-deficient (MCD) diet study and were fed chow (54.4% carbohydrate, 23.6% protein, 5.3% fat, and a vitamin and mineral mixture) or the MCD diet (18.3% methionine-deficient amino acid mix, 10% sucrose, 10% lard, 5% cellulose, 1% choline deficient vitamin mix, 3.5% mineral mix, 53% cornstarch) for 6 weeks (n = 4–5/group). The mice were then sacrificed, and livers were extracted. The animals were handled in accordance with the guidelines for the care and use of experimental animals published by the Japanese Association for Laboratory Animal Science.
Western Blotting
Livers were homogenized in lysis buffer containing 50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 1 mm PMSF, 1 mm orthovanadate, and protease inhibitor mixture. The lysates were then centrifuged at 15,000 rpm for 30 min. After quantitative protein determination, supernatants were mixed with the same volume of 2× sample buffer. Equal amounts of proteins extracted from livers were electrophoresed on SDS-polyacrylamide gel and then transferred to PVDF membranes. After being blocked with 3% BSA in PBST for 1 h, the membranes were reacted with the following antibodies for 1 h at room temperature: anti-β-actin (1:2000), anti-Pin1 (1:1000), anti-PPARα (1:1000), anti-ACO (1:1000), anti-SMA (1:1000), anti-Col1 (1:1000), anti-CTGF (1:500), and anti-rabbit/mouse HRP secondary antibody (1:2000). Membranes were washed with PBST three times, reacted with Supersignal West Pico Substrate (Thermo Scientific), and exposed to x-ray film. Band density was quantified by ChemiDoc XRS+ (Bio-Rad).
Radiation Chimeras
To generate radiation chimeras, mice received 9.5 gray of ionizing radiation, followed by tail vein injection of 2 × 106 bone marrow cells. Mice were maintained for 6 weeks on chow and then received the MCD diet for 8 weeks. Reconstitutions with donor bone marrow were confirmed by PCR using Ex TaqDNA polymerase (Takara, Tokyo, Japan). For detection of the WT allele, PinF (AGCTGGAAGTGTGAGGT) and EndC (TATAGCTAGTGGAAAGAATT) were used. The Pin1 KO allele was detected by a set of EndC/Pin1G3 (GGGCCAGCTCATTCCTCCACTCA) (31). PCR was performed at 94 °C for 10 min, followed by 35 cycles of amplification (94 °C for 1.5 min and 57 °C 1.5 min) and 72 °C for 10 min.
Cell Culture
THP-1 cells were maintained in RPMI medium (Nissui, Tokyo, Japan). For siRNA experiments, cells were cultivated in 12-well plates and 10 ng/ml phorbol 12-myristate 13-acetate were then added for macrophage differentiation. After 24 h, siRNA reagents were added to the culture medium. At 48 h after siRNA treatments, cells were stimulated with 10 ng/ml LPS for 6 h. Then RNA was extracted.
Primary Macrophages
Mouse peritoneal macrophages were prepared by intraperitoneal injection of 2 ml of 5% thioglycollate broth and then plated in 6-well plates at 2 × 105 cells/ml. Macrophages were cultured in RPMI medium with 10% fetal calf serum. Cells were treated with bovine serum albumin-conjugated palmitate for 6 h. Then RNA was extracted.
Morphologic Studies
Livers were fixed with 10% formaldehyde, then dehydrated through ethanol and xylene, and finally embedded in paraffin. Sections were cut and stained with hematoxylin and eosin or Azan. For α-SMA antibody staining, sections were treated with 0.1% Triton X-100 and 10 mm citrate buffer. Next, sections were stained with primary antibody (1:50) at 4 °C overnight and then incubated with diaminobenzidine reagents (Santa Cruz Biotechnology).
For immunofluorescence staining by both Pin1 and F4/80 antibody, sections were treated with 0.1% Triton X-100 followed 10 mm citrate buffer for 10 min. After washing with PBS three times, sections were stained with both anti-Pin1 antibody (1:50) and FITC-conjugated F4/80 antibody (1:50) at 4 °C overnight. Next, sections were reacted with anti-mouse Alexa 548 (1:100) at room temperature for 1 h and then observed under a fluorescence microscope. For Oil Red O staining, livers were frozen in liquid nitrogen and embedded in OTC compound. After staining, these sections were washed and embedded.
Serum Investigations
Serum was collected after a 10-min centrifugation at 8000 rpm. Aspartate transaminase (AST) and alanine aminotransferase (ALT) were assayed with an AST/ALT assay kit (Wako, Tsukuba, Japan), according to the manufacturer's instructions.
Real Time PCR
RNA extraction was performed using Sepasol (Nakalai Tesque, Tokyo, Japan) according to the manufacturer's protocol. Briefly, liver tissues were homogenized in Sepasol, and chloroform was added. After being vortexed, tubes were centrifuged at 15,000 rpm for 10 min. Upper phases were transferred to new tubes, and total RNA was obtained by ethanol precipitation. cDNA was obtained using total RNA employing a Verso cDNA synthesis kit (Thermo Scientific). Reaction solutions contained 5× cDNA synthesis buffer, dNTP mix, oligo(dT) primer, RT enhancer, Verso enzyme mix, and 1 μg of total RNA. The program for the reverse transcription cycle was 42 °C for 30 min and 95 °C for 2 min. Real time quantitative PCR was carried out using a CFX96 real time PCR system (Bio-Rad) with SYBR mix (Takara), according to the manufacturer's protocol. Reaction solutions were 20 μl scale containing SYBR premix EX taq, primers, and template. PCRs were two steps at 95 °C for 5 s and 60 °C for 30 s and were repeated 40 times. Relative mRNA genes were normalized to the GAPDH mRNA levels and relative expression levels determined by the comparative Ct method. The primers used are shown in Table 1.
TABLE 1.
Primer set for real time PCR
F is forward, and R is reverse; m is mouse.
| mPPARα F, atg cca gta ctg ccg ttt tc |
| mPPARα R, ggc ctt gac ctt gtt cat gt (220 bp) |
| mACO F, ttcaagacagagccgtgcaa |
| mACO R, cagagccaagggtcacatcc (221 bp) |
| mCYP4A10 F, cca gca gtt ccc atc acc tc |
| mCYP4A10 R, tga tcg ccc cag aat cac tt (129 bp) |
| mCPT1 F, cca ggc tac agt ggg aca tt |
| mCPT1 R, gaa ctt gcc cat gtc ctt gt (199 bp) |
| mCollagen 1a1 F, gag cgg aga gta ctg gat cg |
| mCollagen 1a1 R, gct tct ttt cct tgg ggt tc (158 bp) |
| mCollagen 1a2 F, ccg tgc ttc tca gaa cat ca |
| mCollagen 1a2 R, gag cag cca tcg act agg ac (168 bp) |
| mTGFβ F, ggaaggacctgggttggaag |
| mTGFβ R, ggacaactgctccaccttgg (233 bp) |
| mCTGF F, agggcctcttctgcgatttc |
| mCTGF R, catttcccaggcagcttgac (247 bp) |
| mSREBP1 F, tag agc ata tcc ccc agg tg |
| mSREBP1 R, ggt acg ggc cac aag aag ta (245 bp) |
| mTIMP1 F, att caa ggc tgt ggg aaa tg |
| mTIMP1 R, ctc aga gta cgc cag gga ac (183 bp) |
| mMCP-1 F, agg tcc ctg tga tgc ttc tg |
| mMCP-1 R, tct gga ccc att cct tct tg (248 bp) |
| mTNFα F, gaa ctg gca gaa gag gca ct |
| mTNFα R, agg gtc tgg gcc ata gaa ct (193 bp) |
Measurement of Hepatic Triglyceride
Hepatic triglycerides were extracted by sonication in 1 ml of chloroform/methanol. After adding water, samples were centrifuged for 3 min at 3000 rpm. The lower phase was collected, evaporated, and dissolved in 0.5 ml isopropyl alcohol. Triglycerides were measured by triglyceride E-test (Wako).
RESULTS
Pin1 Expression in Liver Is Increased by MCD Diet Feeding
To determine whether Pin1 was involved in NASH progression, we used a mouse model of NASH derived from MCD diet feeding. MCD diet feeding caused the hepatic pathology of NASH, including steatohepatitis, inflammation, and fibrosis. Although body and liver weights were decreased in the MCD diet-fed group, as reported previously (32), no differences were observed between WT and Pin1 KO mice (Fig. 1a). Interestingly, hepatic Pin1 expression in the WT mice was shown to be increased by MCD diet feeding for 6 weeks (Fig. 1b). Mice on the MCD diet showed elevation of serum ALT and AST levels reflecting liver injury, and the degrees of increases due to the MCD diet were significantly lower in Pin1 KO than in WT mice (Fig. 1c). Hematoxylin and eosin staining showed that the livers of mice fed the MCD diet developed marked macrovesicular steatosis (Fig. 1d). In contrast, Pin1 KO mice showed less accumulated fat in their livers than WT mice.
FIGURE 1.
Hepatic Pin1 expressions were increased by MCD diet feeding, althoughPin1 ablation ameliorated liver injury and steatohepatitis. Eight-week-old mice were fed a normal diet (ND) or the MCD diet for 6 weeks, and the proteins in their livers were investigated by immunoblotting. a, body and liver weights were reduced by MCD feeding but did not differ between WT and Pin1 KO mice. b, immunoblotting (IB) with antibodies against Pin1 and actin (left panel) and the amounts of Pin1 and actin were quantified using ChemiDoc XRS (Bio-Rad) (n = 4 for each group). Relative protein levels of Pin1 were normalized to actin. c, serum AST and ALT levels were measured with an AST/ALT assay kit (n = 4, only ND groups show the AST average of two mice). d, paraffin-embedded sections were stained with hematoxylin and eosin. Representative photographs of each group are shown. *, p < 0.05; ***, p < 0.001. Scale bar, 100 μm.
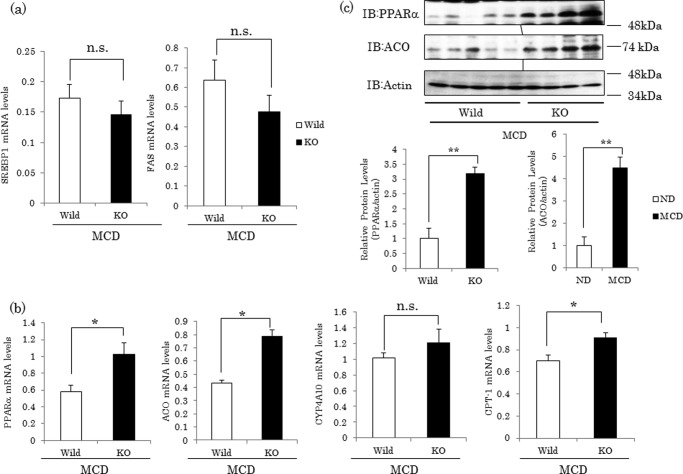
Expressions of PPARα Target Genes Are Higher in Pin1 KO Mice than in WT Mice Fed the MCD Diet
To investigate the mechanism of lipid droplet accumulation, we analyzed the mRNA expressions of genes involved in steatohepatitis. As reported previously (33), expressions of genes involved in fatty acid synthesis such as sterol regulatory element-binding protein (SREBP-1) and fatty-acid synthase were decreased in the MCD diet-fed group, and there were no differences between WT and Pin1 KO mice given the MCD diet (Fig. 2a). However, PPARα regulates lipid metabolism in the liver through the induction of genes that promote fatty acid disposal, and it has been reported that up-regulation of PPARα activity improves hepatic steatosis when animals are fed the MCD diet (34). MCD diet feeding reportedly decreased mRNA expressions of PPARα and its target genes, ACO and CPT-1, which are involved in β-oxidation (35, 36). In this study, expressions of ACO and CPT-1 were significantly higher in Pin1 KO than in WT mice, although expression of CYP4A10, which is involved in ω-oxidation, was unchanged (Fig. 2b). The protein levels of PPARα and ACO were also higher in Pin1 KO than in WT mice (Fig. 2c).
FIGURE 2.
Expressions of PPARα and its downstream genes were higher in Pin1 KO than in WT mice. a, expressions of SREBP1 and fatty-acid synthase mRNA in the liver were decreased by MCD feeding, but there was no difference between WT and Pin1 KO mice (n = 3–5 for each group). b, mRNA levels of PPARα, ACO, CYP4A10, and CPT-1 in livers were determined by real time PCR using primers corresponding to their mRNA sequences (n = 4–5 for each group). Data are shown as means ± S.E. c, protein levels of PPARα and ACO in livers were determined by Western blotting. The amounts of them were quantified using ChemiDoc XRS (Bio-Rad) (n = 4–5 for each group). Relative protein levels of PPARα and ACO were normalized to actin protein. Data are shown as means ± S.E. *, p < 0.05; **, p < 0.01. n.s., not significant; IB, immunoblot.
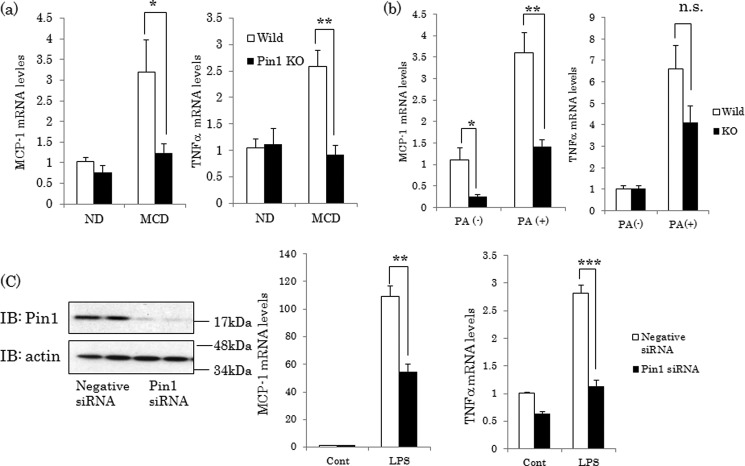
Pin1 Deficiency Reduces Hepatic Inflammation
NASH development was assumed to require the recruitment of macrophages and the release of inflammatory cytokines, especially TNFα. Feeding of the MCD diet increased hepatic TNFα and MCP-1, potent mediators of macrophage activities, mRNA levels in WT mice. In contrast, MCD diet-induced increases in TNFα and MCP-1 mRNAs were almost completely prevented in the livers of Pin1 KO mice (Fig. 3a). To further investigate whether Pin1 has direct effects on these cytokine expressions, we performed experiments using primary macrophages and THP-1, a differentiated macrophage cell line. Incubation of primary macrophages from WT mice with palmitic acid, a TLR4 ligand, for 6 h increased both TNF-α and MCP-1. The up-regulation of MCP-1 was significantly suppressed, whereas that of TNFα tended to be decreased, although not significantly, in Pin1-deficient macrophages (Fig. 3b). Similarly, in THP-1, Pin1 knockdown by siRNA decreased TNF-α and MCP-1 inductions in response to stimulation with lipopolysaccharide (LPS), another TLR4 ligand (Fig. 3c).
FIGURE 3.
Pin1 is involved in cytokine release. a, mRNA levels of TNFα and MCP-1 in livers were determined by real time PCR using primers corresponding to their mRNA sequences (n = 4–5). b, primary macrophages were prepared from WT or Pin1 KO mice. Cells were stimulated with palmitate for 6 h, and mRNA levels of TNFα and MCP-1 were then determined (n = 4). c, THP-1 cells were treated with negative or Pin1 siRNA for 48 h, and the cells were then stimulated with 10 ng/ml LPS for 6 h. The protein levels of Pin1 and actin were determined by immunoblotting (IB) (left panel), and mRNA levels of TNFα and MCP-1 were examined by real time PCR (n = 4). All data are shown as means ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
Ablation of Pin1 Inhibits Liver Fibrosis Because of MCD Diet Feeding
To elucidate the role of Pin1 in liver fibrosis caused by MCD diet feeding, collagen deposition in the liver was estimated by Azan staining. WT mice fed the MCD diet showed liver fibrosis, as reported previously (37, 38), although almost no collagen deposition was observed in the livers of Pin1 KO mice (Fig. 4a). Consistent with the results of Azan staining, mRNA expressions of collagen1a1/1a2 were not increased in the livers of Pin1 KO mice by the MCD diet, although a marked increase was observed in WT mice (Fig. 4b). In good agreement with the mRNA data, collagen1 protein was markedly up-regulated by the MCD diet in WT mouse livers but not in Pin1 KO mouse livers (Fig. 4c).
FIGURE 4.
Pin1 KO mice are resistant to MCD-induced liver fibrosis. a, sections prepared from the livers of normal diet or MCD-diet fed mice were stained with Azan. Representative photographs of each group are shown. Scale bar, 50 μm. b, mRNA levels of Col1a1 and Col1a2 in the livers were determined by real time PCR using primers corresponding to their mRNA sequences (n = 4–5 for each group). c, protein level of Col1a1 and CTGF were determined by immunoblotting (IB). The amounts were quantified using ChemiDoc XRS (Bio-Rad) (n = 3–5 for each group). Relative protein levels of collagen1 and CTGF were normalized to actin protein. Data are shown as means ± S.E. d, mRNA levels of TGFβ and CTGF in these livers were determined by real time PCR using primers corresponding to their mRNA sequences. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
Tumor growth factor β1 (TGFβ1) and CTGF are important profibrogenic cytokines that promote fibrosis. Hepatic mRNA levels of TGFβ1 were not changed in mice fed the MCD diet in this study, although they were significantly reduced in Pin1 KO mice (Fig. 4d). CTGF expressions were markedly increased in the livers of WT mice fed the MCD diet, and ablation of Pin1 strongly decreased the CTGF mRNA up-regulation due to this diet (Fig. 4d). Expression of hepatic CTGF protein was also highly up-regulated in WT mice but was unchanged in Pin1 KO mice (Fig. 4c).
Ablation of Pin1 Inhibits Activation of Hepatic Stellate Cells
Activation of hepatic stellate cells (HSC) is also a key factor promoting liver fibrosis. HSC are reportedly activated by TGF-β1 and endotoxins such as LPS and differentiate into extracellular matrix-producing myofibroblasts (39). To evaluate the involvement of Pin1 in HSC activation, we immunohistochemically analyzed α-SMA, an activated HSC marker. Immunohistochemical analysis revealed that α-SMA accumulated in the livers of WT mice following MCD diet feeding, but not in the livers of Pin1 KO mice (Fig. 5a). Further analysis using real time PCR and Western blotting indicated Pin1 KO mice show only slight HSC activation when fed the MCD diet (Fig. 5, b and c), although marked activation was observed in the WT mice. Moreover, MCD diet feeding increased the expression of the tissue inhibitor of matrix metalloproteinase 1/2 (TIMP1/2), but the level of induction was much lower in Pin1 KO than in WT mice (Fig. 5d).
FIGURE 5.
Genetic Pin1 ablation inhibits the activation of hepatic stellate cells. a, sections prepared from the livers of normal diet or MCD-diet fed mice were stained with α-SMA antibody. Representative photographs of each group are shown. Scale bars, 20 μm. b and c, mRNA and protein levels of α-SMA in these livers were determined by real time PCR using primers corresponding to their mRNA sequences or Western blotting. The amounts of them were quantified using ChemiDoc XRS (Bio-Rad) (n = 3–5 for each group). Relative protein levels of α-SMA were normalized to actin protein. IB, immunoblot. d, mRNA levels of TIMP1 and TIMP2 were determined by real time PCR. Data are shown as means ± S.E. (n = 4–5). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
NASH Induced by the MCD Diet Requires Both Liver and Macrophage Pin1
As shown in Figs. 4 and 5, Pin1 KO mice were clearly resistant to MCD diet-induced liver fibrosis. To determine the contributions of Pin1 in the liver and hematopoietic cells to NASH, we used adoptive transfer to generate mice lacking Pin1 in either nonhematopoietic or hematopoietic cells (Fig. 6a). Four groups of mice were created as follows: WT mice reconstituted with WT bone marrow (WW); WT mice reconstituted with Pin1 KO bone marrow (WK); Pin1 KO mice reconstituted with WT bone marrow (KW); and Pin1 KO mice reconstituted with Pin1 KO bone marrow (KK).
FIGURE 6.
Generation of hematopoietic and nonhematopoietic Pin1 KO mice. a, radiation chimeras used in this study and the experimental design are presented. b, reconstitution of donor bone marrow was confirmed by PCR. c, liver sections were stained with both F4/80 antibody and Pin1 antibody. Representative photographs of each group are shown. d, sections prepared from the livers of normal diet or MCD diet-fed mice were stained with Oil Red O. Representative photographs of each group are shown. e, amount of hepatic triglyceride was measured. f, mRNA levels of TIMP1 and collagen1 in mouse livers were determined by real time PCR using primers corresponding to their mRNA sequences. g, serum ALT levels. Data are shown as means ± S.E. (n = 4–5). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
No differences in body or liver weights were observed among the four groups (data not shown). To confirm reconstitution with donor bone marrow, the DNA allele from the blood of recipient mice was checked by PCR (Fig. 6b). In addition, reconstitution was analyzed by immunofluorescent staining (Fig. 6c). In the livers of WK groups, hepatocytes were well stained with anti-Pin1 antibody, whereas most F4/80-positive cells (more than 80%) were not. On the contrary, in the livers of KW groups, F4/80-positive cells but not hepatocytes were stained with anti-Pin1 antibody.
Oil Red O staining as well as the measurement of hepatic triglyceride levels revealed the suppression of steatosis in the livers of nonhematopoietic Pin1-deficient groups (KW and KK) (Fig. 6, d and e). These data indicate the requirement of Pin1 in nonhematopoietic cells for lipid accumulation in response to the MCD diet. However, MCD diet-induced elevations of the fibrosis marker collagen1, TIMP1, and ALT were completely suppressed in mice lacking Pin1 in either nonhematopoietic or hematopoietic cells (WK, KW, and KK) (Fig. 6, f and g).
DISCUSSION
In this study, Pin1 expression was clearly demonstrated to be markedly up-regulated in the mouse liver with MCD diet feeding. In addition, Pin1 KO mice were shown to be highly resistant to MCD diet-induced NASH, based on our observations of the inductions of fibrosis genes, steatohepatitis, and inflammatory cytokine expressions. Although the details of the mechanism of NASH pathogenesis remain to be determined, a two-hit mechanism has been hypothesized. The first hit is hepatic steatosis, and the second hit involves the actions of inflammatory cytokines, reactive oxygen species, and lipid peroxidation.
Reduced lipolysis rather than lipogenesis appears to contribute to the “first hit,” i.e. the steatosis induced by the MCD diet, although this is still somewhat controversial. In fact, lipogenic genes were not reportedly up-regulated in MCD diet-induced steatosis (40, 41), which was confirmed by suppressed SREBP-1 and fatty-acid synthase expressions. Instead, some reports have suggested reduced PPARα activity to contribute to the development of NASH, whereas administration of PPARα agonists reportedly ameliorated NASH (34, 35, 38). Our results showed that MCD diet feeding decreased PPARα and its downstream gene expressions, which is in good agreement with previous reports. In addition, increased expressions of PPARα and its downstream genes in the Pin1 KO mouse livers may be regarded as one of the mechanisms underlying the resistance of Pin1 KO mice to NASH development.
At present, the molecular mechanism of PPARα expression by Pin1 is unclear, because the same finding was not obtained in cultured cells such as HepG2 cells. In these cultured cells, reduced expression of Pin1 by siRNA enhanced PPARα transcriptional activity without altering the expression level of PPARα (data not shown). Thus, some contradictory results exist between the findings from mouse livers and cultured cells. Considering a previous report showing the suppression of PPARγ transcriptional activity by Pin1 (42), Pin1 may be an important regulator of PPAR family proteins, and further studies are thus required to clarify this issue.
In mouse livers, it is reasonable to theorize that increased Pin1 is involved in the reduced expressions of downstream lipolytic genes regulated by PPARα. Therefore, the Pin1 KO mouse liver, in which the suppression of PPARα activity by Pin1 does not occur, might be protected from steatohepatitis development.
Besides the pathway via PPARα, it should be noted that there are multiple possible mechanisms underlying Pin-1-induced fat accumulation. First, Pin1 enhances insulin signaling through the association with IRS-1, which contributes to increased lipid synthesis (21). Second, Pin1 reportedly suppresses cAMP-activated protein kinase signaling, through its association with the α subunit of cAMP-activated protein kinase (43), which leads to decreased lipolysis. Third, Pin1 associates with Crtc2 and suppresses cAMP-response element transcriptional activity, which potentially contributes to enhanced insulin sensitivity. A previous study revealed that the inhibition of cAMP-response element transcriptional activity enhances the liver triglyceride accumulation (44). Thus, we consider that at least the aforementioned four pathways (PPARα, IRS-1, cAMP-activated protein kinase, and Ctrc2) are involved in lipid accumulation by Pin1 and that Pin1 KO mice are resistant to hepatic fat accumulation. This may be similar to the oncogenic activity of Pin1; Pin1 activates more than 10 molecules to enhance cell proliferation while simultaneously suppressing a similar number of tumor suppressors (6–9).
The “second hit” process of NASH is characterized by inflammation, reactive oxygen species, and resultant fibrosis. TGFβ and CTGF are important profibrogenic cytokines. Elevations of these cytokines were reportedly detected in fibrotic human livers and MCD diet-fed rodents. We found that expressions of TGFβ and CTGF, two important fibrosis mediators, were far lower in Pin1 KO than in WT mice. Shen et al. (45) reported that Pin1 promotes the stability of TGFβ mRNA in eosinophils, and our results are consistent with this observation. Although the mechanism of CTGF induction by MCD-diet feeding has yet to be clarified, a recent report demonstrated that increased p53 induces CTGF synthesis (46), and it is well known that Pin1 promotes p53 transactivation (11, 12). Taken together, these results indicate that, while on the MCD diet, Pin1 may enhance the induction of CTGF expression through p53 activity.
In addition, Pin1 deficiency or Pin1 knockdown attenuated palmitate-induced inflammatory cytokine release from peritoneal macrophages or THP-1 cells. Consistent with our results, it was recently reported that Pin1 promotes cytokine release from bone marrow-derived macrophages by enhancing IRAK1 phosphorylation (19). Thus, Pin1 has an up-regulating effect on inflammatory cytokine expressions.
Finally, to distinguish both the metabolic and the inflammatory functions of Pin1 and to examine whether hematopoietic or nonhematopoietic Pin1 or both are critical for NASH development, mice lacking Pin1 in either nonhematopoietic or hematopoietic cells were produced by bone marrow transplantation between WT and Pin1 KO mice. A recent report showed that approximately one-third of Kupffer cells of recipient mice survived if clodronate was not used prior to radiation (47), although other hematopoietic cells were completely replaced. In our experiments, clodronate was not used, but co-immunostaining with anti-F4/80 and anti-Pin1 antibodies revealed that at least 80% of Kupffer cells had been replaced by radiation and subsequent bone marrow transplantation.
The mice with Pin1 in their hepatocytes (WW and WK in Fig. 6) exhibited fat accumulation in the liver with the MCD diet. It is reasonable to speculate that hepatic Pin1 is directly involved in the fat accumulation in hepatocytes. Interestingly, in WK mice, despite the fat accumulation in hepatocytes being comparable with that of WW mice, no fibrotic changes were detected. Thus, from the comparison between WW and WK mice, it is reasonable to conclude that hematopoietic Pin1 is critical for the advanced stage of NASH, which represents progression from simple fat accumulation to fibrosis. These results are in good agreement with a report that MCP-1-deficient mice were protected from liver fibrosis by the MCD diet but did not show the resistance to steatosis (32). Even considering the survival of some Kupffer cells, the fact that only the mice having both nonhematopoietic and hematopoietic Pin1 suffered from full manifestation of NASH clearly indicates both forms of Pin1 have critical roles. In other words, if either hepatic or hematopoietic Pin1 is deleted or suppressed, NASH does not develop.
In conclusion, this is the first demonstration of the critical role of Pin1 in the pathogenesis of NASH. Based on our present data, we propose Pin1 to be a possible novel target for NASH therapy. Because no abnormal function of the Pin1 KO mouse liver besides mild insulin resistance was reported, we believe this to be a potentially safe treatment strategy. To suppress Pin1 activity, two approaches are possible. One is gene silencing of Pin1 to normalize the up-regulated expression of Pin1 in the liver, whether in hepatocytes or macrophages, using siRNA or shRNA. The other approach is delivery of a Pin1-specific inhibitor to the liver or macrophages. We anticipate that these therapies will yield promising results in NASH rodent models. If so, research on this approach should be pursued by refining the procedures so as to develop administration methods acceptable for human use.
This work was supported by a grant-in-aid for young scientists (B) (to Y. N.), a grant-in-aid for scientific research on innovative areas (to T. A.), a grant-in-aid for scientific research (B) (to T. A.) from the Ministry of Education, Science, Sports, and Culture, Japan, and by Tsuchiya Foundation Grant 2010–2012 (to Y. N.).
- PPIase
- peptidyl-prolyl cis/trans isomerase
- NASH
- nonalcoholic steatohepatitis
- MCD
- methionine choline-deficient
- IRS
- insulin receptor substrate
- SREBP
- sterol regulatory element-binding protein
- TIMP
- tissue inhibitor of metalloproteinases
- MCP-1
- monocyte chemoattractant protein 1
- PPARα
- peroxisome proliferator-activated receptor α
- ACO
- acyl CoA oxidase
- AST
- aspartate transaminase
- ALT
- alanine aminotransferase
- CTGF
- connective tissue growth factor
- HSC
- hepatic stellate cell
- SMA
- smooth muscle actin
- ND
- normal diet.
REFERENCES
- 1. Lu K. P., Finn G., Lee T. H., Nicholson L. K. (2007) Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 3, 619–629 [DOI] [PubMed] [Google Scholar]
- 2. Chao Y., Xing Y., Chen Y., Xu Y., Lin Z., Li Z., Jeffrey P. D., Stock J. B., Shi Y. (2006) Structure and mechanism of the phosphotyrosyl phosphatase activator. Mol. Cell 23, 535–546 [DOI] [PubMed] [Google Scholar]
- 3. Leulliot N., Vicentini G., Jordens J., Quevillon-Cheruel S., Schiltz M., Barford D., van Tilbeurgh H., Goris J. (2006) Crystal structure of the PP2A phosphatase activator. Implications for its PP2A-specific PPIase activity. Mol. Cell 23, 413–424 [DOI] [PubMed] [Google Scholar]
- 4. Magnusdottir A., Stenmark P., Flodin S., Nyman T., Hammarström M., Ehn M., Bakali H. M. A., Berglund H., Nordlund P. (2006) The crystal structure of a human PP2A phosphatase activator reveals a novel fold and highly conserved cleft implicated in protein-protein interactions. J. Biol. Chem. 281, 22434–22438 [DOI] [PubMed] [Google Scholar]
- 5. Lu K. P., Hanes S. D., Hunter T. (1996) A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380, 544–547 [DOI] [PubMed] [Google Scholar]
- 6. Yeh E. S., Means A. R. (2007) PIN1, the cell cycle and cancer. Nat. Rev. Cancer 7, 381–388 [DOI] [PubMed] [Google Scholar]
- 7. Takahashi K., Uchida C., Shin R. W., Shimazaki K., Uchida T. (2008) Prolyl isomerase, Pin1. New findings of post-translational modifications and physiological substrates in cancer, asthma, and Alzheimer's disease. Cell. Mol. Life Sci. 65, 359–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Driver J. A., Lu K. P. (2010) Pin1. A new genetic link between Alzheimer's disease, cancer, and aging. Curr. Aging Sci. 3, 158–165 [DOI] [PubMed] [Google Scholar]
- 9. Lee T. H., Pastorino L., Lu K. P. (2011) Peptidyl-prolyl cis-trans isomerase Pin1 in ageing, cancer, and Alzheimer disease. Expert Rev. Mol. Med. 13, e21. [DOI] [PubMed] [Google Scholar]
- 10. Zhou X. Z., Kops O., Werner A., Lu P. J., Shen M., Stoller G., Küllertz G., Stark M., Fischer G., Lu K. P. (2000) Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and Tau proteins. Mol. Cell 6, 873–883 [DOI] [PubMed] [Google Scholar]
- 11. Zacchi P., Gostissa M., Uchida T., Salvagno C., Avolio F., Volinia S., Ronai Z., Blandino G., Schneider C., Del Sal G. (2002) The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419, 853–857 [DOI] [PubMed] [Google Scholar]
- 12. Zheng H., You H., Zhou X. Z., Murray S. A., Uchida T., Wulf G., Gu L., Tang X., Lu K. P., Xiao Z. X. (2002) The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature 419, 849–853 [DOI] [PubMed] [Google Scholar]
- 13. Lu K. P. (2003) Prolyl isomerase Pin1 as a molecular target for cancer diagnostics and therapeutics. Cancer Cell 4, 175–180 [DOI] [PubMed] [Google Scholar]
- 14. Wulf G., Ryo A., Liou Y. C., Lu K. P. (2003) The prolyl isomerase Pin1 in breast development and cancer. Breast cancer Res. 5, 76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim M. R., Choi H. K., Cho K. B., Kim H. S., Kang K. W. (2009) Involvement of Pin1 induction in epithelial-mesenchymal transition of tamoxifen-resistant breast cancer cells. Cancer Sci. 100, 1834–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan X., Zhou F., Wan J., Hang J., Chen Z., Li B., Zhang C., Shao K., Jiang P., Shi S., Feng X., Lv N., Wang Z., Ling Y., Zhao X., Ding D., Sun J., Xiong M., He J. (2010) Pin1 expression contributes to lung cancer. Prognosis and carcinogenesis. Cancer Biol. Ther. 9, 111–119 [DOI] [PubMed] [Google Scholar]
- 17. Lu P. J., Wulf G., Zhou X. Z., Davies P., Lu K. P. (1999) The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated Tau protein. Nature 399, 784–788 [DOI] [PubMed] [Google Scholar]
- 18. Lim J., Balastik M., Lee T. H., Nakamura K., Liou Y. C., Sun A., Finn G., Pastorino L., Lee V. M., Lu K. P. (2008) Pin1 has opposite effects on wild-type and P301L Tau stability and tauopathy. J. Clin. Invest. 118, 1877–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tun-Kyi A., Finn G., Greenwood A., Nowak M., Lee T. H., Asara J. M., Tsokos G. C., Fitzgerald K., Israel E., Li X., Exley M., Nicholson L. K., Lu K. P. (2011) Essential role for the prolyl isomerase Pin1 in Toll-like receptor signaling and type I interferon-mediated immunity. Nat. Immunol. 12, 733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakatsu Y., Sakoda H., Kushiyama A., Ono H., Fujishiro M., Horike N., Yoneda M., Ohno H., Tsuchiya Y., Kamata H., Tahara H., Isobe T., Nishimura F., Katagiri H., Oka Y., Fukushima T., Takahashi S., Kurihara H., Uchida T., Asano T. (2010) Pin1 associates with and induces translocation of CRTC2 to the cytosol, thereby suppressing cAMP-responsive element transcriptional activity. J. Biol. Chem. 285, 33018–33027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakatsu Y., Sakoda H., Kushiyama A., Zhang J., Ono H., Fujishiro M., Kikuchi T., Fukushima T., Yoneda M., Ohno H., Horike N., Kanna M., Tsuchiya Y., Kamata H., Nishimura F., Isobe T., Ogihara T., Katagiri H., Oka Y., Takahashi S., Kurihara H., Uchida T., Asano T. (2011) Peptidyl-prolyl cis/trans isomerase NIMA-interacting 1 associates with insulin receptor substrate-1 and enhances insulin actions and adipogenesis. J. Biol. Chem. 286, 20812–20822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atkinson G. P., Nozell S. E., Harrison D. K., Stonecypher M. S., Chen D., Benveniste E. N. (2009) The prolyl isomerase Pin1 regulates the NF-κB signaling pathway and interleukin-8 expression in glioblastoma. Oncogene 28, 3735–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryo A., Suizu F., Yoshida Y., Perrem K., Liou Y. C., Wulf G., Rottapel R., Yamaoka S., Lu K. P. (2003) Regulation of NF-κB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 12, 1413–1426 [DOI] [PubMed] [Google Scholar]
- 24. Gelman A. E., Zhang J., Choi Y., Turka L. A. (2004) Toll-like receptor ligands directly promote activated CD4+ T cell survival. J. Immunol. 172, 6065–6073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cannons J. L., Yu L. J., Hill B., Mijares L. A., Dombroski D., Nichols K. E., Antonellis A., Koretzky G. A., Gardner K., Schwartzberg P. L. (2004) SAP regulates T(H)2 differentiation and PKC-θ-mediated activation of NF-κB1. Immunity 21, 693–706 [DOI] [PubMed] [Google Scholar]
- 26. Chiang D. J., Pritchard M. T., Nagy L. E. (2011) Obesity, diabetes mellitus, and liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G697–G702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hebbard L., George J. (2011) Animal models of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 8, 35–44 [DOI] [PubMed] [Google Scholar]
- 28. Schattenberg J. M., Schuppan D. (2011) Nonalcoholic steatohepatitis. The therapeutic challenge of a global epidemic. Curr. Opin. Lipidol. 22, 479–488 [DOI] [PubMed] [Google Scholar]
- 29. Duvnjak M., Tomasic V., Gomercic M., Smircic Duvnjak L., Barsic N., Lerotic I. (2009) Therapy of nonalcoholic fatty liver disease. Current status. J. Physiol. Pharmacol. 60, 57–66 [PubMed] [Google Scholar]
- 30. Satapathy S. K., Sanyal A. J. (2010) Novel treatment modalities for nonalcoholic steatohepatitis. Trends Endocrinol. Metab. 21, 668–675 [DOI] [PubMed] [Google Scholar]
- 31. Fujimori F., Takahashi K., Uchida C., Uchida T. (1999) Mice lacking Pin1 develop normally, but are defective in entering cell cycle from G0 arrest. Biochem. Biophys. Res. Commun. 265, 658–663 [DOI] [PubMed] [Google Scholar]
- 32. Kassel K. M., Guo G. L., Tawfik O., Luyendyk J. P. (2010) Monocyte chemoattractant protein-1 deficiency does not affect steatosis or inflammation in livers of mice fed a methionine-choline-deficient diet. Lab. Invest. 90, 1794–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haque J. A., McMahan R. S., Campbell J. S., Shimizu-Albergine M., Wilson A. M., Botta D., Bammler T. K., Beyer R. P., Montine T. J., Yeh M. M., Kavanagh T. J., Fausto N. (2010) Attenuated progression of diet-induced steatohepatitis in glutathione-deficient mice. Lab. Invest. 90, 1704–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ip E., Farrell G., Hall P., Robertson G., Leclercq I. (2004) Administration of the potent PPARα agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 39, 1286–1296 [DOI] [PubMed] [Google Scholar]
- 35. Tomita K., Oike Y., Teratani T., Taguchi T., Noguchi M., Suzuki T., Mizutani A., Yokoyama H., Irie R., Sumimoto H., Takayanagi A., Miyashita K., Akao M., Tabata M., Tamiya G., Ohkura T., Hibi T. (2008) Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology 48, 458–473 [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi K., Itoh Y., Yokomizo C., Nishimura T., Niimi T., Fujii H., Okanoue T., Yoshikawa T. (2010) Blockade of interleukin-6 signaling enhances hepatic steatosis but improves liver injury in methionine choline-deficient diet-fed mice. Lab. Invest. 90, 1169–1178 [DOI] [PubMed] [Google Scholar]
- 37. Witek R. P., Stone W. C., Karaca F. G., Syn W. K., Pereira T. A., Agboola K. M., Omenetti A., Jung Y., Teaberry V., Choi S. S., Guy C. D., Pollard J., Charlton P., Diehl A. M. (2009) pan-Caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology 50, 1421–1430 [DOI] [PubMed] [Google Scholar]
- 38. Uno M., Kurita S., Misu H., Ando H., Ota T., Matsuzawa-Nagata N., Kita Y., Nabemoto S., Akahori H., Zen Y., Nakanuma Y., Kaneko S., Takamura T. (2008) Tranilast, an antifibrogenic agent, ameliorates a dietary rat model of nonalcoholic steatohepatitis. Hepatology 48, 109–118 [DOI] [PubMed] [Google Scholar]
- 39. Seki E., De Minicis S., Osterreicher C. H., Kluwe J., Osawa Y., Brenner D. A., Schwabe R. F. (2007) TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 [DOI] [PubMed] [Google Scholar]
- 40. Serviddio G., Giudetti A. M., Bellanti F., Priore P., Rollo T., Tamborra R., Siculella L., Vendemiale G., Altomare E., Gnoni G. V. (2011) Oxidation of hepatic carnitine palmitoyltransferase-I (CPT-I) impairs fatty acid β-oxidation in rats fed a methionine-choline deficient diet. PloS ONE 6, e24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Da Silva Morais A., Lebrun V., Abarca-Quinones J., Brichard S., Hue L., Guigas B., Viollet B., Leclercq I. A. (2009) Prevention of steatohepatitis by pioglitazone: implication of adiponectin-dependent inhibition of SREBP-1c and inflammation. J. Hepatol. 50, 489–500 [DOI] [PubMed] [Google Scholar]
- 42. Fujimoto Y., Shiraki T., Horiuchi Y., Waku T., Shigenaga A., Otaka A., Ikura T., Igarashi K., Aimoto S., Tate S., Morikawa K. (2010) Proline cis/trans isomerasePin1 regulates peroxisome proliferator-activated receptor γ activity through the direct binding to the activation function-1 domain. J. Biol. Chem. 285, 3126–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khanal P., Kim G., Yun H. J., Cho H. G., Choi H. S. (2012) The prolyl isomerase Pin1 interacts with and down-regulates the activity of AMPK leading to induction of tumorigenicity of hepatocarcinoma cells. Mol. Carcinog., in press [DOI] [PubMed] [Google Scholar]
- 44. Saberi M., Bjelica D., Schenk S., Imamura T., Bandyopadhyay G., Li P., Jadhar V., Vargeese C., Wang W., Bowman K., Zhang Y., Polisky B., Olefsky J. M. (2009) Novel liver-specific TORC2 siRNA corrects hyperglycemia in rodent models of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 297, E1137–E1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen Z. J., Esnault S., Rosenthal L. A., Szakaly R. J., Sorkness R. L., Westmark P. R., Sandor M., Malter J. S. (2008) Pin1 regulates TGF-β1 production by activated human and murine eosinophils and contributes to allergic lung fibrosis. J. Clin. Invest. 118, 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kodama T., Takehara T., Hikita H., Shimizu S., Shigekawa M., Tsunematsu H., Li W., Miyagi T., Hosui A., Tatsumi T., Ishida H., Kanto T., Hiramatsu N., Kubota S., Takigawa M., Tomimaru Y., Tomokuni A., Nagano H., Doki Y., Mori M., Hayashi N. (2011) Increases in p53 expression induce CTGF synthesis by mouse and human hepatocytes and result in liver fibrosis in mice. J. Clin. Invest. 121, 3343–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klein I., Cornejo J. C., Polakos N. K., John B., Wuensch S. A., Topham D. J., Pierce R. H., Crispe I. N. (2007) Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood 110, 4077–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]