Background: Molecular details of cofactor interaction with the intrinsically disordered N-terminal glucocorticoid receptor (GR) AF1 transactivation domain are poorly understood.
Results: Biochemical and biophysical studies of GR AF1 binding to the N terminus of the coactivator TIF2 are described.
Conclusion: Binding the TIF2 N terminus increases GR AF1 domain α-helical content.
Significance: A novel TIF2-induced conformational reorganization of GR helps explain coactivator activity.
Keywords: Glucocorticoid Receptor, Intrinsically Disordered Proteins, Protein Folding, Steroid Hormone Receptor, Transcription Coactivators
Abstract
Control of gene transcription by glucocorticoid receptors (GRs) is important for many physiological processes. Like other steroid hormone receptors, the regulation of target genes by GR is mediated by two transactivation domains: activation function 1 (AF1) in the N-terminal domain and AF2 in the C-terminal ligand-binding domain (LBD). Full receptor activity requires both AF1 and -2 plus assorted coregulatory proteins. Crystal structures of the ligand-bound LBD have provided insight regarding how AF2 interacts with specific coactivators. However, despite its being the major activation domain of GRs, knowledge of AF1 structure/function has languished. This is mainly because of the highly disorganized structure of the GR N-terminal domain. This lack of AF1 structure is shared by all members of the steroid/nuclear receptor superfamily for which it has been examined and AF1 is thought to allow productive interactions with assorted cofactors via protein-induced changes in secondary/tertiary structures. To date, there are no reports of a classical coactivator altering the secondary/tertiary structure of the GR AF1 domain. Earlier, we reported an N-terminal fragment of the p160 coactivator TIF2, called TIF2.0, that binds the GR N-terminal domain and alters GR transcriptional activity. We therefore proposed that TIF2.0 binding to AF1 changes both its conformation and transcriptional activity. We now report that TIF2.0 interacts with the GR AF1 domain to increase the amount of α-helical structure in the complex. Furthermore, TIF2 coactivator activity is observed in the absence of the GR LBD in a manner that requires the AF1 domain. This contrasts with previous models where TIF2 receptor interaction domains binding to GR LBD somehow alter AF1 conformation. Our results establish for the first time that coactivators can modify the structure of the AF1 domain directly via the binding of a second region of the coactivator and suggest a molecular explanation for how coactivators increase the transcriptional activity of GR-agonist complexes.
Introduction
The control of gene transcription by glucocorticoid receptors (GRs)5 in response to changing concentrations of endogenous steroid hormone is important for many physiological processes including lung maturation, inflammation, autoimmune diseases, and cancer (1–3). The basic steps have been known for many years. After entering the cell by passive diffusion, the steroid binds to GR, which is mostly cytoplasmic (4, 5). After a still poorly understood phenomenon called activation, the receptor-steroid complex concentrates in the nucleus, where it binds to both biologically active and inactive sites on DNA and chromatin (6, 7). This binding sets in motion the recruitment of a large number of cofactors and comodulators that influence the level and nature of gene expression in a cell- and gene-selective manner (8). Chromatin reorganization is often thought to be affected by steroid receptor binding to genomic sites, but recent reports suggest that this may play a minor role for most GR-regulated genes (9–11).
Gene transactivation, as well as repression, by GRs is mediated by two transactivation domains: activation function 1 (AF1) in the N-terminal half of GR and AF2 in the C-terminal ligand-binding domain (LBD). Of the two, the AF1 region is about 5–10 times more active than the AF2 domain (12, 13). X-ray structural analysis has identified the AF2 domain as being a highly ordered three-dimensional pocket, comprising α-helices 3, 4, 5, and 12 of the GR LBD, into which three leucine side chains of the coactivator peptide encompassing the receptor interaction domain (RID) are inserted (14). Even in the absence of associated coactivator, most of the α-helical structures of the GR LBD are retained.
In marked contrast, the N-terminal domain of GR is highly unstructured and exists as an intrinsically disordered (ID) domain. This is not unique to GR but is commonly seen for many transcription factors, including all members of the steroid/nuclear receptor superfamily for which it has been examined (15–17). Even when stabilized in a crystal lattice, the N-terminal region of steroid/nuclear receptors does not assume a unique structure (18). This conformational disorganization appears to be a hallmark of transcriptionally active sequences and AF1 is thought to allow the transactivation domains to form productive interactions with a larger variety of cofactors/comodulators via protein-induced changes in tertiary and quaternary structures (19, 20). ID regions are commonly found in transcription factors and cofactors (16, 17). Recent studies suggest that ID regions promote molecular recognition by forming large interaction surfaces, which allow ID regions to identify and bind many biological targets with high specificity and low binding affinity (16, 17). This is an important phenomenon that frequently occurs during signal transduction, recognition, and regulation events (16, 17). It has been proposed that such interactions are often accompanied by disorder-order transitions resulting in increased structure formation in ID regions (16, 17). This hypothesis has been experimentally supported for GRs, where the α-helical content of the GR AF1 domain was found to increase upon binding the core of the TATA-binding protein (21–23). Similarly, the DNA binding of GRs lacking the LBD but retaining the N-terminal domain and the DNA-binding domain is able to alter the tertiary structure of the N-terminal domain of GR (24). Some proteins also modify the conformation/activity of N-terminal regions of steroid receptors by binding to their DBD (DNA-binding domain)/LBD (25–27). Thus, even regions that do not contact a binding molecule can be affected (20, 28, 29).
To date, there are no reports of a coactivator altering the secondary/tertiary structure of the GR AF1 domain. In fact, evidence that a coactivator interacts directly with the N-terminal domain of GR has emerged only recently. Studies with an N-terminal fragment of the p160 coactivator TIF2, called TIF2.0, unexpectedly revealed that not only does it bind to GRs but also that it associates with the N-terminal half of GR. No interaction of TIF2.0 was detected with GR LBD (30–32), which is where TIF2 was first found to bind for most receptors (33–35). Furthermore, this binding of TIF2.0 to GRs was capable of preventing the interactions of GR with the corepressor NCoR (30–32). This suggests that the formation of TIF2 and GR complexes is more complicated than previously considered and provides greater molecular details for how the ratio of coactivators and corepressors can control the net activity of GR-agonist steroid complexes (30).
In view of the ability of TIF2.0 to bind to the N terminus of GR and modify GR transcriptional activity, it was reasonable to propose that TIF2.0 binds to the AF1 domain of GR, thereby changing the protein conformation and eventually GR transcriptional activity. The purpose of this study was to see if we could obtain direct evidence to support this hypothesis. We now report biophysical evidence that preparations of purified TIF2.0 retaining the previously reported biological activities do interact with the GR AF1 domain to increase the amount of α-helical structure in the complex. These results establish for the first time that a coactivator can directly modify the structure of the most potent activation domain of GRs and suggest a molecular explanation for how coactivators increase the transcriptional activity of GR-agonist complexes.
EXPERIMENTAL PROCEDURES
Antibodies
The following antibodies are available commercially: mouse anti-FLAG M2 monoclonal antibody (Sigma), rabbit anti-GR monoclonal antibodies (Affinity BioReagents), anti-TIF2 mouse monoclonal antibody (BD Biosciences), HRP-conjugated goat anti-rabbit IgG and anti-mouse IgG-HRP (Santa Cruz Biotechnology). A second rabbit anti-GR antibody (against amino acids 150–175 of the human GR) was a gift from Dr. E. Brad Thompson (University of Texas Medical Branch, Galveston).
Plasmids
pSG5 and pBSK+ were purchased from Stratagene. The pM and VP16 vectors were from Clontech (Palo Alto, CA). FLAG/BAP (bovine alkaline phosphatase) was obtained from Sigma. The pFR-Luc reporter, which contains five repeats of the GAL4 binding element fused upstream of a basic TATA promoter and the luciferase reporter, is available from Stratagene (La Jolla, CA). pRL-TS (Renilla) was a kind gift from Drs. Nasreldin M. Ibrahim and Otto Fröhlich (Department of Physiology) and Dr. S. Russ Price (Department of Medicine) at Emory University School of Medicine, Atlanta, GA. pCMX and pCMX-GAL-NCoR-RID (amino acids 1944–2453) were a gift (Mitch Lazar, University of Pennsylvania School of Medicine) as were pSG5/TIF2 and pSG5/TIF2.0 (Hinrich Gronemeyer, Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) and pSVLGR (Keith Yamamoto, University of California, San Francisco). Rat GR (pSG5-GR), pSG5-HA/TIF2.0, pSG5-hSA, pSG5-GRN523, and VP16/GR have been described previously6 (30, 36). pFLAG-CTC was purchased from Sigma. TIF2.0/FLAG was prepared by generating the TIF2.0 cDNA fragment by PCR from pSG5/TIF2 using sense (5′-CCT CGA GAT GAG TGG GAT GGG A-3′) and antisense (5′-AGT GAA TTC CTA GTC AGC TCT-3′) primers flanked at their 5′-ends by XhoI and EcoRI consensus sequences, respectively. The purified PCR product was cloned into the XhoI-EcoRI sites of pFLAG-CTC and confirmed by sequencing.
Bacterial Expression of TIF2.0/FLAG
BL21(DE3) codon plus RIPL (Stratagene) was transformed with pFLAG-CTC/TIF2.0 plasmid following the manufacturer's protocol. A single colony was picked and inoculated into 3 ml of Luria-Bertani broth with 100 μg/ml carbenicillin. After overnight culture, 5 ml of bacterial culture was diluted into 500 ml of Luria-Bertani broth containing 100 μg/ml carbenicillin and shaken at 37 °C until the bacterial density became 0.8. Isopropyl β-d-thiogalactopyranoside was added to the culture to a final concentration 1 mm, and the mixture was shaken at 37 °C until density became 1.2 (2–3 h). The cells were harvested by centrifugation, washed once with PBS, and stored at −80 °C until used.
Extraction and Purification of TIF2.0/FLAG and GR AF1
Bacterial pellets were lysed by suspension in 25 ml of Bugbuster protein extraction reagent (Novagen) containing 1 unit/μl lysozyme (Novagen), 25 units/μl benzonase nuclease (Novagen), and protease inhibitor mixture (Roche Applied Science) and shaken at 37 °C for 20 min followed by centrifugation at 15,000 × g at 4 °C for 20 min. The lysate was affinity-purified on an anti-FLAG M2-agarose column. Briefly, 4 ml of 50% anti-FLAG M2-agarose (Sigma) slurry was incubated with 5 ml of 0.1 m glycine-HCl for 5 min at room temperature with rotation followed by neutralization by adding 5 ml of buffer A (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, pH 7.5) and centrifugation at 500 × g for 2 min at 4 °C. Beads were then washed with buffer A three times and incubated with lysate with rotation over night at 4 °C. The lysate and beads were packed in a mini column (Bio-Rad) with gravity flow. The flow-through was passed back over the column twice followed by washing the column with buffer B (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1% Nonidet P-40). The TIF2.0/FLAG fraction was eluted (1 ml/fraction) with 10 ml of 3 × FLAG peptide (Sigma) (200 μg/ml buffer A). The purity of each fraction was determined by SDS-PAGE. The purer fractions were pooled followed by Microcon YM-100 filtration to remove remaining 3 × FLAG and stored at 4 °C. The human GR AF1 domain (amino acids 77–262) was constructed from human GR cDNA digested with BglII and inserted into expression vector pGEX-4T-1 (Amersham Biosciences) as described (37). The overexpression and purification of AF1 protein is described elsewhere (37, 38). The final protein purity of GR AF1 was greater than 95%, whereas TIF2.0 was ≥90%, and usually ≥93%, as verified by the presence of a single band on SDS-PAGE.
Cell Culture, Transient Transfection, and Reporter Gene Analyses
Triplicate samples of cells were transiently transfected in 24-well plates with luciferase reporter plasmids as described for CV-1 (36). The total transfected DNA was adjusted to 300 ng/well of a 24-well plate with pBluescript II SK (Stratagene). The molar amount of plasmids expressing different protein constructs was kept constant with the addition of empty vector. pRL-TS (Renilla; 10 ng/well of a 24-well plate) was included as an internal control for transfection efficiency. After 24 h, cells were treated with DMEM containing 10% FBS and appropriate hormone dilutions. Sixteen hours later, the cells were lysed and assayed for reporter gene activity using Dual-Luciferase assay reagents according to the manufacturer's instructions (Promega, Madison, WI). Luciferase activity was measured by an EG&G Berthold luminometer (Microlumat LB 96P). The data were normalized to Renilla activity and expressed as a percentage of the maximal response with dexamethasone (Dex; from Sigma-Aldrich) before being plotted ±S.E. unless noted otherwise.
Mammalian Two-hybrid Assay
The recommended procedure for the Mammalian Matchmaker two-hybrid assay kit (Clontech) was modified slightly by changing from a chloramphenicol acetyltransferase reporter to a luciferase reporter pFRLuc (Stratagene), which is under the control of five repeats of the upstream activating sequence for the binding of GAL4. For competition assays, GR was fused to the VP16 activation domain, and NCoR-RID (amino acids 1944–2453) was ligated to the GAL4 DNA-binding domain. TIF2 and TIF2.0 fragments were used without any fused protein.
Cytosolic GR Preparations
COS-7 cells were seeded in 150-mm dishes at 106 cells/dish containing 20 ml of medium plus 5% FCS. On the next day, 15 μg of DNA/dish was transfected with 40 μl of FuGENE 6 (Roche Applied Science) reagent. One day later, cells were treated with fresh medium containing 1 μm Dex for 2 h, washed once with 20 ml of PBS at room temperature, and lysed for 10 min at room temperature with 1.5 ml/150 mm dish of CytoBuster protein extraction reagent (Novagen) containing protease inhibitor mixture (Roche Applied Science). The lysate was collected with a cell scraper and centrifuged for 20 min at 16,000 × g (4 °C). Cytosols were aliquotted and stored at −80 °C until used.
Competitive Pulldown Assays
Bacterial lysates (0.5 ml) containing overexpressed TIF2.0/FLAG and purified TIF2.0/FLAG protein (2 μg/10 μl) were incubated with 20 μl of anti-FLAG M2-agarose beads for 2 h at 4 °C with rotation. The mixture was centrifuged (5000 × g) for 2 min, the supernatant was discarded, and the pellet was washed (4 times with 1 ml each) with 50 mm Tris, pH 7.5, 150 mm NaCl, and 0.1% Nonidet P-40. Each 20 μl sample of immobilized TIF2.0/FLAG was then incubated overnight with 10 μl of recombinant GR AF1 and 100 μl of COS-7 cytosol overexpressing GR, TIF2, GRN523, or HA-TIF2.0 in different combinations at 4 °C. To gauge the effect of sodium molybdate on the GR-TIF2.0 interaction, COS-7 cytosol overexpressing GR was incubated with 20 mm sodium molybdate in the presence of 1 μm Dex or 1 μm RU486 (gift from Etienne Baulieu, Paris, France) for 2.5 h followed by heating at 20 °C for 20 min. This cytosolic mixture was incubated with anti-FLAG M2-agarose prebound with TIF2.0/FLAG overnight at 4 °C with rotation. The matrix was washed (4 times with 1 ml each) with 50 mm Tris, pH 7.5, 150 mm NaCl, and 0.1% Nonidet P-40. The immobilized proteins were removed from the beads by heating at 90 °C for 5 min in 20 μl of 6× SDS loading buffer. The proteins were then separated on 10% SDS-PAGE and visualized by Western blotting.
Surface Plasmon Resonance (SPR) Analysis
The kinetics of TIF2.0 binding to GR AF1 was determined by SPR on a Biacore X-100 Plus (GE Healthcare). The binding reaction was carried out at room temperature in a physiologic buffer (0.01 m HEPES, pH 7.4, 0.15 m NaCl, 50 μm EDTA, 0.05% Tween-20). Purified GR AF1 was immobilized to the Fc2 channel of a CM5 chip by amine coupling as the ligand at 150–200 Response Units. The Fc1 channel was treated equally but without protein as the control. A multi-cycle kinetics procedure was employed to measure the binding. TIF2.0/FLAG at different concentrations (0.1–3.2 μm), used as the analyte, was injected sequentially over Fc1 and Fc2 channels to measure its binding to AF1. The sensor surface was regenerated by 0.3% SDS after each cycle of binding. The flow rate was kept constant at 30 μl/min. Data from 180 s of association and 240 s of dissociation were collected. The sensorgrams were normalized by the subtraction of Fc1 from Fc2. One medium concentration was repeated twice to monitor the reproducibility of the assay. The data were double reference-subtracted and fitted for kinetics using various models depending on the actual binding reaction pattern (BIAevaluation 3.0 software). The dissociation constant (KD) was calculated from the equation KD = kd/ka, where ka is the association rate and kd the dissociation rate.
Circular Dichroism (CD) Spectroscopy
The far-UV CD spectra of the purified recombinant AF1, TIF2.0, and AF1:TIF2.0 mixtures were recorded at 22 °C on a Jasco 815 spectropolarimeter by using a 0.1-cm quartz cell with a bandwidth of 0.5 nm and a scan step of 0.5 nm. The spectra were recorded at a fixed AF1 protein concentration (4.5 μm) and varying concentrations of TIF2.0. All of the spectra recorded were corrected for the contribution of solute concentrations. Each spectrum is a result of five spectra accumulated, averaged, and smoothed. Qualitative analyses of secondary structural elements from CD data were carried out using the K2d algorithm (39).
Temperature-induced Denaturation Monitored by Circular Dichroism
Protein stability was determined by thermal denaturation by monitoring the decrease in α-helical content at a wavelength of 222 nm with increasing temperature. For thermal melting experiments, data points were taken at 1 °C intervals at a scan rate of 60 °C/h. The temperature dependence of the ellipticity (θ) was fitted to obtain a fraction of the unfolded state, fU(t), using a nonlinear least-squares algorithm assuming a two-state unfolding reaction as described (40).
Hydrogen/Deuterium Exchange and Mass Spectrometry
Solution-phase amide HDX was carried out with a fully automated system as described previously (41). Briefly, 4 μl of GR AF1 was diluted to 20 μl with D2O-containing HDX buffer and incubated at 4 °C for 10, 30, 60, 900, or 3600 s. Following the exchange, unwanted forward or back exchange was minimized, and the protein was denatured by dilution to 50 μl with 0.1% (v/v) TFA in 3 m urea and 0.1% n-octyl-β-d-glucoside (held at 1 °C). Samples were then passed across an immobilized pepsin column (prepared in-house (42)) at 50 μl min−1 (0.1% v/v TFA, 15 °C), and the resulting peptides were trapped on a C8 trap cartridge (Hypersil Gold, Thermo Fisher Scientific). Peptides were then gradient-eluted (4% (w/v) CH3CN to 40% (w/v) CH3CN, 0.3% (w/v) formic acid over 5 min at 2 °C) across a 1 × 50 mm C18 HPLC column (Hypersil Gold) and electrosprayed directly into an Orbitrap mass spectrometer (LTQ Orbitrap with ETD, Thermo Fisher Scientific). Peptide ion signals were confirmed if they had a MASCOT score of 20 or greater and had no ambiguous hits using a decoy (reverse) sequence in a separate experiment with a 60-min gradient. The intensity-weighted average m/z value (centroid) of the isotopic envelope of each peptide was calculated with in-house developed software (43) and corrected for back-exchange. To measure the difference in exchange rates, we calculated the average percentage of deuterium uptake for native GR AF1 following 10, 30, 60, 900, and 3600 s of exchange. From this value, we subtracted the average percentage of deuterium uptake measured for the TIF2.0-bound GR AF1.
Limited Proteolytic Digestion
Three sets of purified proteins (AF1, TIF2.0, and AF1:TIF2.0 mixture) were digested by using trypsin or chymotrypsin (Promega). Digestions were carried out at 4 °C by using a protein:enzyme mass ratio of 100:1. Reactions were terminated by adding SDS loading buffer and placing the sample tubes in boiling water. The proteolytic digestion products were resolved on SDS-PAGE followed by either Coomassie Blue R-250 staining or immunoblotting with an antibody for AF1.
Secreted Alkaline Phosphatase (SEAP) Promoter-Reporter Assays
For the promoter-reporter assay, we employed the SEAP reporter system. CV-1 monkey kidney epithelial cells (American Type Culture Collection) were grown at 37 °C in MEM with Earle's salts (Invitrogen) supplemented with 10% (v/v) fetal bovine serum (Atlanta Biologicals, Norcross, GA). Cells were subcultured every 2–3 days. Cells were plated on a 96-well plate 1 day before the transfection; they were transfected using Attractene Transfection Reagent (Qiagen) according to the manufacturer's protocol and maintained at 37 °C in 5% CO2/95% air for the duration of the experiment (24 h). Cells were cotransfected with 0.05 μg of pGRE/SEAP reporter vector, 0.05 μg of pECFP/GR500 or pECFP/GR500(ΔAF1), and 0.05 μg of pSG5/TIF2. The molar amount of plasmids expressing different protein constructs was kept constant with added empty vector. 24 h after transfection, 50 μl of medium was collected and tested for the presence of SEAP. Experiments were performed twice in triplicate. Data from different experiments were normalized to GR500 activity.
RESULTS
Expression and Purification of TIF2.0/FLAG
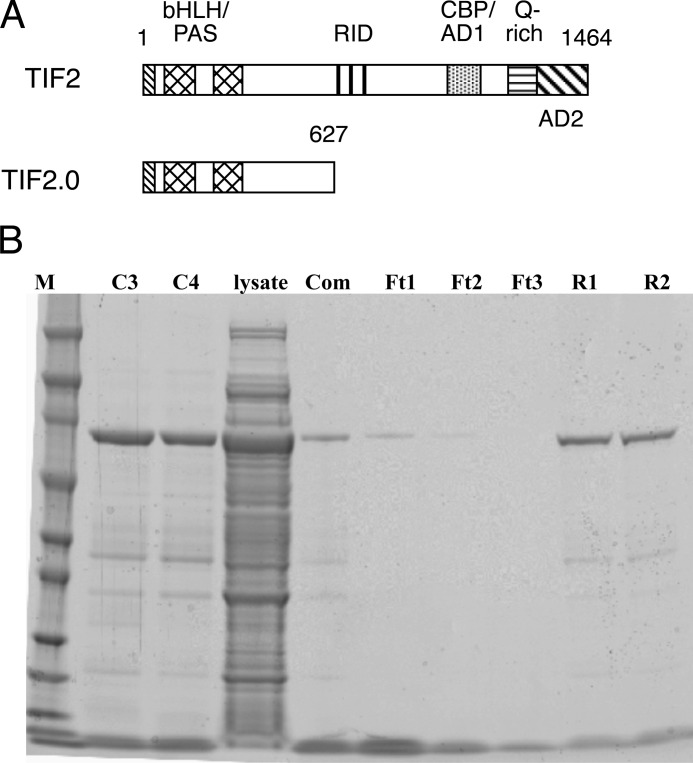
TIF2.0 comprises the N-terminal 627 amino acids of the p160 coactivator, TIF2 (Fig. 1A). The only known active protein sequences in this region are the basic helix-loop-helix (bHLH) and Per-Arnt-Sim (PAS) domains. TIF2.0 lacks the two major activation domains (AD1 and AD2). Most importantly, TIF2.0 is missing the nuclear receptor interaction domain containing three RIDs that are responsible for TIF2 binding via LXXLL sequences to steroid/nuclear receptors. Thus it was surprising to discover that TIF2.0 binds to and inhibits the biological activity of the N-terminal regions of GR and PR (30–32). To examine this in greater detail using purified components, we first constructed a chimera of TIF2.0 fused downstream of GST (GST/TIF2.0). The protein was expressed but was not retained on glutathione beads (data not shown). We therefore prepared TIF2.0 fused at its C terminus to a smaller protein, FLAG. TIF2.0/FLAG was expressed at reasonable levels in Escherichia coli. The recombinant protein was usually isolated in ≥93% purity via a three-step process of affinity chromatography on anti-FLAG columns, elution with FLAG peptide, and microfiltration to separate the FLAG peptide from TIF2.0/FLAG (Fig. 1B).
FIGURE 1.
TIF2.0 purification. A, diagram of domains of WT TIF2 and TIF2.0. B, Coomassie Blue-stained gel of stages of TIF2.0/FLAG purification. The lanes are as follow: M, molecular weight marker; C3 and C4, anti-FLAG M2-agarose column elution fractions 3 and 4; lysate, crude E. coli lysate; Com, combined elution fractions C3 and C4; Ft1–Ft3, YM-100 filtrates of three aliquots of combined C3 and C4 fractions; R1 and R2, YM-100 retentates of the two most concentrated aliquots of combined C3 and C4 fractions, which constitute the final purified material.
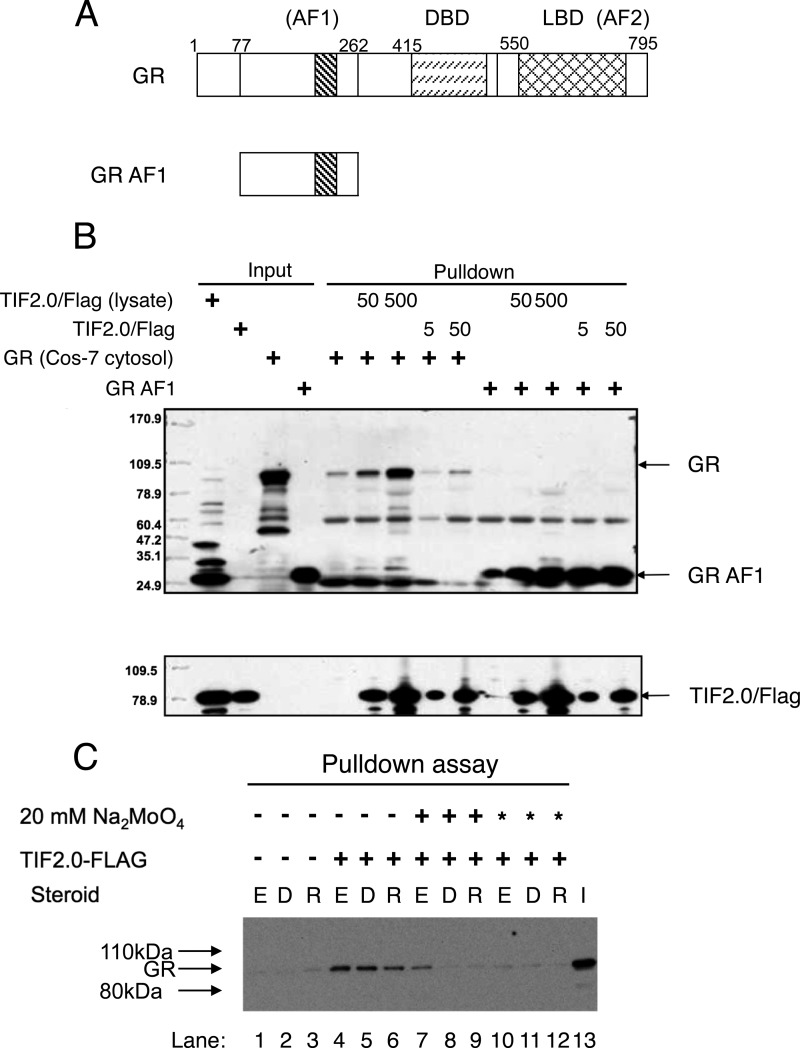
TIF2.0/FLAG binds to Activated GRs
A major question was whether TIF2.0/FLAG is functionally active either before or after purification. To address this question, we examined the binding of TIF2.0/FLAG to both full-length GR and the purified GR AF1 fragment (Fig. 2A). This was accomplished by mixing the various components, immunoadsorbing any complexes with anti-FLAG antibody beads, and then analyzing the isolated material by SDS gel chromatography and Western blotting. As shown in Fig. 2B, both the crude TIF2.0/FLAG bacterial lysate and the purified TIF2.0/FLAG cause co-immunoadsorption of full-length GR and the GR AF1 fragment. For both GR samples, there is some background binding to the beads. However, GR co-adsorption is greatly increased by the presence of TIF2.0/FLAG in a manner that is augmented by higher levels of TIF2.0/FLAG. It is interesting that the co-isolation of the GR AF1 fragment with either preparation of TIF2.0/FLAG (crude lysate or purified material) appears to be more efficient than that with the full-length GR. This may reflect the competition of TIF2.0/FLAG association by other GR-binding proteins in the cytosolic preparations of GR.
FIGURE 2.
TIF2.0/FLAG is functionally active. A, schematic of domains of WT GR and GR AF1 (amino acids 77–262). B, TIF2.0 pulldown of GR. Crude TIF2.0/FLAG lysate or purified TIF2.0/FLAG, was immobilized on anti-FLAG M2-agarose beads before being treated with crude overexpressed WT GR or purified GR AF1. The inputs and eluants of the beads were then analyzed by Western blotting with anti-GR antibody (upper panel) or anti-TIF2 antibody (lower panel). DBD, DNA-binding domain. C, TIF2.0 binding to GR requires activation of GR. Overexpressed WT GR (lanes 1–12) was incubated at 0 °C with vehicle (EtOH (E)), 1 μm dexamethasone (D), or 1 μm RU486 (R) under one of three conditions: −, no added Na2MoO4; +, Na2MoO4 added after steroid addition; and *, Na2MoO4 added before steroid addition. TIF2.0/FLAG (crude bacterial lysate) was then added as indicated. The rest of the assay was conducted as described in B using anti-GR antibody in the final Western blotting. I, input overexpressed GR.
Before GRs can bind to DNA, they must undergo a step called activation, which is accompanied by the dissociation of associated proteins such as Hsp90 from GRs (44). A long established reagent for studying this process is sodium molybdate, which blocks the activation of receptor-steroid complexes in general and GR complexes in particular (45). The binding of steroid-bound GR to the corepressor NCoR also requires activation of the receptor, as shown by the inhibitory effects of sodium molybdate (31). We therefore asked whether activation was required for TIF2.0/FLAG binding to full-length GRs. COS-7 cytosol containing overexpressed full-length GR was incubated with vehicle, Dex, or the antiglucocorticoid RU486 in one of three sequences: (a) without sodium molybdate, (b) immediately before adding 20 mm sodium molybdate to block activation, or (c) immediately after adding 20 mm sodium molybdate. These GR solutions were then incubated with TIF2.0/FLAG and subjected to immunoadsorption by beads coated with anti-FLAG antibody as shown in Fig. 2B. Crude lysate was used instead of purified TIF2.0/FLAG because of the more robust complex formation with unpurified TIF2.0/FLAG (seen in Fig. 2B). As shown in Fig. 2C, sodium molybdate blocks the co-isolation of GR with TIF2.0/FLAG. Furthermore, this inhibition is most effective when sodium molybdate is added before the steroid (Fig. 2C, lanes 10–12 versus 4–6 and 7–9). This is consistent with the significant amount of activation of GR that occurs even at 0 °C (46, 47). Similar experiments were not performed with the GR AF1 fragment, as activation has no meaning for this species, which cannot bind to DNA. These data argue not only that TIF2.0/FLAG is functionally active but also that it's binding to wild type GR requires activation of the receptor.
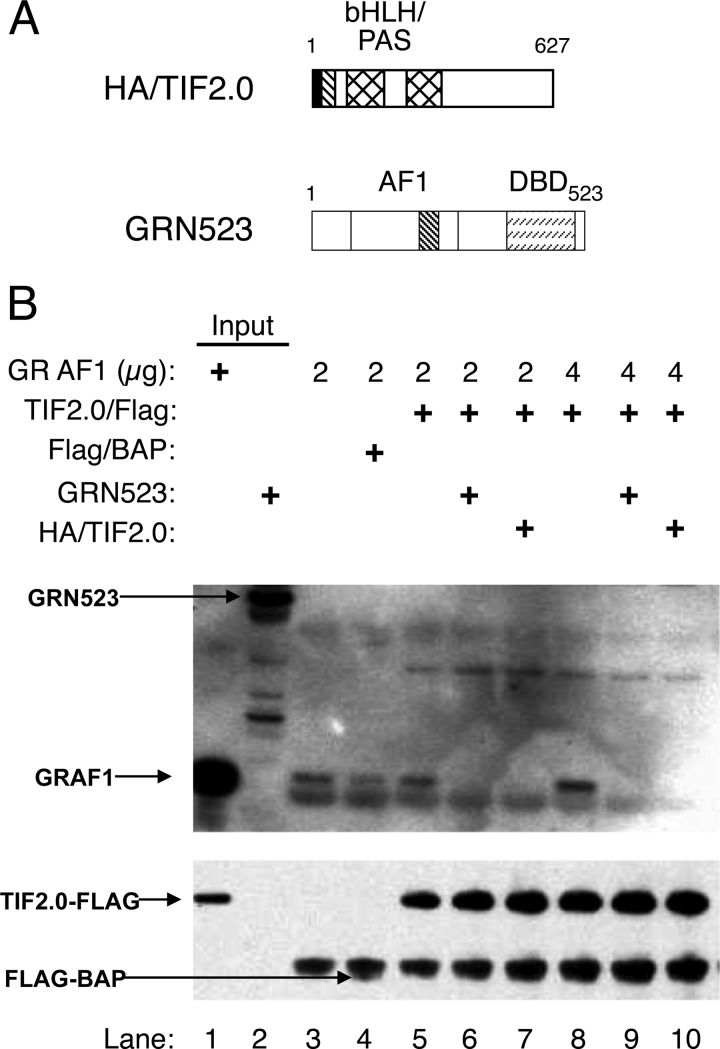
Competition of TIF2.0/FLAG Binding to GR
To obtain further evidence of the activity of TIF2.0/FLAG and of the specificity of its binding to GR, we asked whether these interactions could be competed by HA-tagged TIF2.0 and by a longer form of GR that contains the AF1 domain, i.e. GRN523 (Fig. 3A). For this experiment, purified GR AF1 was incubated with a control protein (FLAG-tagged BAP) or TIF2.0/FLAG without or with added GRN523 or HA/TIF2.0. FLAG/TIF2.0 and associated proteins were removed by anti-FLAG antibody-coated beads, and the proteins were visualized by Western blotting. As shown in Fig. 3B, the binding of a low concentration of GR AF1 to the beads is higher in the presence of TIF2.0/FLAG than the control protein FLAG/BAP (lane 5 versus 4). In the absence of added protein, the nonspecific binding of GR AF1 is significant (Fig. 3B, lane 3). More importantly, added GR523N and HA/TIF2.0 both inhibit the co-immunoprecipitation of GR AF1 by TIF2.0/FLAG. This competition is more pronounced with larger amounts of added GR AF1 (Fig. 3B, lanes 6–8). Thus, excess of either component appears to compete with the binding of GR AF1 to TIF2.0.
FIGURE 3.
GRN523 and HA/TIF2.0 compete for complex formation of GR AF1 and TIF2.0/FLAG. A, schematic of competing proteins HA/TIF2.0 and GRN523. B, competition of GR AF1 and TIF2.0/FLAG complex formation. Anti-FLAG M2-agarose beads were prebound with nothing (lane 3), the control protein FLAG/BAP (lane 4), or TIF2.0/FLAG (lanes 5–10) and then treated with low (2 μg) or high (4 μg) amounts of GR AF1 without or with competitor (GRN523 or HA/TIF2.0) as indicated. Samples were then analyzed as described in the legend for Fig. 2.
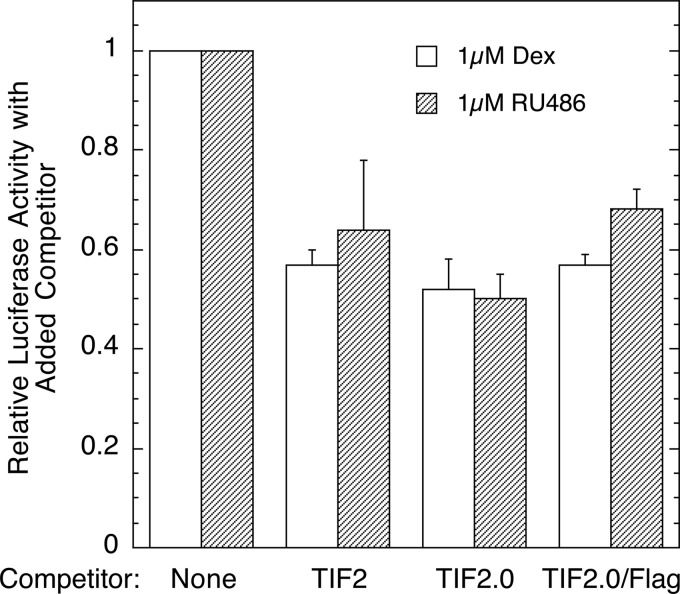
TIF2.0/FLAG Inhibits the Interactions of VP16/GR and GAL/NCoR-RID
The competitive binding shown in Fig. 3B occurred in a cell-free system with partially purified proteins. To determine whether TIF2.0/FLAG was also active in a whole cell environment, we asked whether TIF2.0/FLAG would be as effective as TIF2.0 (28–30) in preventing the functional interactions of GR binding to a corepressor, NCoR. Therefore, we examined the ability of full-length TIF2, TIF2.0, and TIF2.0/FLAG to interfere with the ability of VP16/GR to bind to GAL/NCoR-RID in a mammalian two-hybrid assay and thereby reduce the transcription of luciferase from a GAL-regulated luciferase reporter. Fig. 4 indicates that TIF2.0/FLAG does inhibit the biologically relevant interactions of GR and NCoR in intact cells. Furthermore, with the high concentrations of TIF2 plasmids employed, TIF2.0/FLAG appears to be as effective as TIF2.0 or full-length TIF2 in preventing the association of GR and NCoR to induce transactivation of the target luciferase reporter gene. Therefore, we concluded that TIF2.0/FLAG is functionally active in intact cells. Collectively, the above data argue that purified TIF2.0/FLAG retains biologically relevant functional activity and can be used under cell-free conditions to examine the biophysical properties of TIF2.0 interactions with the AF1 domain of GR.
FIGURE 4.
TIF2.0/FLAG is functionally active as a competitor in intact cells. A mammalian two-hybrid assay was conducted in triplicate as described under “Experimental Procedures,” where the indicated competitors (WT TIF2, TIF2.0, or TIF2.0/FLAG) were examined for their ability to disrupt the capacity of GAL/NCoR-RID and VP16/GR (with or without the agonist Dex or the antagonist RU486) to induce the reporter gene FRLuc. The data for each steroid are plotted relative to the luciferase activity of the uncompeted sample ± S.E. (n = 2).
Kinetics Analysis of GR AF1·TIF2.0 Binding by SPR
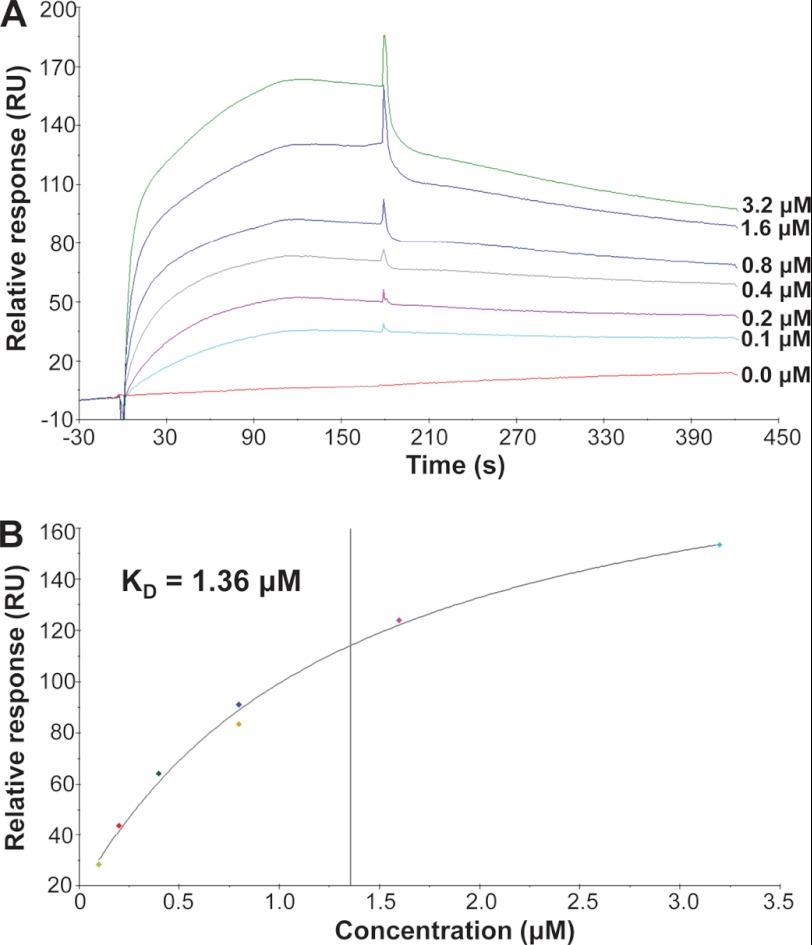
To measure the binding kinetics of AF1 and TIF2.0, we quantitated complex formation via surface plasmon resonance (SPR) using a Biacore X100 Plus. GR AF1 was immobilized onto a CM5 chip by chemical coupling. TIF2.0/FLAG was used as an analyte. A low density of AF1 (150–200 Response Units) was immobilized to eliminate mass transport limitation and heterogenic ligand for the kinetic assays. The regeneration was optimized to remove TIF2.0/FLAG after the binding. TIF2.0 exhibited specific binding to AF1 as indicated by a higher response and different kinetics in the assay channel (Fc2), whereas the control Fc1 channel was just the perturbation due to the analyte (data not shown). The adjusted sensorgrams show a typical dose-dependent response of binding but with a complicated kinetics (Fig. 5A). The spikes at the start and end of association are due to the change in buffer components derived from the analyte (TIF2.0/FLAG) solution, which is common in SPR assays. The overall binding showed a slow association and dissociation pattern. It is important to note that there were two phases within the association, with slight dissociation occurring during the later phase of association, producing decreased total response at the end of association. This result suggested the high probability of a conformational change in AF1 induced by TIF2.0 binding. Concomitantly, fitting the kinetics data with a heterogeneous ligand model was relatively the best among other fitting models, although none of those models seemed satisfied statistically (data not shown). The heterogeneous ligand model might indicate that AF1 underwent gradual conformational change upon the continuous binding to TIF2.0, as AF1 is known to be structurally disordered and tends to form different conformations upon binding to various transcriptional co-factors. To obtain the accurate binding affinity, a steady-state fitting model was used to eliminate the synergistic effect of binding and conformational change. As shown in Fig. 5B, the regression was remarkably optimal, with two independent experiments using 0.8 μm TIF2.0/FLAG as the quality control; the calculated Kd was 1.36 μm.
FIGURE 5.
Analysis of the binding of GR AF1 to TIF2.0 by SPR. Measurement of the binding of TIF2.0 to AF1 by SPR was carried out on a Biacore X-100. AF1 was immobilized to the Fc2 channel of CM5 as the ligand by amine coupling. The Fc1 channel was treated similarly but without AF1 protein (control). TIF2.0/FLAG was used as analyte to flow through both Fc1 and Fc2 channels for binding. A, the adjusted (Fc2 - Fc1) sensorgrams from a series of TIF2.0/FLAG concentrations (shown on the right) are plotted. B, the binding affinity of the AF1-TIF2.0 interaction as calculated by steady-state fitting.
During the development of the method, when FLAG-tagged TIF2.0 was first captured by FLAG antibody immobilized on CM5 chip to measure the binding to AF1 as the analyte, no specific binding was obtained (data not shown). Conversely, when His-tagged AF1 was immobilized on a nitrilotriacetic acid chip as the ligand, again no binding was observed with TIF2.0/FLAG as the analyte (data not shown). These results implied that the binding domains on TIF2.0 and AF1 might localize in the region close to the FLAG or His tags at the C- and N-terminal sequences of TIF2.0 and AF1, respectively, in which case the immobilization through these tags could constrain the movement of ligand and block the access to the analyte. In contrast, chemically induced coupling of AF1 to the chip appears to occur via other regions of the protein, which allows TIF2.0/FLAG binding to occur. Thus, these failed experiments provided important information regarding the structural requirements of TIF2.0-AF1 interaction, meriting a future mapping study of binding sites on TIF2.0 and AF1.
HDX-MS Confirms the Intrinsically Disordered Nature of GR AF1
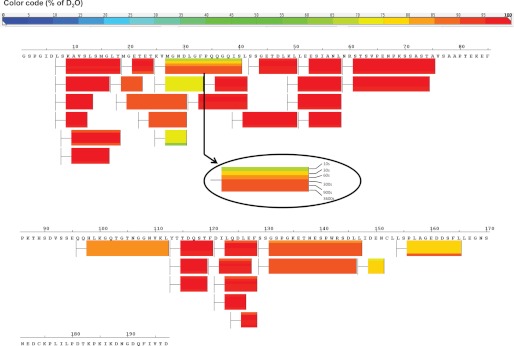
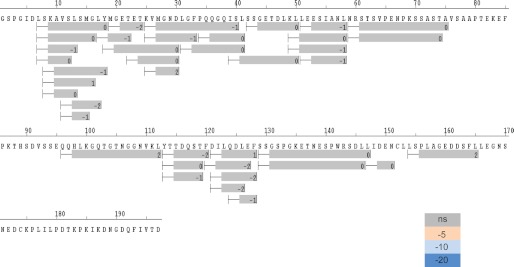
We examined the solution properties of AF1 domain using HDX mass spectrometry. HDX analysis revealed that most of the backbone amides were fully exchanged (>80%) within the smallest time frame (10 s of incubation) with deuterium, which is consistent with the predicted disordered nature of AF1 (Fig. 6). This same phenomenon was observed for TIF2.0-bound GR AF1, as we did not observe any significant protection in the differential HDX experiment (Fig. 7). It could be that the transient helical structures formed upon interaction with TIF2.0 interconvert to unstructured conformers over a faster time scale than the shortest time interval (10 s) we used for our HDX experiment. HDX-MS on a millisecond time scale might provide additional insight into the dynamics of these intrinsically disordered proteins.
FIGURE 6.
Solution properties of AF1 domain using HDX mass spectrometry. HDX protection map of GR-AF1. The bars below the sequence represent the peptide fragments resolved by mass spectrometry, and the color of the bars represents the relative deuterium/hydrogen exchange (color code at top). Each bar has six segments showing six different time points (see inset).
FIGURE 7.
Differential HDX map comparing protection of GR AF1 and TIF2.0-bound GR AF1. The bars below the sequence represent the peptide fragments resolved by mass spectrometry, and the color of the bars represents the relative change in HDX protection. All peptides resolved showed no significant change in protection (color code is in lower right corner; ns, no significant change in protection) between GR AF1 and TIF2.0-bound GR AF1.
Binding of TIF2.0 Induces Structure in the Otherwise Intrinsically Disordered GR AF1 Domain
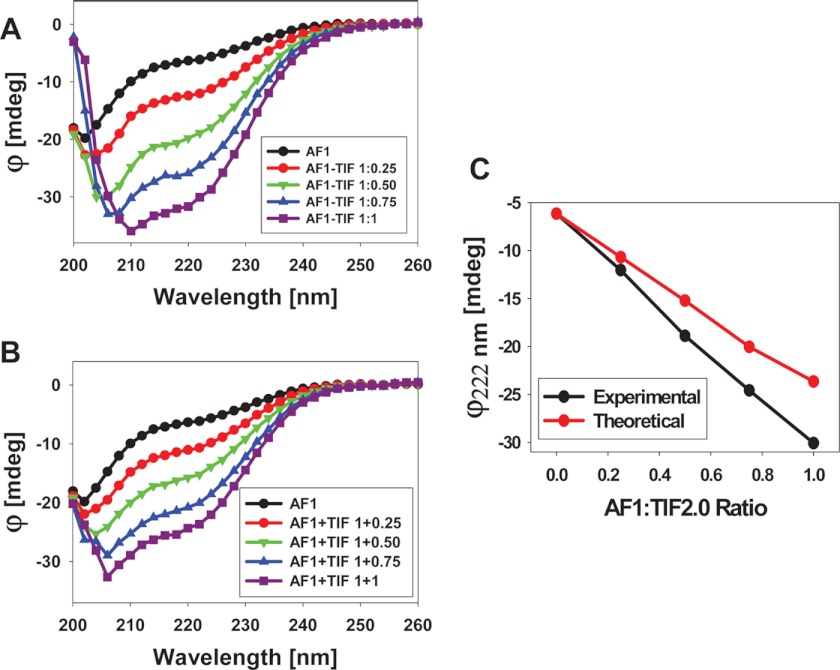
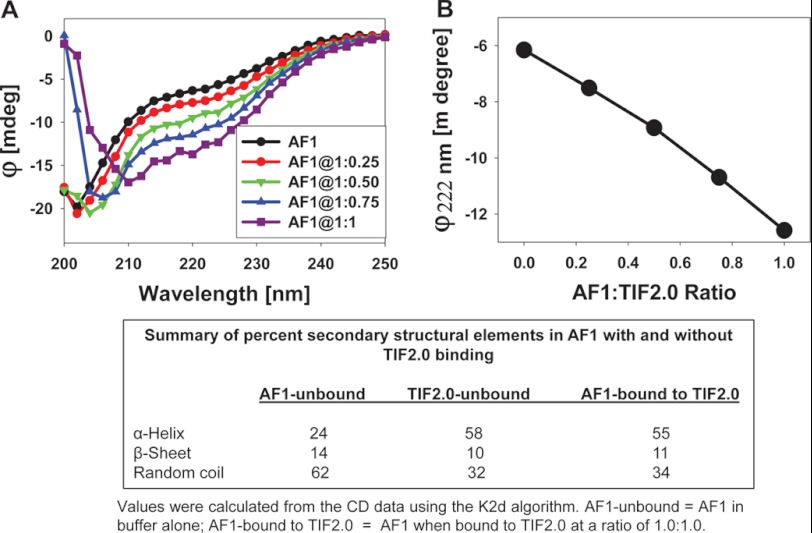
To test the effects of TIF2.0 binding on the conformational changes in GR AF1, we recorded the far-UV CD spectra of purified recombinant AF1, TIF2.0, and a mixture of AF1:TIF2.0 at various ratios ranging from 1:0.25 to 1:1. As expected, AF1 alone showed the characteristics of an ID protein (Fig. 8A), whereas with an increasing concentration of TIF2.0, the complex adopted more secondary structural elements, as evident from the increased ellipticity (i.e. more negative values) at around 222 nm wavelength followed by a red-shift of the maximum ellipticity from about 205 to about 210 nm. When individual data for AF1 and TIF2.0 (at each concentration) are added and the theoretical sum plotted, a series of nested curves with increasingly negative ellipticity are also observed (Fig. 8B). When the theoretical and experimental values of ellipticity at 222 nm (to follow helical contents) are plotted against the increasing concentrations of TIF2.0, a concentration-dependent linear relationship is obtained (Fig. 8C). However, a comparison of the experimental and theoretical sum plots suggests that increasing concentrations of TIF2.0 cause the complex to adopt significantly higher helical content in a concentration-dependent manner, as evident from the differences in ellipticity at 222 nm. This result suggests that the increased helical content in the AF1·TIF2.0 complex is due to formation of the complex and not just to the additive effects of the two proteins.
FIGURE 8.
Far-UV CD spectra of GR AF1.TIF2.0 complex. A, far-UV CD spectra of the GR AF1·TIF2.0 complex (experimental) at a constant AF1 concentration and varying concentrations of TIF2.0/FLAG (as indicated). B, far-UV CD spectra of GR AF1 + TIF2.0 (theoretical sum) at a constant AF1 concentration and varying concentrations of TIF2.0/FLAG (as indicated). C, a comparison of changes in the ellipticity at 222 nm for experimental and theoretical sums with respect to the AF1:TIF2.0 ratio. Each spectrum presented is the result of five spectra that were averaged, corrected for the contribution of the buffer, and smoothed.
To determine whether these structural changes observed in the AF1·TIF2.0 complex reflected conformational differences in AF1, in TIF2.0, or in both proteins, we subtracted the contribution of TIF2.0/FLAG from each spectrum of AF1·TIF2.0 complex and plotted them with respect to AF1 alone (Fig. 9A). This analysis revealed that AF1 adopts a secondary structure with progressively higher helical content when complexed with increasing concentrations of TIF2.0/FLAG (Fig. 9A). The graph in Fig. 9B displays the absolute changes in the ellipticity at 222 nm in AF1 at each concentration of TIF2.0. These results suggest that the helical content in the complex increases approximately linearly up to a ratio of 1:1. Unfortunately, because of high backgrounds at greater concentrations of TIF2.0, we could not carry out our CD experiments beyond a ratio of 1:1 to determine whether a saturation level was indeed attained. A summary of the percentage of secondary structural elements in AF1 with and without TIF2.0/FLAG binding indicates that at a 1:1 ratio of AF1·TIF2.0 complex formation, the helical content in AF1 was increased by more than 2-fold; this increased helical content appears to occur at the expense of a random coil configuration, as indicated by a relative decrease in this population (Fig. 9, data summarized in the box).
FIGURE 9.
GR AF1 adopts higher helical content when complexed with TIF2.0. A, far-UV CD spectra showing AF1 after subtracting the contribution of TIF2.0/FLAG in each case. B, a plot ellipticity at 222 nm of AF1·TIF2.0 minus TIF2.0 with respect to the AF1:TIF2.0 ratio. A summary of percent secondary structural elements in AF1 with and without TIF2.0 binding is shown in the box.
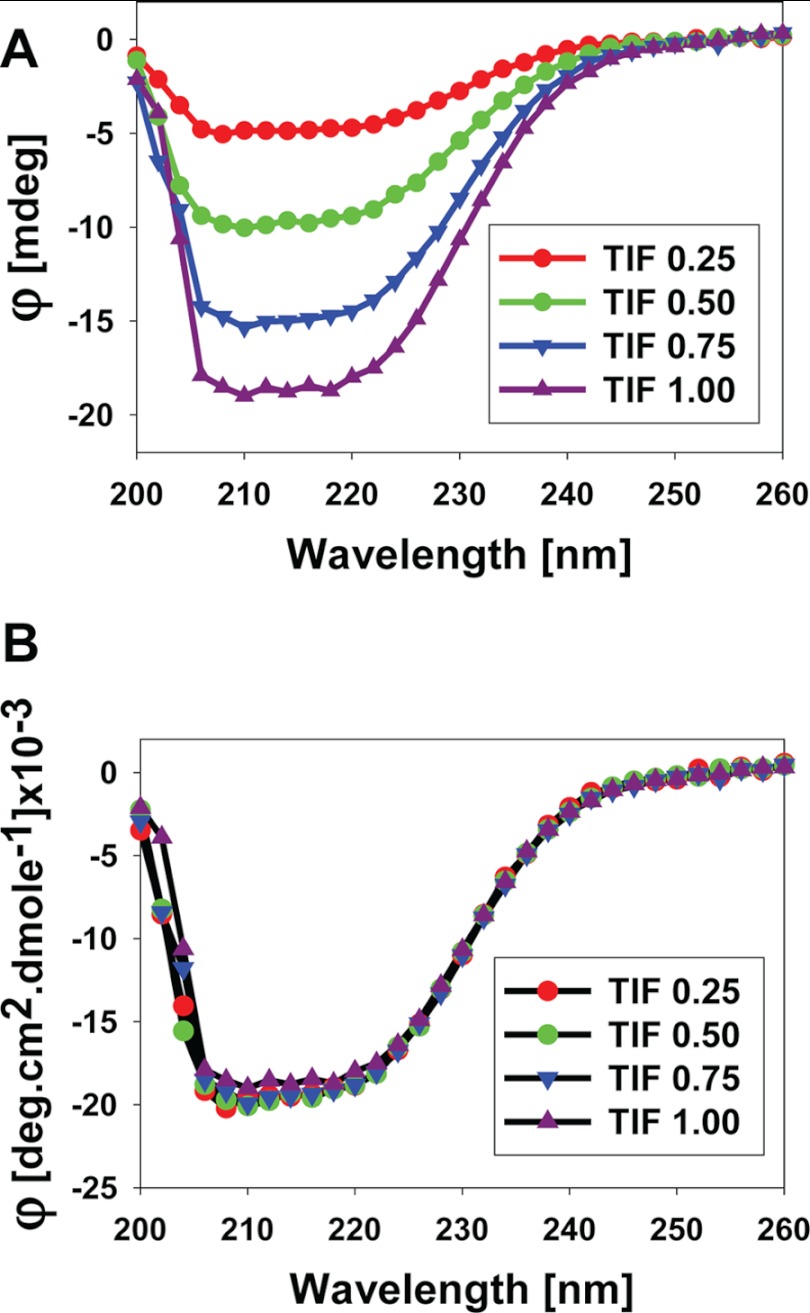
We next asked whether the structural changes observed above in the AF1 protein are actually due to its specific binding to TIF2.0 or merely the result of experimental variations in the TIF2.0 spectrum at higher concentrations of TIF2.0. We therefore plotted the spectra of TIF2.0/FLAG at each concentration, all of which showed a similar overall pattern of CD spectra that increased with the concentration of TIF2.0 (Fig. 10A). To be absolutely sure that there were no structural differences in TIF2.0 at different concentrations, we converted the CD data from each concentration of TIF2.0 into molar concentrations and plotted them as molar ellipticity (Fig 10B). As expected, each spectrum of TIF2.0 shows an overlapping pattern, suggesting that there are no structural differences in TIF2.0 attributable simply to increasing TIF2.0/FLAG concentrations. Together, these results clearly demonstrate that binding of TIF2.0 to GR AF1 results in an induced structure formation in the otherwise disordered AF1 domain without any significant perturbation in TIF2.0/FLAG structure.
FIGURE 10.
Far-UV CD spectra of TIF2.0/FLAG at various concentrations. A, spectra plotted at each concentration of TIF2.0 (as indicated). B, the data from each concentration are converted to a molar scale. Each spectrum presented is the result of five spectra that were averaged, corrected for the contribution of the buffer, and smoothed.
The GR AF1·TIF2.0 Complex Exhibits Increased Structural Stability
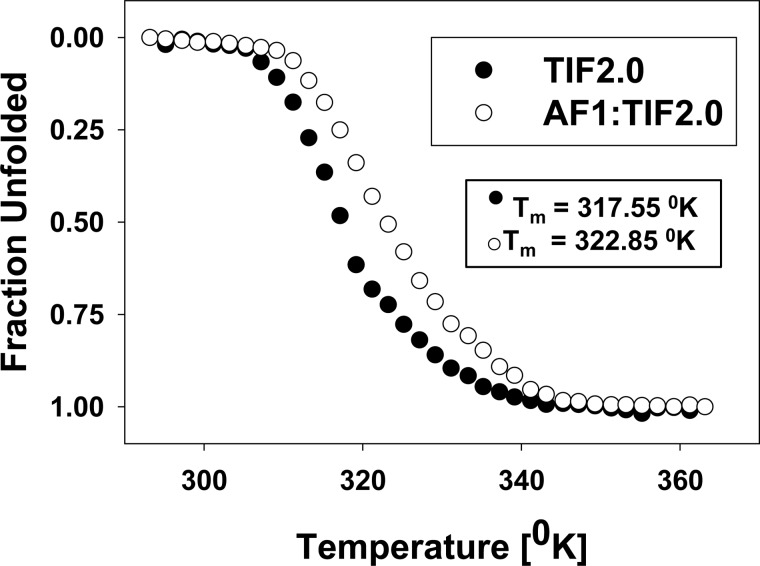
Functionally important protein-protein interactions that result in conformational changes in one or both interacting proteins should induce the formation of a more stable complex. Because the interaction between GR AF1 and TIF2.0 led to an induced conformation in AF1, we sought to determine whether TIF2.0 interaction would affect the stability of the AF1·TIF2.0 complex. To determine the stability of the complex, we verified the change in the thermal melt curve or the temperature at which a protein unfolds. The ellipticities of protein samples containing AF1 or TIF2.0 alone or in combination were monitored at a single wavelength (222 nm) by CD spectroscopy to measure the loss of α-helical structure with respect to increasing temperatures. As expected, we could not record a melt curve for the disordered AF1 alone because it possesses little or no structure that can be destabilized at higher temperatures (data not shown). In contrast, TIF2.0/FLAG undergoes a broad temperature-dependent transition to an unfolded protein with a distinct thermal midpoint (Tm) of 317.55 K (Fig. 11). The AF1·TIF2.0 complex exhibited a Tm of 322.85 K; and, the unfolding of the complex resembled that of a highly cooperative two-state transition from a folded to an unfolded protein. This change in the thermal melt curve indicates that the AF1·TIF2.0 complex is more stable than either protein alone. Despite the disordered nature of GR AF1 alone, the increase in stability indicates that the interaction with TIF2.0 confers conformational stability to the resulting complex.
FIGURE 11.
TIF2.0 interaction with GR AF1 forms a stable complex. TIF2.0/FLAG alone or in a mixture with AF1 was incubated in a temperature-regulated CD cell, and absorbance at 222 nm was monitored as the temperature in the cell was raised from 20 to 90 °C at a controlled rate of 1 °C/min. Tm is the temperature at which half of the sample persists in native conformation and half has unfolded and lost absorbance at 222 nm.
The Structure Induced by AF1·TIF2.0 Interaction Resists Proteolysis
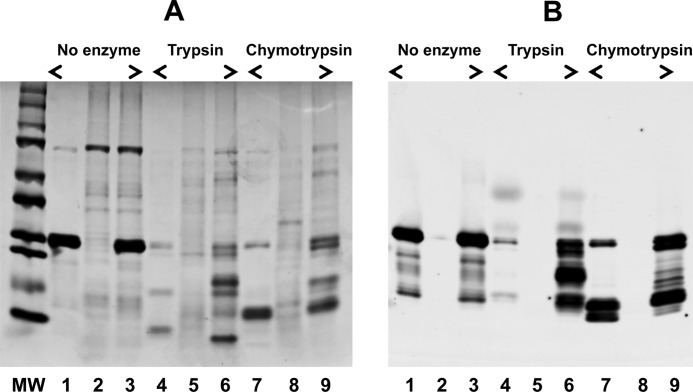
To evaluate the changes in the tertiary structure of the AF1 region brought about by TIF2.0 binding, we carried out experiments involving limited proteolytic digestions. The reasoning for this was that any changes in the tertiary structure of AF1 would be expected to alter the accessibility of and digestion by different proteases. The patterns of proteolytic products of AF1, TIF2.0/FLAG, and the AF1:TIF2.0/FLAG mixture after digestion by trypsin or chymotrypsin are shown in Fig. 12. It is evident from the Coomassie-stained gel (Fig. 12A) that, alone, AF1 and TIF2.0 are mostly digested into small fragments under these conditions of proteolysis (Fig. 12A, compare lanes 1 and 2 with lanes 4 and 5, respectively). In marked contrast, there is partial protection of several bands in the mixture, which are not seen in either AF1 or TIF2.0 when digested alone (Fig. 12A, compare lanes 4 and 5 with lane 6). These results suggest that a more compact tertiary structure is formed in the complex, such that the residues attacked by trypsin are moved to positions that are no longer easily digested by trypsin. Similar protection from chymotrypsin is shown in Fig. 12A, lanes 7–9. To further confirm these proteolytic data, we followed these tryptic digests immunochemically. After the digestion, the products of proteolysis were resolved on an SDS-PAGE and examined by immunoreaction with an anti-AF1 antibody. It is evident from Fig. 12B that a strong immunoreaction for intact AF1 (as assessed by its molecular weight) in undigested samples (lanes 1 and 3) is seen. As expected, there was no immunoreaction with anti-AF1 antibody observed in the sample containing only undigested TIF2.0/FLAG (Fig. 12B, lane 2), consistent with the antibody being specific for AF1. Similarly, no strong protected band was observed when AF1 alone was digested with trypsin (Fig. 12B, lane 4). However, when AF1 is complexed with TIF2.0, the protected AF1 bands can be seen (Fig. 12B, lane 6). Again as expected, no reactivity of anti-GR antibody is seen in lane 5 (Fig. 12B), where only TIF2.0 is present. These observations suggest that AF1 folds into a protease-resistant shape and that it remains in this conformation as long as TIF2.0 is bound. It is evident from these results that the TIF2.0-induced structure in AF1 is not confined to secondary structural elements but that tertiary structural changes are also taking place in the protein.
FIGURE 12.
Limited proteolysis of AF1, TIF2.0/FLAG, and the AF1·TIF2.0/FLAG complex. A, Coomassie Blue-stained gel showing product of proteolytic digestion. MW, molecular weight markers; lanes 1–3, undigested AF1, TIF2.0, and AF1:TIF2.0 mixture, respectively; lanes 4–6, trypsin-digested AF1, TIF2.0, and AF1:TIF2.0 mixture, respectively; lanes 7–9, chymotrypsin-digested AF1, TIF2.0, and AF1·TIF2.0 complex, respectively. B, immunoreactions with an antibody raised against amino acids 150–175 in the human GR AF1 showing products of trypsin and chymotrypsin digestions. Numbering of the lanes is same as in A.
Effect of TIF2 on AF1-driven GR Transcription
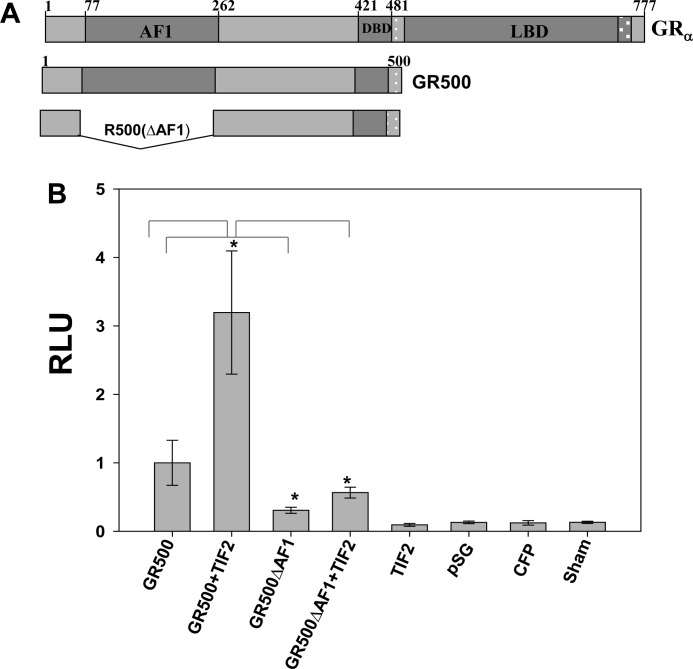
We tested the effects of TIF2.0-induced binding/folding events on AF1-driven transcription using GR-responsive promoters in transient transfection-based reporter assays in GR-deficient CV-1 cells. The promoter-reporter plasmid (GRE-SEAP) contains three GREs upstream from a TATA box and a reporter gene that encodes alkaline phosphatase secreted into the medium. To test the effect of TIF2 on AF1-driven GR transcription, we cotransfected CV-1 cells with the GRE-SEAP reporter gene and a constant amount of GR500 (pECFP/GR500) expression vector (Fig. 13) alone or with added vector expressing TIF2 (pSG5/TIF2). Full-length TIF2 was used because TIF2.0 is devoid of the C-terminal activation domains (AD1 and AD2) that are essential for TIF2 coactivator activity. The GR500 construct instead of full-length GR was chosen to avoid any effects of AF2. Lacking LBD, GR500 is transcriptionally active without steroid and can induce genes and/or apoptosis in cells to nearly the same extent as steroid-bound holoGR (22). Our data indicate that GR500 alone significantly increased reporter activity compared with empty vector alone (Fig. 13), and input of the plasmid expressing the TIF2 gene enhanced the GR500 induction of the GRE-SEAP reporter by ∼3-fold (Fig. 13). These reporter activities were significantly reduced when the GR500 lacking AF1 (pECFP/GR500 (ΔAF1)) was used (Fig. 13). Importantly, removal of the AF1 domain significantly reduced the ability of added TIF2 to increase transactivation by GR500. These results strongly suggest that the enhancement of GR-induced transcription by TIF2 is achieved through TIF2.0 binding to the AF1 region and that a TIF2.0-induced binding/folding event on AF1 plays an important role in GRE-mediated AF1 transcriptional activity.
FIGURE 13.
TIF2 increases GRE-mediated AF1 activity as assessed by SEAP-based promoter-reporter assay in CV-1 cells. A, schematic representation of the GR constructs used in the transient transfection experiments. B, test of GR AF1 activity in the absence of the LBD. CV-1 cells were cotransfected with pECFP/GR500, or pECFP/GR500(ΔAF1) ± pSG5/TIF2 (as described under “Experimental Procedures”). The medium was assayed for SEAP activity 24 h later. The levels of significance were evaluated by a two-tailed paired Student's t test; *, p value of <0.05 was considered significant. RLU, relative light units; DBD, DNA-binding domain.
DISCUSSION
The p160 group of proteins of SRC-1, SRC-2/TIF2, and SRC-3/AIB1/pCIP/ACTR/TRAP1/RAC3 are important and critical coactivators for most of the steroid hormone receptors and have been reported to be essential components of various physiological actions of steroid hormone receptors including GR (48, 49). This study presents biophysical evidence that the previously neglected N-terminal domain of TIF2, in the form of the fragment TIF2.0, is capable of interacting with, and conformationally reorganizing, the unstructured dominant transactivation domain of GR, the AF1 domain. Overexpressed TIF2.0 was purified by means of a C-terminal FLAG tag. Several assays established that the FLAG tag did not alter the biological activity properties of the previously described TIF2.0 (30–32), thereby justifying its use in detailed studies with the purified GR AF1 domain. Under these conditions, we observed a strong interaction of TIF2.0 with GR AF1 that was accompanied by a significant increase in the α-helical content, and an approximately equal decrease in random coil structure of AF1. The fact that complex dissociation is slower than complex association is compatible with a structural rearrangement of AF1 to yield a more stable folded conformation. Further support for a tightly associated TIF2.0·AF1 complex with a new, induced tertiary structure comes from both the higher temperature required to denature the TIF2.0·AF1 complex and the altered protease digestion patterns of the complex compared with the two isolated components. Such interactions of a coactivator with a steroid receptor have not been described previously and offer novel molecular insight regarding the gene-regulatory activity of steroid receptors.
A central region of TIF2 containing three RIDs is well known to bind a pocket in the AF2 domain of the GR LBD (50). Despite the existing body of work examining the interactions of the p160 coactivators with the AF2 domain of steroid hormone receptors, the paucity of structural data has left unanswered many important questions about their role in the physical interaction and regulation of AF1 function, which possesses most of the gene-regulatory activity of GRs (12). Our earlier evidence that the N-terminal region of TIF2 (TIF2.0) interacts with the GR AF1 domain and inhibits the binding of the corepressor to GR (30–32) is fully supported by the present observation that deletion of the AF1 domain of GR500 severely reduces the ability of full-length TIF2 to augment GR500 transactivation activity. Full-length TIF2 contains both C-terminal activation domains (AD1 and AD2) but has been widely thought to bind only to the GR LBD via its central RIDs. The fact that TIF2 augments the activity of GR500, which lacks the LBD, requires an additional binding site in GR. The present studies verify that the GR AF1 domain is such a second site and that it complexes with TIF2.0. We therefore conclude that the greatly reduced coactivator activity of full-length TIF2 with GR500ΔAF1 is due to the absence of the interactions of GR AF1 with the N-terminal sequences of TIF2 found in TIF2.0.
These results are unexpected because the central region of TIF2, which contains three RIDs, has been thought to be the only point of contact with GR (50, 51). It has been reported that TIF2/GRIP1 (lacking N-terminal half of TIF2.0) is required for AF2 but not AF1 activity of GR in yeast (52), but the molecular explanation for this is unknown. The AF1 activity of several other steroid hormone receptors has been found to be accentuated by coactivators (53–59), but it was not clear that GRs would be similarly regulated in mammalian cells. In view of our previous report that TIF2.0 also interacts with the N terminus of progesterone receptors (32), we predict that TIF2.0 can similarly induce conformational changes in the AF1 domain of other steroid receptors. We further note that the currently observed second point of contact between TIF2 and GR would be expected to increase the strength of TIF2-GR complex formation (32).
The historical belief is that the specific functionality of a given protein is determined by its unique three-dimensional structure. However, in recent years, it has become quite evident that many biologically important proteins possess large stretches of amino acid sequence that do not possess a well defined three-dimensional structure (16, 61–63). These unstructured proteins/protein regions have been termed “intrinsically disordered” (or ID) and exist as dynamic ensembles of interconverting conformers that are capable of undergoing disorder/order transition under specific physiological conditions. In this regard, ID AF1 may have functional advantages by allowing it to interact with multiple target molecules that are critical for specific activities. Because AF1 exists as a large collection of highly dynamic and rapidly interconverting conformations, the binding of a particular conformer to a specific cofactor to generate a high affinity N-terminal domain AF1 coregulatory protein interaction may take place. The present study is the first to document a protein-induced conformational change in AF1 by a coactivator and joins TATA-binding protein in being able to accomplish the same feat for GR AF1 (21–23). It will be interesting to determine the similarities and differences of these conformationally induced complexes at a molecular level and whether the changes with TIF2 and TATA-binding protein are additive or exclusionary. There are several examples in which binding sites within the ID regions contain molecular recognition feature; these binding sites consist of a short stretch of amino acid sequences that undergoes a disorder-to-order transition and is stabilized by binding to a partner molecule (64–67). ANCHOR is one method that has been developed for the computational discovery of disorder-based binding regions that undergo disorder-to-order transition at binding (68, 69). In this study we show that complex formation between GR AF1 and TIF2.0 is accompanied by a change in protein conformation. We have not yet been able to identify the location of the sequences involved in TIF2.0 or GR AF1.
Our results provide a potential mechanism by which GR AF1 may regulate the expression of GR target genes. This information is essential to understanding how specific signals are passed from the receptor to target genes, given the fact that the AF1 domain is much more active than the more extensively studied AF2 domain. Our results from this study elucidate one possible mechanism through which ID activation domains form an assembly of critical coactivator proteins and acquire transcriptional activity. These effects can be influenced further by other factors such as interdomain interactions, post-translational modifications, small molecule ligands, and other protein-protein interactions. In view of the similar interactions of TIF2.0 with progesterone receptors (32) and the effects of TIF2 on the activity of AF1 in other receptors, we speculate that this proposed molecular mechanism of coactivator action via conformational reorganizations, which are seen here for TIF2.0 and GR AF1, may be shared by the other steroid receptors.
In summary, the work presented here provides a new structural model for the interaction of GR AF1 with TIF2 and establishes a foundation for the functional adaptability of AF1. The results from this study firmly establish that intrinsic disorder in GR AF1 is the principal physical basis for engaging AF1-coactivator binding. The interaction between GR AF1 and TIF2.0 restricts conformational flexibility and provides structural stability to the N-terminal activation domain of the GR; it may also play similar roles for other steroid hormone receptors, which possess similar intrinsically disordered N-terminal domain AF1 proteins. This phenomenon of altering an unstructured protein to that of a functional structure through the binding of another protein appears to be a general event among the activation domains of numerous transcription factors (60, 70, 71).
This work was supported in part by the National Institutes of Health Intramural Research Program of the NIDDK (to S. S. S., Jr.).
Z. Zhang, Y. Sun, Y.-W. Cho, C. C. Chow, and S. S. Simons, Jr., submitted for publication.
- GR
- glucocorticoid receptor
- AF1 and AF2
- activation function 1 and 2, respectively
- GRE
- glucocorticoid response element
- LBD
- ligand-binding domain
- RID
- receptor interaction domain
- ID
- intrinsically disordered
- SPR
- surface plasmon resonance
- BAP
- bovine alkaline phosphatase
- Dex
- dexamethasone
- HDX
- hydrogen/deuterium exchange
- SEAP
- secreted alkaline phosphatase.
REFERENCES
- 1. Chrousos G. P., Kino T. (2005) Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci. STKE 2005, pe48. [DOI] [PubMed] [Google Scholar]
- 2. Beck I. M., Vanden Berghe W., Vermeulen L., Yamamoto K. R., Haegeman G., De Bosscher K. (2009) Cross-talk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr. Rev. 30, 830–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simons S. S., Jr. (2010) Glucocorticoid receptor cofactors as therapeutic targets. Curr. Opin. Pharmacol. 10, 613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pratt W. B., Galigniana M. D., Morishima Y., Murphy P. J. (2004) Role of molecular chaperones in steroid receptor action. Essays Biochem. 40, 41–58 [DOI] [PubMed] [Google Scholar]
- 5. Pratt W. B. (1993) The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J. Biol. Chem. 268, 21455–21458 [PubMed] [Google Scholar]
- 6. Kumar R., Thompson E. B. (2005) Gene regulation by the 4glucocorticoid receptor: structure:function relationship. J. Steroid Biochem. Mol. Biol. 94, 383–394 [DOI] [PubMed] [Google Scholar]
- 7. Kumar R., Thompson E. B. (2003) Transactivation functions of the N-terminal domains of nuclear hormone receptors: protein folding and coactivator interactions. Mol. Endocrinol. 17, 1–10 [DOI] [PubMed] [Google Scholar]
- 8. John S., Johnson T. A., Sung M. H., Biddie S. C., Trump S., Koch-Paiz C. A., Davis S. R., Walker R., Meltzer P. S., Hager G. L. (2009) Kinetic complexity of the global response to glucocorticoid receptor action. Endocrinology 150, 1766–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. John S., Sabo P. J., Johnson T. A., Sung M. H., Biddie S. C., Lightman S. L., Voss T. C., Davis S. R., Meltzer P. S., Stamatoyannopoulos J. A., Hager G. L. (2008) Interaction of the glucocorticoid receptor with the chromatin landscape. Mol. Cell 29, 611–624 [DOI] [PubMed] [Google Scholar]
- 10. Biddie S. C., John S., Sabo P. J., Thurman R. E., Johnson T. A., Schiltz R. L., Miranda T. B., Sung M. H., Trump S., Lightman S. L., Vinson C., Stamatoyannopoulos J. A., Hager G. L. (2011) Transcription factor AP1 potentiates chromatin accessibility and glucocorticoid receptor binding. Mol. Cell 43, 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. John S., Sabo P. J., Thurman R. E., Sung M. H., Biddie S. C., Johnson T. A., Hager G. L., Stamatoyannopoulos J. A. (2011) Chromatin accessibility predetermines glucocorticoid receptor binding patterns. Nat. Genet. 43, 264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollenberg S. M., Giguere V., Segui P., Evans R. M. (1987) Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell 49, 39–46 [DOI] [PubMed] [Google Scholar]
- 13. Cho S., Kagan B. L., Blackford J. A., Jr., Szapary D., Simons S. S., Jr. (2005) Glucocorticoid receptor ligand binding domain is sufficient for the modulation of glucocorticoid induction properties by homologous receptors, coactivator transcription intermediary factor 2, and Ubc9. Mol. Endocrinol. 19, 290–311 [DOI] [PubMed] [Google Scholar]
- 14. Bledsoe R. K., Montana V. G., Stanley T. B., Delves C. J., Apolito C. J., McKee D. D., Consler T. G., Parks D. J., Stewart E. L., Willson T. M., Lambert M. H., Moore J. T., Pearce K. H., Xu H. E. (2002) Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110, 93–105 [DOI] [PubMed] [Google Scholar]
- 15. Kumar R., Litwack G. (2009) Structural and functional relationships of the steroid hormone receptors' N-terminal transactivation domain. Steroids 74, 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dunker A. K., Uversky V. N. (2010) Drugs for “protein clouds”: targeting intrinsically disordered transcription factors. Curr. Opin. Pharmacol. 10, 782–788 [DOI] [PubMed] [Google Scholar]
- 17. Liu J., Perumal N. B., Oldfield C. J., Su E. W., Uversky V. N., Dunker A. K. (2006) Intrinsic disorder in transcription factors. Biochemistry 45, 6873–6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chandra V., Huang P., Hamuro Y., Raghuram S., Wang Y., Burris T. P., Rastinejad F. (2008) Structure of the intact PPAR-γ-RXR-α nuclear receptor complex on DNA. Nature 29, 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minezaki Y., Homma K., Kinjo A. R., Nishikawa K. (2006) Human transcription factors contain a high fraction of intrinsically disordered regions essential for transcriptional regulation. J. Mol. Biol. 359, 1137–1149 [DOI] [PubMed] [Google Scholar]
- 20. Hilser V. J., Thompson E. B. (2007) Intrinsic disorder as a mechanism to optimize allosteric coupling in proteins. Proc. Natl. Acad. Sci. U.S.A. 104, 8311–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar R., Volk D. E., Li J., Lee J. C., Gorenstein D. G., Thompson E. B. (2004) TATA box-binding protein induces structure in the recombinant glucocorticoid receptor AF1 domain. Proc. Natl. Acad. Sci. U.S.A. 101, 16425–16430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Copik A. J., Webb M. S., Miller A. L., Wang Y., Kumar R., Thompson E. B. (2006) Activation function 1 of glucocorticoid receptor binds TATA-binding protein in vitro and in vivo. Mol. Endocrinol. 20, 1218–1230 [DOI] [PubMed] [Google Scholar]
- 23. Khan S. H., Ling J., Kumar R. (2011) TBP binding-induced folding of the glucocorticoid receptor AF1 domain facilitates its interaction with steroid receptor coactivator-1. PLoS One 6, e21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar R., Baskakov I. V., Srinivasan G., Bolen D. W., Lee J. C., Thompson E. B. (1999) Interdomain signaling in a two-domain fragment of the human glucocorticoid receptor. J. Biol. Chem. 274, 24737–24741 [DOI] [PubMed] [Google Scholar]
- 25. Garza A. S., Khan S. H., Moure C. M., Edwards D. P., Kumar R. (2011) Binding-folding-induced regulation of AF1 transactivation domain of the glucocorticoid receptor by a cofactor that binds to its DNA binding domain. PLoS One 6, e25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kino T., Ichijo T., Amin N. D., Kesavapany S., Wang Y., Kim N., Rao S., Player A., Zheng Y. L., Garabedian M. J., Kawasaki E., Pant H. C., Chrousos G. P. (2007) Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol. Endocrinol. 21, 1552–1568 [DOI] [PubMed] [Google Scholar]
- 27. Hill K. K., Roemer S. C., Jones D. N., Churchill M. E., Edwards D. P. (2009) A progesterone receptor co-activator (JDP2) mediates activity through interaction with residues in the carboxyl-terminal extension of the DNA binding domain. J. Biol. Chem. 284, 24415–24424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tao Y. G., Xu Y., Xu H. E., Simons S. S., Jr. (2008) Mutations of glucocorticoid receptor differentially affect AF2 domain activity in a steroid-selective manner to alter the potency and efficacy of gene induction and repression. Biochemistry 47, 7648–7662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee G. S., Simons S. S., Jr., (2011) Ligand binding domain mutations of the glucocorticoid receptor selectively modify the effects with, but not binding of, cofactors. Biochemistry 50, 356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Q., Blackford J. A., Jr., Song L. N., Huang Y., Cho S., Simons S. S., Jr. (2004) Equilibrium interactions of corepressors and coactivators with agonist and antagonist complexes of glucocorticoid receptors. Mol. Endocrinol. 18, 1376–1395 [DOI] [PubMed] [Google Scholar]
- 31. Wang D., Simons S. S., Jr. (2005) Corepressor binding to progesterone and glucocorticoid receptors involves the activation function-1 domain and is inhibited by molybdate. Mol. Endocrinol. 19, 1483–1500 [DOI] [PubMed] [Google Scholar]
- 32. Wang D., Wang Q., Awasthi S., Simons S. S., Jr. (2007) Amino-terminal domain of TIF2 is involved in competing for corepressor binding to glucocorticoid and progesterone receptors. Biochemistry 46, 8036–8049 [DOI] [PubMed] [Google Scholar]
- 33. Hu X., Lazar M. A. (1999) The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402, 93–96 [DOI] [PubMed] [Google Scholar]
- 34. Nagy L., Kao H. Y., Love J. D., Li C., Banayo E., Gooch J. T., Krishna V., Chatterjee K., Evans R. M., Schwabe J. W. (1999) Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 13, 3209–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perissi V., Staszewski L. M., McInerney E. M., Kurokawa R., Krones A., Rose D. W., Lambert M. H., Milburn M. V., Glass C. K., Rosenfeld M. G. (1999) Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 13, 3198–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He Y., Szapary D., Simons S. S., Jr. (2002) Modulation of induction properties of glucocorticoid receptor-agonist and -antagonist complexes by coactivators involves binding to receptors but is independent of ability of coactivators to augment transactivation. J. Biol. Chem. 277, 49256–49266 [DOI] [PubMed] [Google Scholar]
- 37. Baskakov I. V., Kumar R., Srinivasan G., Ji Y. S., Bolen D. W., Thompson E. B. (1999) Trimethylamine N-oxide-induced cooperative folding of an intrinsically unfolded transcription-activating fragment of human glucocorticoid receptor. J. Biol. Chem. 274, 10693–10696 [DOI] [PubMed] [Google Scholar]
- 38. Kumar R., Lee J. C., Bolen D. W., Thompson E. B. (2001) The conformation of the glucocorticoid receptor AF1/tau1 domain induced by osmolyte binds co-regulatory proteins. J. Biol. Chem. 276, 18146–18152 [DOI] [PubMed] [Google Scholar]
- 39. Andrade M. A., Chacón P., Merelo J. J., Morán F. (1993) Evaluation of secondary structure of proteins from UV circular dichroism using an unsupervised learning neural network. Protein Eng. 6, 383–390 [DOI] [PubMed] [Google Scholar]
- 40. Wardell S. E., Kwok S. C., Sherman L., Hodges R. S., Edwards D. P. (2005) Regulation of the N-terminal transcription activation domain of progesterone receptor by a cofactor-induced protein folding mechanism. Mol. Cell. Biol. 25, 8792–8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chalmers M. J., Busby S. A., Pascal B. D., He Y., Hendrickson C. L., Marshall A. G., Griffin P. R. (2006) Probing protein ligand interactions by automated hydrogen/deuterium exchange mass spectrometry. Anal. Chem. 78, 1005–1014 [DOI] [PubMed] [Google Scholar]
- 42. Busby S. A., Chalmers M. J., Griffin P. R. (2007) Improving digestion efficiency under H/D exchange conditions with activated pepsinogen columns. Int. J. Mass Spectrom. 259, 130–139 [Google Scholar]
- 43. Chalmers M. J., Busby S. A., Pascal B. D., Southern M. R., Griffin P. R. (2007) A two-stage differential hydrogen deuterium exchange method for the rapid characterization of protein/ligand interactions. J. Biomol. Tech. 18, 194–204 [PMC free article] [PubMed] [Google Scholar]
- 44. Pratt W. B., Toft D. O. (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 18, 306–360 [DOI] [PubMed] [Google Scholar]
- 45. Leach K. L., Dahmer M. K., Hammond N. D., Sando J. J., Pratt W. B. (1979) Molybdate inhibition of glucocorticoid receptor inactivation and transformation. J. Biol. Chem. 254, 11884–11890 [PubMed] [Google Scholar]
- 46. Cavanaugh A. H., Simons S. S., Jr. (1990) Glucocorticoid receptor binding to calf thymus DNA. 1. Identification and characterization of a macromolecular factor involved in receptor-steroid complex binding to DNA. Biochemistry 29, 989–996 [DOI] [PubMed] [Google Scholar]
- 47. Cavanaugh A. H., Simons S. S., Jr. (1990) Glucocorticoid receptor binding to calf thymus DNA. Role of a DNA-binding activity factor in receptor heterogeneity and a multistep mechanism of receptor activation. Biochemistry 29, 996–1002 [DOI] [PubMed] [Google Scholar]
- 48. York B., O'Malley B. W. (2010) Steroid receptor coactivator (SRC) family: masters of systems biology. J. Biol. Chem. 285, 38743–38750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu J., Wu R. C., O'Malley B. W. (2009) Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat. Rev. Cancer 9, 615–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Darimont B. D., Wagner R. L., Apriletti J. W., Stallcup M. R., Kushner P. J., Baxter J. D., Fletterick R. J., Yamamoto K. R. (1998) Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 12, 3343–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heery D. M., Kalkhoven E., Hoare S., Parker M. G. (1997) A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387, 733–736 [DOI] [PubMed] [Google Scholar]
- 52. Hong H., Kohli K., Garabedian M. J., Stallcup M. R. (1997) GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol. Cell. Biol. 17, 2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ikonen T., Palvimo J. J., Jänne O. A. (1997) Interaction between the amino- and carboxyl-terminal regions of the rat androgen receptor modulates transcriptional activity and is influenced by nuclear receptor coactivators. J. Biol. Chem. 272, 29821–29828 [DOI] [PubMed] [Google Scholar]
- 54. Onate S. A., Boonyaratanakornkit V., Spencer T. E., Tsai S. Y., Tsai M. J., Edwards D. P., O'Malley B. W. (1998) The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J. Biol. Chem. 273, 12101–12108 [DOI] [PubMed] [Google Scholar]
- 55. Webb P., Nguyen P., Shinsako J., Anderson C., Feng W., Nguyen M. P., Chen D., Huang S. M., Subramanian S., McKinerney E., Katzenellenbogen B. S., Stallcup M. R., Kushner P. J. (1998) Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol. Endocrinol. 12, 1605–1618 [DOI] [PubMed] [Google Scholar]
- 56. Lavinsky R. M., Jepsen K., Heinzel T., Torchia J., Mullen T. M., Schiff R., Del-Rio A. L., Ricote M., Ngo S., Gemsch J., Hilsenbeck S. G., Osborne C. K., Glass C. K., Rosenfeld M. G., Rose D. W. (1998) Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc. Natl. Acad. Sci. U.S.A. 95, 2920–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Norris J. D., Fan D., Stallcup M. R., McDonnell D. P. (1998) Enhancement of estrogen receptor transcriptional activity by the coactivator GRIP-1 highlights the role of activation function 2 in determining estrogen receptor pharmacology. J. Biol. Chem. 273, 6679–6688 [DOI] [PubMed] [Google Scholar]
- 58. Garza A. M., Khan S. H., Kumar R. (2010) Site-specific phosphorylation induces functionally active conformation in the intrinsically disordered N-terminal activation function (AF1) domain of the glucocorticoid receptor. Mol. Cell. Biol. 30, 220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roeder R. G. (2005) Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 579, 909–915 [DOI] [PubMed] [Google Scholar]
- 60. Kumar R., McEwan I. J. (2012) Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation. Endocrin. Rev. 33, 271–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Babu M. M., van der Lee R., de Groot N. S., Gsponer J. (2011) Intrinsically disordered proteins: regulation and disease. Curr. Opin. Struct. Biol. 21, 432–440 [DOI] [PubMed] [Google Scholar]
- 62. Nilsson J., Grahn M., Wright A. P. (2011) Proteome-wide evidence for enhanced positive Darwinian selection within intrinsically disordered regions in proteins. Genome Biol. 12, R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tompa P. (2011) Unstructural biology coming of age. Curr. Opin. Struct. Biol. 21, 419–425 [DOI] [PubMed] [Google Scholar]
- 64. Dyson H. J., Wright P. E. (2002) Coupling of folding and binding for unstructured proteins. Curr. Opin. Struct. Biol. 12, 54–60 [DOI] [PubMed] [Google Scholar]
- 65. Babu M. M., Kriwacki R. W., Pappu R. V. (2012) Versatility from protein disorder. Science 337, 1460–1461 [DOI] [PubMed] [Google Scholar]
- 66. Mohan A., Oldfield C. J., Radivojac P., Vacic V., Cortese M. S., Dunker A. K., Uversky V. N. (2006) Analysis of molecular recognition features (MoRFs). J. Mol. Biol. 362, 1043–1059 [DOI] [PubMed] [Google Scholar]
- 67. Walsh I., Martin A. J., Di Domenico T., Vullo A., Pollastri G., Tosatto S. C. (2011) CSpritz: accurate prediction of protein disorder segments with annotation for homology, secondary structure, and linear motifs. Nucleic Acids Res. 39, W190–W196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dosztányi Z., Mészáros B., Simon I. (2009) ANCHOR: Web server for predicting protein binding regions in disordered proteins. Bioinformatics 25, 2745–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mészáros B., Simon I., Dosztányi Z. (2009) Prediction of protein binding regions in disordered proteins. PLoS Comput. Biol. 5, e1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kumar R., Serrette J. M., Khan S. H., Miller A. L., Thompson E. B. (2007) Effects of different osmolytes on the induced folding of the N-terminal activation domain (AF1) of the glucocorticoid receptor. Arch. Biochem. Biophys. 465, 452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Khan S. H., Kumar R. (2009) An overview of the importance of conformational flexibility in gene regulation by the transcription factors. J. Biophys. 2009, 210485. [DOI] [PMC free article] [PubMed] [Google Scholar]