Background: An in vitro model for studying BBB opening and entry of impermeable substances was constructed.
Results: A brain capillary endothelial cell monolayer was disrupted and opened to the penetration of impermeable therapeutic agents by C-TAT peptides.
Conclusion: Experimental conditions enabling the blood to brain entry of impermeant therapeutic agents were established.
Significance: Approaches toward overcoming states of major brain disorders were initiated.
Keywords: Drug Delivery, Endothelial Cell, Neurobiology, Peptide Interactions, Protein Domains, BBB, Permeability, TAT, TEER
Abstract
Most chemotherapeutic agents are blood-brain barrier (BBB) impermeants. HIV-1-derived TAT protein variants contain a transmembrane domain, which may enable them to cross the BBB and reach the brain. Here we synthesized CAYGRKKRRQRRR, a peptide containing a cysteine moiety attached to the N terminus of the transmembrane domain (C-TAT peptide), and studied its effects in an in vitro BBB model, which we found to reflect penetration by a receptor-independent pathway. Incubation of the brain capillary endothelial cell monolayer with 0.3–0.6 μmol/ml of this C-TAT peptide, for a period of 1–2 h, destabilizes brain capillary endothelial cell monolayer and introduces the ability of impermeant therapeutic agents including high molecular weight proteins to penetrate it substantially. The cysteinyl moiety at position 1 of the C-TAT peptide contributes largely to the destabilizing potency and the penetration efficacy of impermeant substances. The destabilizing effect was reversed using heparin. In summary, experimental conditions allowing a significant increase in entry of impermeant low and high molecular weight substances from the luminal (blood) to the abluminal side (brain) were found in an in vitro BBB model reflecting in vivo protein penetrability by a receptor-independent pathway.
Introduction
A large variety of therapeutic agents with potential for treating brain disorders are not in clinical use because they are unable to cross the blood-brain barrier (BBB)4 following peripheral administration (1). Even small bioactive peptides penetrate the BBB only if highly lipophilic (2). Furthermore, therapeutically relevant proteins such as herceptin (transtuzumab, a humanized IgG1 molecule) become ineffective, when HER-2-positive metastases have reached the brain (3).
The BBB is formed in part by tight junctions between adjacent endothelial cells that make up the cerebral capillaries. Tight junction proteins connect neighboring endothelial cells to each other in a noncovalent fashion (4). Several pathological conditions are known to result in marked disruption of the tight junctions of the BBB (5–8). Such disruptions can lead to undesirable pathological consequences but might permit the penetration of therapeutic agents relevant for brain disorders. Clinically, all attempts so far to enhance penetration of therapeutic proteins via the BBB in quantities sufficient to treat major brain disorders were unsuccessful (9).
A truncated form (71 amino acids) of the HIV-1 TAT (transactivator of transcription) protein was found to have the unique ability to enter cells through the plasma membrane and accumulate intracellularly (10, 11). This is believed to take place through a plasma membrane bilayer component, in a receptor- and transporter-independent fashion (12). This feature was attributed to a nine-amino acid stretch of basic amino acids of sequence RKKRRQRRRR, located in region IV of TAT protein (13). Peptide fragments containing this protein transduction domain preserve this feature as well (14, 15). Studies in rodents in vivo revealed that both TAT protein variants and peptides or fragments containing this protein transduction domain are capable of penetrating the BBB and accumulate in brain tissue (16). This was the rationale to covalently link the peptide to biologically active proteins for delivering them either into peripheral tissues or to the CNS (13–15). Indeed such peptide-protein conjugates did reach the brain (17), but in quantities far below that required to treat a major brain disorder such as brain tumors (for instance glioblastoma multiforme (18)). Thus, conditions that allow the efficient penetration of therapeutic proteins from the periphery to the brain are still needed (19).
In this study, we have synthesized cysteine-containing 13-amino acid fragments, based on the protein transduction domain of the TAT protein, postulating that the efficacy of this protein to cross the plasma membrane and to disrupt BBB tightness are interrelated. Efforts were then undertaken to modify this peptide to a form that preserves its BBB disrupting property but with reduced penetrability into cell interiors. We have used an in vitro experimental system composed of tight junction-linked primary porcine brain endothelial cells (PBEC) grown in culture (20, 21). This in vitro experimental system reflects well BBB in vivo with regard to permeability (Pe) in agreement with findings made in rodents in vivo (22). The tightness of this monolayer is determined by measuring the transendothelial electrical resistance (TEER). As relevant for Pe, this parameter is recognized as one of the most accurate and sensitive measures of BBB integrity (23). As for the BBB in mammals, major tight and adherence junction proteins are expressed when endothelial cells contact each other (20, 21). This experimental system allowed us to determine the disruption efficacy and the permeation potency of a large variety of therapeutic agents, including high molecular weight proteins. Our efforts in this direction are described in this report.
EXPERIMENTAL PROCEDURES
Materials
C-TAT peptide (cysteine- and TAT-containing peptide, CAYGRKKRRQRRR) was synthesized by the manual conventional synthesis. An Fmoc (N-(9-fluorenyl)methoxycarbonyl) strategy was employed throughout the peptide chain assembly. The calculated mass of the C-TAT peptide is 1734.07 Da, found by electrospray single quadropole mass spectroscopy analysis: ES (electrospray) = 1733.53 ± 0.04 Da. HSA, 5,5′-dithiobis (2-nitrobenzoic acid), N-ethylmaleimide, and fluresceine isothiocyanate were purchased from Sigma. PEG5-MAL (a 5-kDa PEG chain-containing maleimide moiety; CH3O-PEG5-C2H4-maleimide) was a product of Rapp Polymere (Tubingen, Germany). Na125I (carrier free) was obtained from New England Nuclear, and herceptin was purchased from Rosh (Basel, Switzerland). All other materials used in this study were of analytical grade.
Chemical Synthesis
C-TAT-MAL (C-TAT peptide in which N-ethylmaleimide was linked to the cysteine moiety) was prepared by reacting the C-TAT peptide with N-ethylmaleimide. The peptide (8.7 mg, 5 μmol) dissolved in 1.0 ml of 0.1 m Hepes buffer, pH 7.4. N-Ethylmaleimide (20 μl) from a fresh solution of 0.5 m in N,N-dimethylformamide was then added (2 molar excess over the peptide). The reaction was continued for 5 min at 25 °C. The product thus obtained was dialyzed for 10 h against H2O and lyophilized. C-TAT-MAL is 5,5′-dithiobis (2-nitrobenzoic acid)-negative, and the calculated mass for C-TAT-MAL is 1859 Da. Found by electrospray single quadropole mass spectroscopy analysis: ES = 1858.49 ± 0.21.
HO3S-C-TAT (a C-TAT peptide in which the cysteine moiety was converted to cysteic acid) was prepared by performic acid oxidation of the peptide. C-TAT peptide (12.2 mg, 7 μmol) was dissolved in 0.45 ml of formic acid. Hydrogen peroxide (50 μl) was then added, and the reaction was carried out at 25 °C over a period of 2 h. Performic acid was then removed by evaporation. The product obtained was dissolved in H2O and lyophilized. This procedure was repeated three times. HO3S-C-TAT peptide contains cysteic acid moiety (mol/mole) as verified by amino acid analysis following acid hydrolysis. The calculated mass is 1782 Da, found by electrospray single quadropole mass spectroscopy analysis: ES = 1781.52 ± 0.06 Da.
Acetylated C-TAT peptide was prepared by dissolving 12.2 mg of C-TAT in a mixture of 1:1:0.5 of H2O, pyridine and acetic anhydride. The reaction was carried out at 0 °C. The medium was evaporated, and the product was obtained, dissolved in H2O, and lyophilized. This procedure was repeated three times. Acetylated C-TAT peptide was then dissolved in 0.1 m Na2CO3 (pH 10.3, 1 h, 25 °C) for deacetylating the tyrosyl and the cysteinyl moieties of this derivative. The pH was then dropped to 6.0 with HCl, and the product was frozen until used.
Fluresceine-labeled HSA was prepared by dissolving 67 mg of HSA (1 μmol) in 0.5 ml of 0.1 m Na2CO3 (pH 10.3). Fluresceine isothiocyanate 1.4 mg (3.6 molar excess over HSA) was then added, and the reaction was carried out for 1 h at 25 °C. The reaction mixture was then loaded on a Sephadex G-50 column (1.7 × 14 cm), equilibrated, and run in the same buffer. The tubes containing Fluresceine-labeled HSA were pooled, dialyzed against H2O, and lyophilized. Flures-HSA (HSA derivative containing 1.7 mol of fluresceine/mol of HSA) prepared by this procedure contains 1.7 ± 0.2 mol of fluresceine/mol of HSA as determined by its absorbance at 500 nm using ϵ500 = 62,000.
Cationized Flures-HSA was prepared by dissolving 20 mg of Flures-HSA in 1.0 ml of 1 m 1,3-diaminopropane or in 1 ml of 1 m arginine-amide. 1-Ethyl-3-(3-dimethyl aminopropyl) carbodiimide (70 mg) was then added. The pH was adjusted to 6.0 ± 0.1. The reaction was carried out for 2 h. The derivatives thus obtained were dialyzed against H2O for 2 days with several changes of H2O and lyophilized. Flures-HSA that was cationized with arginine-amide contains additional 64 ± 3 moieties of arginine-amide/mol of Flures-HSA as determined by amino acid analysis following acid hydrolysis. The protein concentration was calculated according to alanine (62 residues) and glycine (12 residues).
Biological and Chemophysical Procedures
Radiolabeling with Na125I
Radiolabeling of peptides and proteins with Na125I was carried out essentially by the procedure of Hunter and Greenwood (24) with slight modifications that were described in detail in Ref. 25.
Primary Cultures of Brain Endothelial Cells
Primary cultures of brain endothelial cells were isolated from freshly collected porcine brains as described previously (21, 26). These PBEC were validated as true BBB endothelial cells in previous studies (21, 26). The purity of the culture was confirmed by specific staining for von Willebrand factor (20).
In Vitro BBB Models and Transendothelial Electrical Resistance Measurements
In a typical experiment, PBEC were seeded at a density of 100,000 PBEC/cm2 on a microporous membrane of a Transwell insert (Corning Costar, Acton, MA) placed into a 12-well plates. The cells were cultured in plating medium for up to 3 days until they reached confluency. The medium was replaced with a serum-free medium (assay medium) for an additional 24–48 h, and the integrity of this cellular barrier was determined by measuring TEER. A decrease in TEER reflects an increase in permeability and a loss of barrier function. TEER of the filter insert was recorded using an Endohm chamber connected to an EVOM resistance meter (World Precision Inst., Inc., Sarasota, FL). The TEER of each filter insert was calculated by subtracting the TEER of the microporous membrane without PBEC and is reported as Ωcm2. When using a co-culture BBB in vitro system, Transwell inserts seeded with glial cells at the bottom were used as blanks. The co-culture system was used in a “contact” configuration as described in detail in Ref. 20. For testing the effects of the different compounds on TEER, they were diluted in assay medium at the desired concentrations and added to the luminal (to mimic blood to brain passage) or abluminal (to mimic brain to blood passage) side of the inserts.
Media
Plating medium is composed of newborn calf serum (10%), l-glutamine (2 mm), penicillin (100 units/ml), streptomycin (0.1 mg/ml), and gentamicin (0.1 mg/ml), all dissolved in Earl's medium 199 (Sigma). The assay medium consists of l-glutamine (2 mm), penicillin (100 units/ml), streptomycin (0.1 mg/ml), gentamicin (0.1 mg/ml), and hydrocortisone (550 nm) in DMEM diluted 1:1 in Ham's F-12 medium (Biological Industries, Beit Haemek, Israel).
Permeability Measurements
The PBEC permeability of this in vitro BBB model to radiolabeled compounds ([14C]sucrose, [125I]HSA, and [125I]Herceptin) and fluorescently labeled compounds (doxorubicin or Fluores-HSA) was carried out as described in detail in Refs. 20 and 27 with slight modifications. The tested compounds were added at the luminal side of the Transwell inserts. During the transport assay, the cells were incubated at 37 °C. Samples (500 μl) were collected every 10 min over a period of 40 min from the abluminal side and measured for their 14C or 125I content or for their fluorescent intensity. Pe values of filters having no PBEC seeded on them were subtracted. Pe was obtained from the slope of the calculated clearance curve as described in Ref. 27.
RESULTS
Cationized Albumin Permeates through the PBEC Monolayer
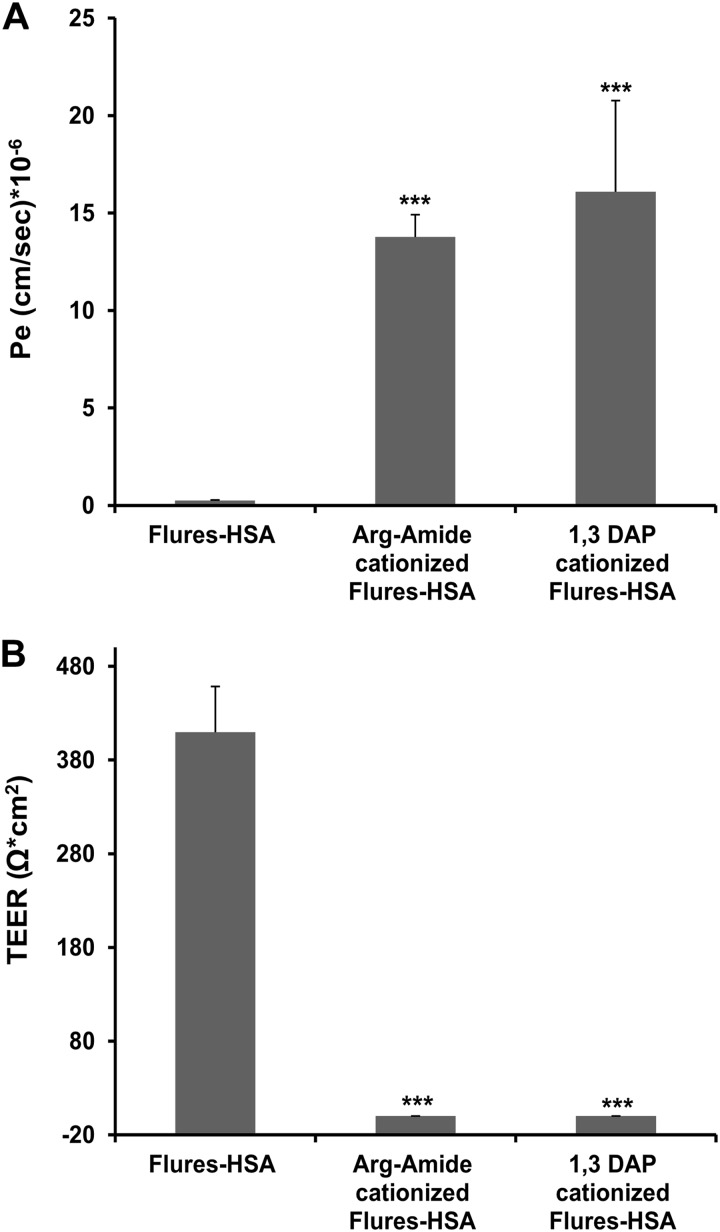
We prepared Flures-HSA and further cationized it by linking either 1,3-diaminopropane or arginine-amide, converting a major part of the carboxylate moieties (∼60%) into positively charged residues (“Experimental Procedures”). Both versions of fluorescently labeled cationized HSA penetrated this PBEC monolayer efficiently (14–17 cm/s × 10−6; Fig. 1A). Both derivatives had a marked effect in reducing TEER (Fig. 1B), suggesting that penetrability is secondary to destabilizing the tightness of this monolayer. Noncationized HSA neither penetrated nor destabilized this PBEC monolayer (Fig. 1). Thus, this in vitro experimental system resembles BBB penetrability in vivo, with regard to proteins that can enter this barrier by absorptive mediated transendocytosis (1, 28).
FIGURE 1.
Cationized derivatives of HSA decrease tightness and permeate across PBEC monolayer. A, Flures-HSA and Flures-HSA that was cationized with either arginine-amide or with 1,3-diaminopropane (9 mg/ml of each) were placed at the luminal side, and Pe values were measured over a period of 40 min. B, TEER measurements of PBEC 40 min after placing Flures-HSA or its cationized derivatives. The results are expressed as the means ± S.E. (n = 3–7 inserts). ***, p < 0.001 versus Flures-HSA.
Synthesizing and Characterizing a Peptide That Opens BBB, Based on TAT Protein Variants
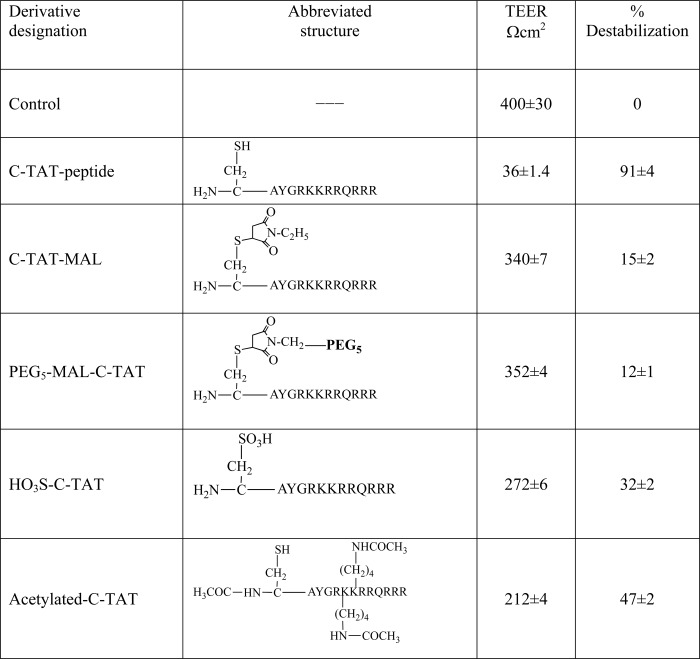
Several TAT-containing peptide versions were synthesized: of these, we have selected as the first prototype a version containing a single cysteinyl moiety at position 1 combined with fragment 46–57 of TAT protein, which contains the transduction domain (13). Table 1 summarizes several of the properties of this C-TAT peptide (CAYGRKKRRQRRR). It migrates as a single peak on HPLC column with a retention time value of 4.605 min. It absorbs at 278 nm with extinction coefficient (ϵ278) of 2400 ± 50, a value that is 1.71 times higher than that expected from a peptide containing a single tyrosyl moiety (ϵ278 = 1400). Mass spectrum analysis revealed the corrected mass as calculated (1734.07 Da). Performic acid oxidation of this peptide yielded 1.0 ± 0.1 mol/mol cysteic acid, as determined by amino acid analysis following acid hydrolysis. C-TAT peptide is highly soluble in H2O (>20 mg/ml).
TABLE 1.
Chemical features of C-TAT peptide
| Characteristic | |
|---|---|
| Migration on analytical HPLC retention timea | Single peak, 4.605 min |
| Absorbance at 278 nmb | ϵ278 = 2400 ± 50 |
| Mass spectrac | Calculated 1734.07 daltons, found (ES) 1733.53 ± 0.04 daltons |
| Cysteic acid content following performic acid oxidationd | 1.0 ± 0.1 mol/mol |
| Solubility in H2O | > 20 mg/ml |
a Conducted with a linear gradient from 0 to 100% of solution A (0.1% TFA) to solution B (acetonitrile H2O 75:25 in 0.1% TFA) using a chromolith Rp 18e (100 × 4 mm) column.
b Determined by UV spectroscopy. Peptide concentration was determined by acid hydrolysis of 20-μl aliquot followed by amino acid analysis and calculated according to alanine (one residue) and glycine (one residue).
c Mass spectroscopy was determined by the electrospray ionization technique.
d Cysteic acid content was determined by acid hydrolysis and amino acid analysis of an aliquot following performic acid oxidation (“Experimental Procedures”). Peptide concentration was calculated according to alanine and glycine (one residue of each).
C-TAT Peptide Decreases TEER in a Concentration- and Time-dependent Fashion
Fig. 2A shows the effect of several concentrations of C-TAT peptide placed at the Transwell luminal side, which resembles the blood side of the BBB, caused by the asymmetry alignment of the cells grown on collagen-coated inserts (26), on TEER over a period of 2 h. At a C-TAT peptide concentration of 0.6 μmol/ml, TEER values were decreased in a time-dependent fashion amounted to 47, 13, and 2% of initial value following 0.5, 1.0, and 2 h of incubation, respectively. At 0.3 μmol/ml C-TAT peptide, TEER decreased to 48, 27, and 16% of initial value following 0.5, 1.0, and 2 h of incubation, respectively. A lower concentration of the C-TAT peptide (0.06 μmol/ml) decreased TEER by 26%, following 1 h of incubation with no further decrease at 2 h (Fig. 2A).
FIGURE 2.
Time- and dose-dependent effect of the C-TAT peptide on destabilizing BBB in vitro systems. A, the indicated concentrations of the C-TAT peptide were added to the luminal or abluminal side of the Transwells with PBEC cultured in monolayers, and TEER values were monitored over a period of 2 h. The data are presented as the means ± S.E. (n = 4–9 inserts). B, side by side comparison of TEER and Pe for monoculture versus co-culture BBB in vitro model. C-TAT at 0.6 mm was applied to the luminal side of either a monoculture or co-culture (with the addition of glial cells at the bottom of the Transwell insert (20)). The TEER was then monitored for 2 h. The results are expressed as the means ± S.E. for three inserts each. The data used in this figure were driven only from a side by side comparison from cells (PBEC and glial) prepared in the same preparation.
We also studied the destabilizing effect of the C-TAT peptide placed at the abluminal side (“brain” side). TEER value has decreased as well with time, although at a lower rate, as compared with the same peptide concentration placed at the luminal side. However, in both cases TEER values decreased to ∼2–7% of initial value following 2 h of incubation.
Lastly, we have compared the destabilizing effect of the C-TAT peptide, side by side on a monoculture of PBEC as described above and a contact co-culture made of endothelial cells on one side of the insert and glial cells seeded on the bottom of the Transwell inserts (20). TEER was ∼1.5 times higher in the co-culture (707 ± 16 versus 458 ± 30 Ω*cm2, respectively). C-TAT peptide, however, was nearly equipotent in decreasing TEER with time (t½ = 0.4 ± 0.02 h; Fig. 2B). Thus, the added glial cells enhance significantly BBB tightness but not the efficacy of the C-TAT peptide to disrupt it, suggesting that the effect of C-TAT on the barrier properties is primarily on the endothelial cells.
Two Patterns of BBB Opening by C-TAT Peptide
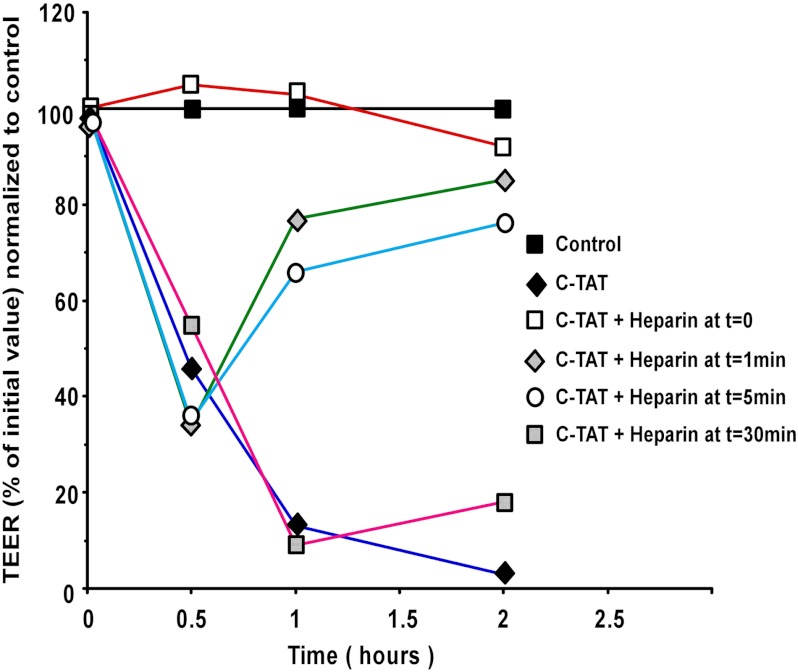
Heparin was documented to associate with TAT protein transduction domain (29). Fig. 3 shows that heparin associates with the C-TAT peptide as well. Heparin, at a 2:1 molar ratio suppresses the peptide efficacy to reduce TEER over a period of 2 h (Fig. 3). We therefore added heparin at 0, 1, 5, and 30 min following the addition of the C-TAT peptide to the PBEC monolayer (PBEC-M), and TEER values were monitored over a period of 2 h. Exposure of PBEC-M to the C-TAT peptide for a period of 1 min prior to neutralizing it with heparin reduced TEER by ∼60%, and this was followed by nearly full recovery of TEER within a period of 2 h. Nearly the same pattern is seen following 5 min of exposure of PBEC-M to the peptide prior to neutralizing it. More prolonged exposure (30 min) of PBEC-M to the peptide prior to neutralizing it, yielded large decrease in TEER with little restoring efficacy in the 2-h time frame shown in Fig. 3, although after 24 h, PBEC-M has restored to large extent its TEER values (data not shown). Thus, C-TAT peptide appears to yield two distinct patterns of BBB opening. The first one takes place within a short (1 min) transitory exposure and is nearly fully reversible, where the second pattern takes place following prolonged (30 min) exposure of the PBEC-M to the peptide, yielding a more extensive and less reversible BBB opening pattern.
FIGURE 3.
PBEC monolayer recovers after neutralization of C-TAT with heparin. C-TAT was added to the luminal side of the in vitro BBB model at 0.6 mm, and heparin was added at 2:1 molar ratio in several time points afterward. TEER values were then monitored. The data are expressed as percentages of initial TEER values normalized to control inserts and presented as the means ± S.E. values (n = 3 inserts).
Cysteine 1 of the C-TAT Peptide Contributes Significantly to the PBEC-M Destabilizing Potency of the Peptide
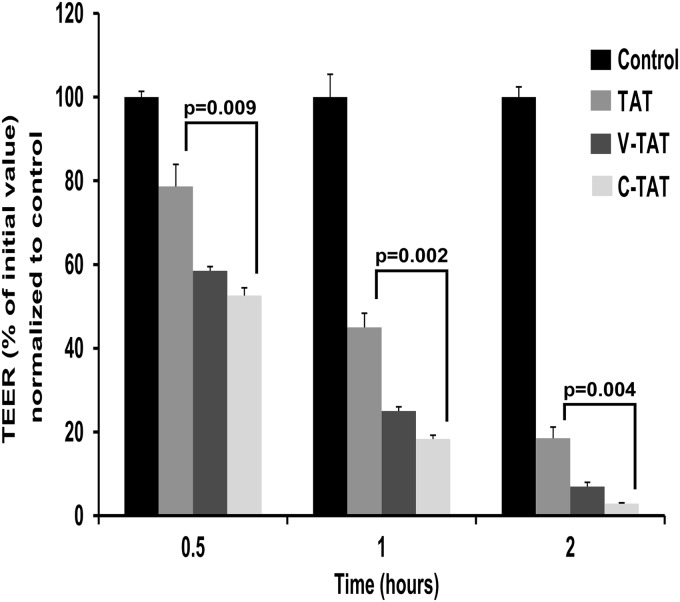
Initially we elongated the C-TAT peptide by adding additional arginine moiety at the C-terminal position. This 14-amino acid peptide (CAYGRKKRRQRRRR) was as effective as the shorter C-TAT version (not shown), indicating that additional positive charge at the C-terminal end is not required for enhancing its destabilizing efficacy. Subsequently we prepared two additional versions of this longer C-TAT peptide. The first one lacks its cysteinyl moiety at position 1 (AYGRKKRRQRRRR; TAT peptide), and in the second version the cysteinyl moiety was replaced with valine (VAYGRKKRRQRRRR; V-TAT peptide). Valine has rather similar atomic volume and degree of hydrophobicity as cysteine but lacks the weakly acidic and the reducing efficacy of cysteine. As shown in Fig. 4, the C-TAT peptide is approximately twice as potent as the TAT peptide in disrupting PBEC-M, as well as capable of fully reducing TEER, 2 h after addition. V-TAT peptide was nearly as potent as the C-TAT peptide in this respect (Fig. 4). Thus, the destabilizing efficacy of TAT containing peptides is significantly enhanced if either cysteine or valine are present at the N-terminal end. It appears that a hydrophobic side chain moiety, e.g., -CH2-SH (cysteine) or -CH(CH3)2 (valine), is responsible for this increase in potency. Further derivatization of the sulfhydryl moiety of the C-TAT peptide yielded inactive derivatives.
FIGURE 4.
Cysteine 1 of the C-TAT peptide contributes significantly to the peptide PBEC-M destabilizing potency. Two versions of the longer C-TAT peptide were synthesized. The first one lacks its cysteinyl moiety at position 1 (AYGRKKRRQRRRR; TAT), and in the second version the cysteinyl moiety was replaced with valine (VAYGRKKRRQRRRR; V-TAT). The peptides were added to the luminal side of the Transwells with PBEC-cultured in monolayers, and TEER values were monitored over a period of 2 h. The data are presented as the means ± S.E. (n = 3 inserts).
Structure-Function Relationships
Table 2 shows the potencies of several C-TAT peptide derivatives to reduce TEER value of PBEC monolayer following 1 h of incubation with 0.6 μmol/ml of each derivative. Derivatization of the free cysteinyl moiety with NEM, linking to it PEG5-MAL, or oxidizing it to cysteic acid, yielded derivatives having reduced BBB disrupting potency. Interestingly, acetylation of the C-TAT peptide under conditions that preserve the cysteinyl-moiety in its reduced form yielded a C-TAT peptide derivative that preserved ∼47% of its destabilizing potency (Table 2). Thus, the positive charges of the two lysine moieties in this peptide can be replaced by noncharged amino acid moieties.
TABLE 2.
Structure-function studies: effect of modified derivatives of C-TAT peptide on destabilizing PBEC-M
C-TAT peptide and the indicated derivatives of the C-TAT peptide (0.6 μmol/ml of each) were placed at the luminal side of the Transwell, and TEER values were determined following 1 h of incubation at 37 °C. The data are presented as the means ± S.E. (n = 5).
C-TAT Peptide Turns PBEC Monolayer Penetrable to Low Molecular Weight Substances
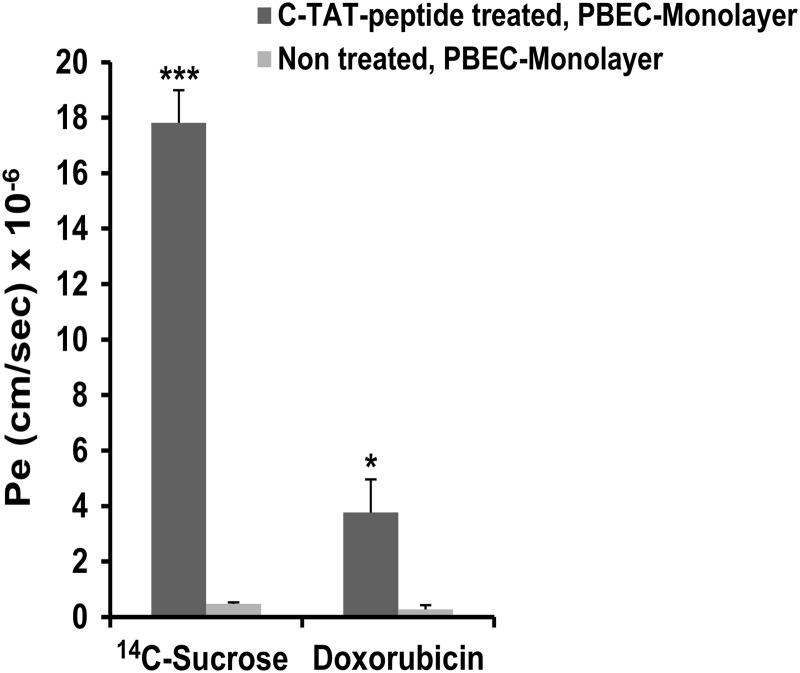
We incubated the PBEC monolayer with 0.6 μmol/ml C-TAT peptide for 2 h at 37 °C. [14C]Sucrose (molecular weight = 342) or doxorubicin HCl (molecular weight = 580) was then added to the Transwell luminal side, and the extent of permeability after a period of 2 h was quantitated. Both substances penetrated the PBEC monolayer (Fig. 5). Pe values were 17.8 ± 1.8 × 10−6 and 3.8 ± 1.8 × 10−6 cm/s for [14C]sucrose and doxorubicin, respectively, exceeding the Pe values obtained in untreated PBEC monolayer by 38- and 14-fold, respectively (Fig. 5). Thus, incubation with the C-TAT peptide alters PBEC in a way that allows the entrance of low molecular weight substances from the luminal to the abluminal side to a considerable extent.
FIGURE 5.
Effect of C-TAT peptide treatment on penetration of low molecular weight substances. PBEC-M in Transwells were incubated with medium alone or with medium containing C-TAT peptide (0.6 μmol/ml) for 2 h at 37 °C. [14C]Sucrose or doxorubicin HCl were then added at the luminal side, and their permeability across PBEC-M to the abluminal side was determined by measuring Pe values every 10 min over a period of 40 min. The results are expressed as the means ± S.E. of the independent assays (n = 3–4 inserts). *, p < 0.05; ***, p < 0.001 versus untreated.
Permeation of High Molecular Weight Proteins Following PBEC Monolayer Treatment with C-TAT Peptide
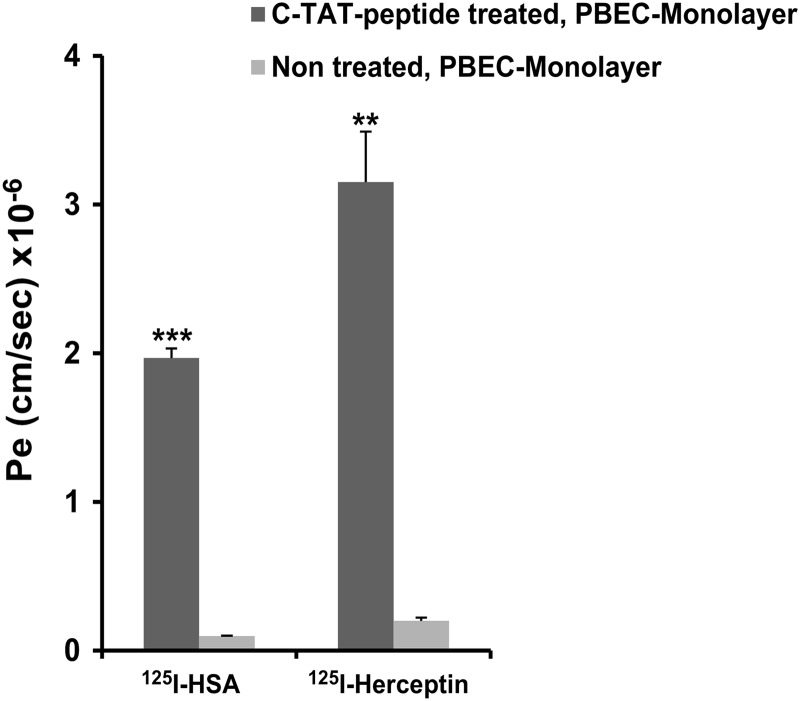
Next, we have studied the permeability of [125I]HSA (molecular mass = 66 kDa) and of [125I]herceptin (molecular mass = 145 kDa) following the incubation of the PBEC monolayer with C-TAT peptide under the same conditions used in Fig. 5. Both proteins penetrated the treated PBEC monolayer to a significant extent (Fig. 6). Penetrability amounted to 1.9–3.2 × 10−6 cm/s, exceeding 15–18 times that found in control PBEC monolayer. Thus, the destabilizing effect of the C-TAT peptide enabled the entrance of high molecular weight proteins via this PBEC monolayer from the luminal to the abluminal side.
FIGURE 6.
Effect of C-TAT peptide treatment on penetration of high molecular weight substances. PBEC-M in Transwells were incubated with medium alone or with medium containing C-TAT peptide (0.6 μmol/ml) for 2 h at 37 °C. [125I]HSA or [125I]herceptin was then added at the luminal side, and their permeability across PBEC-M to the abluminal side was determined by measuring Pe values every 10 min over a period of 40 min. The results are expressed as the means ± S.E. of the independent assays (n = 4–6 inserts). **, p < 0.01; ***, p < 0.001 versus untreated.
As shown in the co-culture experimental system, the addition of glial cells largely enhanced the tightness of the endothelial cell monolayer as judged by increased TEER (Fig. 2B). To assess whether this increase in BBB integrity imposes a profound decrease in protein penetrability following disruption with the C-TAT peptide, we compared [125I]herceptin penetrability with both systems under identical experimental conditions. Pe of [125I]herceptin in the co-culture system amounted to 43 ± 3% of that obtained in the monoculture system. However, the same extent of penetration was reached by increasing the incubation time with the C-TAT peptide to 3 h or doubling its concentration (data not shown).
DISCUSSION
In this study we were interested in finding conditions that permit significant permeations of impermeant substances including therapeutic proteins from the periphery to the brain. Major attempts have been undertaken worldwide in this direction. A common approach is to link or fuse BBB-impermeable peptide or protein to a “shuttle protein” that enters the BBB by receptor-mediated transendocytosis (30). Such approaches may deliver low (pmol) amounts of a peptide or a protein from the periphery to the CNS. From a clinical standpoint, however, this approach appears inadequate. Other directions were therefore attempted. Brown et al. (31) have demonstrated opening of the BBB in patients with malignant brain tumors using hyperosmolar mannitol. This procedure increased to some extent BBB penetrability of 86Rb+ and of [14C]sucrose but had little or no effect in enhancing penetrability of high molecular weight proteins.
In this study we have used an in vitro model of the BBB, composed of PBECs grown in culture (20, 21). Initially we have analyzed whether this experimental system reflects BBB penetrability of high molecular weight proteins as well. As validated in rodents in vivo, we found that HSA (molecular mass = 66 kDa), an impermeant molecule, becomes permeable following cationization (Fig. 1). This opened to us the options of finding conditions that weakened BBB tightness and allowed penetration of therapeutic proteins, two parameters that are extremely problematic for evaluation in vivo in a quantitative fashion.
Several mechanisms have been proposed by which TAT protein disrupts BBB, including the decrease of tight junction proteins expression and relocalization (32–34). It is reasonable to assume that the C-TAT peptide may have effects similar to those of the TAT protein on the expression of tight junction proteins. This would suggest a paracellular passage after C-TAT peptide treatment. In designing our BBB-disrupting peptide prototype, we hypothesized that in addition to the transduction domain, additional site(s) located in other regions of the TAT protein may be required to extend the BBB disruption potency of a transduction domain-containing fragment. Postulating that such an additional site must be a conserved entity among TAT protein variants, we have selected a cysteinyl moiety as a representative of the seven very conserved cysteines of region II. NMR studies suggested that the flexibility of the C-TAT protein allows regions II and IV to be in close proximity to each other (35, 36). For that reason we have placed the cysteinyl moiety at position 1 for allowing its approach to the vicinity of the transduction domain if the appropriate structural alterations do take place at aqueous physiological media. Indeed the inclusion of a cysteinyl moiety at position 1 yielded a TAT-containing peptide that is considerable more potent in destabilizing the PBEC monolayer (Fig. 4). A free cysteinyl side chain moiety (-CH2-SH) appears to be required. Its derivatization yields less potent derivatives (Table 2). A low concentration of the C-TAT peptide (0.3–0.6 μmol/ml) destabilizes PBEC monolayer within a period of 1.5–2 h (Fig. 2). Short (1 min) exposure of PBEC-M to the peptide followed by its neutralization with heparin yielded 60% decrease in TEER within 30 min followed by nearly full TEER recovery within the next 2 h (Fig. 3). This desirable transitory opening feature is important for clinical implications.
Although not definitive yet, our efforts to obtain a peptide derivative capable of disrupting BBB but lacking its ability to penetrate into cell interiors were not successful so far. For example, PEG5-MAL-C-TAT peptide (a conjugate of PEG5-MAL linked to the cysteine moiety of the C-TAT peptide) is impermeable into cell interiors5 and ineffective as well in destabilizing the PBEC monolayer (Table 2). It therefore seems that these two functions may be interrelated and cannot be dissociated. From a clinical point of view, this implies that in vivo experimental conditions need to be found to bring the C-TAT peptide into close proximity to the BBB, to minimize its uptake by peripheral tissue. Considering the usage of cationized proteins as BBB-opening agents, the efficiency is dependent on the dosage and the type of cationization. Although difficult to compare, we found on a molar basis that 1,3-diaminopropane-cationized HSA is ∼3–5 times more potent than the C-TAT peptide in disrupting and opening PBEC-M to impermeant substances (data not shown).
Finally, we stress that acetylation of the C-TAT peptide under conditions that leave the cysteinyl moiety nonmodified yielded a derivative that preserved ∼47% of its BBB-disrupting potency (Table 2). Thus, these two lysyl moieties can be replaced, derivatized, or modified upon developing a second generation of BBB opening peptides with improved therapeutic efficacies.6
In summary, we have used here an in vitro BBB model that appears to reflect BBB in vivo by at least the following five criteria: very low penetrating capacity of [14C]sucrose; high TEER value (>400 Ω*cm) (20, 21); similar expression pattern of major tight and adherence junction proteins (20); negligible penetrability of doxorubicin, [125I]HSA, and [125I]herceptin (Figs. 5 and 6); and the conversion of fluorescein-HSA into a penetrable species following cationization (Fig. 1). This model assisted us in developing, experimental conditions that destabilizes BBB and allows the delivery of therapeutic agents including high molecular weight proteins, from the periphery to the brain in quantities that might be sufficient to treat major CNS disorders and malignant brain tumors.
This work was supported by a joint Weizmann Institute of Science-Sheba Medical Center grant for Biomedical Research.
I. Cooper, K. Sasson, V. I. Teichberg, M. Schnaider-Beeri, M. Fridkin, and Y. Shechter, unpublished observation.
I. Cooper, K. Sasson, V. I. Teichberg, M. Schnaider-Beeri, M. Fridkin, and Y. Shechter, manuscript in preparation.
- BBB
- blood-brain barrier
- ES
- electrospray
- HSA
- human serum albumin
- TEER
- transendothelial electrical resistance
- Pe
- permeability
- PBEC
- porcine brain endothelial cell
- MAL
- maleimide
- PBEC-M
- PBEC monolayer.
REFERENCES
- 1. Pardridge W. M. (2010) Biopharmaceutical drug targeting to the brain. J. Drug Target 18, 157–167 [DOI] [PubMed] [Google Scholar]
- 2. van de Waterbeemd H., Camenisch G., Folkers G., Chretien J. R., Raevsky O. A. (1998) Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J. Drug Target 6, 151–165 [DOI] [PubMed] [Google Scholar]
- 3. Doolittle N. D., Peereboom D. M., Christoforidis G. A., Hall W. A., Palmieri D., Brock P. R., Campbell K. C., Dickey D. T., Muldoon L. L., O'Neill B. P., Peterson D. R., Pollock B., Soussain C., Smith Q., Tyson R. M., Neuwelt E. A. (2007) Delivery of chemotherapy and antibodies across the blood-brain barrier and the role of chemoprotection, in primary and metastatic brain tumors. Report of the Eleventh Annual Blood-Brain Barrier Consortium Meeting. J. Neurooncol. 81, 81–91 [DOI] [PubMed] [Google Scholar]
- 4. Huber J. D., Egleton R. D., Davis T. P. (2001) Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 24, 719–725 [DOI] [PubMed] [Google Scholar]
- 5. Huber J. D., Hau V. S., Borg L., Campos C. R., Egleton R. D., Davis T. P. (2002) Blood-brain barrier tight junctions are altered during a 72-h exposure to λ-carrageenan-induced inflammatory pain. Am. J. Physiol. Heart Circ. Physiol. 283, H1531–H1537 [DOI] [PubMed] [Google Scholar]
- 6. Mooradian A. D. (1988) Effect of aging on the blood-brain barrier. Neurobiol. Aging 9, 31–39 [DOI] [PubMed] [Google Scholar]
- 7. Stone L. A., Smith M. E., Albert P. S., Bash C. N., Maloni H., Frank J. A., McFarland H. F. (1995) Blood-brain barrier disruption on contrast-enhanced MRI in patients with mild relapsing-remitting multiple sclerosis. Relationship to course, gender, and age. Neurology 45, 1122–1126 [DOI] [PubMed] [Google Scholar]
- 8. Yang G. Y., Betz A. L. (1994) Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke 25, 1658–1665 [DOI] [PubMed] [Google Scholar]
- 9. Gabathuler R. (2010) Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 37, 48–57 [DOI] [PubMed] [Google Scholar]
- 10. Campbell G. R., Watkins J. D., Esquieu D., Pasquier E., Loret E. P., Spector S. A. (2005) The C terminus of HIV-1 TAT modulates the extent of CD178-mediated apoptosis of T cells. J. Biol. Chem. 280, 38376–38382 [DOI] [PubMed] [Google Scholar]
- 11. Jeang K. T., Xiao H., Rich E. A. (1999) Multifaceted activities of the HIV-1 transactivator of transcription, TAT. J. Biol. Chem. 274, 28837–28840 [DOI] [PubMed] [Google Scholar]
- 12. Derossi D., Calvet S., Trembleau A., Brunissen A., Chassaing G., Prochiantz A. (1996) Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271, 18188–18193 [DOI] [PubMed] [Google Scholar]
- 13. Brooks H., Lebleu B., Vivès E. (2005) TAT peptide-mediated cellular delivery. Back to basics. Adv. Drug Deliv. Rev. 57, 559–577 [DOI] [PubMed] [Google Scholar]
- 14. Ezhevsky S. A., Nagahara H., Vocero-Akbani A. M., Gius D. R., Wei M. C., Dowdy S. F. (1997) Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc. Natl. Acad. Sci. U.S.A. 94, 10699–10704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagahara H., Vocero-Akbani A. M., Snyder E. L., Ho A., Latham D. G., Lissy N. A., Becker-Hapak M., Ezhevsky S. A., Dowdy S. F. (1998) Transduction of full-length TAT fusion proteins into mammalian cells. TAT-p27Kip1 induces cell migration. Nat. Med. 4, 1449–1452 [DOI] [PubMed] [Google Scholar]
- 16. Banks W. A., Robinson S. M., Nath A. (2005) Permeability of the blood-brain barrier to HIV-1 TAT. Exp. Neurol. 193, 218–227 [DOI] [PubMed] [Google Scholar]
- 17. Schwarze S. R., Ho A., Vocero-Akbani A., Dowdy S. F. (1999) In vivo protein transduction. Delivery of a biologically active protein into the mouse. Science 285, 1569–1572 [DOI] [PubMed] [Google Scholar]
- 18. Jarajapu Y. P., Baltunis J., Knot H. J., Sullivan S. M. (2005) Biological evaluation of penetration domain and killing domain peptides. J. Gene Med. 7, 908–917 [DOI] [PubMed] [Google Scholar]
- 19. Doolittle N. D., Petrillo A., Bell S., Cummings P., Eriksen S. (1998) Blood-brain barrier disruption for the treatment of malignant brain tumors. The National Program. J. Neurosci. Nurs. 30, 81–90 [DOI] [PubMed] [Google Scholar]
- 20. Cohen-Kashi Malina K., Cooper I., Teichberg V. I. (2009) Closing the gap between the in-vivo and in-vitro blood-brain barrier tightness. Brain Res. 1284, 12–21 [DOI] [PubMed] [Google Scholar]
- 21. Cooper I., Cohen-Kashi Malina K., Cagnotto A., Bazzoni G., Salmona M., Teichberg V. I. (2011) Interactions of the prion peptide (PrP 106–126) with brain capillary endothelial cells. Coordinated cell killing and remodeling of intercellular junctions. J. Neurochem. 116, 467–475 [DOI] [PubMed] [Google Scholar]
- 22. Abbott N. J., Dolman D. E., Patabendige A. K. (2008) Assays to predict drug permeation across the blood-brain barrier, and distribution to brain. Curr. Drug Metab. 9, 901–910 [DOI] [PubMed] [Google Scholar]
- 23. Rutten M. J., Hoover R. L., Karnovsky M. J. (1987) Electrical resistance and macromolecular permeability of brain endothelial monolayer cultures. Brain Res. 425, 301–310 [DOI] [PubMed] [Google Scholar]
- 24. Hunter W. M., Greenwood F. C. (1962) Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature 194, 495–496 [DOI] [PubMed] [Google Scholar]
- 25. Shechter Y., Tsubery H., Fridkin M. (2002) N-[(2-Sulfo)-9-fluorenylmethoxycarbonyl](3)-gentamicin C(1) is a long-acting prodrug derivative. J. Med. Chem. 45, 4264–4270 [DOI] [PubMed] [Google Scholar]
- 26. Franke H., Galla H., Beuckmann C. T. (2000) Primary cultures of brain microvessel endothelial cells. A valid and flexible model to study drug transport through the blood-brain barrier in vitro. Brain Res. Brain Res. Protoc. 5, 248–256 [DOI] [PubMed] [Google Scholar]
- 27. Dehouck M. P., Méresse S., Delorme P., Fruchart J. C., Cecchelli R. (1990) An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J. Neurochem. 54, 1798–1801 [DOI] [PubMed] [Google Scholar]
- 28. Dakwar G. R., Abu Hammad I., Popov M., Linder C., Grinberg S., Heldman E., Stepensky D. (2012) Delivery of proteins to the brain by bolaamphiphilic nano-sized vesicles. J. Control Release 160, 315–321 [DOI] [PubMed] [Google Scholar]
- 29. Ziegler A., Seelig J. (2004) Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate. Binding mechanism and thermodynamic parameters. Biophys. J. 86, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pardridge W. M. (2008) Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug. Chem. 19, 1327–1338 [DOI] [PubMed] [Google Scholar]
- 31. Brown R. C., Egleton R. D., Davis T. P. (2004) Mannitol opening of the blood-brain barrier. Regional variation in the permeability of sucrose, but not 86Rb+ or albumin. Brain Res. 1014, 221–227 [DOI] [PubMed] [Google Scholar]
- 32. András I. E., Pu H., Tian J., Deli M. A., Nath A., Hennig B., Toborek M. (2005) Signaling mechanisms of HIV-1 TAT-induced alterations of claudin-5 expression in brain endothelial cells. J. Cereb. Blood Flow Metab. 25, 1159–1170 [DOI] [PubMed] [Google Scholar]
- 33. Xu R., Feng X., Xie X., Zhang J., Wu D., Xu L. (2012) HIV-1 TAT protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Res. 1436, 13–19 [DOI] [PubMed] [Google Scholar]
- 34. Zhong Y., Zhang B., Eum S. Y., Toborek M. (2012) HIV-1 TAT triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J. Neurosci. 32, 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grégoire C., Péloponèse J. M., Jr., Esquieu D., Opi S., Campbell G., Solomiac M., Lebrun E., Lebreton J., Loret E. P. (2001) Homonuclear 1H-NMR assignment and structural characterization of human immunodeficiency virus type 1 TAT Mal protein. Biopolymers 62, 324–335 [DOI] [PubMed] [Google Scholar]
- 36. Péloponèse J. M., Jr., Grégoire C., Opi S., Esquieu D., Sturgis J., Lebrun E., Meurs E., Collette Y., Olive D., Aubertin A. M., Witvrow M., Pannecouque C., De Clercq E., Bailly C., Lebreton J., Loret E. P. (2000) 1H-13C nuclear magnetic resonance assignment and structural characterization of HIV-1 TAT protein. C. R. Acad. Sci. III 323, 883–894 [DOI] [PubMed] [Google Scholar]