Background: Integrin α2β1 is a platelet collagen receptor.
Results: Novel sulfonamide derivatives are conformation-selective inhibitors of α2β1, especially when tested under shear stress conditions. Only inhibitors that block non-activated integrins inhibit platelet binding to collagen.
Conclusion: Non-activated α2β1 integrin plays an important role in platelet binding to collagen.
Significance: We propose an alternative model for α2β1 activation during thrombosis.
Keywords: Adhesion, Collagen, Integrin, Platelets, Shear Stress
Abstract
The interaction between α2β1 integrin (GPIa/IIa, VLA-2) and vascular collagen is one of the initiating events in thrombus formation. Here, we describe two structurally similar sulfonamide derivatives, BTT-3033 and BTT-3034, and show that, under static conditions, they have an almost identical effect on α2-expressing CHO cell adhesion to collagen I, but only BTT-3033 blocks platelet attachment under flow (90 dynes/cm2). Differential scanning fluorimetry showed that both molecules bind to the α2I domain of the recombinant α2 subunit. To further study integrin binding mechanism(s) of the two sulfonamides, we created an α2 Y285F mutant containing a substitution near the metal ion-dependent adhesion site motif in the α2I domain. The action of BTT-3033, unlike that of BTT-3034, was dependent on Tyr-285. In static conditions BTT-3034, but not BTT-3033, inhibited collagen binding by an α2 variant carrying a conformationally activating E318W mutation. Conversely, in under flow conditions (90 dynes/cm2) BTT-3033, but not BTT-3034, inhibited collagen binding by an α2 variant expressing E336A loss-of-function mutation. Thus, the binding sites for BTT-3033 and BTT-3034 are differentially available in distinct integrin conformations. Therefore, these sulfonamides can be used to study the biological role of different functional stages of α2β1. Furthermore, only the inhibitor that recognized the non-activated conformation of α2β1 integrin under shear stress conditions effectively blocked platelet adhesion, suggesting that the initial interaction between integrin and collagen takes place prior to receptor activation.
Introduction
Integrin function is strictly regulated. In platelets, individual variations in the expression level of α2β1 integrin can lead to pathological bleeding (1) or unwanted thrombosis (2). In addition to the number of integrins on the cell surface, receptor clustering and structural modifications are also considered to play critical roles in the regulation of cell adhesion. Integrin α2β1 belongs to a subset of integrins in which the α subunit contains an extra domain, called an inserted domain (I domain), or A domain based on its structural similarity to the von Willebrand factor A domain (3). In vertebrates, four of the αI domain integrins (α1, α2, α10, and α11) partner with a β1 subunit to act as collagen receptors, whereas the other five (αD, αE, αL, αM, αX) are leukocyte integrins that associate with β2 or, in one case, with a β7 subunit. The αI domain harbors a metal ion-dependent adhesion site (MIDAS)2 and is responsible for ligand recognition and binding. The activity of the integrin αI domain can be regulated at the structural level. The “closed” α2I domain conformation has a lower affinity for many ligands, including collagens, compared with the “open” α2I conformation.
Extensive studies of the structural basis of β2- and β3-integrin function have unveiled fundamental changes in the conformation of integrin “leg” parts, both before and after ligand binding (4, 5). Atomic structures of crystallized heterodimeric αVβ3, αIIbβ3, and αXβ2 ectodomains, as well as electron micrographs of αVβ3, αIIbβ3, αXβ2, and αLβ2, have indicated that the legs of the integrins contain “knees” that allow the receptors to bend (6–10). In the bent form, the ligand-binding site of the receptor is facing the plasma membrane, and many studies have suggested that the ability of this conformation to bind to large ligands is limited. Physiological signals can activate intracellular regulatory pathways and induce the binding of specific proteins, such as talin or kindlins, to the intracellular domains of integrin β subunits (11). As a result of this inside-out regulation, the integrin “stands up” taking on an extended conformation that is capable of binding large ligands. Alternatively, some experimental evidence has led to speculation that ligand binding to a bent integrin may also induce extension of the receptor (4).
In leukocytes, the integrin αI conformation is linked to the conformation of the β-subunit by a specific glutamate residue that can act as an intrinsic ligand for the MIDAS in the βI domain and mediate structural changes related to both inside-out and outside-in signaling (12–14). Structural studies of αXβ2 integrin have indicated that the connection between the αI domain and the rest of the heterodimer is flexible and may allow the αI domain to move relatively freely (15).
The β1-integrins are thought to function in a manner similar to β2- and β3-integrins. Still, direct evidence for most details of this process is lacking. We have previously shown that the Glu-336 residue may act as a link between α2I and β1I domains (16) in a manner similar to that of Glu-320 in αM (12, 13) and Glu-310 in αL (14) and regulate the activity of the receptor. However, the function of α2β1 does not seem to be entirely dependent on conformational activation because α2β1 can bind even a large ligand (human echovirus-1) in a non-activated conformation (17).
To develop novel α2β1 integrin inhibitors, we used a ligand-based drug discovery approach to combine properties of the integrin-binding RKKH peptide (18, 19) with sulfonamide inhibitors (20, 21) of α2β1 integrin. The urea moiety was found to have properties that appropriately mimicked the arginine of the RKKH peptide. Taking advantage of several urea-substituted integrin ligands that have been previously discovered (22, 23), we generated a novel class of urea-substituted sulfonamide derivatives. Here, we describe two molecules that have different α2I domain-binding mechanisms and distinct functional properties. On the basis of our experiments utilizing α2 loss-of-function and gain-of-function mutations, we propose that the two sulfonamides are selective for different α2β1 integrin conformations, especially under shear stress conditions. Accordingly, they can be used to test the biological role of distinct functional stages of α2β1. Our data support the idea that β1-integrins also have several different activation stages, although there are differences compared with those of β2-integrins. Collectively, our results suggest a re-evaluation of the role of non-activated α2β1 under shear stress conditions.
EXPERIMENTAL PROCEDURES
Cell Assays under Static Conditions
CHO, PC-3 (human prostate cancer), and MG-63 and Saos-2 (human osteosarcoma) cells were obtained from the American type Culture Collection (ATCC). CHO cells were stably transfected with expression constructs of wild-type human α2 integrin (CHO-α2wt), α2 integrin variants (CHO-α2Y285F, CHO-α2E336A, CHO-α2E318W), or α1 integrin expression constructs (17, 24, 25). In adhesion assays, cells (1.5 × 105 cells/well) were allowed to attach to plates coated with rat tail collagen I, collagen IV (BD Biosciences), laminin-332, vitronectin, or α-chymotryptic fragments (120 or 40 kDa) of fibronectin (10 μg/ml; Chemicon) for 2 h at 37 °C. Prior to adhesion, cells were incubated for 10 min at 37 °C with tetradecanoylphorbol acetate (TPA; 100 nm; Sigma). The number of adherent cells was measured using the cell proliferation reagent WST-1 (Roche Applied Science) according to the manufacturer's protocol. EC50 (concentration required for half-maximal effect) and Emax (maximum inhibitory effect) values were determined using Graph Pad Prism (Graph Pad Software, Inc.). Cytotoxicity assays using CytoTox-ONE (Promega) were performed following the manufacturer's protocol.
For continuous measurement of cell adhesion, CHO-α2wt, CHO-α2E318W, and CHO-α2E336A cells (5 × 104 cells/well) were plated on rat tail collagen I-coated (Sigma) E-plates (Roche Applied Science) and monitored for 3 h with xCelligence (Roche Applied Science), which measures changes in impedance at the bottom of a cell culture well caused by cell attachment and spreading.
Platelet Adhesion under Flow Conditions
A Cellix microfluidic platform (Cellix, Ltd.), a dynamic set-up that mimics physiological flow conditions, was used to measure mouse and human platelet adhesion on collagen I (Nycomed)- or convulxin (Coatech)-coated chips (Cellix, Ltd.) under flow. Whole-blood samples were collected from mice (C57Bl/6) into containers containing 40 μm d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone (Calbiochem) and 7.5 units/ml heparin (Leo Pharma) as anticoagulants, and from humans using 40 μm d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone (Calbiochem) as an anticoagulant. Whole blood was stained with 1 μm 3,3′-dihexyloxacarbocyanine iodide (Invitrogen), and adhesion of platelets to fibrillar collagen (60 μg/ml)-or convulxin (20 μg/ml)-coated capillaries (Cellix, Ltd.) was detected with a fluorescence microscope (Carl Zeiss, Inc.) at 20× magnification. Whole blood was incubated with or without the inhibitors, BTT-3033 or BTT-3034 (10 μm), and a neutralizing antibody (8 μg/ml) against mouse GPVI (JAQ-1; Emfret Analytics) for 5 min. Blood was run through capillaries at a constant shear rate of 90 and 120 dynes/cm2 for human and mouse whole blood, respectively (Mirus 1.0 Nanopump; Cellix, Ltd.) for 5 min (human) or 4 min (mouse). Platelet adhesion to capillary walls was analyzed with DucoCell software (Cellix, Ltd.).
CHO Cell Adhesion under Flow Conditions
Adhesion of CHO cells to collagen I-coated chips under flow was measured using a Cellix microfluidic platform (Cellix Ltd.). Cells (5 × 106 cells/ml) were stained with 1 μm 3,3′-dihexyloxacarbocyanine iodide (Invitrogen) and adhesion to fibrillar collagen-coated (100 μg/ml) capillaries (Cellix, Ltd.) was detected with a fluorescence microscope (Carl Zeiss, Inc.) at 20× magnification. Cells were incubated with or without the inhibitors BTT-3033 or BTT-3034 (EC50) and a neutralizing antibody (10 μg/ml) against human α2 (P1H5; Santa Cruz Biotechnology) for 5 min and run through the capillary at a constant shear rate of 90 or 0.01 dynes/cm2 (Mirus 1.0 Nanopump; Cellix, Ltd.) for 5 min. Cell adhesion to the capillary wall was analyzed with DucoCell software (Cellix, Ltd.).
Analysis of Integrin Expression Levels
Integrin α2 expression levels on the CHO cell surface were determined by flow cytometry. Cells (3 × 105 cells/ml) were incubated with mouse anti-human CD49b primary antibody (7.5 μg/ml; BD Biosciences) for 1 h at 4 °C, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgG secondary antibody (115 μg/ml; DAKO) for 30 min at 4 °C. Cells were washed and analyzed with FACScan (BD Biosciences). Secondary antibody only or α2-negative cells were used as negative controls. Cytometry data were analyzed with Flowing Software 2 (Turku, Finland).
Binding Site Studies
CHO-α2wt cells (106 cells/ml) were incubated with the indicated concentrations of BTT-3033 or BTT-3034 for 30 min, after which the compound, CBL027 (50 μm), which exhibits context-dependent (Tyr-285) fluorescence (26), was added, and incubation was continued for 1 h. Fluorescently labeled cells were detected by flow cytometry (BD LSR II; BD Biosciences).
Protein thermal stability was determined using a Bio-Rad C1000 Thermal cycler and CFx96 real-time system. Protein unfolding was monitored over a range of 20 °C-95 °C at a rate of 0.5 °C/30 s by measuring the fluorescence of the environment-sensitive fluorescent dye, SYPRO Orange (5×; Invitrogen). The final protein concentration in the samples was 7 μm, and the concentrations of BTT-3033 and BTT-3034 were 42 and 49 μm, respectively. The total volume of samples was 25 μl.
Statistics
Continuous data are reported as means with S.E. using GraphPad Prism (GraphPad Software, San Diego). To evaluate the differences between groups, Wilcoxon Rank-Sum test or Student's t test was utilized.
RESULTS
Two Novel Sulfonamide Derivatives Selectively Block Collagen Binding by α2β1 Integrin
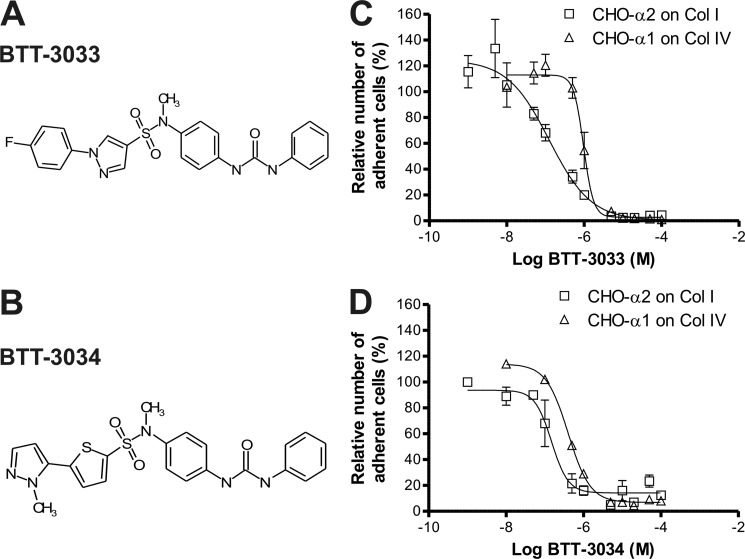
To understand the role of different substituents in the sulfonamide, we developed novel structural analogs based on previously identified α2β1 integrin modulator molecules (20, 21, 24, 26). First, the keto group in the benzophenone moiety (20, 21, 26) at the amide site was replaced with urea to test the effect of a slightly bulkier substituent at that site. Second, the bi-phenyl moiety of BTT-3016 (20) was replaced with analogs that have a similar shape. Third, all developed analogs were tested with and without amide methylation. A cell-based assay utilizing CHO-α2wt cells was used to test two potential α2β1 integrin-binding molecules, BTT-3033 and BTT-3034 (Fig. 1, A and B). BTT-3033 and BTT-3034 inhibited cell adhesion to rat tail collagen I with EC50 values of 130 and 160 nm, respectively, and corresponding Emax values of 97 and 86% (Fig. 1, C and D). The relative selectivity for α2β1 and α1β1 integrins was determined in an assay using CHO cells stably overexpressing wild-type human α1 (CHO-α1wt) and measuring attachment to collagen IV (Fig. 1, C and D). A comparison of EC50 values in CHO-α1wt/collagen IV assays and CHO-α2wt/collagen I assays showed that BTT-3033 selectivity for α2β1 integrin (8-fold) was greater than that of BTT-3034 (2-fold). Neither BTT-3033 nor BTT-3034 was cytotoxic at concentrations up to 200 μm (data not shown). The chemical characteristics of the synthesized compounds, BTT-3033 and BTT-3034, are summarized in supplemental Table 1. The potential inhibitory effects of BTT-3033 and BTT-3034 on the function of various integrins were tested using MG-63 and PC3 cells in adhesion assays utilizing different matrices. Neither BTT-3033 nor BTT-3034 (at EC50 concentrations) inhibited the adhesion of MG-63 cells to vitronectin, 120-kDa fibronectin or 40-kDa fibronectin, assays that measured αV, α5β1, and α4β1 integrin function, respectively (supplemental Table 2). Furthermore, BTT-3033 did not inhibit adhesion of PC3 cells to laminin-332 (previously termed laminin-5; LN-332), indicating that this sulfonamide does not interact with α3β1 integrin at its EC50 concentration. BTT-3034, however, did slightly inhibit PC3 cell adhesion to LN-332 (20% inhibition) at its EC50 concentration (supplemental Table 2).
FIGURE 1.
BTT-3033 and BTT-3034 are potent inhibitors of α2β1 integrin. Chemical structures of BTT-3033 (A) and BTT-3034 (B) are shown. The inhibitory effects and selectivity of BTT-3033 (C) and BTT-3034 (D) were tested by measuring CHO-α2β1 (□) and CHO -α1β1 (Δ) cell adhesion to collagen I and collagen IV, respectively. Cells were allowed to attach to collagen matrices for 2 h, and adherent cells were detected with the WST-1 reagent. BTT-3033 and BTT-3034 inhibited the adhesion of CHO-α2wt cells on collagen I with EC50 values of 130 and 160 nm, respectively, and corresponding Emax values of 97 and 86%. The selectivity versus α1β1 integrin was determined by comparing EC50 values in CHO-α1wt/collagen IV assay to those in CHO-α2wt/collagen I assays. The selectivity of BTT-3033 for α2β1 integrin (8-fold) was greater than that of BTT-3034 (2-fold).
The Sulfonamide Derivative BTT-3033, but Not BTT-3034, Inhibits Platelet Binding to Collagen I under Flow
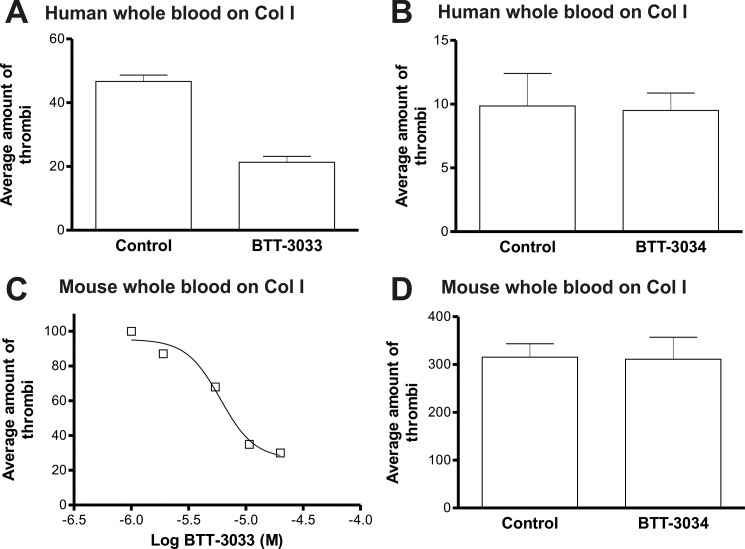
The effects of BTT-3033 and BTT-3034 on platelet aggregation in capillaries coated with collagen I was studied using a Cellix platform (Cellix, Ltd.). This technology allows the function of platelets to be tested under near-physiological conditions. BTT-3033 (10 μm) inhibited human platelet adhesion to collagen I-coated capillaries under flow (Fig. 2A). (Note that the free concentration of the sulfonamide derivative was remarkably reduced (to 1–3% of the total concentration) in the presence of plasma proteins.) With mouse whole blood, the EC50 value for BTT-3033 was determined to be 6 μm (Fig. 2C). In sharp contrast to BTT-3033, BTT-3034 (10 μm) had no inhibitory effect on platelet adhesion to collagen I-coated capillaries under flow (Fig. 2, B and D).
FIGURE 2.
The sulfonamide derivative BTT-3033, but not BTT-3034, blocks platelet binding to collagen under flow. The effect of BTT-3033 and BTT-3034 on platelet aggregation in capillaries coated with collagen I was studied using a Cellix microfluidic platform. A. BTT-3033 (10 μm) inhibited human platelet adhesion to collagen I-coated capillaries at a constant shear rate. C. The EC50 value for BTT-3033 using mouse whole blood was 6 μm. (B, D) BTT-3034 (10 μm) had no inhibitory effect on human (B) or mouse (D) platelet adhesion to collagen I-coated capillaries at a constant shear rate.
The two major platelet collagen receptors, α2β1 integrin and GPVI, have been shown to activate each other when platelets adhere to collagen I (27). We have previously shown that the sulfonamide inhibitors have no effect on platelets derived from α2 integrin-deficient mice (20), suggesting that they do not affect GPVI-mediated adhesion to collagen I. Here, we also coated capillaries with convulxin, a C-type lectin from rattlesnake venom that is a ligand for GPVI, but not α2β1 (28). BTT-3033 (10 μm) did not inhibit human platelet aggregation or binding to convulxin; unexpectedly, it actually slightly increased binding (supplemental Fig. 1). Mouse platelets did not show significant binding to convulxin when tested at a high flow rate (120 dynes/cm2). However, under these conditions, a large concentration (8 μg/ml) of a specific antibody against mouse GPVI (JAQ1) inhibited platelet binding to collagen (by 27%), suggesting that GPVI may still participate in the binding process (data not shown). Importantly, at 120 dynes/cm2, the maximal inhibition by BTT-3033 was ∼75% (Fig. 2C). These data indicate that GPVI-related mechanisms cannot explain the differential effects of the two sulfonamide derivatives on platelet binding to collagen I under flow.
The Two Sulfonamide Derivatives Bind α2β1 Integrin through Distinct Mechanisms
To obtain a more detailed view of the α2β1 binding sites and the mechanisms of BTT-3033 and BTT-3034, we first tested the ability of these agents to inhibit collagen binding by CHO-α2Y285F cells. The Y285F mutation in the α2I domain has no effect on collagen binding but prevents MIDAS binding of some α2β1 inhibitors, including the previously described sulfonamide derivative, BTT-3016 (Fig. 3A) (20). The function of BTT-3033, unlike that of BTT-3034, was shown to be fully dependent on Tyr-285 (Fig. 3A).
FIGURE 3.
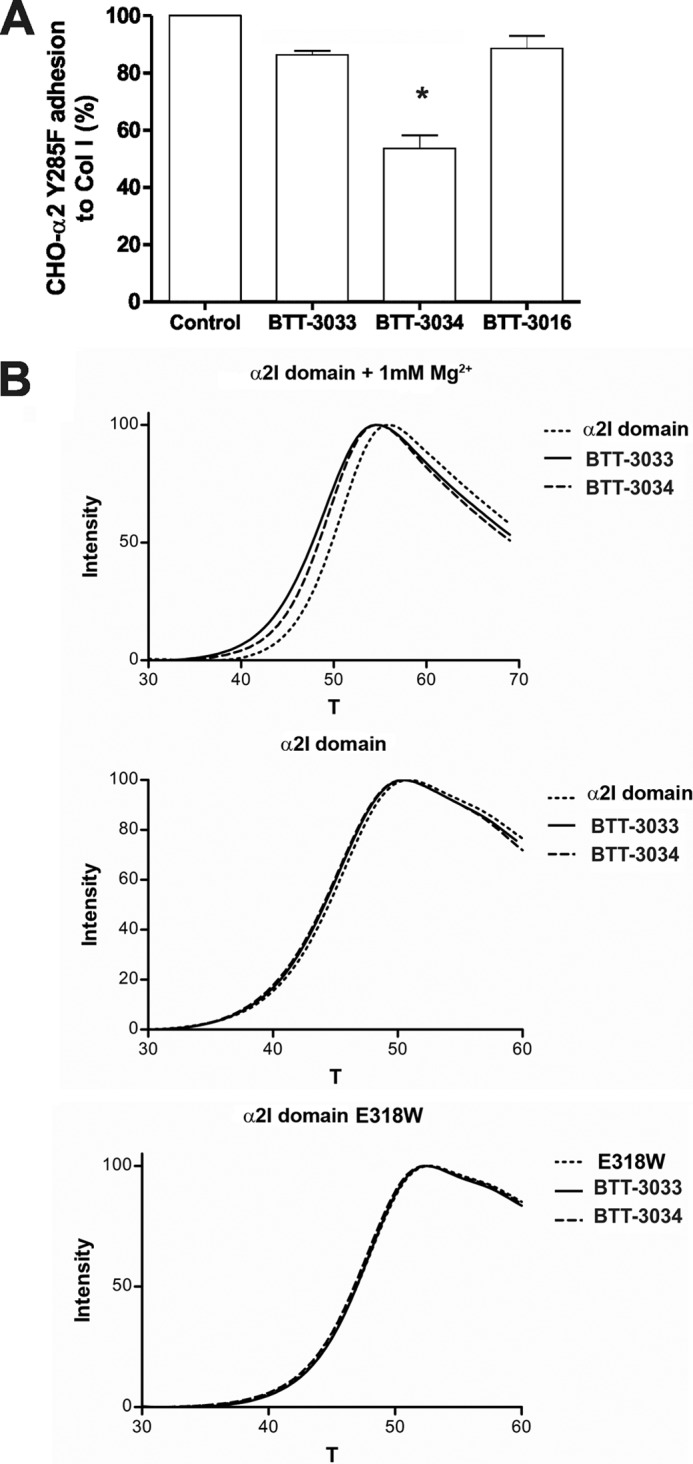
Both BTT-3033 and BTT-3034 bind to the α2I domain but only the function of BTT-3033 is dependent on Tyr-285. A, the adhesion of CHO-α2Y285F cells to collagen I in the presence or absence of the integrin inhibitors BTT-3033, BTT-3034, or BTT-3016 (at EC50 concentrations) was evaluated. Adherent cells were detected using the WST-1 reagent. BTT-3034, unlike BTT-3033 or BTT-3016, inhibited the adhesion of CHO-α2Y285F cells to collagen I, indicating that BTT-3033 and BTT-3016 bind to the α2I MIDAS motif. The data expressed are the mean ± S.E. of three independent experiments. Significant difference between BTT-3033 and BTT-3034 is indicated by an asterisk (Student's t test, *, p = 0.05). B, the thermal stability of wt and E318W recombinant α2I domains in the presence or absence of BTT-3033 (42 μm) or BTT-3034 (49 μm) and 1 mm Mg2+ was studied using differential scanning fluorimetry. Both BTT-3033 and BTT-3034 decreased the thermal stability of the α2I wt domain in the presence of Mg2+.
The results of differential scanning fluorimetry (29) showed that both BTT-3033 and BTT-3034 bound to the recombinant α2I domain in a metal-dependent manner and reduced the thermal stability of the α2I domain (ΔTm, −2.0 °C and −1.4 °C for BTT-3033 and BTT-3034, respectively; Fig. 3B; supplemental Table 3). Importantly, these differences between BTT-3033 and BTT-3034 suggest that BTT-3033 binds more efficiently to the α2I domain. We did not obtain similar results with a recombinant α2I domain containing the gain-of-function E318W mutation (Fig. 3B; supplemental Table 3), which is thought to promote adoption of an open/active α2I domain conformation (17, 30). The distinct binding sites for BTT-3033 and BTT-3034 were confirmed in competition assays using CBL027, a compound that fluoresces in a Tyr-285-dependent manner (26). BTT-3033 almost completely eliminated CBL027 fluorescence, indicating that BTT-3033 and CBL027 have the same binding site; in contrast, BTT-3034 only partially competed with CBL027 (supplemental Fig. 2). Taken together, our results suggest that BTT-3033 and BTT-3034 have distinct binding preferences and that whereas BTT-3033 favors a site close to the α2 MIDAS motif, BTT-3034 acts at least partially through another as yet uncharacterized allosteric site.
The Sulfonamide Derivatives Recognize Different Conformations of α2β1 Integrin
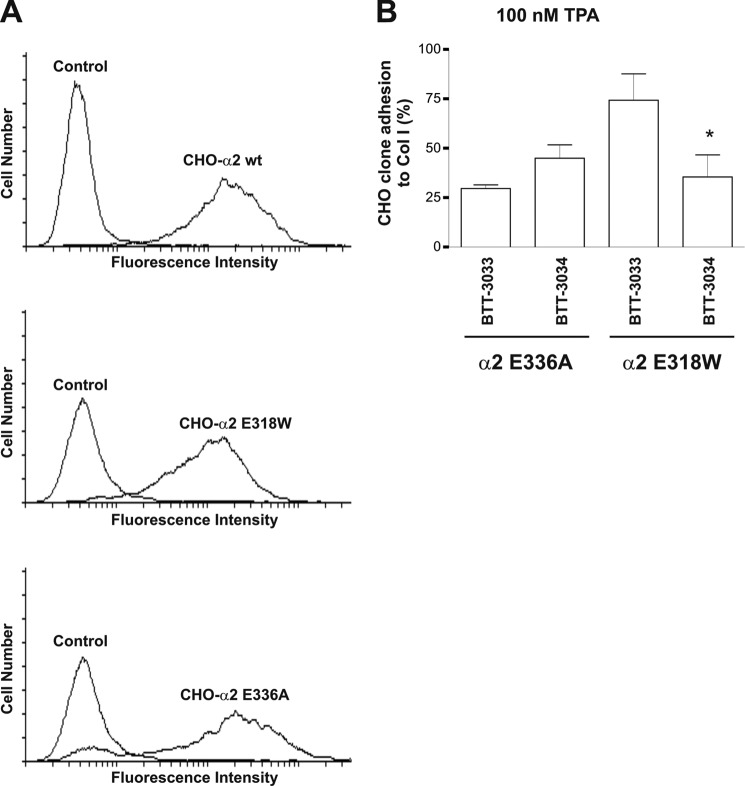
To gain further insight into the specificity of BTT-3033 and BTT-3034, we compared their effects on CHO cell clones transfected with different α2 variants (Fig. 4A). The E318W mutation activates α2β1 integrin at the α2I-domain level (17, 30), whereas the E336A loss-of-function mutation prevents cross-talk between the α2I domain and the β1 subunit. Equivalent loss-of-function mutations, namely E310A in αL and E320A in αM (12–14), are thought to force the integrin into a bent conformation. Before assaying the effects of sulfonamides, we tested the ability of CHO cells expressing variant integrins to attach and spread on collagen I. For this purpose, CHO-α2wt, CHO-α2E336A, and CHO-α2E318W cell clones were plated on collagen I and continuously monitored for 3 h using xCelligence technology (supplemental Fig. 3A), which measures changes in impedance at the bottom of a cell culture well caused by cell attachment and spreading and allows detailed temporal comparisons between cell clones. In general, CHO-α2E318W and CHO-α2wt cells showed similar attachment properties when plated on collagen I (supplemental Fig. 3A), whereas CHO-α2E336A cells attached less effectively to collagen I (supplemental Fig. 3A). Interestingly, the early adhesion and spreading of CHO-α2E318W cells to collagen I was consistently slightly less than that of CHO-α2wt cells (supplemental Fig. 3B) but was always greater at the end of the 3-h period. These results suggest that, under static conditions, preactivation of integrins plays a minor role in the early attachment phase but makes the final adhesion stronger.
FIGURE 4.
BTT-3033 and BTT-3034 recognize different α2β1 integrin conformations. A, integrin α2 expression levels on the CHO cell surface were determined by flow cytometry using anti-human CD49b and FITC-labeled secondary antibodies. B, the inhibitors (at EC50 concentrations) were tested by assaying CHO-α2E336A and CHO-α2E318W cell adhesion to collagen I in the presence of 100 nm TPA under static conditions. Adherent cells were detected using the WST-1 reagent. The data expressed are the mean ± S.E. of three to four independent experiments. BTT-3034 was a potent inhibitor of TPA-activated CHO-α2E318W cells, whereas BTT-3033 was not. Significant inhibition by BTT-3034 is indicated by an asterisk (Wilcoxon Rank-Sum test, one-tailed, *, p = 0.034; paired t test, p = 0.002).
The ability of the two sulfonamide derivatives to inhibit collagen binding by CHO-α2E318W and CHO-α2E336A cells was tested under static conditions and after TPA (100 nm) treatment (Fig. 4B). TPA is a phorbol ester that is known to induce ligand-independent clustering of α2β1 integrin (16). It also induces a transient shift in the wt α2I domain from a closed to an open state (16). BTT-3034 was a potent inhibitor of CHO-α2E318W cells, whereas BTT-3033 was not (Fig. 4B). In these conditions, BTT-3033 inhibited 74% and BTT-3034 53% of binding by CHO-α2E336A cells (difference not statistically significant).
Under Shear Stress the Sulfonamide Derivatives Have Distinct Effects on the E336A-induced Conformation of α2β1 Integrin
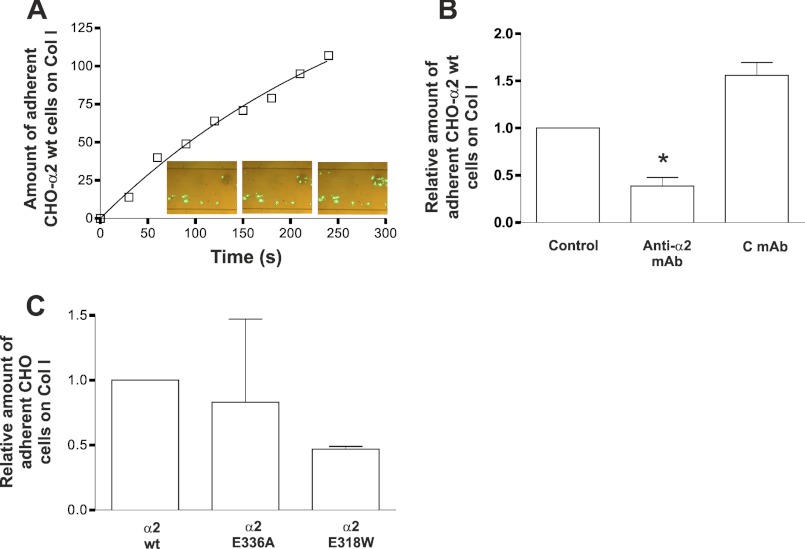
To directly study the function of different integrin conformations under flow conditions, we measured the attachment of transfected CHO cell clones to collagen I-coated capillary walls under flow using a Cellix microfluidic platform (Fig. 5A). A comparison of low-flow (0.01 dynes/cm2) and high-flow (90 dynes/cm2) conditions showed that CHO-α2wt cells adhered more effectively to collagen I-coated capillary wall at low than at high shear rates (not shown). The adhesion of CHO-α2wt cells to collagen I-coated capillaries was inhibited by an α2 integrin neutralizing antibody, indicating that, under the test conditions used, attachment is mediated almost entirely by α2 (Fig. 5B). Importantly, when cells were tested under fast flow conditions, CHO α2E336A cells could bind to collagen, whereas E318W mutation in α2 integrin did not improve the binding (Fig. 5C). Similar with the results obtained with E318W integrins, TPA did not activate the binding of CHO-α2wt cells when the shear rate was 90 dynes/cm2 (data not shown). Using the lower shear rate, some increase in cell adhesion was detected (data not shown).
FIGURE 5.
Under flow, preactivation of α2β1 integrin does not promote CHO cell adhesion to collagen I. Cell adhesion on capillaries coated with collagen I was studied using a Cellix microfluidic platform. A, the linearity of time-dependent accumulation of CHO-α2wt cells to collagen I-coated capillary walls at a flow rate of 90 dynes/cm2 was demonstrated. B, an integrin α2 neutralizing antibody (anti-α2 mAb, 10 μg/ml) inhibited the adhesion of CHO-α2wt cells to collagen I-coated capillaries at flow rate of 90 dynes/cm2. Control antibody (C mAb, 10 μg/ml) had no effect on the adhesion of CHO-α2wt cells to collagen I-coated capillaries at a high shear rate. The data expressed are the mean ± S.E. of seven independent experiments. Significant difference between control antibody and anti-α2 mAb is indicated by an asterisk (Wilcoxon Rank-Sum test; *, p = 0.018). C, binding of CHO-α2wt, CHO-α2E318W, or CHO-α2E336A cells to collagen I at shear rate of 90 dynes/cm2. The data expressed are the mean ± S.E. of three independent experiments.
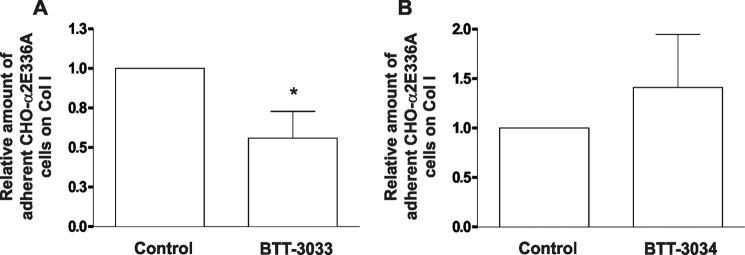
Thus, these results suggest that in under flow conditions, the “closed,” loss-of-function α2β1 variant can bind to collagen. Preactivation of the integrins, either by E318W mutation (Fig. 5C) or TPA-induced inside-out signaling (data not shown) may even decrease adhesion. Under shear stress conditions (90 dynes/cm2), BTT-3033 inhibited (maximally 70%) the binding of CHO-α2wt cells to collagen I, an effect not observed with BTT-3034 (data not shown). Thus, CHO-α2wt cells behaved in a similar manner as platelets. Importantly, BTT-3033 inhibited adhesion of CHO-α2E336A cells to collagen I (p = 0.028; Fig. 6A), whereas BTT-3034 had no inhibitory effect (Fig. 6B). Thus, BTT-3033 and BTT-3034 recognize different α2β1 integrin conformations, especially under high shear stress conditions. On the basis of these data, it is possible to propose a model for α2β1 action under flow conditions in which the initial interaction with collagen is mediated by a non-activated integrin conformation.
FIGURE 6.
BTT-3033, but not BTT-3034, blocks CHO-α2E336A cell binding to collagen under flow. The selectivity of BTT-3033 for the nonactivated α2β1 integrin was confirmed in assays testing CHO-α2E336A cell binding under flow conditions (90 dynes/cm2) using a Cellix microfluidic platform. A, BTT-3033 (at EC50 concentration) inhibited CHO-α2E336A cell adhesion to collagen I. The data expressed are the mean ± S.E. of 13 independent experiments. Significant difference between Control and BTT-3033 is indicated by an asterisk (Wilcoxon Rank-Sum test, *, p = 0.028). B, BTT-3034 (at EC50 concentration) had no inhibitory effect on CHO-α2E336A cell adhesion to collagen I at a high shear rate. The data expressed are the mean ± S.E. of nine independent experiments.
DISCUSSION
Integrin α2β1 is an αI domain-containing collagen receptor that is abundantly expressed in human tissues. It has a potential role in inflammation, cancer, and thrombosis and, as such, is a target of many active drug development projects. The structure-function relationship of leukocyte αI domain integrins, especially αLβ2 and αMβ2, has been extensively studied (4, 5). However, much less is known about the conformational regulation of the β1-integrins, including the αI domain-containing collagen receptors.
In this study, we describe two new sulfonamides that can be used to probe the structural regulation of α2β1 function. BTT-3033 and BTT-3034 have almost identical effects on CHO-α2wt cell adhesion to collagen I, but only BTT-3033 blocks platelet attachment under flow. Our experiments suggest that the function of BTT-3033 is dependent on α2 Tyr-285, whereas that of BTT-3034 is not, despite the fact that BTT-3034 also binds to the α2I domain. Thus, BTT-3033 may bind close to MIDAS, whereas BTT-3034 appears to bind to other allosteric sites. We have shown that BTT-3034 interacts with the α2I domain, but we cannot exclude the possibility that BTT-3034 also binds other sites (e.g. on the β1I domain-α2 subunit interface). The exact binding mechanism of BTT-3034 remains to be solved, but the existence of a potential allosteric regulatory site in the α2I domain has been described previously (23). Importantly, the binding sites for BTT-3033 and BTT-3034 appear to be differentially available in distinct integrin conformations. This was shown using CHO cells (which normally have no collagen receptors) transfected with cDNAs encoding variant α2 integrins (17). In the α2 subunit, amino acid residue Glu-336 corresponds to Glu-310 in αL and Glu-320 in αM (12–14). These glutamate residues may act as intrinsic ligands that mediate conformational regulation between α and β I-domains. Mutation of αL Glu-310 changes the balance of integrin conformations on the cell surface toward the bent stage (31). In general, it is not known whether β1-integrins can adopt a bent conformation, and there is no direct evidence that the E336A substitution in α2β1 leads to a shift from an extended to a bent structure. However, the obvious inactivation of α2β1, which we have noted in the E336A mutant (16), is difficult to explain in any other way. Mutation of this residue may also prevent preactivation of the α2I domain by inside-out signals (16); however, in collagen receptors, closed αI domains also bind to their ligands with relatively high avidity (30, 32–35). Thus, collagen receptors should not be critically dependent on preactivation at the αI-domain level.
Another mutation in the α2I domain, namely E318W, breaks an intradomain salt bridge (Arg-288/Glu-318) that regulates the shift between closed (non-activated) and open (activated) αI domain conformation (30, 35). When the two sulfonamides were tested with variant integrins, it was suggested that BTT-3034 is a more effective inhibitor of the gain-of-function α2E318W mutant. This difference was seen in assays with transfected cells, but not with recombinant α2I domains. Conversely, under flow the inhibition of E336A variant by BTT-3033 was statistically significant, whereas BTT-3034 had no effect.
These data indicate that sulfonamide derivatives can be used to study the biological roles of preactivated and non-activated integrins, especially under shear stress conditions. Surprisingly, only the inhibitor that was selective for non-activated conformation could block platelet-collagen interactions. This contradicts reports proposing that platelet α2β1 must be preactivated by inside-out signals before binding to collagen (36, 37). We evaluated the significance of α2β1 preactivation further, testing CHO cells in a Cellix microfluidic platform at high (90 dynes/cm2) flow rates. With fast flow, CHO-α2E336A cells could clearly bind to collagen I. Surprisingly, α2E318W mutation did not improve cell attachment. Thus, we conclude that preactivation of α2 provides no advantage in collagen binding under flow. The results from the Cellix experiments were in accordance with measurements performed using xCelligence technology. In these latter experiments, integrin activation seemed to be more important for strengthening the final adhesion sites than for the initial attachment.
Our results suggest a model in which, under flow, non-activated integrins are capable of low-affinity interactions with collagen. This may generate signals that further activate integrins and lead to firm adhesion. Importantly, the initial interaction between non-activated integrins and collagen seems to be essential for the entire process of integrin-mediated attachment. Low-avidity interactions, leading to platelet rolling but not firm adhesion, may play an important role in platelet-vessel wall interaction (38). The molecular basis for platelet rolling is not completely understood, but the interaction between GPIbα and immobilized von Willebrand factor can at least partially explain this phenomenon (38). Leukocytes exhibit an analogous rolling on an endothelial cell layer before attachment and extravasation (38). This process was originally shown to be dependent on the action of P- or E-selectin, whereas activated αLβ2 integrin (LFA-1) was proposed to mediate firm adhesion only (39). More recently, several reports have indicated that leukocyte integrins may also participate in this process and stabilize selectin-mediated rolling (40). In αLβ2 integrin, the transition from rolling to firm adhesion is associated with a change in the αI domain from a closed to an open or intermediate conformation (41, 42). However, the overall mechanism of αLβ2 action seems to be different compared with that of α2β1 integrin: The E310A mutation in αL results in an inactive receptor that can support neither rolling nor firm adhesion (31), whereas our experiments showed that the corresponding mutation (E366A) in α2 only weakens collagen binding but does not completely prevent it. In conclusion, in a continuation of our previous research approach (20), we have developed two novel α2β1 inhibitors that show selectivity for non-activated and activated α2β1 under flow. These inhibitors were used to test the role of different α2β1 conformations during cell and platelet adhesion to collagen I. The results support the idea that, under flow, no preactivation of α2β1 integrin is needed for collagen recognition.
Acknowledgments
We are grateful to Dr. Pekka Rappu for help in statistical analysis. The expert technical assistance of Karin Laurén, Anu Lillbacka, and Marjut Bäcklund is acknowledged.
This work was supported by grants from the Academy of Finland, the Sigrid Juselius Foundation, and the Finnish Cancer Association. L. N., J. N., M. P., and A. M. are/have been employees of BioTie Therapies Corp., a biotechnology company that has developed sulfonamide compounds.

This article contains supplemental Tables 1–3 and Figs. 1–3.
- MIDAS
- metal ion-dependent adhesion site
- EC50
- concentration required for half-maximal effect
- Emax
- maximum inhibitory effect
- TPA
- tetradecanoylphorbol acetate
- GPVI
- platelet collagen receptor glycoprotein VI.
REFERENCES
- 1. Nieuwenhuis H. K., Akkerman J. W., Houdijk W. P., Sixma J. J. (1985) Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature 318, 470–472 [DOI] [PubMed] [Google Scholar]
- 2. Carlsson L. E., Santoso S., Spitzer C., Kessler C., Greinacher A. (1999) The α2 gene coding sequence T807/A873 of the platelet collagen receptor integrin α2β1 might be a genetic risk factor for the development of stroke in younger patients. Blood 93, 3583–3586 [PubMed] [Google Scholar]
- 3. Lee J. O., Rieu P., Arnaout M. A., Liddington R. (1995) Crystal structure of the A domain from the α subunit of integrin CR3 (CD11b/CD18). Cell 80, 631–638 [DOI] [PubMed] [Google Scholar]
- 4. Arnaout M. A., Mahalingam B., Xiong J. P. (2005) Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 21, 381–410 [DOI] [PubMed] [Google Scholar]
- 5. Luo B. H., Carman C. V., Springer T. A. (2007) Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiong J. P., Stehle T., Diefenbach B., Zhang R., Dunker R., Scott D. L., Joachimiak A., Goodman S. L., Arnaout M. A. (2001) Crystal structure of the extracellular segment of integrin α Vβ3. Science 294, 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiong J. P., Mahalingham B., Alonso J. L., Borrelli L. A., Rui X., Anand S., Hyman B. T., Rysiok T., Müller-Pompalla D., Goodman S. L., Arnaout M. A. (2009) Crystal structure of the complete integrin αVβ3 ectodomain plus an α/β transmembrane fragment. J. Cell Biol. 186, 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu J., Luo B. H., Xiao T., Zhang C., Nishida N., Springer T. A. (2008) Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell 32, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishida N., Xie C., Shimaoka M., Cheng Y., Walz T., Springer T. A. (2006) Activation of leukocyte β2 integrins by conversion from bent to extended conformations. Immunity 25, 583–594 [DOI] [PubMed] [Google Scholar]
- 10. Takagi J., Petre B. M., Walz T., Springer T. A. (2002) Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–511 [DOI] [PubMed] [Google Scholar]
- 11. Moser M., Legate K. R., Zent R., Fässler R. (2009) The tail of integrins, talin, and kindlins. Science 324, 895–899 [DOI] [PubMed] [Google Scholar]
- 12. Alonso J. L., Essafi M., Xiong J. P., Stehle T., Arnaout M. A. (2002) Does the integrin αA domain act as a ligand for its βA domain? Curr. Biol. 12, R340–342 [DOI] [PubMed] [Google Scholar]
- 13. Shimaoka M., Xiao T., Liu J. H., Yang Y., Dong Y., Jun C. D., McCormack A., Zhang R., Joachimiak A., Takagi J., Wang J. H., Springer T. A. (2003) Structures of the α L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang W., Shimaoka M., Salas A., Takagi J., Springer T. A. (2004) Intersubunit signal transmission in integrins by a receptor-like interaction with a pull spring. Proc. Natl. Acad. Sci. U.S.A. 101, 2906–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie C., Zhu J., Chen X., Mi L., Nishida N., Springer T. A. (2010) Structure of an integrin with an αI domain, complement receptor type 4. EMBO J. 29, 666–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connors W. L., Jokinen J., White D. J., Puranen J. S., Kankaanpää P., Upla P., Tulla M., Johnson M. S., Heino J. (2007) Two synergistic activation mechanisms of α2β1 integrin-mediated collagen binding. J. Biol. Chem. 282, 14675–14683 [DOI] [PubMed] [Google Scholar]
- 17. Jokinen J., White D. J., Salmela M., Huhtala M., Käpylä J., Sipilä K., Puranen J. S., Nissinen L., Kankaanpää P., Marjomäki V., Hyypiä T., Johnson M. S., Heino J. (2010) Molecular mechanism of α2β1 integrin interaction with human echovirus 1. EMBO J. 29, 196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ivaska J., Käpylä J., Pentikäinen O., Hoffrén A. M., Hermonen J., Huttunen P., Johnson M. S., Heino J. (1999) A peptide inhibiting the collagen binding function of integrin α2I domain. J. Biol. Chem. 274, 3513–3521 [DOI] [PubMed] [Google Scholar]
- 19. Pentikäinen O., Hoffrén A. M., Ivaska J., Käpylä J., Nyrönen T., Heino J., Johnson M. S. (1999) “RKKH” peptides from the snake venom metalloproteinase of Bothrops jararaca bind near the metal ion-dependent adhesion site of the human integrin α(2) I-domain. J. Biol. Chem. 274, 31493–31505 [DOI] [PubMed] [Google Scholar]
- 20. Nissinen L., Pentikäinen O. T., Jouppila A., Käpylä J., Ojala M., Nieminen J., Lipsanen A., Lappalainen H., Eckes B., Johnson M. S., Lassila R., Marjamäki A., Heino J. (2010) A small-molecule inhibitor of integrin α2 β1 introduces a new strategy for antithrombotic therapy. Thromb Haemost 103, 387–397 [DOI] [PubMed] [Google Scholar]
- 21. Koivunen J. T., Nissinen L., Juhakoski A., Pihlavisto M., Marjamäki A., Huuskonen J., Pentikäinen O. T. (2011) Blockage of collagen binding to integrin α2β1: structure-activity relationship of protein-protein interaction inhibitors. Med. Chem. Commun. 2, 764–770 [Google Scholar]
- 22. Choi S., Vilaire G., Marcinkiewicz C., Winkler J. D., Bennett J. S., DeGrado W. F. (2007) Small molecule inhibitors of integrin α2β1. J. Med. Chem. 50, 5457–5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller M. W., Basra S., Kulp D. W., Billings P. C., Choi S., Beavers M. P., McCarty O. J., Zou Z., Kahn M. L., Bennett J. S., DeGrado W. F. (2009) Small-molecule inhibitors of integrin α2β1 that prevent pathological thrombus formation via an allosteric mechanism. Proc. Natl. Acad. Sci. U.S.A. 106, 719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Käpylä J., Pentikäinen O. T., Nyrönen T., Nissinen L., Lassander S., Jokinen J., Lahti M., Marjamäki A., Johnson M. S., Heino J. (2007) Small molecule designed to target metal binding site in the α2I domain inhibits integrin function. J. Med. Chem. 50, 2742–2746 [DOI] [PubMed] [Google Scholar]
- 25. Nykvist P., Tu H., Ivaska J., Käpylä J., Pihlajaniemi T., Heino J. (2000) Distinct recognition of collagen subtypes by α(1)β(1) and α(2)β(1) integrins. α(1)β(1) mediates cell adhesion to type XIII collagen. J. Biol. Chem. 275, 8255–8261 [DOI] [PubMed] [Google Scholar]
- 26. Koivunen J. T., Nissinen L., Käpylä J., Jokinen J., Pihlavisto M., Marjamäki A., Heino J., Huuskonen J., Pentikäinen O. T. (2011) Fluorescent small molecule probe to modulate and explore α2β1 integrin function. J. Am. Chem. Soc. 133, 14558–14561 [DOI] [PubMed] [Google Scholar]
- 27. Auger J. M., Kuijpers M. J., Senis Y. A., Watson S. P., Heemskerk J. W. (2005) Adhesion of human and mouse platelets to collagen under shear: a unifying model. FASEB J. 19, 825–827 [DOI] [PubMed] [Google Scholar]
- 28. Polgár J., Clemetson J. M., Kehrel B. E., Wiedemann M., Magnenat E. M., Wells T. N., Clemetson K. J. (1997) Platelet activation and signal transduction by convulxin, a C-type lectin from Crotalus durissus terrificus (tropical rattlesnake) venom via the p62/GPVI collagen receptor. J. Biol. Chem. 272, 13576–13583 [DOI] [PubMed] [Google Scholar]
- 29. Pantoliano M. W., Petrella E. C., Kwasnoski J. D., Lobanov V. S., Myslik J., Graf E., Carver T., Asel E., Springer B. A., Lane P., Salemme F. R. (2001) High-density miniaturized thermal shift assays as a general strategy for drug discovery. J. Biomol. Screen 6, 429–440 [DOI] [PubMed] [Google Scholar]
- 30. Aquilina A., Korda M., Bergelson J. M., Humphries M. J., Farndale R. W., Tuckwell D. (2002) A novel gain-of-function mutation of the integrin α2 VWFA domain. Eur. J. Biochem. 269, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 31. Salas A., Shimaoka M., Kogan A. N., Harwood C., von Andrian U. H., Springer T. A. (2004) Rolling adhesion through an extended conformation of integrin αLβ2 and relation to αI and βI-like domain interaction. Immunity 20, 393–406 [DOI] [PubMed] [Google Scholar]
- 32. Kamata T., Takada Y. (1994) Direct binding of collagen to the I domain of integrin α2 β1 (VLA-2, CD49b/CD29) in a divalent cation-independent manner. J. Biol. Chem. 269, 26006–26010 [PubMed] [Google Scholar]
- 33. Käpylä J., Ivaska J., Riikonen R., Nykvist P., Pentikäinen O., Johnson M., Heino J. (2000) Integrin α(2)I domain recognizes type I and type IV collagens by different mechanisms. J. Biol. Chem. 275, 3348–3354 [DOI] [PubMed] [Google Scholar]
- 34. Tulla M., Pentikäinen O. T., Viitasalo T., Käpylä J., Impola U., Nykvist P., Nissinen L., Johnson M. S., Heino J. (2001) Selective binding of collagen subtypes by integrin α1I, α2I, and α10I domains. J. Biol. Chem. 276, 48206–48212 [DOI] [PubMed] [Google Scholar]
- 35. Tulla M., Lahti M., Puranen J. S., Brandt A. M., Käpylä J., Domogatskaya A., Salminen T. A., Tryggvason K., Johnson M. S., Heino J. (2008) Effects of conformational activation of integrin α1I and α2I domains on selective recognition of laminin and collagen subtypes. Exp. Cell Res. 314, 1734–1743 [DOI] [PubMed] [Google Scholar]
- 36. Jung S. M., Moroi M. (2000) Signal-transducing mechanisms involved in activation of the platelet collagen receptor integrin α(2)β(1). J. Biol. Chem. 275, 8016–8026 [DOI] [PubMed] [Google Scholar]
- 37. Jung S. M., Moroi M. (2001) Platelet collagen receptor integrin α2β1 activation involves differential participation of ADP-receptor subtypes P2Y1 and P2Y12 but not intracellular calcium change. Eur. J. Biochem. 268, 3513–3522 [DOI] [PubMed] [Google Scholar]
- 38. McEver R. P., Zhu C. (2010) Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 26, 363–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawrence M. B., Springer T. A. (1991) Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65, 859–873 [DOI] [PubMed] [Google Scholar]
- 40. Dunne J. L., Ballantyne C. M., Beaudet A. L., Ley K. (2002) Control of leukocyte rolling velocity in TNF-α-induced inflammation by LFA-1 and Mac-1. Blood 99, 336–341 [DOI] [PubMed] [Google Scholar]
- 41. Salas A., Shimaoka M., Chen S., Carman C. V., Springer T. (2002) Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin lymphocyte function-associated antigen-1. J. Biol. Chem. 277, 50255–50262 [DOI] [PubMed] [Google Scholar]
- 42. Salas A., Shimaoka M., Phan U., Kim M., Springer T. A. (2006) Transition from rolling to firm adhesion can be mimicked by extension of integrin αLβ2 in an intermediate affinity state. J. Biol. Chem. 281, 10876–10882 [DOI] [PMC free article] [PubMed] [Google Scholar]